Abstract
Individual-level epidemiologic studies of pregnancy outcomes after maternal influenza are limited in number and quality and have produced inconsistent results. We used a time-series design to investigate whether fluctuation in influenza virus circulation was associated with short-term variation in population-level rates of preterm birth, stillbirth, and perinatal death in Ontario between 2003 and 2012. Using Poisson regression, we assessed the association between weekly levels of circulating influenza virus and counts of outcomes offset by the number of at-risk gestations during 3 gestational exposure windows. The rate of preterm birth was not associated with circulating influenza level in the week preceding birth (adjusted rate ratio = 1.01, 95% confidence interval: 1.00, 1.02) or in any other exposure window. These findings were robust to alternate specifications of the model and adjustment for potential confounding. Stillbirth and perinatal death rates were similarly not associated with gestational exposure to influenza circulation during late pregnancy. We could not assess mortality outcomes relative to early gestational exposure because of missing dates of conception for many stillbirths. In this time-series study, population-level influenza circulation was not associated with short-term variation in rates of preterm birth, stillbirth, or perinatal death.
Keywords: influenza, pregnancy, preterm birth, stillbirth, time-series study
Editor's Note:An invited commentary on this article appears on page 187.
Pregnant women have long been considered vulnerable to serious influenza disease and related complications. Numerous studies in which investigators documented excess influenza-related mortality in pregnant women during pandemics (1–3) and higher rates of hospitalization for influenza during seasonal epidemics (4–6) contributed to recommendations for universal influenza immunization of pregnant women in many countries (7). Although the primary goal of these recommendations is to protect pregnant women from influenza disease, the benefits have more recently been shown to extend to neonates (8).
Safety reviews (9, 10) have not identified evidence of any adverse association of influenza vaccine on pregnancy outcomes. On the contrary, potential benefits of influenza immunization against adverse pregnancy outcomes reported in some observational studies (11) have generated public health interest because they suggest additional value of maternal immunization programs beyond prevention of maternal and neonatal influenza (12). Theoretically, benefits to the fetus from averted influenza are plausible insofar as maternal influenza disease increases the risk of adverse outcomes; however, individual-level epidemiologic studies on this topic have yielded inconsistent results (13–18).
Studying the relationship between maternal influenza and perinatal outcomes is challenging. Prospective cohort studies with laboratory confirmation of infection are ideal but tend to be hindered by low numbers of events, particularly for outcomes such as fetal death. Although existing birth registries can facilitate large cohort studies, they do not routinely collect measures of influenza infection, and linkages to other health databases to identify medically attended influenza result in the underestimation of milder disease (19) because individuals with mild or subclinical infection may never seek medical care, laboratory testing is not always ordered during clinical consultations, and viral detection may not be possible if there is a lag between the primary infection and seeking care. Moreover, exposure measures based on seeking medical care also risk introducing diagnostic bias if women who have a higher baseline risk or exhibit signs of impending adverse pregnancy outcomes are differentially assessed and diagnosed with influenza (20).
An alternate approach that can avoid some of these challenges with individual-level measurement of influenza is a time-series study. Such designs can be useful for assessing the public health impact of an exposure when exposures and outcomes vary temporally and when individual-level exposure data are unavailable or difficult to obtain (21). Because influenza epidemics affect the population and viral circulation is routinely measured through surveillance, we used a time-series design to investigate whether fluctuation in influenza circulation was associated with short-term variation in rates of adverse perinatal outcomes.
METHODS
Study population and data sources
We included all livebirths and stillbirths at 20 weeks’ gestation or later in hospitals in Ontario from 2003 to 2012 recorded in the Discharge Abstract Database (22). We linked birth records with maternal records to obtain information on maternal age. For analyses of the birth cohort according to date of conception, we excluded births to mothers whose last menstrual period was more than 20 weeks before the start of the study period or less than 43 weeks before the end to avoid over- and under-representing longer gestations at the beginning and end of our cohort, respectively (Figure 1) (23). We used Statistics Canada's Postal Code Conversion File software to assign residential postal codes to census dissemination areas (24) and extracted data on neighborhood family income and educational level from the 2006 Canadian Census (additional details provided in Web Appendix 1 and Web Tables 1 and 2, available at http://aje.oxfordjournals.org/).
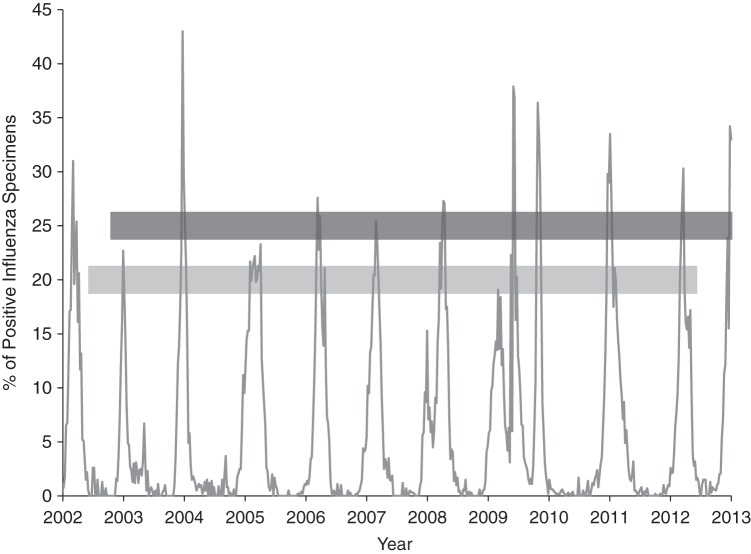
Figure 1.
Weekly percentage of positive influenza specimens in the study sample, Ontario, Canada, 2002–2013. The dark gray horizontal line represents the study cohort of births at 20 weeks of gestation or later from January 1, 2003, to December 31, 2012. The light gray horizontal line represents corresponding conceptions.
Measures
Provincial virologic surveillance data were obtained from FluWatch, Canada's national influenza surveillance system. Sixteen sentinel laboratories in Ontario submit information on influenza virus circulation to the FluWatch system; however, only the aggregated provincial-level data are made publicly available (25). We defined exposure to circulating influenza as a continuous variable that represented the proportion of laboratory specimens in each surveillance week that tested positive for influenza A or B viruses, and we considered the beginning and end of each influenza season as the first and last occurrence of 2 consecutive weeks in which at least 5% of specimens that were submitted to provincial surveillance laboratories tested positive for influenza A or B (26). Although evidence suggests that pregnant women are at highest risk for influenza-related complications during the third trimester (4, 5), susceptible time windows for fetal exposure to influenza are unknown. Following the hypothesis that influenza could provoke an acute adverse outcome or predispose persons to later adverse outcomes (27, 28), we assessed 2 exposure windows to evaluate a potential acute association with circulating influenza (final week and final month of gestation) and one to assess a delayed association (first month of gestation) (28). Our unit of observation was influenza surveillance week; thus, each measure represented an average over the exposure window relative to each week (Web Table 3).
