Prior instances of bacteremia and sepsis substantially increase the 5-year risk of stroke or transient ischemic attack and myocardial infarction.
Keywords: bloodstream infection, causal inference, inverse probability weighting, marginal structural model, time-varying confounding
Abstract
Background. The long-term and cumulative effect of multiple episodes of bacteremia and sepsis across multiple hospitalizations on the development of cardiovascular (CV) events is uncertain.
Methods. We conducted a longitudinal study of 156 380 hospitalizations in 47 009 patients (≥18 years old) who had at least 2 inpatient admissions at an academic tertiary care center in St Louis, Missouri, from 1 January 2008 through 31 December 2012. We used marginal structural models, estimated by inverse probability weighting (IPW) of bacteremia or sepsis and IPW of censoring, to estimate the marginal causal effects of bacteremia and sepsis on developing the first observed incident CV event, including stroke, transient ischemic attack, and myocardial infarction (MI), during the study period.
Results. Bacteremia and sepsis occurred during 4923 (3.1%) and 5544 (3.5%) hospitalizations among 3932 (8.4%) and 4474 (9.5%) patients, respectively. CV events occurred in 414 (10.5%) and 538 (12.0%) patients with prior episodes of bacteremia or sepsis, respectively, vs 3087 (7.2%) and 2963 (7.0%) patients without prior episodes of bacteremia or sepsis. The causal odds of experiencing a CV event was 1.52-fold (95% confidence interval [CI], 1.21- to 1.90-fold) and 2.39-fold (95% CI, 1.88- to 3.03-fold) higher in patients with prior instances of bacteremia or sepsis, respectively, compared to those without. Prior instances of septic shock resulted in a 6.91-fold (95% CI, 5.34- to 8.93-fold) increase in the odds of MI.
Conclusions. Prior instances of bacteremia and sepsis substantially increase the 5-year risk of CV events.
Cardiovascular (CV) disease affects one-quarter of the US population, costs the healthcare system more than $315.4 billion dollars annually, and is a leading cause of death [1]. Although traditional risk factors for CV disease are well documented, identifying unrecognized factors that may trigger CV events and are amenable to interventions is vitally important [2]. A targeted intervention on potential preventable causes of CV events could have a significant impact on public health and clinical practice.
Several studies suggest an association between infection and subsequent CV events [3–14]. These investigations found a substantial (2-fold to >20-fold) increase in the risk of CV events shortly after acute infection [8, 10–13], and additionally, found that the increased risk may persist for many years [3, 4, 14]. Short- and long-lived alterations in systemic inflammation as well as demand ischemia, endothelial dysfunction, and procoagulant changes in the blood provide a biological basis for the associations between infections and increased CV disease risk [15, 16].
There are some limitations to these previous observational studies, however. Studies that reported the long-term effects of infection on the risk of CV events only considered a single episode of infection rather than the cumulative effects of repeated infections [3–5, 9–11, 14]. Some studies relied on case-crossover designs [17] that are not suited to study the effects of exposures, such as infections, that may not be transient [5–9]. Finally, few studies validated infection status with positive microbiology cultures [7, 8, 12].
The study objective is to estimate the effects of bacteremia and sepsis on 5-year risk of CV events. There are >1.7 million hospitalizations per year in the United States involving sepsis [18]. Despite reports on the short-term effects of bacteremia [12] and sepsis [10, 13] on the risk of CV events, no study has characterized the effects of multiple episodes of bacteremia and sepsis across multiple hospitalizations over a long-term period.
METHODS
Study Design
We conducted a longitudinal study of all patients ≥18 years of age admitted to Barnes-Jewish Hospital (BJH), between 1 January 2008 and 31 December 2012. BJH is an academic tertiary care referral center affiliated with the Washington University School of Medicine in St Louis, Missouri. Clinical, laboratory, pharmacy, and hospital administrative data were extracted from the medical informatics repository of the BJC HealthCare's Center for Clinical Excellence. The study was approved by the Human Research Protection Office of the Washington University School of Medicine with a waiver of written informed consent.
