Abstract
Chalcone synthase catalyzes the initial step of that branch of the phenylpropanoid pathway that leads to flavonoids. A lack of chalcone synthase activity has a pleiotropic effect in maize and petunia mutants: pollen fertility as well as flavonoid synthesis is disrupted. Both maize and petunia mutants are self-sterile due to a failure to produce a functional pollen tube. The finding that the mutant pollen is partially functional on wild-type stigmas led to the isolation and identification of kaempferol as a pollen germination-inducing constituent in wild-type petunia stigma extracts. We show that adding micromolar quantities of kaempferol to the germination medium or to the stigma at pollination is sufficient to restore normal pollen germination and tube growth in vitro and full seed set in vivo. Further we show that the rescue ability resides in particular structural features of a single class of compounds, the flavonol aglycones. This finding identifies another constituent of plant reproduction and suggests that addition or removal of the flavonol signal during pollen germination and tube growth provides a feasible way to control plant fertility.
Full text
PDF

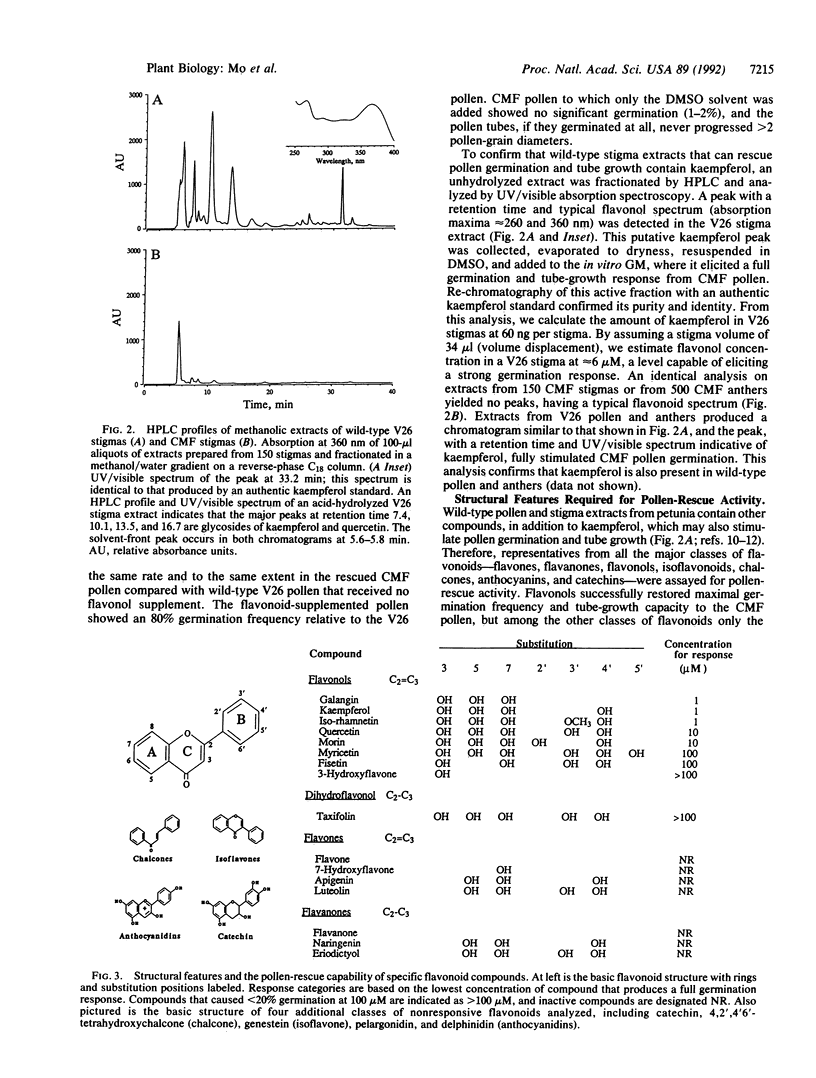
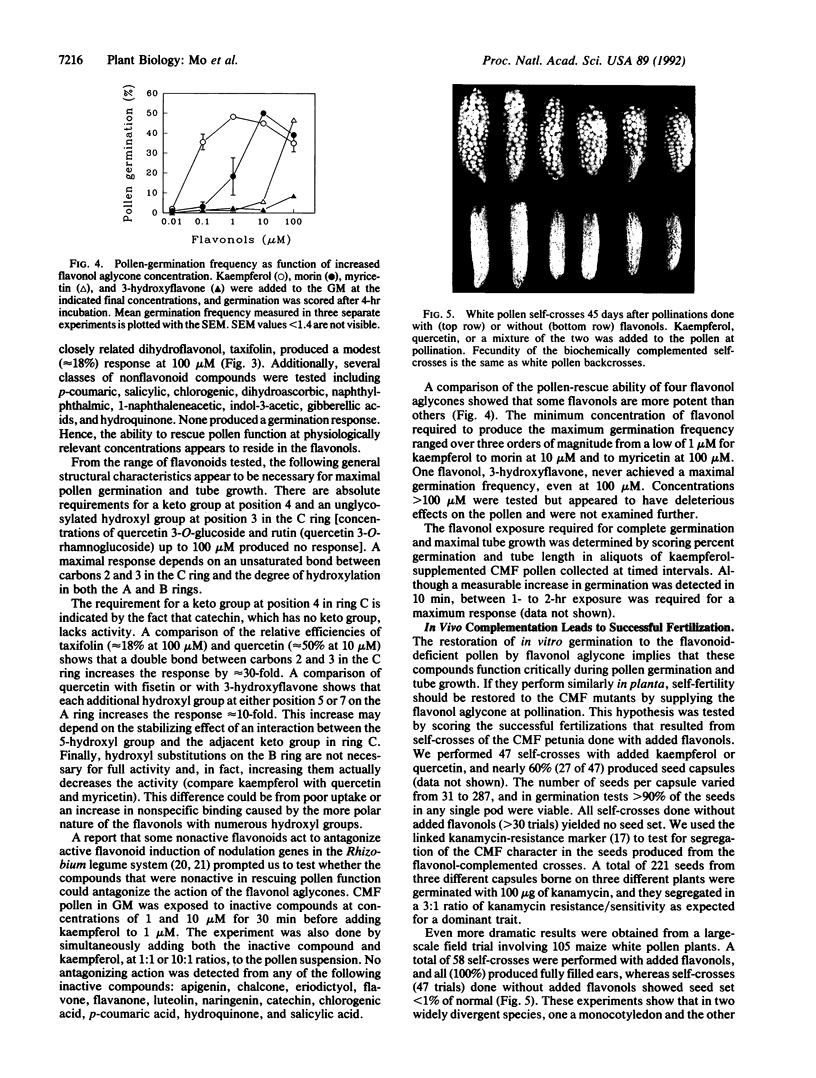

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish E. C., Anderson M. A., Clarke A. E. Molecular aspects of fertilization in flowering plants. Annu Rev Cell Biol. 1988;4:209–228. doi: 10.1146/annurev.cb.04.110188.001233. [DOI] [PubMed] [Google Scholar]
- Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987 May;6(5):1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnen W., Lush W. M., Clarke A. E. Inhibition of in Vitro Pollen Tube Growth by Isolated S-Glycoproteins of Nicotiana alata. Plant Cell. 1989 May;1(5):501–510. doi: 10.1105/tpc.1.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J. P. The Male Gametophyte of Flowering Plants. Plant Cell. 1989 Jul;1(7):657–664. doi: 10.1105/tpc.1.7.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990 Apr;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah J. B., Nasrallah M. E. The molecular genetics of self-incompatibility in Brassica. Annu Rev Genet. 1989;23:121–139. doi: 10.1146/annurev.ge.23.120189.001005. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Long S. R. Alfalfa Root Exudates and Compounds which Promote or Inhibit Induction of Rhizobium meliloti Nodulation Genes. Plant Physiol. 1988 Oct;88(2):396–400. doi: 10.1104/pp.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]





