For adult patients with intermediate-risk acute myelogenous leukemia (AML) in first complete remission (CR1), allogeneic stem cell transplantation (allo-SCT) has been demonstrated to be the best therapeutic option. The ideal donor is a HLA-genoidentical sibling. In the absence of a genoidentical donor, a HLA 10/10 or 8/8 matched unrelated donor (URD) is presently considered to be the best choice, taking advantage of the 25 million volunteer donors registered worldwide.1 Although the probability of finding an unrelated donor has recently reached almost 80% for white Europeans, it still remains at around only 41% for Chinese patients.2 Furthermore, while waiting for a volunteer donor, the risk of early relapse remains, highlighting the need for alternative sources of donors.
Initially, haploidentical donor transplantation was pioneered by the Perugia team,3 using selected megadose stem cells and highly myeloablative pre-transplant regimens. Recently, in the USA and then in Europe, a considerable improvement has occurred with the shift to T-cell replete grafts and the introduction of high-dose cyclophosphamide post-graft infusion for the prevention of graft versus host disease (GvHD).4 In China, the “Beijing group” has pioneered a different approach with T-cell replete HID, intensive myeloablation, a combination of G-CSF-primed bone marrow plus peripheral blood as the stem cell source, and anti-thymocyte globulins (ATGs).5 This team has reported a large series of haploidentical transplants for AML in CR1, with leukemia-free survival (LFS) as high as 70% and a very low incidence of both acute and chronic GvHD.
A recent study in the USA6 using the CIBMTR database, compared 198 haploidentical versus 1982 HLA 8/8 matched unrelated donor transplants in AML patients. In this study, patients were in various disease stages (CR1, CR2 and more advanced), and the preparative regimens were either non-myeloablative or myeloablative. Results for overall survival were similar, at around 50%. In another more recent study from EBMT (Piemontese S, et al. unpublished results, 2016), 273 patients receiving an HID transplant while in CR1 or CR2 were pair-matched with 273 patients receiving a MUD 10/10 and 273 patients receiving a MMUD 9/10 transplant. Patients showed better outcomes if transplanted from a MUD 10/10, but there was no significant difference in outcome between transplants from MMUD 9/10 and HID, suggesting that both can be safely used in the absence of a 10/10 MUD.
Regarding the Beijing approach,5 the LFS of around 70% reported in AML in CR1 has been questioned as being superior to the present outcome of patients transplanted with HID in many other centers throughout Europe.7 A possible explanation is that the Beijing patient population may have been highly selected for good prognostic factors, such as younger age and longer interval from diagnosis to transplant. The Acute Leukemia Working Party of the EBMT therefore decided to use the EBMT registry to compare HID carried out in Beijing and 10/10 matched unrelated donor transplants for patients with intermediate-risk AML in CR1 in a matched pair analysis.
The study inclusion criteria included adult (>=18 years old) patients, with de novo AML with intermediate-risk cytogenetics transplanted in CR1, exclusively following a chemotherapy-based myeloablative conditioning regimen (with no TBI), in the period from January 2008 to December 2012. The data from 160 patients with T-cell replete HID from Beijing were compared with 241 patients present in the EBMT-ALWP database who received a fully matched 10/10 URD transplant. Because the patient population was small, a 1:1 ratio matched pair analysis was implemented with the following matching factors: (1) age ± 5 years, (2) interval from CR1 to transplant less than or greater than 6 months, and (3) numbers of induction courses to reach CR1 of one or more than one. The primary endpoint was LFS, and the secondary endpoints were overall survival (OS), relapse incidence (RI), and non-relapse mortality (NRM).
We were able to match 87 HID with 87 URD patients. The two matched groups were comparable in donor and patient sex, year of transplant, number of induction courses to reach CR1, and time interval from diagnosis to transplant. Donors in the HID group were older (40.5 versus 34.1 years, P=0.02) since most of them were parents. There was less CMV patient (4.6% versus 46.4%, P<0.01) and donor (4.6% versus 58.6%, P<0.01) negative serology in the HID group (Table 1). All patients in the HID group received the combination of primed BM plus PB as graft, while about 50% of the URD patients received either BM alone or PB alone as graft.
Table 1.
Patient characteristics.