In order to assess gestational exposure to circulating influenza during the first month of gestation, we analyzed our data according to date of conception, which was estimated by subtracting gestational age from the date of birth and adding 14 days (28). Preterm birth was evaluated using 2 cutoffs (<37 weeks and <32 weeks) and was restricted to singleton livebirths. Stillbirth was defined as fetal death after 20 weeks’ gestation among all births, and perinatal mortality included stillbirths and neonatal deaths before 7 days of age. Because fetal deaths before 20 weeks’ gestation were not ascertained in the source database, we included a secondary outcome examining monthly birth rates per 10,000 females 15–49 years of age relative to early pregnancy exposure to the peak of the 2009 H1N1 pandemic (29) (Web Table 4).
Seasonal patterns in births, conceptions, pregnancy outcomes, and aggregated individual-level characteristics can introduce temporal confounding in time-series studies (30). On the basis of data availability, we assessed possible confounding by maternal age, neighborhood income and educational level, and gestational age. We also adjusted for respiratory syncytial virus, a common cause of respiratory infection with temporal circulation similar to that of influenza (31).
Statistical analyses
We used time-series plots to assess temporal patterns in births and their corresponding conceptions among singleton livebirths. Data were aggregated by calendar month for this purpose. To better visualize nonlinear patterns, we fitted nonparametric, locally weighted regression smoothers (27, 32). Because there was a higher proportion of missing information in earlier years of the study, analyses of sociodemographic characteristics were restricted to 2006–2012 (Web Table 5). For each calendar month and year, we examined the proportion of births in the lowest (<20 years) and highest (≥35 years) categories of maternal age, neighborhood income (lowest and highest quintile), and education (lowest and highest quintile).
Daily numerator counts for each outcome were aggregated to influenza surveillance week by date of birth for late-pregnancy exposure windows and by date of conception for the early-exposure window. For preterm birth before 37 weeks’ gestation relative to late-pregnancy exposure, the gestations-at-risk denominators were aggregated by date of birth. Fetuses entered the risk set at 20 weeks (the earliest gestation at which they could be ascertained) and exited on the date of preterm birth or on reaching 37 completed weeks. For the early-pregnancy exposure, we used the weekly number of conceptions that resulted in in singleton livebirth as the denominator. For preterm birth before 32 weeks’ gestation, the at-risk gestations were censored upon completing 32 weeks. The denominator for stillbirth and perinatal mortality in late-pregnancy exposure windows included all ongoing gestations that were at 20 weeks or later (resulting in livebirth or stillbirth) aggregated by date of birth. Records of stillbirths with missing gestational age were included in the numerators but not denominators because we could not determine when the fetus entered the risk set. We did not evaluate mortality outcomes in relation to circulating influenza during the early-pregnancy exposure window because it required aggregation by date of conception, which was missing for 30% of stillbirth records.
We modeled study outcomes as weekly rates, using Poisson generalized linear models with an offset for the gestations-at-risk denominators. Rate ratios and 95% confidence intervals were calculated to estimate the relative change in the expected rate of each outcome and expressed per 10–percentage point increase in the proportion of positive influenza specimens. We included cubic splines to account for background temporal trends and selected the best-fitting number of knots based on minimizing the Akaike information criterion (33, 34). Residual autocorrelation was assessed using autocorrelation and partial autocorrelation functions (33, 35), whereas model fit was assessed using the ratio of deviance to degrees of freedom and residual plots (36). In sensitivity analyses, we varied the number and location of knots in our cubic splines (34) and modeled lagged exposure variables (37). In our models for preterm birth, we assessed the potential for seasonal confounding by maternal age and gestational age by including terms representing the weekly proportion of women in the lowest and highest maternal age groups and the weekly proportion of ongoing gestations in the highest gestational age category of the risk set (e.g., proportion of fetuses that were 20–36 weeks of age in week 36). We also assessed the subset of preterm births that occurred after spontaneous onset of labor. To accommodate a possible nonlinear exposure-outcome relationship, we classified exposure as a binary variable indicating whether each week fell within an influenza season and as an ordinal categorical variable representing the proportion of positive specimens and fit a restricted cubic spline function to the continuous influenza measure.
For our secondary outcome, we first generated a series of 120 monthly (observed) birth rates and then computed a 3-year centered moving average after excluding the 2009 H1N1 pandemic time period. For each of the 120 time points, we calculated the difference between the observed and centered moving average birth rates and then computed the average difference by calendar month (i.e., January through December). We used a linear regression model to derive an expected birth rate in each of the 120 time points based on the centered moving average (representing long-term patterns in birth rates) and the average difference (representing seasonal patterns in birth rates). We examined the residuals generated from this model to assess whether there were any consecutive months with excess deviation after the 2009 H1N1 pandemic (29).
We conducted all analyses using SAS, version 4.3 (SAS Institute, Inc., Cary, North Carolina). The cubic spline basis functions were generated using the “frencurv” command in Stata SE, version 12.1 (StataCorp LP, College Station, Texas). The McGill University Faculty of Medicine Institutional Review Board and the Children's Hospital of Eastern Ontario Research Ethics Board approved this study.
RESULTS
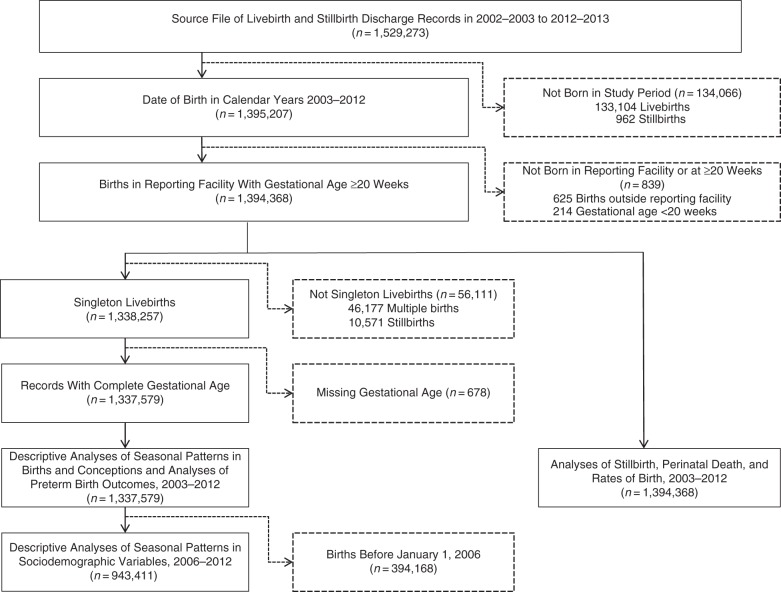
Figure 2 illustrates the steps used to obtain the final records for analysis. We identified 1,395,207 livebirths and stillbirths between 2003 and 2012 and then excluded 625 births that took place outside the reporting hospital (because pertinent information on the births was missing from these records) and 214 records for births at a gestational age less 20 weeks. Among the 1,394,368 remaining, 97.5% of livebirths and 83.0% of stillbirths were linked to a maternal record. Characteristics of the study population are provided in Table 1. The distribution of study variables was similar across each of the subgroups used for different aspects of the analyses.
