Injectable antiretrovirals are anticipated for preexposure prophylaxis for human immunodeficiency virus prevention, but their cost-effectiveness in resource-limited settings is unclear. Our model-based analyses suggest that prioritized scale-up of injectable preexposure prophylaxis in KwaZulu-Natal, South Africa, could be very cost-effective and potentially cost-saving.
Keywords: HIV infection, preexposure prophylaxis (PrEP), HIV prevention intervention, mathematical model, cost-effectiveness
Abstract
Background. Long-acting injectable antiretrovirals such as rilpivirine (RPV) could promote adherence to preexposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) prevention. However, the cost-effectiveness of injectable PrEP is unclear.
Methods. We constructed a dynamic model of the heterosexual HIV epidemic in KwaZulu-Natal, South Africa, and analyzed scenarios of RPV PrEP scale-up for combination HIV prevention in comparison with a reference scenario without PrEP. We estimated new HIV infections, life-years and costs, and incremental cost-effectiveness ratios (ICERs), over 10-year and lifetime horizons, assuming a societal perspective.
Results. Compared with no PrEP, unprioritized scale-up of RVP PrEP covering 2.5%–15% of adults prevented up to 9% of new infections over 10 years. HIV prevention doubled (17%) when the same coverage was prioritized to 20- to 29-year-old women, costing $10 880–$19 213 per infection prevented. Prioritization of PrEP to 80% of individuals at highest behavioral risk achieved comparable prevention (4%–8%) at <1% overall coverage, costing $298–$1242 per infection prevented. Over lifetime, PrEP scale-up among 20- to 29-year-old women was very cost-effective (<$1600 per life-year gained), dominating unprioritized PrEP, while risk prioritization was cost-saving. PrEP's 10-year impact decreased by almost 50% with increases in ICERs (up to 4.2-fold) in conservative base-case analysis. Sensitivity analysis identified PrEP's costs, efficacy, and reliability of delivery as the principal drivers of uncertainty in PrEP's cost-effectiveness, and PrEP remained cost-effective under the assumption of universal access to second-line antiretroviral therapy.
Conclusions. Compared with no PrEP, prioritized scale-up of RPV PrEP in KwaZulu-Natal could be very cost-effective or cost-saving, but suboptimal PrEP would erode benefits and increase costs.
In 2014 alone, there were 2 million new human immunodeficiency virus (HIV) infections and 1.2 million AIDS-related deaths globally [1]. With >6 million infected individuals, South Africa bears an outsized share of the global burden, especially in provinces like KwaZulu-Natal where HIV prevalence among adults approaches 30% [2].
Antiretroviral therapy (ART) reduces HIV morbidity, mortality, and transmission [3, 4]. While ART scale-up is gaining momentum [5], only a third of South African HIV-infected persons had access to ART and more than half were unaware of their infection in 2012 [2]. Male medical circumcision (MMC) and condoms are efficacious for HIV prevention, but their demand and suitability may be limited [2, 6]. Thus, there is an unmet need for interventions to prevent HIV. Antiretroviral preexposure prophylaxis (PrEP) is a safe and efficacious biomedical intervention against HIV acquisition [7–9]. However, implementation of PrEP in resource-limited settings is slow [10], primarily due to concerns about suboptimal adherence and costs, alongside uncertainty regarding optimal scale-up strategies.
Long-acting injectable antiretrovirals that require infrequent dosing, such as rilpivirine (RPV), a second-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), are being investigated as PrEP agents [11, 12]. In addition to potentially improving adherence, long-acting PrEP would be a novel, discreet HIV prevention method; however, its role in combination HIV prevention is unclear, particularly in resource-limited settings. Given the above rationale, we constructed and analyzed a mathematical model to estimate the health outcomes, costs, and cost-effectiveness of RPV PrEP scale-up in KwaZulu-Natal, South Africa. We simulated optimistic and conservative base-case scenarios of RPV PrEP scale-up in combination with ART and MMC, using strategies of PrEP implementation in the general or specific at-risk populations. We compared the health outcomes and costs with a reference scenario without PrEP, and examined the sensitivity of model outputs to uncertainty in model inputs and modeling assumptions.
METHODS
Model Design
We developed a detailed mathematical model to simulate the HIV epidemic in KwaZulu-Natal. The model population was stratified by sex, age (15–54 years), sexual behavior (4 sexual activity levels, the highest representing female sex workers and male clients), infection status, HIV disease progression (6 stages, stratified by CD4 cell counts), intervention status, and HIV drug susceptibility (drug-sensitive wild-type or resistant to first-generation NNRTIs, to RPV, or to both, ie, cross-resistant). HIV transmission was represented through heterosexual contact influenced by mixing patterns and behavioral factors including condom use. The model was calibrated using Bayesian methods to longitudinal HIV incidence [13] and prevalence [14] data from KwaZulu-Natal and cross-sectional behavioral risk-stratified HIV prevalence data from South Africa [15]. Model details are provided in the Supplementary Data.
Interventions
Our reference scenario was based on South Africa's National Strategic Plan [16] and HIV management guidelines [17]—that is, scaling up to 80% coverage of MMC by 2017 and ART by 2020 using a CD4 threshold ≤500 cells/µL, with maintenance thereafter. We assumed that MMC reduced the risk of HIV acquisition in men by 60% [18] and that suppressive ART reduced the transmission risk by 73%–99% [3] and prolonged survival among HIV-infected persons [4]. PrEP scenarios assumed the scale-up of PrEP in combination with ART and MMC.