Definition of Outcomes and Exposures
The primary outcome of interest was the first observed CV event, defined as stroke, transient ischemic attack (TIA), or myocardial infarction (MI) during the study period. Identification of CV events was based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis codes for stroke (430, 431, 433.x1, and 434.x1), TIA (435.x), and MI (410.x).
Bacteremia was defined as having at least 1 positive blood culture during a hospitalization for a known bacterial or fungal pathogen, excluding common skin contaminants such as Bacillus species, coagulase-negative staphylococci, Corynebacterium species, Micrococcus species, and Propionibacterium species. Sepsis, severe sepsis, and septic shock were identified by the ICD-9-CM codes 995.91, 995.92, and 785.52, respectively. Additionally, a composite exposure of “any sepsis”, which included all 3 ICD-9-CM codes, was created for the purpose of sensitivity analysis.
Covariates
Covariates included demographics and risk factors for CV disease and sepsis, based on ICD-9-CM discharge diagnosis codes developed by Elixhauser et al [19] and enhanced by Quan et al [20], including hypertension, diabetes, chronic pulmonary disease, renal failure, liver disease, congestive heart failure, cardiac arrhythmias, valvular heart disease, pulmonary circulation disorders, peripheral vascular disorders, coagulopathy, AIDS or human immunodeficiency virus infection, metastatic cancer, lymphoma, obesity, and alcohol abuse. Comorbidities are defined as preexisting nonacute conditions that are unrelated to the primary reason for hospitalization.
Other covariates included statin usage; administration of any vasopressors during the hospitalization; use of nonsteroidal anti-inflammatory drugs; use of antihypertensive medications including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, diuretics, hydralazine, and nitrates; length of hospital stay; admission or transfer to an intensive care unit (ICU); any surgical procedure classified by ICD-9-CM procedure codes of the National Healthcare Safety Network [21] that was performed during the course of a hospitalization; prior 30-day hospital admission; and patient residency with St Louis City/County based upon home zip codes, as it was assumed to be less likely to return to BJH if a patient's residence moved farther away during the study.
The systemic inflammatory response syndrome (SIRS) was defined as having at least 2 of the 4 following criteria on a calendar day: heart rate >90 beats per minute; respiratory rate >20 breaths per minute; body temperature <36°C or >38.3°C; and white blood cell count <4000 cells/µL or >12 000 cells/µL [22]. To mitigate for frequent transient changes in heart rate, respiratory rate, and temperature, at least 2 out-of-range measurements of these vital signs had to occur on a given calendar day to be considered counting toward SIRS criteria [23].
Description of Study Cohort
Participants were required to have at least 2 hospitalizations at BJH, which allowed us to record patients' covariates and comorbidities before a CV event (if any). Thus, patients with a CV event at their first BJH hospitalization were excluded. Patients were censored due to dropout (loss to follow-up) or death, or at the end of follow-up if they did not experience a CV event.
Analytic Approach
Distribution of the study covariates grouped by bacteremia or sepsis hospitalizations was compared using t test and a χ2 test, or their nonparametric alternatives, as appropriate. We used marginal structural models (MSMs) [24] to estimate the marginal causal effects of bacteremia and sepsis on a subsequent CV event. MSMs are a class of causal models that are fitted to expected counterfactual outcomes, that is, the potential outcomes that are expected if all patients were exposed to the condition of interest, compared to if they were unexposed. We estimated parameters of MSMs through a weighted regression model following the inverse probability weighting (IPW) of exposure of interest [25]. Weighting by IPW method creates a pseudo-population, where each hospitalization is assigned a weight, proportional to the probability that bacteremia or sepsis occurs given the measured confounders and comorbidities. Consequently, in the pseudo-population, bacteremia and sepsis become independent of the measured confounders, which are factors that influence the development of bacteremia and sepsis and could also influence the occurrence of CV events. This redistribution of the population by weighting results in removing the confounding bias from the MSMs that are used to estimate average causal effects of exposures on the outcome in the original study population [24, 25].
