Abstract
After a cluster of fatal toxoplasmosis among stem cell transplant recipients at 2 hospitals, surveillance with polymerase chain reaction (PCR) (blood) was instituted. Rate of reactivation among seropositive recipients was 2.2 and 16%. Parasitemia was successfully managed with preemptive treatment. For seropositive recipients unable to take prophylaxis, toxoplasma PCR surveillance should be routinely performed.
Keywords: toxoplasmosis, stem cell transplant, reactivation, T-cell depleted, CD34+ selected
Toxoplasma gondii (T. gondii) is an intracellular parasite that is estimated to infect one third of the world's population [1]. Humans can acquire the infection by ingesting infected undercooked meat, contaminated water, or handling feces from recently infected felids. Primary and reactivation toxoplasmosis in the immunocompromised host usually manifests as a central nervous system disease and less frequently with multivisceral involvement. Most infections in the post-transplant setting are attributed to reactivation; primary or reinfections are rare. Among recipients of stem cell transplant (SCT), risk of Toxoplasma reactivation after transplant varies by regional seroprevalence [2]. Reports from European centers found that toxoplasma reactivation occurs in 8.7%–16% of seropositive recipients. Overall incidence of disease is 2.9%–6% [3, 4]. Mortality among transplant recipients with Toxoplasma disease is high (63%–80%) [5, 6]. With an overall lower seroprevalence of toxoplasma in the United States compared to Europe, varied screening approaches are used across transplant centers in the United States.
This study describes a cluster of fatal toxoplasmosis cases at 2 SCT centers in New York City and reports outcomes after implementation of polymerase chain reaction (PCR)-based surveillance.
METHODS
The institutional review board at both institutions reviewed the study and granted a Health Insurance Portability and Accountability Act waiver of authorization.
The study was conducted at 2 neighboring transplant centers in New York City among recipients of allogenic SCT as follows: 1. Pre-surveillance: Retrospective review of toxoplasmosis cases from December 2012 to September 2013. 2. Post-surveillance: Prospective serial testing for detection of Toxoplasma in blood from July 2013 to December 2014. Relevant data were obtained from clinical databases. Annually, 150–200 allogeneic SCT are performed at Memorial Sloan Kettering Cancer Center (Hospital A) and 90–100 at New York Presbyterian Hospital Weill Cornell Medicine (Hospital B). Toxoplasma serological testing (immunoglobulin M and immunoglobulin G [IgG]) for donor (D) and recipients (R) was routinely performed at Hospital A and for recipients only at Hospital B throughout.
Transplant Approach for Pre-surveillance Cluster
At Hospital A, recipients of T-cell depleted (TCD) grafts underwent myeloablative conditioning and received CD34+-selected grafts obtained with the CliniMACS system (Miltenyi Biotech, Germany) [7]. All patients received anti-thymocyte globulin to promote engraftment. Both patients at Hospital B underwent myeloablative conditioning and received a single unit cord blood transplant with a CD34+ selected graft from a haplo-identical donor.
Post-Surveillance
Toxoplasma PCR
In response to the cluster, screening in high-risk patients using whole blood PCR was implemented. In July 2013, Hospital A began surveillance for recipients with positive pretransplant Toxoplasma serology (D+ or R+), twice weekly starting at day +14 until discharge. After discharge, weekly testing was conducted until day +100 or as clinically indicated. In October 2013, Hospital B implemented surveillance for all allogeneic transplants regardless of donor or recipient serostatus at weekly intervals from transplant until clinician discretion. Toxoplasma PCR assay for both hospitals was performed at the same reference laboratory (Viracor-IBT, Lenexa, Kansas). This PCR assay targets a 529 bp repeated element.
Prophylaxis
At Hospital A, seropositive recipients (R+) received prophylaxis with trimethoprim/sulfamethoxazole (TMP-SMX) during conditioning (days –7 until –2). Prophylaxis was resumed on day +14 with atovaquone, until TMP-SMX could be reintroduced (1 double-strength [DS] 3 times a week). At Hospital B, TMP-SMX was given from day –7 to day –2 and reintroduced on day +30 (1 DS twice a day on weekends). For patients allergic to TMP-SMX, atovaquone was used.
Statistical Analysis
The incidence of toxoplasmosis was the cumulative probability of Toxoplasma reactivation at 4 months after transplant. Fisher exact test was used to compare categorical variables. All reported P values are 2-sided. A P value <.05 was considered statistically significant.
RESULTS
Pre-Surveillance (Cluster)
The baseline average number of toxoplasmosis cases at Hospital A was 0–1 case/year (2008–2012). No cases occurred at Hospital B. From December 2012 to September 2013, 4 cases were diagnosed at hospital A and 2 at Hospital B (5 R+; 1 seronegative recipient). Four cases occurred within 2 months. The mean age was 58; 3 were males. Average duration from transplant was 55 days (median 38; range 23–135). Five patients had fever at onset and rapidly developed fatal hypoxic respiratory failure. Five patients had absolute lymphocyte count <500/mm3; peak levels of lactate dehydrogenase were >1000 U/L in 4 patients. Diffuse interstitial pneumonitis and multisystem organ failure were noted in all fatal cases.