Model-Based Analyses
Base-Case Analysis
We defined 2 PrEP scenarios: optimistic and conservative (Table 1). For the optimistic scenario we assumed the following: 90% PrEP efficacy [8, 9]; 80% PrEP reliability of delivery (ie, 80% of injections successfully yielded efficacious drug levels, whereas 20% were nonsuccessful); potential selection of PrEP resistance with breakthrough infection after a successful injection [21], but not after a nonsuccessful injection; 40% cross-resistance prevalence between the NNRTI component of ART and RPV [12, 20]; and 0%–50% PrEP efficacy against PrEP-resistant virus. We made less optimistic assumptions for the conservative scenario: 70% efficacy, reliability and cross-resistance prevalence; and potential selection of resistance with breakthrough infection after both successful and nonsuccessful injections (Table 1). Within each PrEP scenario, we simulated 3 different PrEP scale-up strategies: unprioritized PrEP for 2.5%–15% of uninfected adults regardless of age, sex, or sexual behavior; age-prioritized PrEP for 2.5%–15% of uninfected adults, achieving 15%–85% corresponding coverage among women aged 20–29 years (as our model and data [2, 14] suggest that HIV incidence is highest among women aged 20–29 in KwaZulu-Natal [approximately 4%] and South Africa [3.12%; 95% confidence interval, 2.75%–3.50%]); and risk-prioritized PrEP that covered 80% of uninfected female sex workers and male clients but reached only 0.8% in overall (population-level) coverage due to the group's small size (0.4% of women and 2.1% of men) and high HIV prevalence (57% at 2015). Persons enrolled in PrEP received 6 injections per year for 5 years, though a cumulative 40% dropped out early. HIV testing occurred at PrEP enrollment and twice annually thereafter; persons with detected HIV stopped PrEP immediately. PrEP scale-up began at 2015, reached its coverage target over 5 years, and was then maintained until 2025.
Table 1.
Key Intervention-Related Parameters
| Parameter | Base-Case Value Optimistic, Conservative | LHS Range | Source |
|---|---|---|---|
| MMC | |||
| Target coverage among sexually active men, % | 80 | 23–80 | [2, 16] |
| Effectiveness against male HIV acquisition, % | 60 | 60 | [18] |
| ART | |||
| ART coverage of CD4 ≤500 cells/µL at 2020, % | 80 | 65–80 | [16, 17] |
| ART effectiveness against HIV transmission while suppressed, % | 96 | 73–99 | [3] |
| PrEP | |||
| Year PrEP scale-up begins | 2015 | 2015–2020 | Assumed |
| Time to reach target coverage, y | 5 | 2.5–7.5 | Assumed |
| Intended duration of personal use, y | 5 | 2.5–7.5 | Assumed |
| Proportion who drop out of early, % | 40 | 5–60 | [19] |
| Injection frequency, per year | 6 | 6 | [19] |
| HIV testing frequency in the PrEP program, per year | 2 | 1–6 | Assumed |
| Reliability (proportion of injections that are efficacious), % | 80, 70 | 50–99 | Assumed |
| Efficacy | |||
| Efficacy against wild-type HIV or ART-resistant HIV without PrEP cross-resistance, % | 90, 70 | 50–99 | [8, 9] |
| Efficacy against ART-resistant HIV with PrEP cross-resistance (≥10-fold change in RPV IC90), % | 0 | 0 | [20] |
| Efficacy against ART-resistant HIV with PrEP cross-resistance (3- to 9-fold change in RPV IC90), % | 45, 35 | 0–50 | [20] |
| Efficacy against PrEP-resistant HIV, % | 22.5, 0 | 0–50 | Assumed |
| Time until resistance emergence in a cohort on PrEP | |||
| Time until emergence if breakthrough infection occurs after a successful injection, wk | 4 | 2–6 | [21] |
| Rate resistance emerges if breakthrough occurs after a nonsuccessful injection, relative to after successful injection, % | 0, 100 | 0–100 | Assumed |
| Cost | |||
| PrEP costs (drug costs plus diagnostics and overhead), $ per person-year | 250 | 150–350 | [22] |
| MMC cost, per surgery | 110 | 70–150 | [23] |
| ART costs (drug costs plus diagnostics and overhead), $ per person-year | 750 | 460–1040 | [24] |
Additional ART-related inputs and cost parameters are given in Supplementary Table 3.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IC90, inhibitory concentration required to reduce virus replication 90%; LHS, Latin hypercube sampling; MMC, male medical circumcision; PrEP, preexposure prophylaxis; RPV, rilpivirine.
Uncertainty and Sensitivity Analyses
We conducted multivariate sensitivity analysis to determine the effect of random variation in model inputs on projected outcomes [25]. For this we performed 20 000 simulations of the reference scenario and each PrEP strategy, with intervention-related inputs drawn via Latin hypercube sampling. Using data from these simulations, we determined outcomes' medians and interquartile ranges (IQRs), to measure output uncertainty. Next, we calculated standardized regression coefficients, to measure the influence of model inputs on outputs. Finally, we estimated response surfaces using bivariate linear regression with interaction terms, to visualize the interactions between key inputs.
Our base-case analysis assumed only first-line ART scale-up, as second-line access is currently limited in sub-Saharan Africa, including South Africa [26]. As this may change, we performed a structural sensitivity analysis in which base-case simulations assumed second-line ART scale-up reaching universal access by 2020 [5].
Outcomes and Costs
We assumed a modified societal perspective that excluded time and productivity costs, and 2 different simulation time horizons: projections over a 10-year period of PrEP intervention and projections over a lifetime. Lifetime horizon outcomes were determined among the population extant during the intervention period, embedded within the overall population. We estimated cumulative new HIV infections, life-years lived, and costs. We included costs associated with MMC, HIV testing, HIV-related care and treatment, and baseline medical costs using published literature from South Africa [23, 24]. PrEP costs included the costs of HIV testing, laboratory testing, facilities and personnel costs, and drug-related costs. The per-person annual cost of injectable PrEP is currently unknown; hence, we assumed this conservatively as equivalent to the cost of generic current oral daily PrEP ($250 per person-year) [22]. We did not include the costs of identifying and reaching specific populations. We employed gross domestic product (GDP) deflators for South Africa [27] and the average 2012 exchange rate for converting all currencies to 2012 US dollars [28].