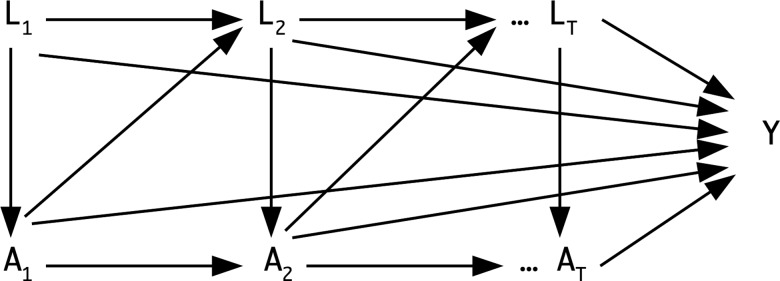
The IPW approach allows consistent estimation of causal effects in the presence of time-varying confounders [24, 26] that are affected by previous occurrences of exposure. This allows for studying long-term effects and potentially multiple episodes of an exposure by inclusion of its entire observed history, that is, including all longitudinal history of a patient's evolving characteristics and exposures prior to an occurrence of CV event or censoring. Figure 1 presents a directed acyclic graph [27] characterizing the concept of a time-varying confounder. We also estimated the IPW of censoring, which accounted for the effect of time-varying confounders and covariates on dropout from the study before the end of the follow-up period at the final hospitalization or administrative censoring at the end of study follow-up. For critical exposures such as bacteremia or sepsis and life-threatening conditions such as CV events, it is assumed that patients will be hospitalized. The IPW for censoring assigns a weight to each hospitalization, which is proportional to the probability of remaining uncensored and having the observed covariates recorded [28]. The final weight for each patient was the product of weights for all time points from the baseline to the last hospitalization for that patient. Weights were truncated at the 1st and 99th percentiles [25]. The covariates included for calculating IPW and summaries of the final stabilized weights are presented in Supplementary Tables 1 and 2, respectively.
Figure 1.
A directed acyclic graph for the time-varying confounding effects of risk factors, comorbidities, medications, and covariates that are also intermediate variables for the effects of bacteremia and sepsis on cardiovascular events. Time-varying exposures, time-varying confounders, and outcome are indicated by A, L, and Y, respectively. Subscripts indicate the time points.
Finally, we fitted weighted pooled logistic MSMs, which asymptotically approximate Cox regression models with time-dependent covariates [29], to estimate the causal effects of bacteremia and sepsis on the first occurrence of a CV event [30, 31]. In addition to the composite outcome of any first CV event, we considered first stroke or TIA, and first MI for comparison.
To test the sensitivity of our results to potential misclassification of bacteremia and sepsis status in instances where the outcome and exposures occurred during the same hospitalization, we required an order for blood cultures within 24 hours of admission to consider bacteremia and sepsis status as positive if they occurred during the same hospitalization as a CV event. We performed a second sensitivity analysis, where we defined bacteremia to also include blood cultures positive for common skin contaminants to minimize the possibility of underestimating bacteremia. All statistical analyses were performed using R language and environment for statistical computing, version 3.2.0 (R Foundation, Vienna, Austria) via the “ipw” library [32].
RESULTS
The study included 156 380 hospitalizations for 47 009 patients. Bacteremia occurred during 4923 (3.1%) hospitalizations among 3932 (8.4%) patients. There was a total of 5544 (3.5%) hospitalizations (in 4474 patients [9.5%]) with a diagnosis of sepsis of any severity; specifically, 2600 (1.7%) hospitalizations with sepsis, 1192 (0.8%) with severe sepsis, and 1752 (1.1%) with septic shock, in 2281 (4.9%), 1125 (2.4%), and 1597 (3.4%) patients, respectively.