Figure 2.
Flow chart of records and structure of study data, Ontario, Canada, 2003–2012. Individual categories may not sum to the total because some records were excluded for more than 1 reason.
Table 1.
Descriptive Characteristics of Study Population, Ontario, Canada, 2003–2012
| Characteristic | Total Births, 2003–2012 (n = 1,394,368) |
Singleton Livebirths |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyses by Date of Birth |
Analyses by Estimated Date of Conceptiona |
|||||||||
| 2003–2012b (n = 1,337,579) |
2006–2012c (n = 943,411) |
2003–2012b (n = 1,281,808) |
2006–2012c (n = 886,649) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Livebirths | 1,383,797 | 99.2 | 1,337,579 | 100 | 943,411 | 100 | 1,281,808 | 100 | 886,649 | 100 |
| Stillbirths | 10,571 | 0.8 | ||||||||
| Gestational length, weeks | 38.8 (2.1)d | 38.9 (1.9)e | 38.9 (1.9)e | 38.9 (1.9)e | 38.9 (1.9)e | |||||
| Completed gestational weeks | 35.0 (6.7)f | |||||||||
| Multiple births | 46,177 | 3.3g | ||||||||
| Preterm birth at <37 weeks | 86,597 | 6.5 | 61,218 | 6.5 | 83,092 | 6.5 | 57,456 | 6.5 | ||
| Preterm birth at <32 weeks | 12,257 | 0.9 | 8,381 | 0.9 | 12,257 | 1.0 | 8,381 | 0.9 | ||
| Calendar year of birth | ||||||||||
| 2003 | 135,048 | 9.7 | 129,877 | 9.7 | 84,247 | 6.6 | ||||
| 2004 | 137,056 | 9.8 | 131,834 | 9.9 | 131,834 | 10.3 | ||||
| 2005 | 137,870 | 9.9 | 132,457 | 9.9 | 132,457 | 10.3 | ||||
| 2006 | 139,337 | 10.0 | 133,940 | 10.0 | 133,940 | 14.2 | 133,940 | 10.4 | 87,319 | 9.8 |
| 2007 | 141,805 | 10.2 | 136,168 | 10.2 | 136,168 | 14.4 | 136,168 | 10.6 | 136,168 | 15.4 |
| 2008 | 142,040 | 10.2 | 136,433 | 10.2 | 136,433 | 14.5 | 136,433 | 10.6 | 136,433 | 15.4 |
| 2009 | 141,694 | 10.2 | 135,651 | 10.1 | 135,651 | 14.4 | 135,651 | 10.6 | 135,651 | 15.3 |
| 2010 | 139,720 | 10.0 | 133,700 | 10.0 | 133,700 | 14.2 | 133,700 | 10.4 | 133,700 | 15.1 |
| 2011 | 139,529 | 10.0 | 133,350 | 10.0 | 133,350 | 14.1 | 133,350 | 10.4 | 133,350 | 15.0 |
| 2012 | 140,269 | 10.1 | 134,169 | 10.0 | 134,169 | 14.2 | 124,028 | 9.7 | 124,028 | 14.0 |
| Calendar year of estimated conceptionh | ||||||||||
| 2002 | 94,817 | 6.8 | 92,164 | 6.9 | 46,534 | 3.6 | ||||
| 2003 | 135,619 | 9.8 | 131,296 | 9.8 | 131,296 | 10.3 | ||||
| 2004 | 137,727 | 9.9 | 133,278 | 10.0 | 133,278 | 10.4 | ||||
| 2005 | 137,132 | 9.9 | 132,422 | 9.9 | 94,992 | 10.1 | 132,422 | 10.3 | 48,371 | 5.5 |
| 2006 | 140,697 | 10.1 | 135,369 | 10.1 | 135,369 | 14.4 | 135,369 | 10.6 | 135,369 | 15.3 |
| 2007 | 142,214 | 10.2 | 136,614 | 10.2 | 136,614 | 14.5 | 136,614 | 10.7 | 136,614 | 15.4 |
| 2008 | 142,366 | 10.2 | 136,498 | 10.2 | 136,498 | 14.5 | 136,498 | 10.7 | 136,498 | 15.4 |
| 2009 | 139,275 | 10.0 | 133,231 | 10.0 | 133,231 | 14.1 | 133,231 | 10.4 | 133,231 | 15.0 |
| 2010 | 140,044 | 10.1 | 134,023 | 10.0 | 134,023 | 14.2 | 134,023 | 10.5 | 134,023 | 15.1 |
| 2011 | 139,259 | 10.0 | 133,531 | 10.0 | 133,531 | 14.1 | 133,531 | 10.4 | 133,531 | 15.1 |
| 2012 | 41,410 | 3.0 | 39,153 | 2.9 | 39,153 | 4.2 | 29,012 | 2.3 | 29,012 | 3.3 |
| Maternal age, yearsi | ||||||||||
| <20 | 48,762 | 3.6 | 47,622 | 3.7 | 33,374 | 3.6 | 45,635 | 3.6 | 31,337 | 3.5 |
| 20–34 | 1,024,162 | 75.4 | 986,294 | 75.7 | 707,450 | 75.3 | 950,115 | 75.7 | 664,913 | 75.3 |
| ≥35 | 285,144 | 21.0 | 269,421 | 20.7 | 198,326 | 21.1 | 259,724 | 20.7 | 186,751 | 21.1 |
| Missing | 36,300 | 34,242 | 4,261 | 26,334 | 3,648 | |||||
| Neighborhood income quintilei,j | ||||||||||
| 1 (lowest) | 329,907 | 24.4 | 317,993 | 24.5 | 223,430 | 24.3 | 304,949 | 24.5 | 209,932 | 24.2 |
| 2 | 264,217 | 19.5 | 254,017 | 19.6 | 179,965 | 19.5 | 243,427 | 19.5 | 169,209 | 19.5 |
| 3 | 275,027 | 20.3 | 263,895 | 20.3 | 188,465 | 20.5 | 253,577 | 20.4 | 177,437 | 20.5 |
| 4 | 274,831 | 20.3 | 263,128 | 20.3 | 189,414 | 20.6 | 253,364 | 20.3 | 178,232 | 20.6 |
| 5 | 208,665 | 15.4 | 198,818 | 15.3 | 140,005 | 15.2 | 190,846 | 15.3 | 131,628 | 15.2 |
| Missing | 41,721 | 39,728 | 22,132 | 35,645 | 20,211 | |||||
| Neighborhood education quintilei | ||||||||||
| 1 (lowest) | 271,313 | 20.1 | 260,858 | 20.1 | 184,208 | 20.0 | 250,153 | 20.1 | 173,162 | 20.0 |
| 2 | 272,634 | 20.2 | 261,884 | 20.2 | 184,970 | 20.1 | 251,241 | 20.2 | 174,204 | 20.1 |
| 3 | 272,151 | 20.1 | 261,248 | 20.1 | 185,266 | 20.1 | 250,815 | 20.1 | 174,237 | 20.1 |
| 4 | 278,358 | 20.6 | 266,926 | 20.6 | 189,162 | 20.5 | 256,375 | 20.6 | 177,732 | 20.5 |
| 5 | 258,191 | 19.1 | 246,935 | 19.0 | 177,673 | 19.3 | 237,579 | 19.1 | 167,103 | 19.3 |
| Missing | 41,721 | 39,728 | 22,132 | 35,645 | 20,211 | |||||
a Births to women whose last menstrual period was more than 20 weeks before the start of the study period or less than 43 weeks before the end of the study period were not included in analyses by estimated date of conception.