We computed incremental cost-effectiveness ratios (ICERs) as the change in cost divided by the change in health outcome (infections and life-years lived) for the different PrEP scenarios compared with the reference scenario without PrEP. We used South Africa's 2012 GDP ($7500 [29]) to define PrEP interventions as cost-effective (ICER for life-year gained <3 times the GDP) or very cost-effective (ICER for life-year gained less than the GDP). A PrEP intervention was deemed cost-saving if it decreased total costs and increased life-years. For estimating ICERs, we discounted costs, infections and life-years, at 3% annually.
RESULTS
Intervention Horizon
Health Outcomes
Without PrEP expansion, our model projected that 0.7 million undiscounted new infections would occur in KwaZulu-Natal during 2015–2025. At 15% (uppermost) overall coverage, optimistic unprioritized PrEP prevented 9.1% of new infections (Table 2), which approximately doubled (17.2%) with age prioritization (covering 85% of women aged 20–29 years). PrEP prioritized to 80% of the individuals at highest behavioral risk had impact (8.1%) comparable to unprioritized PrEP, but with <1% overall coverage. Prevention was almost halved with conservative PrEP: 5.5% when unprioritized, 10.3% when age-prioritized, and 4.4% when risk-prioritized. All PrEP strategies improved overall survival compared to the reference scenario, but 10-year gains were modest (<13 000 discounted life-years) owing to the lag in survival benefit from HIV prevention.
Table 2.
Ten-Year Costs and Outcomes of Base-Case Preexposure Prophylaxis Strategies
| Scenario | HIV Infectionsa |
Life-yearsb |
Cost, Million $b |
ICERb |
||||
|---|---|---|---|---|---|---|---|---|
| New Cases, No. | IP, % | Lived, Thousands | LYG, No. | Total Cost | Cost Increase | $/IP | $/LYG | |
| Reference (no PrEP) | 682 875 | 55 480 | 20 004 | |||||
| Optimistic scenario | ||||||||
| Unprioritized PrEP, 2.5% | 672 053 | 1.6 | 55 481 | 1065 | 20 193 | 188 | 20 905 | 176 755 |
| Unprioritized PrEP, 5% | 661 434 | 3.1 | 55 482 | 2121 | 20 382 | 377 | 21 124 | 177 749 |
| Unprioritized PrEP, 10% | 640 791 | 6.2 | 55 484 | 4207 | 20 761 | 756 | 21 569 | 179 739 |
| Unprioritized PrEP, 15% | 620 926 | 9.1 | 55 486 | 6257 | 21 142 | 1137 | 22 022 | 181 734 |
| Age-prioritized PrEP, 2.5% | 663 108 | 2.9 | 55 482 | 2130 | 20 184 | 180 | 10 880 | 84 418 |
| Age-prioritized PrEP, 5% | 643 476 | 5.8 | 55 484 | 4261 | 20 365 | 361 | 10 945 | 84 624 |
| Age-prioritized PrEP, 10% | 604 433 | 11.5 | 55 488 | 8547 | 20 729 | 725 | 11 046 | 84 820 |
| Age-prioritized PrEP, 15% | 565 261 | 17.2 | 55 493 | 12 832 | 21 097 | 1092 | 11 094 | 85 105 |
| Risk-prioritized PrEP | 627 257 | 8.1 | 55 484 | 4341 | 20 018 | 13.6 | 298 | 3144 |
| Conservative scenario | ||||||||
| Unprioritized PrEP, 2.5% | 676 308 | 1.0 | 55 480 | 695 | 20 197 | 192 | 35 090 | 276 605 |
| Unprioritized PrEP, 5% | 669 885 | 1.9 | 55 481 | 1384 | 20 389 | 385 | 35 494 | 278 240 |
| Unprioritized PrEP, 10% | 657 454 | 3.7 | 55 483 | 2739 | 20 776 | 771 | 36 310 | 281 510 |
| Unprioritized PrEP, 15% | 645 558 | 5.5 | 55 484 | 4069 | 21 163 | 1159 | 37 137 | 284 781 |
| Age-prioritized PrEP, 2.5% | 670 743 | 1.8 | 55 481 | 1405 | 20 192 | 187 | 18 429 | 133 428 |
| Age-prioritized PrEP, 5% | 658 795 | 3.5 | 55 483 | 2807 | 20 380 | 376 | 18 595 | 133 808 |
| Age-prioritized PrEP, 10% | 635 344 | 7.0 | 55 485 | 5604 | 20 758 | 754 | 18 893 | 134 532 |
| Age-prioritized PrEP, 15% | 612 623 | 10.3 | 55 488 | 8357 | 21 138 | 1134 | 19 213 | 135 695 |
| Risk-prioritized PrEP | 652 662 | 4.4 | 55 482 | 2690 | 20 036 | 31.1 | 1242 | 11 568 |
Abbreviations: HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; IP, infections prevented; LYG, life-years gained; PrEP, preexposure prophylaxis.
a Undiscounted new infections and infections prevented are shown.
b Costs and life-years shown are discounted 3% annually. Cost and health outcomes are discounted 3% annually in ICER calculations. ICERs are calculated relative to the reference scenario.
Drug Resistance
ART scale-up generated 440 000 prevalent drug-resistant infections (33% of prevalent HIV infections) at 2025 in the reference scenario. PrEP strategies decreased prevalent drug-resistant infections by 0.1%–4.5% in the optimistic scenario, but increased resistance by 0.3%–5.1% in the conservative scenario (Supplementary Table 4). Unprioritized conservative PrEP produced 1 additional drug-resistant infection for every 854–892 person-years of PrEP. These ratios were less favorable (372–389) for age-prioritized PrEP, whereas risk-prioritized PrEP yielded 1 additional drug-resistant infection for every 13 person-years of PrEP deployed.