In the final study population, first CV events were observed in 3501 (2.2%) hospitalizations (7.4% of patients); thus, the remaining 43 508 (92.6%) patients remained event-free during the study period. These included 1766 (1.1%) hospitalizations with stroke or TIA, 1834 (1.2%) hospitalizations with MI, and 99 (0.1%) hospitalizations with both stroke/TIA and MI. The proportion of patients who experienced a CV event and had at least 1 prior instance of bacteremia was 10.5% (414/3932), compared with 7.2% (3087/43 077) of patients without any prior instance of bacteremia. Among patients with at least 1 prior instance of sepsis of any severity, 12.0% (538/4474) experienced a CV event, compared with 7.0% (2963/42 535) without any prior instance of sepsis of any severity.
The characteristics of the study population are presented in Table 1. Hospitalizations in which bacteremia or any sepsis occurred were more frequent among patients with comorbidities (Table 1). Except for alcohol abuse, hospitalizations in which sepsis developed had a statistically significantly higher burden of comorbidities. There was no statistically significant difference in the frequency of hospitalizations with bacteremia or sepsis in patients who were residents of St Louis City/County and those who were potentially referred from outside the area to this tertiary care center.
Table 1.
Characteristics of the Study Population (N = 156 380)
| Characteristic | Hospitalizations With Bacteremia (n = 4923) | Hospitalizations Without Bacteremia (n = 151 457) | P Value | Hospitalizations With Sepsis (n = 5544) | Hospitalizations Without Sepsis (n = 150 836) | P Value |
|---|---|---|---|---|---|---|
| Age, y, mean, median, IQR | 56.1, 57.0, 21.0 | 53.9, 55.0, 26.0 | <.01 | 57.8, 59.0, 20.0 | 53.9, 55.0, 26.0 | <.01 |
| Male sex | 2682 (54.5) | 69 368 (45.8) | <.01 | 2983 (53.8) | 69 067 (45.8) | <.01 |
| Race/ethnicity | ||||||
| African American | 1794 (36.4) | 57 568 (38.0) | .03 | 1859 (33.5) | 57 503 (38.1) | <.01 |
| Asian | 48 (0.98) | 1182 (0.78) | .15 | 45 (0.81) | 1185 (0.79) | .89 |
| Other | 54 (1.10) | 2295 (1.52) | .02 | 68 (1.2) | 2281 (1.5) | .10 |
| White | 3027 (61.5) | 90 412 (59.7) | .01 | 3572 (64.4) | 89 867 (59.6) | <.01 |
| SIRS | 4002 (81.3) | 53 707 (35.5) | <.01 | 4975 (89.7) | 52 734 (35.0) | <.01 |
| Length of stay, d, mean, median, IQR | 16.5, 10.0, 16.0 | 5.2, 3.0, 4.0 | <.01 | 17.0, 10.6, 16.2 | 5.2, 3.0, 4.0 | <.01 |
| ICU admitted or transferred | 2053 (41.7) | 18 905 (12.5) | <.01 | 3847 (69.4) | 17 111 (11.3) | <.01 |
| Surgery | 858 (17.4) | 28 467 (18.8) | .02 | 1081 (19.5) | 28 244 (18.7) | .15 |
| Prior 30-day admission | 1853 (37.6) | 38 372 (25.3) | <.01 | 2264 (40.8) | 37 961 (25.2) | <.01 |
| St Louis City/County resident | 1236 (25.1) | 36 730 (24.3) | .17 | 1346 (24.3) | 36 620 (24.3) | 1.00 |
| Risk factors for CV disease and sepsis | ||||||
| Diabetes | 1459 (29.6) | 37 182 (24.5) | <.01 | 1616 (29.1) | 37 025 (24.5) | <.01 |
| Hypertension | 3303 (67.1) | 93 568 (61.8) | <.01 | 3851 (69.5) | 93 020 (61.7) | <.01 |
| Congestive heart failure | 962 (19.5) | 24 672 (16.3) | <.01 | 1322 (23.8) | 24 312 (16.1) | <.01 |