b Singleton livebirths in 2003–2012 were used to study seasonal patterns in births, their corresponding conceptions, maternal age, and gestational age and to study preterm birth outcomes.
c Singleton livebirths in 2006–2012 were used to study seasonal patterns in sociodemographic characteristics (i.e., neighborhood income and educational level).
d Value is expressed as mean (standard deviation) among livebirths. Information on gestational age was missing from 682 of 1,394,368 records (0.05%).
e Values are expressed as mean (standard deviation) among singleton livebirths.
f Value is expressed as mean (standard deviation) among stillbirths. Information on gestational age was missing from 3,126 of 10,751 records (29.6%).
g The denominator used to calculate percentage excluded 5,602 records with missing plurality information.
h The denominator used to calculate percentages excluded 3,808 records with missing gestational age information for which the date of conception could not be estimated.
i The denominator used to calculate percentages excluded records with missing information.
j Cutpoints for neighborhood median family income quintiles (in Canadian dollars): quintile 1, $1–$54,026; quintile 2: $54,027–$68,081; quintile 3: $68,082–$81,283; quintile 4: $81,284–$97,797; and quintile 5: ≥$97,798.
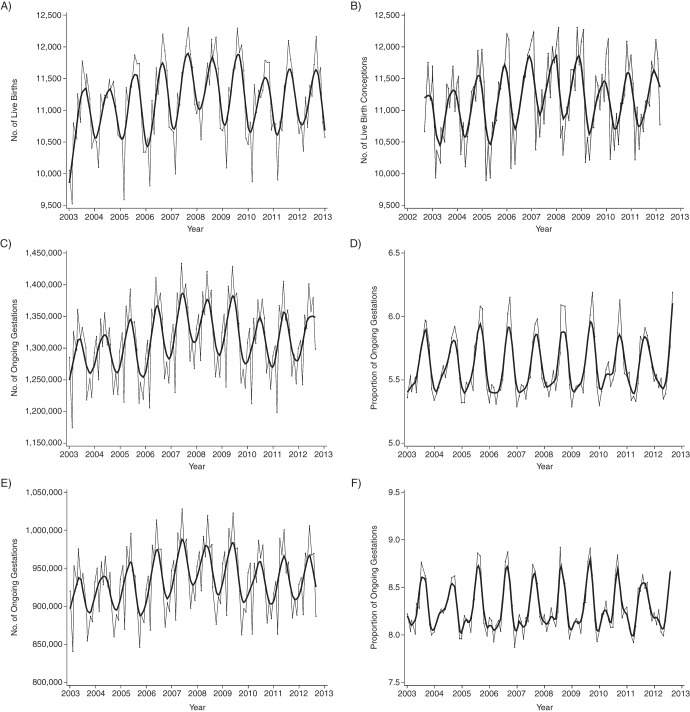
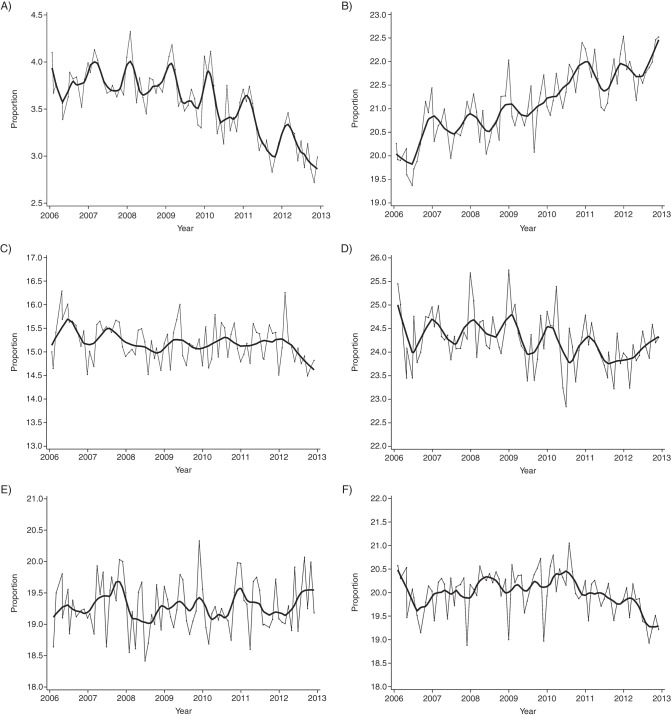
We included 1,337,579 singleton livebirths in our descriptive analyses of seasonal patterns in births and their corresponding conceptions. There was a consistent annual peak in livebirths in September and a low in December–January, whereas temporal patterns for conceptions were shifted 8–9 months earlier, peaking in October–December (Figure 3A and 3B). The denominators of ongoing gestations at risk for preterm birth at less than 37 weeks and less than 32 weeks showed strong seasonality (Figure 3C and 3E), and the proportion of gestations in the highest category of each risk set consistently peaked in the same months each year (Figure 3D and 3F). The proportion of births to women younger than 20 years of age showed a long-term decrease and modest seasonal fluctuation, whereas the proportion of births to women 35 years of age or older showed a long-term increase but no consistent seasonality (Figure 4A and 4B). Births to women in the lowest neighborhood income quintile decreased slightly and demonstrated mild seasonal fluctuation, but no clear patterns were evident in the highest neighborhood income quintile or the lowest or highest education quintiles (Figure 4C–4F). Based on this descriptive assessment, we concluded there were strong seasonal patterns in births and conceptions, but among sociodemographic variables, only maternal age showed consistent seasonality. Because we planned a priori to include cubic splines in our models to account for dominant temporal patterns in our data, we opted to formally assess adjustment for potential confounding by maternal age and gestational age only.