Average duration from symptom onset to death was 7.5 days. Four patients died within 40 days of transplant. Three patients were taking atovaquone for prophylaxis against Pneumocystis jiroveci pneumonia but reported noncompliance. One R− patient was suspected to have developed acute infection. Diagnosis of toxoplasmosis was made by blood PCR (n = 4 patients at Hospital A, range 45 100–4 900 000 genomic copies/mL) or autopsy findings (n = 2 at Hospital B).
None of the patients had common donors for graft, red blood cell (RBC) or platelet units. Residual RBC units tested were negative for Toxoplasma by PCR. No common lot numbers for infusion products were identified. Procedural review of the ex-vivo T-cell depletion method did not reveal any lapses. The Centers for Disease Control and Prevention (CDC) were notified. CDC performed a nationwide query through the Emerging Infection Network that did not recognize additional transplant centers with a similar problem.
Post-Surveillance
Hospital A
From July 2013 to December 2014 (18 months), 274 patients underwent allogeneic SCT 46/274 (17%) patients were IgG+ on screening (R+). Additionally, 23 (8%) recipients received grafts from seropositive donors (D+/R−). Surveillance tests for a follow-up period of at least 4 months were included (until April 2015). A summary of Toxoplasma testing is shown in Table 1. The characteristics of the seropositive recipients are shown in Supplementary Table 1. Among 617 surveillance tests in R+ patients (n = 46), one case of reactivation occurred (1/46; 2.2%): 65 year-old male with AML with CD34+ selected SCT from an unrelated donor had Toxoplasma parasitemia (4400 copies/mL) on day +115. He was asymptomatic and known to be noncompliant with prophylaxis. Treatment with pyrimethamine and sulfadiazine was initiated, and blood Toxoplasma PCR became undetectable 15 days later.
Table 1.
Summary of Toxoplasma Surveillance Testing at Hospital A and B for Transplants Performed During the Post-surveillance Period
| Total | Recipient Negative /Donor Positive |
Recipient Positive |
|||||
|---|---|---|---|---|---|---|---|
| D+/R− | D+ or D−/R+ | ||||||
| Hospital A | |||||||
| Transplant type | Number of patients | Number of patients | Number of tests | Parasitemia detected | Number of patients | Number of tests | Parasitemia detected (%) |
| Conventional | 96 | 12 | 121 | 0/12 | 20 | 208 | 0/20 |
| CD34+ selected PB | 140 | 11 | 148 | 0/11 | 19 | 277 | 0/19 |
| Cord blood +/−CD34+ selected PB | 38 | 0 | 0 | N/A | 7 | 132 | 1/7 (14%) |
| Total | 274 | 23 (8%) | 269 | 0/23 | 46 (17%) | 617 | 1/46 (2.2%) |
| Total | Recipient negativea | Recipient positive | |||||
| R− | R+ | ||||||
| Hospital B | |||||||
| Transplant type | Number of patients | Number of patients | Number of tests | Parasitemia detected | Number of patients tested | Number of tests | Parasitemia detected (%) |
| Cord±Haplo- identical CD34+ | 47 | 35 | 778 | 0/35 | 12 | 313 | 2/12 (16.7%)* |
| Alemtuzumab- TCD PB | 73 | 60 | 1259 | 0/60 | 13 | 278 | 2/13 (15.4%)** |
| Total | 120 | 95 (79%) | 2037 | 0/95 | 25 (21%) | 591 | 4/25 (16%) |
Abbreviations: N/A, not applicable; PB, peripheral blood; TCD, T-cell depleted.
a Includes patients with equivocal titres.
For hospital A routine surveillance was recommended for Toxoplasma seropositive recipients (R+) who underwent TCD PB and cord. Other groups were tested at clinician discretion. For hospital B, all transplants were included in surveillance testing.
*P value =.06, **P value =.02.