| Cardiac arrhythmias | 1145 (23.3) | 23 624 (15.6) | <.01 | 1766 (31.9) | 23 003 (15.3) | <.01 |
| Valvular heart disease | 390 (7.9) | 8891 (5.9) | <.01 | 485 (8.7) | 8796 (5.8) | <.01 |
| Pulmonary circulation disorders | 414 (8.4) | 7690 (5.1) | <.01 | 568 (10.2) | 7536 (5.0) | <.01 |
| Peripheral vascular disorders | 322 (6.5) | 8556 (5.6) | .01 | 449 (8.1) | 8429 (5.6) | <.01 |
| Chronic pulmonary disease | 1193 (24.2) | 36 065 (23.8) | .51 | 1484 (26.8) | 35 774 (23.7) | <.01 |
| Renal failure | 1189 (24.2) | 24 305 (16.0) | <.01 | 1457 (26.3) | 24 037 (15.9) | <.01 |
| Liver disease | 630 (12.8) | 10 229 (6.8) | <.01 | 898 (16.2) | 9961 (6.6) | <.01 |
| Coagulopathy | 965 (19.6) | 8575 (5.7) | <.01 | 1348 (24.3) | 8192 (5.4) | <.01 |
| AIDS/HIV | 140 (2.8) | 1639 (1.1) | <.01 | 86 (1.6) | 1693 (1.1) | <.01 |
| Metastatic cancer | 514 (10.4) | 13 620 (9.0) | <.01 | 554 (10.0) | 13 580 (9.0) | .01 |
| Lymphoma | 406 (8.2) | 5744 (3.8) | <.01 | 486 (8.8) | 5664 (3.8) | <.01 |
| Obesity | 400 (8.1) | 13 495 (8.9) | .06 | 549 (9.9) | 13 346 (8.8) | <.01 |
| Alcohol abuse | 289 (5.9) | 8408 (5.6) | .35 | 298 (5.4) | 8399 (5.6) | .56 |
| Medication use | ||||||
| ACE inhibitors | 946 (19.2) | 33 230 (21.9) | <.01 | 895 (16.1) | 33 281 (22.1) | <.01 |
| ARBs | 225 (4.6) | 10 516 (6.9) | <.01 | 208 (3.8) | 10 533 (7.0) | <.01 |
| β-blockers | 488 (9.9) | 15 094 (10.0) | .92 | 569 (10.3) | 15 013 (10.0) | .46 |
| CCBs | 1078 (21.9) | 31 011 (20.5) | .02 | 1149 (20.7) | 30 940 (20.5) | .71 |
| Diuretics | 2406 (48.9) | 50 069 (33.1) | <.01 | 3000 (54.1) | 49 475 (32.8) | <.01 |
| Hydralazine | 717 (14.6) | 17 352 (11.5) | <.01 | 770 (13.9) | 17 299 (11.5) | <.01 |
| Nitrates | 108 (2.2) | 2873 (1.9) | .15 | 142 (2.6) | 2839 (1.9) | <.01 |
| NSAIDs | 4395 (89.3) | 128 548 (84.9) | <.01 | 4784 (86.3) | 128 159 (85.0) | <.01 |
| Statins | 1015 (20.6) | 37 694 (24.9) | <.01 | 1129 (20.4) | 37 580 (24.9) | <.01 |
| Vasopressors | 1604 (32.6) | 13 214 (8.7) | <.01 | 2979 (53.7) | 11 839 (7.8) | <.01 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CV, cardiovascular; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; n, number of hospitalizations; NSAID, nonsteroidal anti-inflammatory drug; SIRS, systemic inflammatory response syndrome.
Table 2 presents the estimates of the marginal causal effects, expressed as odds ratios and adjusted for all confounders and comorbidities by IPW (Supplementary Table 1), of bacteremia and sepsis for CV events. Bacteremia and sepsis of any severity resulted in higher odds of experiencing all CV events. The causal odds of experiencing a CV event was 1.52-fold (95% confidence interval [CI], 1.21- to 1.90-fold) higher in patients with prior instances of bacteremia compared to those without (Table 2). Prior development of sepsis with any severity resulted in a 2.39-fold (95% CI, 1.88- to 3.03-fold) increase in the odds of experiencing a CV event, with the highest odds ratio of 6.91 (95% CI, 5.34–8.93) for the development of MI in patients with prior instances of septic shock (Table 2).
Table 2.