Figure 3.
Temporal patterns in monthly proportions of ongoing gestations among singleton livebirths that occurred at 20 weeks’ gestation or later, Ontario, 2003–2012. A) Number of births; B) number of conceptions; C) number of ongoing gestations at 20–36 weeks; D) total number of ongoing pregnancies in any given month that were between the gestational ages of 20 and 36 weeks in week 36; E) number of ongoing gestations at 20–31 weeks; and F) total number of ongoing pregnancies in any given month that were between the gestational ages of 20 and 31 weeks in week 31. The gray lines represent observed data; the black lines represent smoothed data.
Figure 4.
Temporal patterns in monthly proportion of births among singleton livebirths that occurred at 20 weeks’ gestation or later by maternal characteristics, Ontario, Canada, 2006–2012. A) Women younger than 20 years of age; B) women 35 years of age or older; C) women in the highest neighborhood income quintile; D) women in the lowest neighborhood income quintile; E) women in the highest neighborhood education quintile; and F) women in the lowest neighborhood education quintile. The gray lines represent observed data; the black lines represent smoothed data.
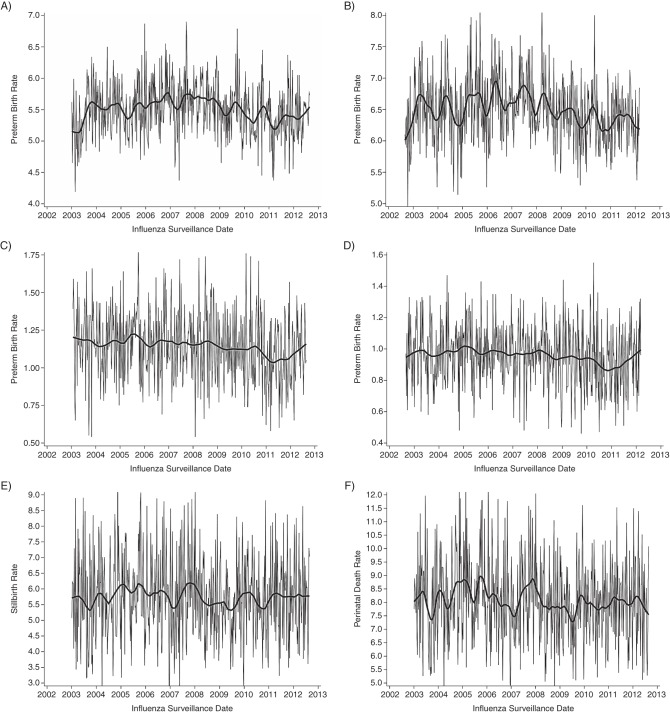
With the exception of the 2009 H1N1 pandemic, influenza seasons started between November and January, with peak viral circulation between December and April. There were 2 pandemic waves—the first occurring in the spring and the second in the fall, as opposed to the usual midwinter timing of nonpandemic seasons. Preterm birth (<37 weeks’ gestation) per 10,000 at-risk gestations showed no obvious temporal patterns when aggregated by surveillance week of birth (Figure 5A). When data were aggregated by week of conception, there was visible seasonality, with a tendency to peak in the winter months (Figure 5B). No obvious seasonal patterns were visible in the plots of preterm birth rates before 32 weeks (Figure 5C and 5D). Rates of stillbirth (Figure 5E) and perinatal death (Figure 5F) were only aggregated by surveillance week of birth and, although both displayed some temporal features, there was little consistency in the plots.
Figure 5.
Temporal patterns in weekly rates of preterm birth, stillbirth, and perinatal death, Ontario, Canada, 2003–2012. A) Rate of preterm births at less than 37 weeks’ gestation per 10,000 ongoing at-risk gestations; B) rate of preterm births at less than 37 weeks’ gestation per 100 livebirth conceptions; C) rate of preterm births at less than 32 weeks’ gestation per 10,000 ongoing at-risk gestations; D) rate of preterm births at less than 32 weeks’ gestation per 100 livebirth conceptions; E) rate of stillbirths per 100,000 ongoing at-risk gestations; and F) rate of perinatal deaths per 100,000 ongoing at-risk gestations. The gray lines represent observed data; the black lines represent smoothed data.
There was no association between the proportion of positive influenza specimens during the preceding week and rates of preterm birth, either in the crude model or in models that included cubic splines and adjustment for respiratory syncytial virus (adjusted risk ratio = 1.01, 95% confidence interval: 1.00, 1.02; Table 2). Additional adjustment for the proportion of fetuses at 36 weeks’ gestation in the risk set for preterm birth did not alter the results (adjusted risk ratio = 1.01, 95% confidence interval: 0.99, 1.02), nor did adjustment for the maternal age distribution in each week (Web Table 6). Similarly, we found no relationship between rates of preterm birth and exposure to circulating influenza during the final month of gestation or during the first month of gestation (Table 2). In sensitivity analyses, these findings were robust to different model specifications, including variation in the number and location of spline knots and adjustment for potential seasonal confounding by maternal age and gestational age (Web Table 6). Model diagnostics provided no indication of residual autocorrelation (Web Figure 1), and model fit was adequate (Web Figure 2). Replacing the main exposure variable with lagged versions of the exposure (by 1 or 2 weeks) did not qualitatively alter the results for rates of preterm birth before 37 weeks (Web Table 7). In models of preterm birth before 32 weeks, we similarly observed no associations with the level of circulating influenza virus in any pregnancy exposure window (Table 2 and Web Table 8). There was no association between circulating influenza and mortality outcomes in relation to late-pregnancy exposure windows (Table 2 and Web Tables 9 and 10).
Table 2.