Cost-effectiveness
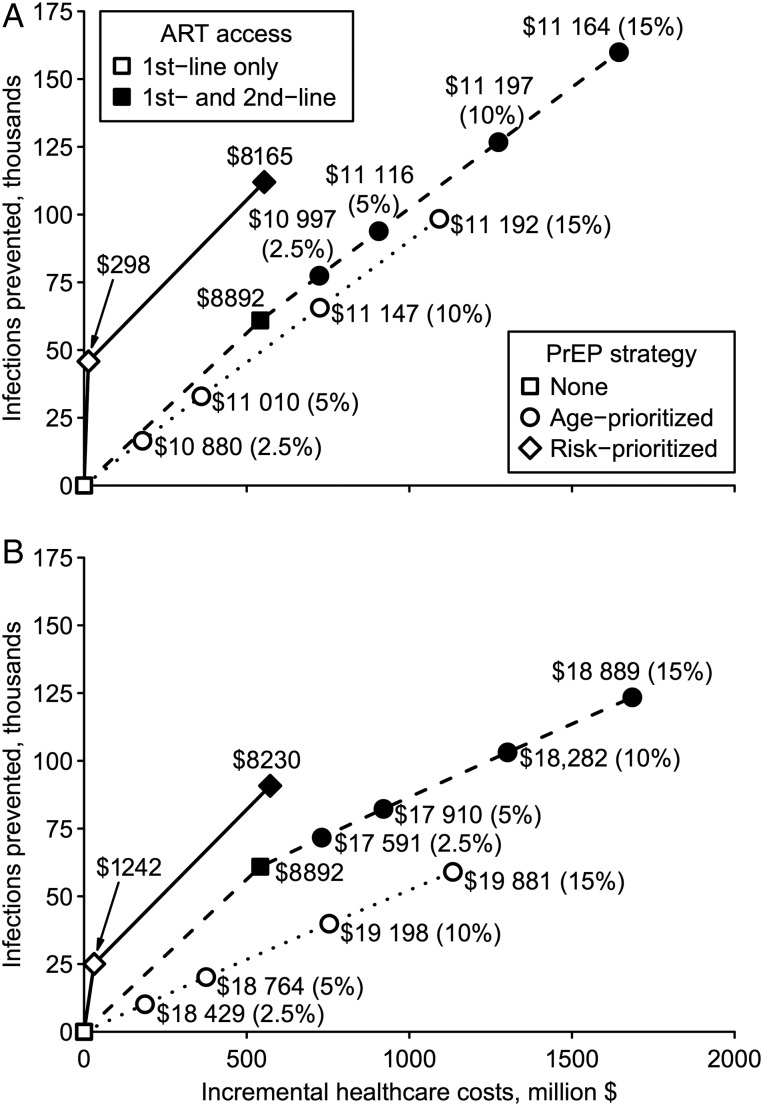
Age-prioritized PrEP dominated unprioritized scale-up considering costs per either infections prevented or life-years gained, while ICERs were lowest for risk-prioritized PrEP (Figure 1; Supplementary Figure 2). Compared to the reference scenario, 2.5%–15% unprioritized PrEP coverage cost $20 905–$37 137 per infection prevented across optimistic and conservative scenarios. By contrast, 2.5% optimistic (conservative) age-prioritized PrEP coverage cost $10 880 ($18 429) per infection prevented; ICERs rose modestly with increasing age-prioritized coverage levels, reaching $11 094 ($19 213) per infection prevented at 15% coverage. Risk prioritization minimized the cost per infection prevented (optimistic: $298; conservative: $1242). ICERs for life-years gained were considerably higher than those for HIV prevention. Unprioritized PrEP cost $176 755–$284 781 per life-year gained, while age-prioritized PrEP cost $84 418–$135 695, nevertheless, risk-prioritized PrEP was cost-effective ($11 568 per life-year gained) when conservative, and very cost-effective ($3144) when optimistic (Table 2).
Figure 1.
Cost-effectiveness of preexposure prophylaxis (PrEP) over 10 years. Incremental costs and infections prevented are plotted for each PrEP strategy in optimistic (A) and conservative (B) scenarios. Optimistic (conservative) scenario assumptions are: 90% (70%) PrEP efficacy vs wild-type human immunodeficiency virus (HIV), 0%–50% relative efficacy vs rilpivirine-resistant HIV, 80% (70%) PrEP reliability, 40% (70%) cross-resistance between antiretroviral therapy (ART) and PrEP, and successful (all) PrEP injections select drug-resistant HIV after breakthrough infection. The origin corresponds to the reference scenario without PrEP. Lines correspond to the cost-effectiveness frontiers, labeled with incremental cost per infection prevented relative to the next best strategy. Frontiers are shown for base-case simulations (dotted line), structural sensitivity analysis including second-line ART (dashed line), and risk-prioritized PrEP (solid line). Age-prioritized PrEP coverage levels are stated in parentheses. Interventions not on these frontiers are not shown.
Lifetime Horizon
Health Outcomes
PrEP's preventive benefit decreased by 50%–70% following cessation of PrEP implementation at 2025, whereas the survival benefit from PrEP continued to increase (Table 3). At 15% coverage, 528 065 life-years were gained with optimistic unprioritized PrEP, which nearly doubled (1 032 275 life-years gained) when age-prioritized; these gains were about half as large (253 610 and 489 307 life-years, respectively) with conservative PrEP. Gains from risk prioritization were 467 454 and 147 148 life-years in optimistic and conservative scenarios, respectively.
Table 3.