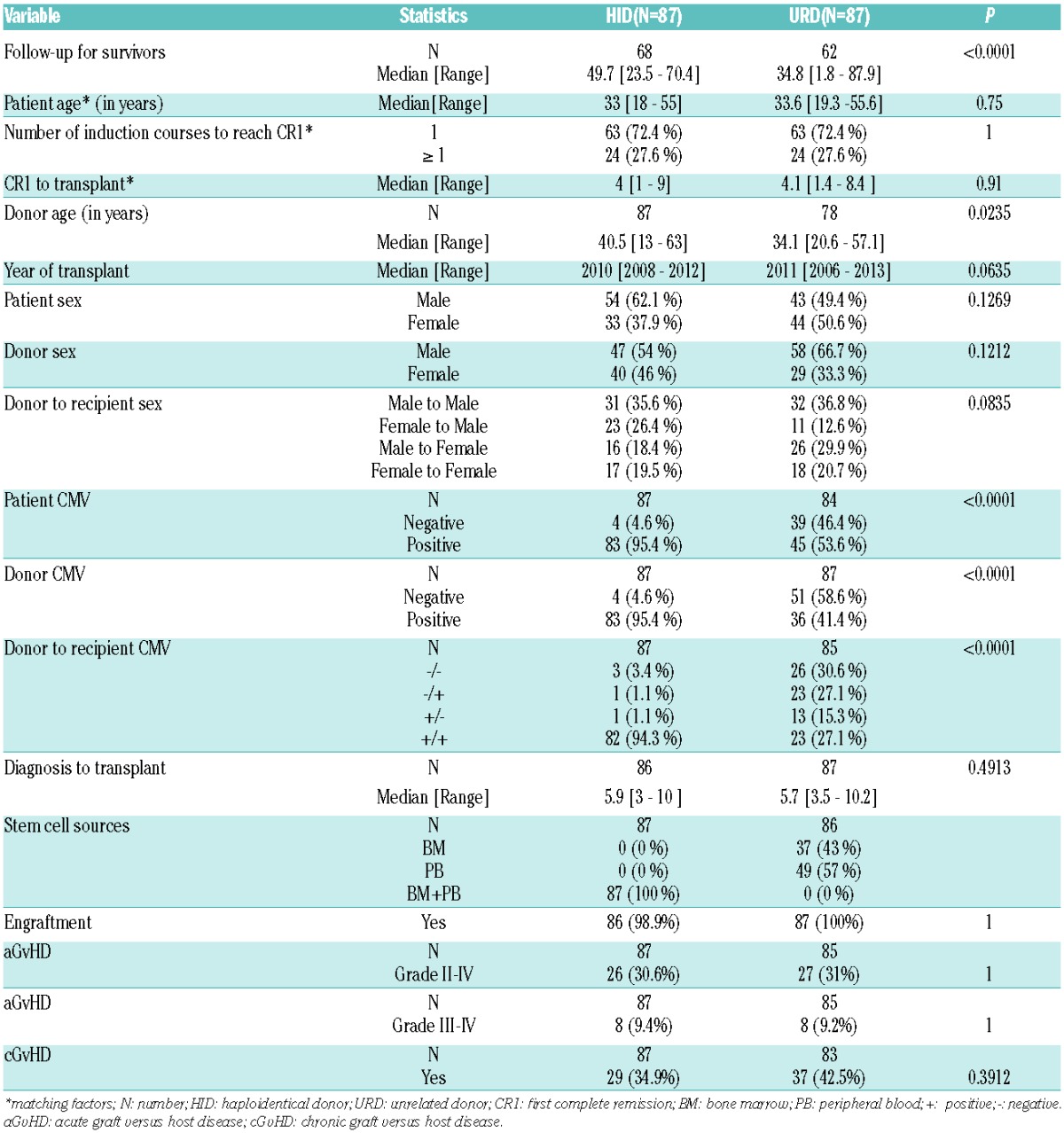
URD and HID patients had similar outcomes (Table 2): the 5 year LFS was 60.3% in the URD group versus 73.5% in the HID group (P=0.15), the OS was 63.6% versus 78.2% (P=0.15) (Figure 1), the RI was 24% versus 12.7% (P=0.08), and the NRM was 15.7% versus 13.8% (P=0.96), respectively (Figure 1). The prevalence of severe grade III–IV acute GvHD (9.4% versus 9.2%, P=1) and chronic GvHD (34.9% versus 42.5%, P=0.39) were also comparable in the two groups. All patients in the HID group engrafted, and only 1 patient in the URD group failed to engraft.
Table 2.
Comparison of outcomes at 5-years after transplant.
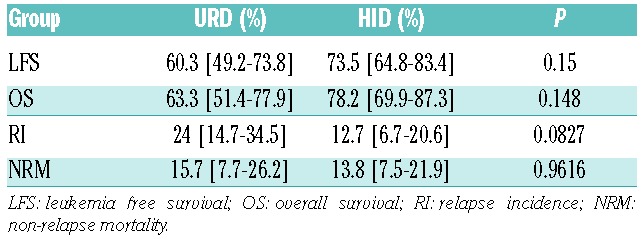
Figure 1.