Rate Ratios for the Association Between the Proportion of Positive Influenza Specimens and the Primary Study Outcomes During Early and Late Pregnancy Exposure Windows, Ontario, Canada 2003–2012
| Outcome and Exposure Window | Unadjusted RRa | 95% CI | Adjusted RRa,b | 95% CI |
|---|---|---|---|---|
| Preterm birth at <37 weeksc | ||||
| Final week of gestation | 1.00 | 0.99, 1.01 | 1.01 | 1.00, 1.02 |
| Final month of gestation | 0.99 | 0.98, 1.00 | 1.01 | 0.99, 1.02 |
| First month of gestation | 1.00 | 0.99, 1.01 | 1.00 | 0.99, 1.02 |
| Preterm birth at <32 weeksd | ||||
| Final week of gestation | 0.98 | 0.96, 1.00 | 0.98 | 0.97, 1.00 |
| Final month of gestation | 0.98 | 0.96, 1.01 | 0.99 | 0.97, 1.01 |
| First month of gestation | 1.00 | 0.98, 1.03 | 1.00 | 0.98, 1.03 |
| Stillbirthe | ||||
| Final week of gestation | 0.98 | 0.96, 1.01 | 0.98 | 0.96, 1.01 |
| Final month of gestation | 0.99 | 0.97, 1.02 | 0.99 | 0.96, 1.02 |
| Perinatal deathf | ||||
| Final week of gestation | 1.00 | 0.98, 1.02 | 1.00 | 0.98, 1.02 |
| Final month of gestation | 1.01 | 0.98, 1.03 | 1.01 | 0.99, 1.03 |
Abbreviations: CI, confidence interval; RR, rate ratio.
a Estimates represent the relative change in the rate for a 10–percentage point increase in the proportion of positive influenza specimens.
b Adjusted for long-term and seasonal trends via cubic splines and for the proportion of positive respiratory syncytial virus specimens in corresponding time period.
c For late-pregnancy exposure windows, the number of preterm births (<37 weeks’ gestation) per influenza surveillance week was aggregated by the date of birth and offset by the number of gestations at risk during the corresponding week (i.e., ongoing gestations at 20–36 weeks). For the early-pregnancy exposure window, the number of preterm births (<37 weeks’ gestation) per influenza surveillance week was aggregated by the estimated date of conception and offset by the total number of conceptions during the corresponding week that resulted in livebirths.
d For late-pregnancy exposure windows, the number of preterm births (<32 weeks’ gestation) per influenza surveillance week was aggregated by the date of birth and offset by the number of gestations at risk during the corresponding week (i.e., ongoing gestations at 20–31 weeks). For the early-pregnancy exposure window, the number of preterm births (<32 weeks’ gestation) per influenza surveillance week was aggregated by the estimated date of conception and offset by the total number of conceptions during the corresponding week that resulted in livebirths.
e For late-pregnancy exposure windows, the number of stillbirths per influenza surveillance week was aggregated by the date of birth and offset by the number of gestations at risk during the corresponding week (i.e., ongoing gestations at ≥20 weeks). Outcomes could not be assessed during the early-pregnancy exposure window.
f For late-pregnancy exposure windows, the number of perinatal deaths per influenza surveillance week was aggregated by the date of birth and offset by the number of gestations at risk during the corresponding week (i.e., ongoing gestations at ≥20 weeks). Outcomes could not be assessed during the early-pregnancy exposure window.
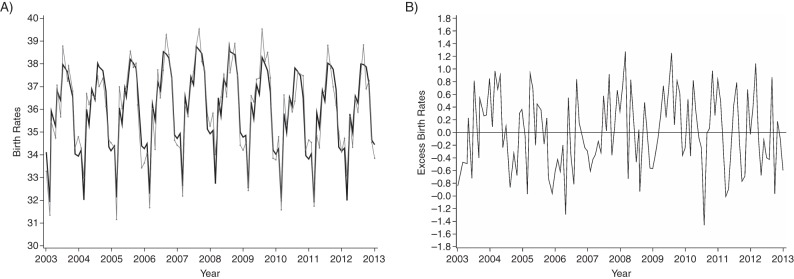
The observed monthly birth rate demonstrated very strong seasonality, peaking during the summer months each year (Figure 6A). Although the residual plot showed that the largest excess deviation between observed and expected monthly birth rates occurred in July 2010, approximately 8 months after the November 2009 peak of the H1N1 pandemic, the residuals immediately before and after this time point approximated zero; thus, the decrease was not sustained (Figure 6B).
Figure 6.
Birth rates among the study population by year, Ontario, Canada, 2003–2012. A) Observed (gray line) and expected (black line) monthly birth rates per 10,000 females 15–49 years of age; B) monthly excess birth rates.
DISCUSSION
In the present time-series study across 9 seasonal influenza epidemics and a contemporary influenza pandemic, circulating influenza was not associated with any short-term variation in rates of preterm birth, stillbirth, or perinatal death. Our findings were robust in sensitivity analyses that included alternate model specifications, adjustment for potential seasonal confounding, and assessment of the 2009 H1N1 pandemic distinct from epidemic influenza seasons. We also found no sustained excess reduction in expected monthly birth rates in the months after the peak of the 2009 H1N1 pandemic, suggesting that exposure to the pandemic was not associated with early pregnancy interruption.
Although time-series designs have been used to examine health outcomes associated with circulating influenza (21) and perinatal outcomes associated with environmental exposures (27, 28, 35, 38, 39), we are aware of only one other time-series study of perinatal outcomes and circulating influenza. Luteijn et al. (40) recently studied the association between ecologic exposure to the influenza season during the critical development window for several nonchromosomal congenital anomalies and found no association with seasonal or pandemic influenza. Bloom-Feshbach et al. (29) used a different time-series approach to examine patterns in monthly birth rates relative to the 1918–1919 influenza pandemic and concluded that a sustained depression in birth rates 6 months after peak pandemic activity was consistent with an excess of first trimester miscarriages. Following this line of thinking, we hypothesized that we might see a similar depression in birth rates after the 2009 H1N1 pandemic if there was an excess of first trimester miscarriages or intentionally delayed conceptions among women planning a pregnancy. Our findings did not support this hypothesis, possibly indicating different pathogenicity of the 1918–1919 and 2009 pandemic viruses, susceptibility of the host population of pregnant women and fetuses, or methodologies. For example, we had a lower number of months available for establishing expected baseline birth rates.