Estimates of the Causal Odds Ratio, Adjusted for Measured Confounders and Comorbidities by the Inverse Probability Weighting, of Bacteremia and Sepsis for Subsequent Any Cardiovascular Event, Stroke or Transient Ischemic Attack, and Myocardial Infarction
| Exposure | Causal Odds Ratioa (95% CI) |
||
|---|---|---|---|
| Any CV Event | Stroke or TIA | MI | |
| Bacteremia | 1.52 (1.21–1.90) | 1.00 (.70–1.44) | 2.00 (1.55–2.60) |
| Any sepsis | 2.39 (1.88–3.03) | 1.24 (.86–1.79) | 3.29 (2.49–4.36) |
| Sepsis | 1.99 (1.50–2.63) | 1.15 (.75–1.77) | 2.63 (1.89–3.65) |
| Severe sepsis | 3.60 (2.59–5.00) | 1.53 (1.06–2.21) | 4.99 (3.35–7.45) |
| Septic shock | 4.55 (3.58–5.78) | 1.64 (1.03–2.63) | 6.91 (5.34–8.93) |
Abbreviations: CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; TIA, transient ischemic attack.
a Covariates included for calculating inverse probability weighting are presented in Supplementary Table 1.
There were 620 (0.4%) and 1062 (0.7%) hospitalizations in which bacteremia and sepsis of any severity developed concurrent with a CV event, respectively. To increase the likelihood that exposures preceded a CV event, we considered 69 (0.04%) and 76 (0.05%) hospitalizations in patients who developed bacteremia and sepsis of any severity without a physician's order for blood culture within 24 hours of admission as negative for bacteremia in our sensitivity analysis, respectively. The results of the first sensitivity analysis suggested the same conclusion (ie, same direction) as the results of the primary analysis, except for the effect of nonsevere sepsis and bacteremia on the development of stroke or TIA that did not reach statistical significance (Supplementary Table 3). In the second sensitivity analysis, we considered an additional 3920 (2.5%) hospitalizations in patients with a positive blood culture for a common skin contaminant as having bacteremia. Including these patients resulted in bacteremia occurring during 8843 (5.7%) hospitalizations among 6646 (14.1%) patients in the second sensitivity analysis. The results of the second sensitivity analysis also indicated the same conclusions as the results of the primary analysis; however, the estimates for the causal odds ratios were marginally higher in the sensitivity analysis (Supplementary Table 4).
DISCUSSION
We found that development of either bacteremia or sepsis resulted in a 1.52- to 6.91-fold increase in the odds of developing subsequent CV events, with the largest effect observed for septic shock and its impact on subsequent MI. This elevated risk of developing a subsequent CV event was consistent across all sepsis severity levels as well as with confirmed bacteremia, and, with the exception of nonsevere sepsis or bacteremia and stroke/TIA, was robust to the sensitivity analyses.
These findings are important, given reports indicating that the incidence of CV events is sometimes insufficiently explained by traditional risk factors, which may be lacking in some populations such as adults ≤45 years of age [33]. Prevention or rigorous monitoring for sepsis and bacteremia therefore represents an additional area for targeted interventions, to prevent CV events especially in high-risk groups such as elderly persons, patients receiving immunosuppressant drugs, or transplant recipients. The added burden of CV events as outcomes of sepsis should be taken into account for defining strategies and policies to prevent sepsis. The downstream effects of sepsis not only manifest in increased rates of disability and cognitive impairment, but also may have untoward effects on CV and neurological health, as we have shown, which could have a significant impact on public health in the United States.
One distinct feature of this study is that we accounted for the contribution of all instances of bacteremia or sepsis prior to a CV event, rather than restricting the analysis to the first or the last instance of exposure. We appropriately adjusted for the time-varying confounding effects of evolving comorbidities and covariates [24, 26]. We included a large patient population that was not limited to an ICU, which allowed us to classify CV events as stroke/TIA and MI in addition to studying the composite outcome of any CV event. In addition to the explicit ICD-9-CM codes for sepsis, our study included bacteremia based on positive blood cultures, which, despite its imperfect accuracy, is not subject to the inherent inaccuracy of medical coding in administrative billing data [34, 35].