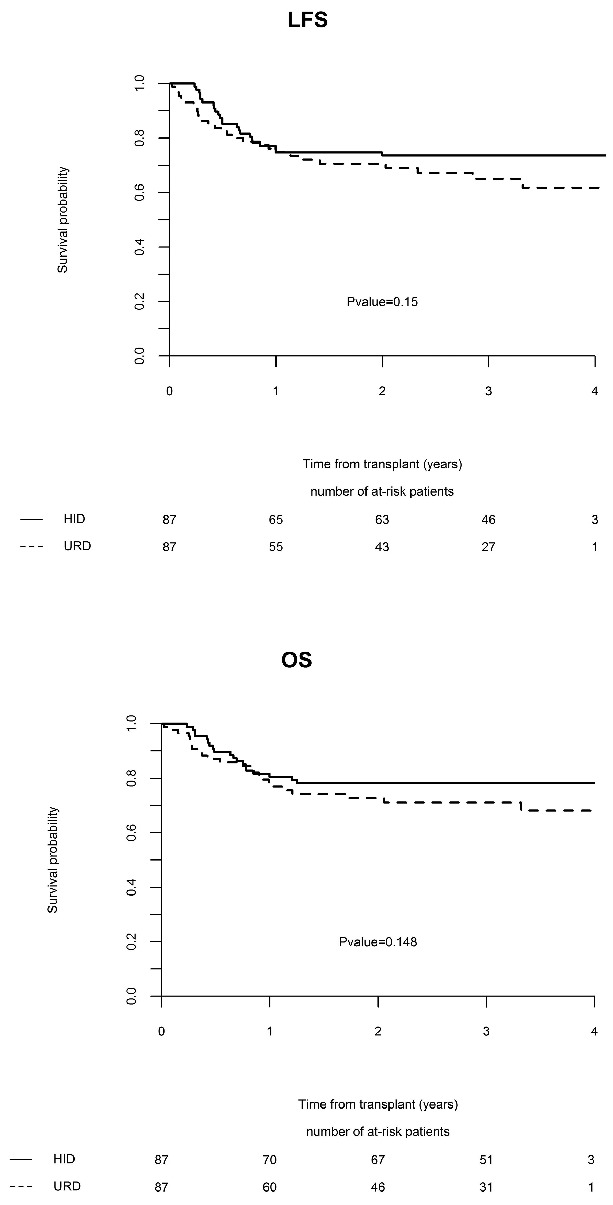
Comparisons of outcome between HID group and URD group (adjusted). OS: Overall survival; LFS: leukemia-free survival.
This retrospective study, specifically concerning intermediate-risk AML adult patients in CR1, suggests that HID transplantation can achieve results similar to those obtained with a fully matched 10/10 URD. Recent years have witnessed major improvements in haploidentical transplantation: first T-cell depletion protocols in the nineties, and now T-cell repletion. There are presently two major T-cell replete approaches for HID: the “Beijing model” using the combination of G-CSF primed bone marrow plus peripheral blood, and the “Baltimore method” with post-transplant cyclophosphamide. Both methods have achieved good results. Several previous studies have already compared HID with URD,8–11 and concluded that T-cell replete haploidentical transplantation resulted in outcomes similar to URD. However, these studies have included heterogeneous populations of patients with mixed hematological malignancies receiving various transplant protocols.
In the present study, we focused on a very uniform population of intermediate-risk AML patients in CR1, which is presently not considered by all teams as a primary indication for an alternative donor transplant, and in this population, we compared the outcome post HID in Beijing with the outcome of a pair-matched population of patients who received a 10/10 matched unrelated donor transplant following a myeloablative regimen. Not surprisingly, the number of patients was small and we could only do a one to one matched pair analysis. We found that the HID “Beijing model” achieved results similar to transplantation with a fully matched unrelated donor, regarding engraftment, GvHD and survival. Our results are in accordance with the recent CIBMTR registry-based study which showed that T-cell replete HID using post-transplant cyclophosphamide also achieved survival comparable with URD, and was also associated with less GvHD.6 Therefore, HID appears to be a good alternative choice for patients without a matched sibling donor.
HID transplantation is still improving over time. Several strategies have demonstrated additional efficacy, such as the risk-stratified GvHD prevention with low-dose glucocorticoids as well as the prediction and prevention strategy of relapse based on minimal residual disease monitoring. Several recent studies have shown that HID can even achieve comparable outcome with matched sibling donors,5,9,10 and a comparable quality of life.11
However, we have to admit that this preliminary study has several limitations: the first one is related to the fact that this study is a comparison between a single center and a registry. However, since this HID protocol is almost exclusively used in China (especially in Beijing) and the results reported are encouraging, we felt it was of interest to compare the results achieved in this single center with the URD transplants reported to the EBMT registry using a carefully matched pair analysis. Of note, a comparison with URD transplants done in China was not possible, URD transplants being infrequent in this country.