The remaining literature on this topic is dominated by case-series reports of unknown representativeness, often with incomplete information on pregnancy outcomes (41). Findings from individual-level epidemiologic studies are inconsistent, and many studies are limited by an inadequate number of events (particularly for fetal death) and methodological weaknesses. Heterogeneity in measurement of influenza exposure may account for some of the inconsistency in results—for instance, among studies in which increased risks of preterm birth (13–15) and fetal death (13, 16) were reported after maternal 2009 pandemic H1N1 influenza illness, exposure was based on severe influenza disease in all but one (16) (i.e., all or most women were hospitalized or laboratory testing was selectively carried out only on women with clinical symptoms of severe illness). Similar to our ecologic results, results from studies in which investigators ascertained milder maternal pandemic H1N1 influenza disease have not shown an association with preterm birth (16, 17); the same is true for results from high-quality studies from nonpandemic influenza seasons (17, 18). Although laboratory testing is considered the gold standard for influenza diagnosis (42), specimens are only collected among women who seek medical attention. If pregnant women at higher risk for adverse pregnancy outcomes are more likely to seek care for influenza illness, be selected for confirmatory influenza testing, or be admitted to hospital on a precautionary basis, exposure classification will differ by outcome, potentially biasing point estimates away from the null value. This potential for diagnostic ascertainment bias and other challenges in individual-level exposure measurement were our primary rationale for using a time-series approach.
Time-series designs offer several practical and methodological advantages. Because they include the entire population, they are not susceptible to selection bias (27) and can be valuable for assessing the public health impact of a population-wide exposure (43). Because routine collection of influenza surveillance and birth outcome data already exists, a time-series design is efficient and relatively inexpensive (43). Factors that act as confounders between maternal influenza and perinatal outcomes at the individual level cannot confound the relationship at the ecologic level unless they co-vary with exposure and outcome across an extended time period and affect a large proportion of the population (34, 44). In our study population, models that were adjusted for temporal patterns in the distribution of maternal age and gestational age did not differ from the main analyses, implying no confounding by these factors at the ecologic level.
Several study limitations require consideration. Pregnancy losses before 20 weeks were not ascertained in the source database (45). If influenza in early pregnancy were associated with death of susceptible fetuses before 20 weeks, we could have underestimated a potentially harmful relationship between circulating influenza and rates of study outcomes, although findings from our secondary outcome suggest this was not the case. We presume some misclassification of preterm birth because gestational age estimates vary according to dating methods (46), but this is likely to be independent of temporal variation in influenza circulation. We restricted our descriptive analyses and preterm birth analyses to singleton livebirths because of missing gestational age information in many stillbirth records. The fact that preterm stillbirth is a competing risk for preterm livebirth could have introduced bias if exposure prevented pregnancies from surviving to become a preterm livebirth. Given the small number of gestations resulting in stillbirths relative to the number of livebirths, we expect that any such influence would be unlikely to change our findings. We studied population exposure to influenza virus circulation, which is not equivalent to individual exposure and could explain our null findings. Influenza may cause adverse perinatal outcomes; however, considering the multifactorial etiology of both preterm birth and fetal death (47–49) and the low prevalence of influenza during pregnancy (8, 14, 16, 50, 51), an association may not be detectable in a time-series analysis at the population level. Despite the large size of the obstetrical population in our study, our statistical power may not have been sufficient to rule out a small ecologic association. Finally, we were unable to assess the impact of adjustment for influenza vaccination rates because of a lack of data on vaccination in pregnancy in the Ontario obstetrical population.
In summary, understanding the population impact of influenza on adverse perinatal outcomes is essential for informing prevention and treatment strategies for pregnant women during epidemics and pandemics. Inconsistent findings and limitations in the epidemiologic literature arising from methods used to assess and ascertain individual-level influenza exposure motivated us to explore this topic at the population level. Although we observed temporal variation in rates of preterm birth, stillbirth, and perinatal death, we found no evidence that any short-term variation was associated with pregnancy exposure to circulating influenza at the population level.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Better Outcomes Registry & Network (BORN) Ontario, Children's Hospital of Eastern Ontario Research Institute, Ottawa, Ontario, Canada (Deshayne B. Fell); Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Québec, Canada (Deshayne B. Fell, David L. Buckeridge, Robert W. Platt, Jay S. Kaufman, Olga Basso); Department of Obstetrics and Gynecology, McGill University, Montreal, Québec, Canada (Olga Basso); Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada (Kumanan Wilson); Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Ontario, Canada (Kumanan Wilson); and Institute for Clinical Evaluative Sciences, Ottawa, Ontario, Canada (Kumanan Wilson).
This work was not supported by external funding. D.B.F. was supported by a Canadian Institutes of Health Research Doctoral Award while this study was being conducted. R.W.P. is supported by a Chercheur-National (National Scholar) award from the Fonds de Recherche du Quebec—Santé (FRQ-S), as well as core support to the Research Institute of the McGill University Health Centre from FRQ-S.
We thank the Better Outcomes Registry & Network (BORN) Ontario, Ottawa, Canada for providing data access.
This work was presented as a poster at the Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, June 14–15, 2015, Denver, Colorado, and as an oral presentation at the 2nd Annual Canadian National Perinatal Research Meeting, February 24–27, 2015, Montebello, Quebec, Canada.
Conflict of interest: none declared.