Some past studies have suggested an association between infection and stroke and coronary syndromes [15, 16]. Walkey et al reported a 2.7-fold increase in the odds of in-hospital stroke in patients with severe sepsis who developed atrial fibrillation [10]. Donzé et al reported a 3.3- and 5.7-fold increase in the odds of postoperative thromboses in patients with preoperative sepsis and severe sepsis, respectively [13]. Community-acquired bacteremia has been reported to increase the risk of short-term CV events by 20.9-fold [12]. Unlike the longitudinal design in our study, other studies used case-crossover [5–9], case-control [3, 5], or cohort [4, 8, 10–13] designs that are less suitable to study the long-term and cumulative effects of bacteremia and sepsis on subsequent CV events.
Our work can be extended, for example, to study the effects of specific infectious agents or specific clinical conditions such as pneumonia, urinary tract infection, or postsurgical infection on more specific outcomes such as stroke type. Another important area of research is to identify the specific causal pathways that lead bacteremia and sepsis to exert their long-term effects on CV events through a causal mediation analysis [36]. Moreover, it is important to identify factors such as genetic factors or comorbid conditions that may have mechanistic interactions with bacteremia and sepsis for occurrences of CV events, especially those factors that may act as sufficient causes [37, 38].
Our study had some limitations. We relied on administrative data for identification of comorbidities and CV events; however, we used validated algorithms [1, 20] whenever possible to minimize potential misclassification bias. Our analytic approach was not directed to and could not compare the timing of (early vs late) CV events after a specific episode of bacteremia or sepsis. It was not possible to compare the risk of CV events following a single episode with those with multiple episodes of bacteremia or sepsis. Validity of estimates in MSMs relies on the correct specification of exposure, censoring, and final structural models to satisfy the conditional exchangeability (ie, no unmeasured confounding), consistency (ie, well-defined treatment), and positivity (ie, positive probability of exposure at every level of confounders) assumptions [25]. Similar to the traditional regression models for outcomes, our analytic approach is not robust to model misspecification and unmeasured confounders [39]. Potential unmeasured confounders such as proinflammatory markers or imperfect adherence to outpatient-administered medications could bias the risk estimates. Even though we used the existing consensus definition, the consistency assumption may be harder to conceptualize for a clinical condition such as sepsis. We could not rule out previous CV events prior to the first hospitalization at our hospital. Our study population was limited to our tertiary care academic center. However, we theoretically adjusted for the fact that some patients might have developed sepsis or bacteremia or experienced CV events in (or were referred to) other healthcare facilities using IPW for censoring. Finally, we note that our study is a single-center study that is not subject to the random variations and clustering effects (eg, differential care quality in academic/specialty vs community hospitals) that could inherently arise in a multicenter observational study.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Author contributions. S. R. J. and B. S. T. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S. R. J., B. S. T., and V. J. F. conceptualized and designed the study. S. R. J. drafted the manuscript. All authors critically revised the manuscript for important intellectual content. V. J. F., J. G., and D. K. W. supervised the study.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH).
Financial support. This work was supported by the Prevention Epicenters Program of the CDC (grant number U54 CK000162) and the Washington University Institute of Clinical and Translational Sciences from the National Center for Advancing Translational Sciences (grant number UL1 TR000448). V. J. F. reports grant funding from the CDC and the NIH.