Lifetime Costs and Outcomes of Base-Case Preexposure Prophylaxis Strategies
| Scenario | HIV Infectionsa |
Life-yearsb |
Cost, Million $b |
ICERb |
||||
|---|---|---|---|---|---|---|---|---|
| New Cases, No. | IP, % | Lived, Thousands | LYG, No. | Total Cost | Cost Increase | $/IP | $/LYG | |
| Reference (no PrEP) | 1 715 802 | 163 595 | 54 759 | |||||
| Optimistic scenario | ||||||||
| Unprioritized PrEP, 2.5% | 1 702 971 | 0.7 | 163 686 | 91 480 | 54 893 | 134 | 12 982 | 1470 |
| Unprioritized PrEP, 5% | 1 690 331 | 1.5 | 163 776 | 181 562 | 55 029 | 270 | 13 153 | 1489 |
| Unprioritized PrEP, 10% | 1 665 622 | 2.9 | 163 952 | 357 560 | 55 305 | 546 | 13 502 | 1528 |
| Unprioritized PrEP, 15% | 1 641 672 | 4.3 | 164 123 | 528 065 | 55 587 | 828 | 13 857 | 1568 |
| Age-prioritized PrEP, 2.5% | 1 693 442 | 1.3 | 163 767 | 171 926 | 54 839 | 81 | 4420 | 470 |
| Age-prioritized PrEP, 5% | 1 671 067 | 2.6 | 163 938 | 343 418 | 54 921 | 163 | 4451 | 474 |
| Age-prioritized PrEP, 10% | 1 626 063 | 5.2 | 164 281 | 686 382 | 55 087 | 328 | 4485 | 479 |
| Age-prioritized PrEP, 15% | 1 580 162 | 7.9 | 164 627 | 1 032 275 | 55 251 | 493 | 4459 | 477 |
| Risk-prioritized PrEP | 1 647 881 | 4.0 | 164 062 | 467 454 | 54 443 | –315 | CS | CS |
| Conservative scenario | ||||||||
| Unprioritized PrEP, 2.5% | 1 709 004 | 0.4 | 163 639 | 44 543 | 54 918 | 159 | 27 866 | 3578 |
| Unprioritized PrEP, 5% | 1 702 352 | 0.8 | 163 683 | 88 151 | 55 078 | 320 | 28 261 | 3628 |
| Unprioritized PrEP, 10% | 1 689 476 | 1.5 | 163 767 | 172 637 | 55 403 | 644 | 29 061 | 3731 |
| Unprioritized PrEP, 15% | 1 677 151 | 2.3 | 163 848 | 253 610 | 55 731 | 973 | 29 873 | 3836 |
| Age-prioritized PrEP, 2.5% | 1 703 761 | 0.7 | 163 680 | 84 941 | 54 881 | 123 | 11 941 | 1444 |
| Age-prioritized PrEP, 5% | 1 691 899 | 1.4 | 163 763 | 168 507 | 55 005 | 247 | 12 106 | 1465 |
| Age-prioritized PrEP, 10% | 1 668 632 | 2.7 | 163 927 | 331 820 | 55 258 | 500 | 12 401 | 1506 |
| Age-prioritized PrEP, 15% | 1 646 111 | 4.1 | 164 084 | 489 307 | 55 517 | 758 | 12 721 | 1549 |
| Risk-prioritized PrEP | 1 690 608 | 1.5 | 163 742 | 147 148 | 54 610 | –149 | CS | CS |
Abbreviations: CS, cost-saving; HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; IP, infections prevented; LYG, life-years gained; PrEP, preexposure prophylaxis.
a Undiscounted new infections and infections prevented are shown.
b Costs and life-years shown are discounted 3% annually. Cost and health outcomes are discounted 3% annually in ICER calculations. ICERs shown are calculated relative to the reference scenario.
Cost-effectiveness
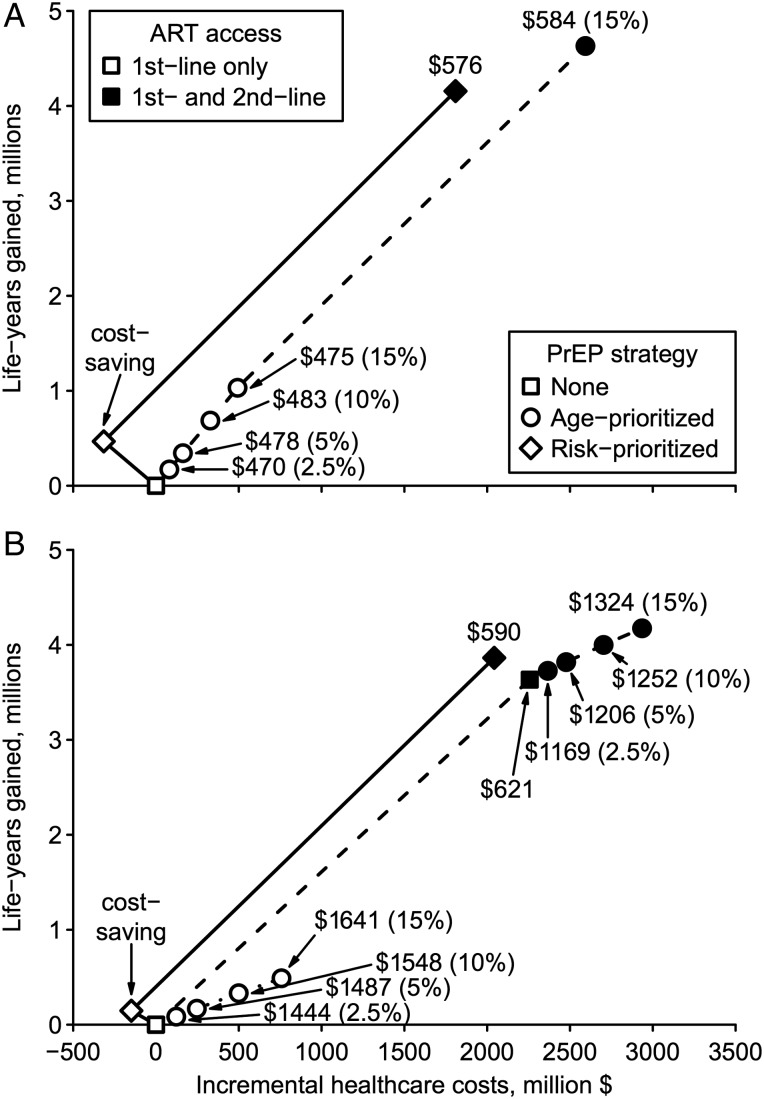
Survival cost-effectiveness ratios dramatically improved over the lifetime (Table 3) compared to intervention horizon (Table 2). Age-prioritized PrEP was very cost-effective at $470–$1549 per life-year gained relative to the reference scenario, dominating unprioritized PrEP (Figure 2; Supplementary Figure 3). Meanwhile, risk-prioritized PrEP reduced total costs compared to the reference scenario, and thus was cost-saving in both optimistic and conservative scenarios.