REFERENCES
- 1.Harris J. Influenza occurring in pregnant women. J Am Med Assoc. 1919;7214:978–980. [Google Scholar]
- 2.Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–1175. [DOI] [PubMed] [Google Scholar]
- 3.Siston AM, Rasmussen SA, Honein MA et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;30315:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodds L, McNeil SA, Fell DB et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;1764:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuzil KM, Reed GW, Mitchel EF et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;14811:1094–1102. [DOI] [PubMed] [Google Scholar]
- 6.Mertz D, Kim TH, Johnstone J et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz JR, Englund JA, Neuzil KM. Influenza vaccine for pregnant women in resource-constrained countries: a review of the evidence to inform policy decisions. Vaccine. 2011;2927:4439–4452. [DOI] [PubMed] [Google Scholar]
- 8.Madhi SA, Cutland CL, Kuwanda L et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;37110:918–931. [DOI] [PubMed] [Google Scholar]
- 9.Keller-Stanislawski B, Englund JA, Kang G et al. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;3252:7057–7064. [DOI] [PubMed] [Google Scholar]
- 10.Moro PL, Tepper NK, Grohskopf LA et al. Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines. 2012;118:911–921. [DOI] [PubMed] [Google Scholar]
- 11.Fell DB, Platt RW, Lanes A et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG. 2015;1221:17–26. [DOI] [PubMed] [Google Scholar]
- 12.Steinhoff MC, MacDonald N, Pfeifer D et al. Influenza vaccine in pregnancy: policy and research strategies. Lancet. 2014;3839929:1611–1613. [DOI] [PubMed] [Google Scholar]
- 13.Pierce M, Kurinczuk JJ, Spark P et al. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ. 2011;342:d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle TJ, Goodin K, Hamilton JJ. Maternal and neonatal outcomes among pregnant women with 2009 pandemic influenza A(H1N1) illness in Florida, 2009–2010: a population-based cohort study. PLoS One. 2013;810:e79040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naresh A, Fisher BM, Hoppe KK et al. A multicenter cohort study of pregnancy outcomes among women with laboratory-confirmed H1N1 influenza. J Perinatol. 2013;3312:939–943. [DOI] [PubMed] [Google Scholar]
- 16.Håberg SE, Trogstad L, Gunnes N et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;3684:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen C, Desai S, Bredfeldt C et al. A large, population-based study of 2009 pandemic Influenza A virus subtype H1N1 infection diagnosis during pregnancy and outcomes for mothers and neonates. J Infect Dis. 2012;2068:1260–1268. [DOI] [PubMed] [Google Scholar]
- 18.McNeil SA, Dodds LA, Fell DB et al. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol. 2011;204(6 suppl 1):S54–S57. [DOI] [PubMed] [Google Scholar]
- 19.Gilca R, De Serres G, Skowronski D et al. The need for validation of statistical methods for estimating respiratory virus-attributable hospitalization. Am J Epidemiol. 2009;1707:925–936. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO Global Technical Consultation: Global Standards and Tools for Influenza Surveillance. Geneva, Switzerland: WHO Press; 2011. http://apps.who.int/iris/bitstream/10665/70724/1/WHO_HSE_GIP_2011.1_eng.pdf Accessed July 2, 2016. [Google Scholar]
- 21.Jackson ML. Confounding by season in ecologic studies of seasonal exposures and outcomes: examples from estimates of mortality due to influenza. Ann Epidemiol. 2009;1910:681–691. [DOI] [PubMed] [Google Scholar]
- 22.Canadian Institute for Health Information. Data Quality Documentation, Discharge Abstract Database—Multiyear Information. Ottawa, Canada: Canadian Institute for Health Information; 2012. [Google Scholar]
- 23.Strand LB, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkins R. PCCF+ Version 5E Users Guide. Automated Geographic Coding Based on the Statistics Canada Postal Code Conversion Files, Including Postal Codes Through March 2009. Ottawa, Canada: Statistics Canada; 2010. http://data.library.utoronto.ca/datapub/codebooks/cstdli/pccf_health/pccf5f/MSWORD.PCCF5F.pdf Accessed July 2, 2016. [Google Scholar]
- 25.Public Health Ontario. Ontario Influenza and Respiratory Infection Surveillance Program. http://www.publichealthontario.ca/en/eRepository/Influenza_Respiratory_Surveillance_Program_2012-2013.pdf Accessed April 7, 2015.
- 26.Izurieta HS, Thompson WW, Kramarz P et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;3424:232–239. [DOI] [PubMed] [Google Scholar]
- 27.Sagiv SK, Mendola P, Loomis D et al. A time-series analysis of air pollution and preterm birth in Pennsylvania, 1997–2001. Environ Health Perspect. 2005;1135:602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darrow LA, Klein M, Flanders WD et al. Ambient air pollution and preterm birth: a time-series analysis. Epidemiology. 2009;205:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloom-Feshbach K, Simonsen L, Viboud C et al. Natality decline and miscarriages associated with the 1918 influenza pandemic: the Scandinavian and United States experiences. J Infect Dis. 2011;2048:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darrow LA, Strickland MJ, Klein M et al. Seasonality of birth and implications for temporal studies of preterm birth. Epidemiology. 2009;205:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall CB, Weinberg GA, Iwane MK et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;3606:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleveland WS, Devlin DS. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1998;83403:596–610. [Google Scholar]
- 33.Bhaskaran K, Gasparrini A, Hajat S et al. Time series regression studies in environmental epidemiology. Int J Epidemiol. 2013;424:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng RD, Dominici F, Louis TA. Model choice in time series studies of air pollution and mortality. J R Stat Soc Ser A Stat Soc. 2006;1692:179–203. [Google Scholar]
- 35.Lee SJ, Hajat S, Steer PJ et al. A time-series analysis of any short-term effects of meteorological and air pollution factors on preterm births in London, UK. Environ Res. 2008;1062:185–194. [DOI] [PubMed] [Google Scholar]
- 36.Cameron AC, Trivedi PK. Essentials of count data regression. In: Baltagi B, ed. A Companion to Theoretical Econometrics. Malden, MA: Blackwell Publishing Ltd; 2003:331–348. [Google Scholar]
- 37.Centers for Disease Control and Prevention. Value of pharmacy-based influenza surveillance—Ontario, Canada, 2009. Morb Mortal Wkly Rep. 2013;6220:401–404. [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf J, Armstrong B. The association of season and temperature with adverse pregnancy outcome in two German states, a time-series analysis. PLoS One. 2012;77:e40228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strand LB, Barnett AG, Tong S. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol. 2012;1752:99–107. [DOI] [PubMed] [Google Scholar]
- 40.Luteijn JM, Addor M-C, Arriola L et al. The association of H1N1 pandemic influenza with congenital anomaly prevalence in Europe: an ecological time series study. Epidemiology. 2015;266:853–861. [DOI] [PubMed] [Google Scholar]
- 41.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;2051:10–18. [DOI] [PubMed] [Google Scholar]
- 42.Petric M, Comanor L, Petti CA. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis. 2006;194(suppl 2):S98–S110. [DOI] [PubMed] [Google Scholar]
- 43.Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health. 1995;16:61–81. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg MS, Burnett RT, Brook JR. Counterpoint: time-series studies of acute health events and environmental conditions are not confounded by personal risk factors. Regul Toxicol Pharmacol. 2008;512:141–147. [DOI] [PubMed] [Google Scholar]
- 45.Schisterman EF, Cole SR, Ye A et al. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;275:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morin I, Morin L, Zhang X et al. Determinants and consequences of discrepancies in menstrual and ultrasonographic gestational age estimates. BJOG. 2005;1122:145–152. [DOI] [PubMed] [Google Scholar]
- 47.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;3626:529–535. [DOI] [PubMed] [Google Scholar]
- 48.Kramer MS, Papageorghiou A, Culhane J et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;2062:108–112. [DOI] [PubMed] [Google Scholar]
- 49.Gravett MG, Rubens CE, Nunes TM. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Preg Childbirth. 2010;10(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yates L, Pierce M, Stephens S et al. Influenza A/H1N1v in pregnancy: an investigation of the characteristics and management of affected women and the relationship to pregnancy outcomes for mother and infant. Health Technol Assess. 2010;1434:109–182. [DOI] [PubMed] [Google Scholar]
- 51.Mahmud SM, Becker M, Keynan Y et al. Estimated cumulative incidence of pandemic (H1N1) influenza among pregnant women during the first wave of the 2009 pandemic. CMAJ. 2010;18214:1522–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.