Potential conflicts of interest. D. K. W. has received fees for consultancy from Centene Corporation and Worrell, Inc., and acted as a subinvestigator on a industry-sponsored study outside this submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Balady GJ, Criqui MH et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation 1998; 97:1876–87. [DOI] [PubMed] [Google Scholar]
- 3.Roivainen M, Viik-Kajander M, Palosuo T et al. Infections, inflammation, and the risk of coronary heart disease. Circulation 2000; 101:252–7. [DOI] [PubMed] [Google Scholar]
- 4.Smieja M, Gnarpe J, Lonn E et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2003; 107:251–7. [DOI] [PubMed] [Google Scholar]
- 5.Paganini-Hill A, Lozano E, Fischberg G et al. Infection and risk of ischemic stroke: differences among stroke subtypes. Stroke J Cereb Circ 2003; 34:452–7. [DOI] [PubMed] [Google Scholar]
- 6.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004; 351:2611–8. [DOI] [PubMed] [Google Scholar]
- 7.Corrales-Medina VF, Fatemi O, Serpa J et al. The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis 2009; 41:511–4. [DOI] [PubMed] [Google Scholar]
- 8.Corrales-Medina VF, Serpa J, Rueda AM et al. Acute bacterial pneumonia is associated with the occurrence of acute coronary syndromes. Medicine (Baltimore) 2009; 88:154–9. [DOI] [PubMed] [Google Scholar]
- 9.Elkind MSV, Carty CL, O'Meara ES et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke J Cereb Circ 2011; 42:1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011; 306:2248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation 2012; 125:773–81. [DOI] [PubMed] [Google Scholar]
- 12.Dalager-Pedersen M, Søgaard M, Schønheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation 2014; 129:1387–96. [DOI] [PubMed] [Google Scholar]
- 13.Donzé JD, Ridker PM, Finlayson SRG, Bates DW. Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ 2014; 349:g5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrales-Medina VF, Alvarez KN, Weissfeld LA et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313:264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 2010; 10:83–92. [DOI] [PubMed] [Google Scholar]
- 16.Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. Infectious causes of stroke. Lancet Infect Dis 2014; 14:869–80. [DOI] [PubMed] [Google Scholar]
- 17.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133:144–53. [DOI] [PubMed] [Google Scholar]
- 18.Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. hospitals, 2009: statistical brief no. 122. In: Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Health Care Policy and Research, 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK65391/ Accessed 27 March 2014.
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 21.Edwards JR, Pollock DA, Kupronis BA et al. Making use of electronic data: the National Healthcare Safety Network eSurveillance Initiative. Am J Infect Control 2008; 36:S21–6. [DOI] [PubMed] [Google Scholar]
- 22.Bone RC, Balk RA, Cerra FB et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–55. [DOI] [PubMed] [Google Scholar]
- 23.Kaukonen K-M, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; 372:1629–38. [DOI] [PubMed] [Google Scholar]
- 24.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
- 25.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008; 168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel RM, Cousens SN, De Stavola BL, Kenward MG, Sterne JAC. Methods for dealing with time-dependent confounding. Stat Med 2013; 32:1584–618. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10:37–48. [PubMed] [Google Scholar]
- 28.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013; 22:278–95. [DOI] [PubMed] [Google Scholar]
- 29.D'Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 1990; 9:1501–15. [DOI] [PubMed] [Google Scholar]
- 30.Abbott RD. Logistic regression in survival analysis. Am J Epidemiol 1985; 121:465–71. [DOI] [PubMed] [Google Scholar]
- 31.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 32.van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. J Stat Softw 2011; 43:1–23. [Google Scholar]
- 33.Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nat Rev Neurol 2010; 6:681–94. [DOI] [PubMed] [Google Scholar]
- 34.Jafarzadeh SR, Thomas BS, Marschall J, Fraser VJ, Gill J, Warren DK. Quantifying the improvement in sepsis diagnosis, documentation, and coding: the marginal causal effect of year of hospitalization on sepsis diagnosis. Ann Epidemiol 2016; 26:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas BS, Jafarzadeh SR, Warren DK, McCormick S, Fraser VJ, Marschall J. Temporal trends in the systemic inflammatory response syndrome, sepsis, and medical coding of sepsis. BMC Anesthesiol 2015; 15:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol 2012; 176:190–5. [DOI] [PubMed] [Google Scholar]
- 37.Vanderweele TJ, Vansteelandt S, Robins JM. Marginal structural models for sufficient cause interactions. Am J Epidemiol 2010; 171:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanderWeele TJ, Tchetgen Tchetgen EJ. Attributing effects to interactions. Epidemiology 2014; 25:711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.