Figure 2.
Lifetime cost-effectiveness of preexposure prophylaxis (PrEP). Incremental costs and life-years gained are plotted for each PrEP strategy in optimistic (A) or conservative (B) scenarios. Optimistic (conservative) scenario assumptions are: 90% (70%) PrEP efficacy vs wild-type human immunodeficiency virus (HIV), 0%–50% relative efficacy vs rilpivirine-resistant HIV, 80% (70%) PrEP reliability, 40% (70%) cross-resistance between antiretroviral therapy (ART) and PrEP, and successful (all) PrEP injections select drug-resistant HIV after breakthrough infection. The origin corresponds to the reference scenario without PrEP. Lines correspond to the cost-effectiveness frontiers, labeled with incremental cost per life-year gained relative to the next best strategy. Frontiers are shown for base-case simulations (dotted line), structural sensitivity analysis including second-line ART (dashed line), and risk-prioritized PrEP (solid line). Age-prioritized PrEP coverage levels are stated in parentheses. Interventions not on these frontiers are not shown.
Prediction Uncertainty
Intervention Horizon
Compared to the reference scenario, 2.5%–15% unprioritized PrEP coverage prevented a median 3.9% (IQR, 2.5%–5.6%) of undiscounted new infections at a median (discounted) cost per infection prevented of $21 122 (IQR, $15 797–$28 197). When age-prioritized, these same coverage levels prevented more infections (median, 7.1%; IQR, 4.5%–10.1%), reducing the cost per infection prevented to $11 402 (IQR, $8442–$15 278). Risk-prioritized PrEP covering 50%–90% of female sex workers and clients prevented 4.6% (IQR, 3.5%–6.1%) of new infections, and was cost-saving in 15% of simulations (Supplementary Table 5), otherwise its median cost per infection prevented was just $616 (IQR, $317–$1043). Considering survival over 10 years, only risk prioritization was cost-effective, costing $4967 (IQR, $2550–$8345) per life-year gained. PrEP increased prevalent drug-resistant infections compared to the reference scenario in ≥80% of uncertainty simulations (Supplementary Table 6).
Lifetime Horizon
PrEP increased cumulative costs and life-years lived compared to the reference scenario in >90% of unprioritized and age-prioritized PrEP simulations (Supplementary Table 5). A median of 258 300 (IQR, 151 900–402 900) discounted life-years was gained from unprioritized PrEP at a cost of $1756 (IQR, $1131–$2734) per life-year gained. Compared with unprioritized PrEP, age-prioritized PrEP gained approximately twice as many life-years (median, 485 700; IQR, 284 000–759 700), and was very cost-effective at $697 (IQR, $385–$1179) per life-year gained. In contrast, risk prioritization was cost-saving in 84% of simulations; it gained a median of 289 800 (IQR, 148 000–444 500) life-years and decreased total costs by $184.5 million (IQR, $297.8–$90.3 million).
Sensitivity of Outcomes
Regression Analysis
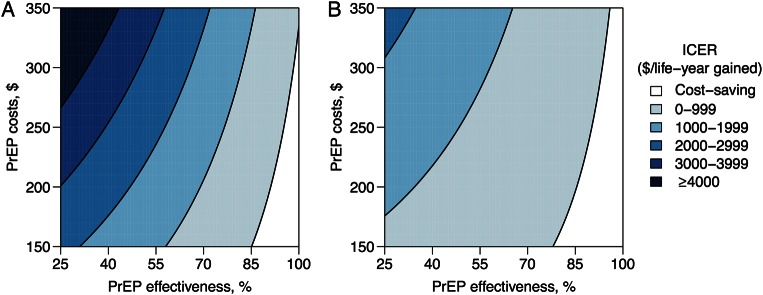
PrEP's costs, efficacy, and reliability emerged as the principal determinants of PrEP's cost-effectiveness. Detailed results are given in the Supplementary Data. While unprioritized and age-prioritized PrEP usually increased total costs compared with the reference scenario, these strategies were cost-saving when PrEP was less expensive and its effectiveness (defined as the product of its reliability and wild-type efficacy) was high (Figure 3). At costs of $150 per person-year, unprioritized PrEP was cost-saving at ≥85% effectiveness (Figure 3A), while age-prioritized PrEP was cost-saving at ≥78% effectiveness (Figure 3B). Higher effectiveness was required to achieve cost-savings with more expensive PrEP. While ICERs for either unprioritized or age-prioritized PrEP increased with more expensive or less effective PrEP, these increases were less pronounced for age-prioritized PrEP.
Figure 3.
Cost-effectiveness of preexposure prophylaxis (PrEP) as a function of PrEP effectiveness and cost. These response surfaces show the incremental cost per life-year gained over lifetime in uncertainty analysis simulations of unprioritized (A) or age-prioritized (B) PrEP scale-up, compared with a reference scenario without PrEP. Risk-prioritized PrEP is not shown; it was cost-saving (reduced cost and increased life-years) in 84% of simulations. Inputs shown are the annual per-capita cost of PrEP and PrEP's effectiveness, defined as the product of its efficacy and reliability. Abbreviation: ICER, incremental cost-effectiveness ratio.
Second-line ART
We examined the cost-effectiveness of universal access to second-line ART, either scaled up alone or in combination with PrEP. Compared with the reference scenario, second-line ART scale-up cost $8892 per infection prevented over 10 years and $621 per life-year gained over lifetime (Supplementary Tables 9 and 10); however, risk-prioritized PrEP was more cost-effective by either measure. Scaled up separately, age-prioritized PrEP was less cost-effective than second-line ART over 10 years; over lifetime, second-line ART was less cost-effective than optimistic age-prioritized PrEP, whereas the converse was true assuming conservative PrEP (Figures 1 and 2). Nevertheless, combined scale-up of conservative age-prioritized PrEP and second-line ART was very cost-effective ($1169 per life-year gained) compared with second-line ART alone (Figure 2B).