A second limitation is that we have no details on the induction regimens used in China to achieve CR, since patients were sent to Beijing as a referral center for transplantation after achievement of complete remission. However, following guidelines published in 2011 in China, Chinese centers have routinely used regimens similar to those used within EBMT, i.e. the “3 + 7”, mostly daunomycin (DNR) +ARA-C or idarubicin (IDA) + ARA-C, with doses of DNR of 45–90mg/m2/d ×3d, doses of IDA of 8–12 mg/m2/d×3d, and doses of ARA-C of 100–200mg/m2/d ×7d; further, we pair-matched for the number of induction courses to reach CR1 (one or more than one) and the interval from CR1 to transplant as a surrogate marker for the number of consolidation courses before transplant (less than or greater than 6 months).
A third limitation is related to the low statistical power which resulted from the small patient population involved.
A fourth limitation of this study is the lack of molecular information (such as FLT3-ITD and NPM1) for the Chinese patients, which also precluded matching for this important prognostic factor. Therefore, we cannot be sure that the two populations were comparable regarding molecular markers. Indeed, there have been two recent series of Chinese patients with AML evaluated for molecular biology markers. In a first cohort of 1185 patients analyzed in 201112 as well as in a second cohort of 269 Chinese patients analyzed in 2015,13 there was a lower frequency than in other European and USA series of several markers, such as DNMT3A, NRAS, NF1 and TP53. The frequency of FLT3/ITD mutations, however, varied from 10% in the first study to 23% in the second one, similar to the 25% reported from the UK MRC AML 10 and 12 trials.
Finally, it has also been recently shown that susceptibility to severe acute GvHD is lower in Asian patients than in Caucasian patients,14 which may account for the more successful outcomes witnessed in Beijing. Despite the limitations of our study, it as well as others suggest that HID may replace URD, at least in some countries such as China.
In fact, the good outcomes observed in Beijing reflect an entire transplant strategy which combines a unique conditioning regimen of 4 drugs (cytarabine, busulfan, cyclophosphamide and methyl-CCNU) without TBI, the combination of G-CSF-mobilized blood and marrow cells for grafting, and the use of 4 different immunosuppressive medications (rabbit ATG, cyclosporine, mycophenolate mofetil and methotrexate) for GvHD prevention. We cannot, of course, fully understand what is the individual contribution of each of these factors to the successful outcomes achieved.
In conclusion, this retrospective study suggests that HID transplantation may be an alternative to allogeneic transplantation using a fully matched 10/10 URD transplantation in patients with AML in CR1.
Acknowledgments
We would like to thank all the participating centers who have contributed patients to this study.
Footnotes
Funding: Dr. Yuqian Sun was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (20120001120059) and the Chinese Scholarship Council for this study.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kekre N, Antin JH. Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist. Blood. 2014;124(3):334–343. [DOI] [PubMed] [Google Scholar]
- 2.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aversa F, Martelli MF, Velardi A. Haploidentical hematopoietic stem cell transplantation with a megadose T-cell-depleted graft: harnessing natural and adaptive immunity. Semin Oncol. 2012;39(6):643–652. [DOI] [PubMed] [Google Scholar]
- 4.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–3962. [DOI] [PubMed] [Google Scholar]
- 6.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with post-transplant cyclophosphamide versus matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015; 126(8):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piemontese S, Ciceri F, Labopin M, et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(5):1069–1075. [DOI] [PubMed] [Google Scholar]
- 8.Xiao-Jun H, Lan-Ping X, Kai-Yan L, et al. Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res. 2009;15(14):4777–4783. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Xiao H, Lai X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124(17):2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013; 31(10):1310–1316. [DOI] [PubMed] [Google Scholar]
- 11.Mo XD, Xu LP, Liu DH, et al. Patients receiving HLA-haploidentical/partially matched related allo-HSCT can achieve desirable health-related QoL that is comparable to that of patients receiving HLA-identical sibling allo-HSCT. Bone Marrow Transplant. 2012; 47(9):1201–1205. [DOI] [PubMed] [Google Scholar]
- 12.Shen Y, Zhu YM, Fan X, et al. Gene mutation patterns and their prognostic impact in a cohort of 1185 patients with acute myeloid leukemia. Blood. 2011;118(20):5593–5603. [DOI] [PubMed] [Google Scholar]
- 13.Yin J, Xie X, Zhang F, et al. Low frequency of mutations in Chinese with acute myeloid leukemia: Different disease or different aetiology¿ Leukemia Res. 2015;39(6):646–648. [DOI] [PubMed] [Google Scholar]
- 14.Kanda J, Brazauskas R, Hu ZH, et al. GVHD after HLA-matched sibling BMT or PBSCT: Comparison of North American Caucasian and Japanese Populations. Biol Blood Marrow Transplant. 2016; 22(4):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]