DISCUSSION
In this study, we have evaluated the health outcomes, costs, and cost-effectiveness [25, 30] of injectable RPV PrEP, for combination HIV prevention in KwaZulu-Natal, South Africa. Our model-based analyses demonstrated that RPV PrEP could substantially improve survival and reduce HIV transmission in KwaZulu-Natal, at a compelling economic value, if prioritized to key populations at high risk for HIV infection. The main findings from this study were the following. First, the preventive benefit of a time-limited PrEP intervention was realized during the intervention period; however, the survival benefit and cost-effectiveness were fully appreciated only over lifetime [31]. Second, the cost-effectiveness of PrEP improved when prioritized (compared with unprioritized) to groups having high HIV incidence [32]. Third, prioritizing PrEP to persons at highest behavioral risk (risk-prioritized PrEP; administered to female sex workers and their clients) was a cost-saving/very cost-effective intervention, but drug resistance could undermine its long-term impact. Fourth, prioritizing PrEP to women aged 20–29 years (age-prioritized PrEP) was very cost-effective over lifetime. Fifth, the principal determinants of PrEP's cost-effectiveness were PrEP's efficacy, delivery-reliability, and costs. Finally, PrEP's cost-effectiveness was realized despite assuming high ART and MMC coverage (80%), and universal access to second-line ART.
Base-case analysis showed that HIV prevention increased in proportion to the level of PrEP coverage. However, compared with unprioritized PrEP, prevention doubled with age-prioritized PrEP at the same overall coverage levels (2.5%–15%), and was similar in magnitude with risk-prioritized PrEP at a fraction of coverage (<1%). A reciprocal trend was reflected in the costs per infection prevented, with the lowest estimate being $298 at 2025, for optimistic risk-prioritized PrEP. Considering survival, due to limited life-years gained, only risk-prioritized PrEP emerged as very cost-effective or cost-effective over the intervention period (ICER compared with reference scenario ranged from $3144 to $11 568). In contrast, when assessed over lifetime, age-prioritized PrEP was very cost-effective ($470–$1549), while risk-prioritized PrEP was cost-saving. Uncertainty analysis confirmed the above cost-effectiveness trends. The cost-effectiveness of an intervention by conventional standards may not translate into its affordability in resource-limited settings due to scarce resources and competing healthcare priorities. Our analyses showed that risk-prioritized PrEP decreased lifetime overall costs, while age-prioritized PrEP increased total costs; 2.5% coverage cost approximately $18 million per year over 10 years, about twice KwaZulu-Natal's budget for MMC. Thus inclusion of PrEP in the HIV/AIDS response will require upfront/ongoing investment and strategic planning.
While our optimistic PrEP strategies decreased the number of prevalent drug-resistant infections, resistance increased in most simulations that included more conservative PrEP-related assumptions, with increases more likely with age or risk prioritization, highlighting the importance of implementing highly effective PrEP with resistance monitoring to circumvent the spread of drug resistance from PrEP scale-up. However, we and others previously determined that, for combined scale-up of PrEP and ART, PrEP's contribution to drug resistance prevalence was modest, while most resistance was generated from ART use [33–36].
Published data specific for injectable RVP PrEP are not available, and estimates of PrEP's cost-effectiveness are highly variable and difficult to compare, due to differences in epidemiological context and modeling assumptions [37]. Walensky et al [38] projected cost-savings over a lifetime from scale-up of an injectable PrEP among young (<26 years), high-incidence (5%/year) South African women. Their projections were qualitatively similar to our previous [32] and current findings for risk-prioritized PrEP; however, their simulation approach focused on at-risk cohorts and did not provide population-level outcomes. As a result, we cannot directly compare their findings to ours for age-prioritized PrEP under different modeling assumptions. Our data were also similar to those of Alistar et al [39], who predicted that unprioritized oral PrEP may cost $1172 per quality-adjusted life-year gained in South Africa whereas risk-prioritized PrEP was potentially cost-saving. Pretorius et al [40] predicted that 90% effective PrEP prioritized to South African women aged 25–35 years may cost over $20 000 per infection prevented over 10 years. This estimate is more conservative than ours, likely due to lower HIV incidence in the adult population modeled.
This study has several caveats. Injectable PrEP is under evaluation; thus, actual estimates of RPV PrEP's efficacy, reliability of delivery, and drug price were unavailable for this study. If we underestimated efficacy or reliability or overestimated PrEP's price, then we likely underestimated PrEP's cost-effectiveness, and vice versa. Nevertheless, we employed a plausible range of input estimates in base-case analysis and explored a wider range in uncertainty and sensitivity analyses. We assumed delivery of regular PrEP injections with programmatic dropout, but maintained the desired coverage level once achieved. In reality, if PrEP injections are irregular or PrEP coverage drops, then we may have overstated PrEP's impact on HIV prevention. We did not simulate PrEP scale-up among men who have sex with men or injection drug users, as HIV is predominantly transmitted heterosexually in South Africa [15]; however, these at-risk populations may also benefit from focused PrEP [37]. We evaluated PrEP scale-up for combination prevention specifically for KwaZulu-Natal, South Africa. Although our quantitative estimates may not apply to other epidemiological contexts, our qualitative findings would likely hold for other similarly mature and generalized HIV epidemics in low- and middle-income countries.
In conclusion, scale-up of RPV PrEP in KwaZulu-Natal was very cost-effective among 20- to 29-year-old women, and cost-saving among individuals at highest behavioral risk.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the Bill & Melinda Gates Foundation (grant number OPP1005974).
Potential conflicts of interest. R. L. G., U. M. P., G. H., K. J. P., J. W. M., and U. L. A. report grants from the Bill & Melinda Gates Foundation during the conduct of the study. J. W. M. reports personal fees from the University of Pittsburgh and Gilead Sciences, has stock options in Cocrystal Pharma, Inc, and has been issued patent number 8 815 829. E. B. reports grant support from the National Institute on Drug Abuse of the National Institutes of Health (R01-DA15612). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Joint United Nations Programme on HIV/AIDS. Fact sheet 2015 global statistics. Geneva, Switzerland: UNAIDS, 2015. [Google Scholar]
- 2.Shisana O, Rehle T, Simbayi L et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town, South Africa: HSRC Press, 2014. [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson LF, Mossong J, Dorrington RE et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013; 10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS. Ambitious treatment targets: writing the final chapter of the AIDS epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 6.Ramjee G, Daniels B. Women and HIV in sub-Saharan Africa. AIDS Res Ther 2013; 10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdool Karim Q, Abdool Karim SS, Frohlich JA et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO expands recommendation on oral pre-exposure prophylaxis of HIV infection (PrEP). Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 11.Spreen WR, Margolis DA, Pottage JC Jr. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 2013; 8:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azijn H, Tirry I, Vingerhoets J et al. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 2010; 54:718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339:966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossong J, Grapsa E, Tanser F, Bärnighausen T, Newell M-L. Modelling HIV incidence and survival from age-specific seroprevalence after antiretroviral treatment scale-up in rural South Africa. AIDS 2013; 27:2471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.South African Centre for Epidemiological Modelling and Analysis. The modes of transmission of HIV in South Africa. Stellenbosch: South African Centre for Epidemiological Modelling and Analysis, 2009. [Google Scholar]
- 16.South Africa National Department of Health. National strategic plan on HIV, STIs and TB, 2012–2016. Pretoria: South Africa National Department of Health, 2012. [Google Scholar]
- 17.South Africa National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria: South Africa National Department of Health, 2014. [Google Scholar]
- 18.Mehta SD, Moses S, Agot K et al. The long-term efficacy of medical male circumcision against HIV acquisition. AIDS 2013; 27:2709–899. [DOI] [PubMed] [Google Scholar]
- 19.The HIV Prevention Trials Network. HPTN 076: Phase II safety and acceptability of an investigational injectable product, TMC278 LA, for pre-exposure prophylaxis (PrEP). Available at: http://www.hptn.org/network_information/076_documents.htm. Accessed 25 May 2016. [Google Scholar]
- 20.Penrose KJ, Wallis CL, Scoulos-Hanson M, Viana R, Mellors JW, Parikh UM. High prevalence of cross-resistance to rilpivirine in subtype C HIV-1 isolates from first-line ART failures in South Africa. AIDS Res Hum Retroviruses 2014; 30(suppl 1):A166. [Google Scholar]
- 21.Penrose KJ, Parikh UM, Hamanishi KA et al. Selection of rilpivirine-resistant HIV-1 in a seroconverter from the SSAT 040 trial who received the 300-mg dose of long-acting rilpivirine (TMC278LA). J Infect Dis 2016; 213:1013–7. [DOI] [PubMed] [Google Scholar]
- 22.Bill & Melinda Gates Foundation. Oral PrEP in South Africa. Bottom-up cost model. Available at: http://www.gatesfoundation.org/grantseeker/Documents/program-cost-model-rsa.xls Accessed 21 April 2016.
- 23.Kripke K, Mayise T, Palmer E et al. Impact and cost of HIV/AIDS prevention and treatment in KwaZulu-Natal, South Africa 2011–2025. Washington, DC: Futures Group, Health Policy Initiative, Costing Task Order, 2013. [Google Scholar]
- 24.Meyer-Rath G, Miners A, Santos AC, Variava E, Venter WDF. Cost and resource use of patients on antiretroviral therapy in the urban and semiurban public sectors of South Africa. J Acquir Immune Defic Syndr 2012; 61:e25–32. [DOI] [PubMed] [Google Scholar]
- 25.Briggs AH, Weinstein MC, Fenwick EAL et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health 2012; 15:835–42. [DOI] [PubMed] [Google Scholar]
- 26.Venter F. Second line and future regimens in sub-Saharan Africa: what's coming? In: XXV International HIV Drug Resistance Workshop, Boston, MA, 2016. [Google Scholar]
- 27.World Bank. GDP deflator. Available at: http://data.worldbank.org/indicator/NY.GDP.DEFL.ZS Accessed 20 April 2016.
- 28.USForex. Historical exchange rates. Available at: http://www.usforex.com/forex-tools/historical-rate-tools/historical-exchange-rates Accessed 29 April 2016.
- 29.World Bank. GDP per capita. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD Accessed 20 April 2016.
- 30.Husereau D, Drummond MF, Petrou S et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013; 16:231–50. [DOI] [PubMed] [Google Scholar]
- 31.NICE International. Bill & Melinda Gates Foundation Methods for Economic Evaluation Project (MEEP). Available at: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-International/projects/MEEP-report.pdf Accessed 29 April 2016.
- 32.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS One 2007; 2:e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbas UL, Glaubius R, Mubayi A, Hood G, Mellors JW. Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. J Infect Dis 2013; 208:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Vijver DAMC, Nichols BE, Abbas UL et al. Preexposure prophylaxis will have a limited impact on HIV-1 drug resistance in sub-Saharan Africa: a comparison of mathematical models. AIDS 2013; 27:2943–51. [DOI] [PubMed] [Google Scholar]
- 35.Abbas UL, Glaubius R, Hood G, Mellors JW. Antiretroviral treatment, preexposure prophylaxis, and drug resistance in sub-Saharan Africa: a consensus among mathematical models. J Infect Dis 2014; 209:164–6. [DOI] [PubMed] [Google Scholar]
- 36.Supervie V, Barrett M, Kahn JS et al. Modeling dynamic interactions between pre-exposure prophylaxis interventions and treatment programs: predicting HIV transmission & resistance. Sci Rep 2011; 1:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med 2013; 10:e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walensky RP, Jacobsen MM, Bekker L-G et al. Potential clinical and economic value of long-acting preexposure prophylaxis for South African women at high-risk for HIV infection. J Infect Dis 2016; 213:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alistar S, Grant P, Bendavid E. Comparative effectiveness and cost-effectiveness of antiretroviral therapy and pre-exposure prophylaxis for HIV prevention in South Africa. BMC Med 2014; 12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pretorius C, Stover J, Bollinger L, Bacaër N, Williams BG. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One 2010; 5:e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.