Hospital B
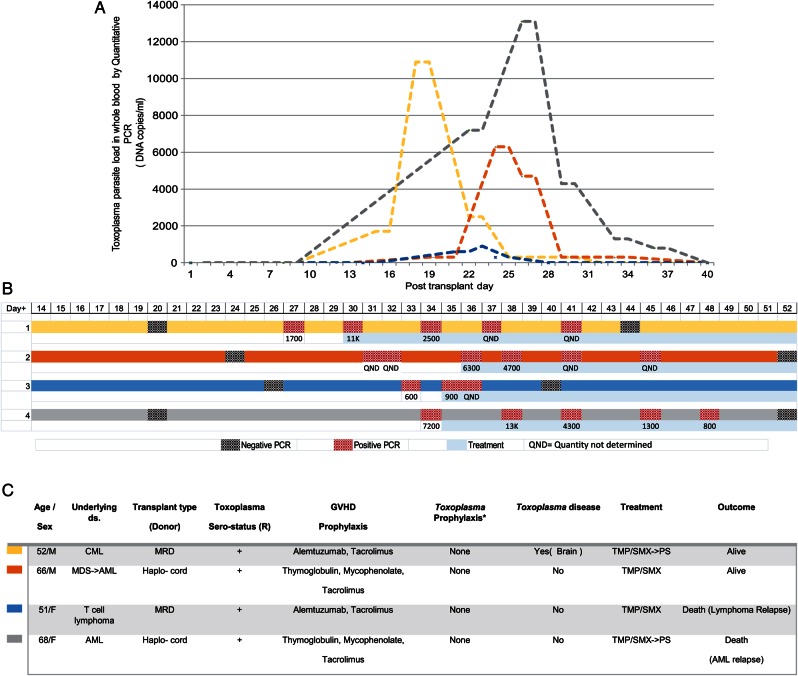
From October 2013 to December 2014 (15 months), 120 allogeneic transplants were performed, 25 (20.8%) were R+ (Table 1). In total, 591 surveillance tests were performed among this cohort (Table 1). Parasitemia was detected in 4 patients with an overall infection rate of 16%; 1 had Toxoplasma disease. The clinical characteristics of the 4 cases are shown in Figure 1. All patients responded to treatment and no Toxoplasma related deaths occurred.
Figure 1.
Hospital B: Toxoplasma infection in 4 patients detected during the surveillance period. A, Graphic representation of Toxoplasma parasite load in blood, relative to day of transplant. B, Timing of surveillance testing and treatment for the four patients. C, Clinical and transplant characteristics. Color bars are coordinated to depict the corresponding patient in part (A, B) and embedded table C. *All received TMP-SMX from day −7 until day −2. Abbreviations: AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; GVHD, graft versus host disease; MDS, myelodysplastic syndrome; MRD, male related donor; PCR, polymerase chain reaction; PS, pyrimethamine sulfadiazine; R, recipient; TMP-SMX, trimethoprim sulfamethoxazole.
DISCUSSION
Early symptoms of toxoplasmosis among SCT patients are nonspecific, making diagnosis challenging. In a recent review of intensive care unit admissions for disseminated toxoplasmosis in France, 1 of 21 allogeneic SCT recipients survived [5], emphasizing that outcomes of Toxoplasma-related illness in this population is dismal [8]. We report a cluster of 6 cases among SCT recipients at 2 neighboring institutions in New York City. All of our patients were at high risk for toxoplasmosis and experienced a fulminant course. A detailed epidemiologic investigation did not reveal any common link. Although the etiology for the increased incidence of toxoplasmosis could not be elucidated, endogenous reactivation is most likely. Recent reports from European centers have suggested PCR screening for toxoplasmosis in high-risk patients [9]. As a prevention strategy, prophylaxis for toxoplasmosis with TMP-SMX is effective. However, noncompliance is common as occurred among 3/6 patients in the reported cluster.
We describe different approaches adopted by two transplant centers that perform a large number of TCD and cord transplants but have different transplant related practices. Hospital A implemented early prophylaxis based on timing of cases in the cluster. Surveillance results from this center show an overall low rate of parasitemia (2.2%), with one late breakthrough due to noncompliance with prophylaxis. Hospital B implemented an extended surveillance that included seronegative recipients; TMP-SMX prophylaxis was started at day +30. Parasitemia was detected in 4/25 patients (16%), between days 27 and 34. All patients who developed parasitemia at Hospital B had received prophylaxis with TMP-SMX during conditioning but developed parasitemia before prophylaxis could be reintroduced after transplant. All reactivations were identified and successfully treated without subsequent relapse. Differences in clinical practices, including method of T-cell depletion (CD34+ selection in Hospital A vs Alemtuzumab in Hospital B), and timing of post-transplant prophylaxis may account for these differences.
Notably, none of the D− or D+/ R− had evidence of Toxoplasma infection; the cost of surveillance in this group should be weighed against the rare reports of Toxoplasma reactivation or acute infection among seronegative recipients. Although screening is not warranted, a high index of suspicion should be maintained for clinically compatible illness in seronegative recipients due to risk of acute infection. Our case series is limited by the small number of cases, retrospective design, and limitations of PCR diagnosis [4, 10, 11]. Heterogeneous transplant related practices were used across the 2 centers; compliance with prophylaxis could not be assessed [4].
Disseminated toxoplasmosis is a devastating disease in SCT recipients. Prophylaxis with TMP-SMX is effective for toxoplasma reactivation but despite best efforts, assuring adherence can be challenging. Our report shows that PCR is an effective surveillance method for detection of T. gondii infection, and preemptive treatment of parasitemia improves clinical outcomes [12]. PCR surveillance targeting seropositive recipients unable to take prophylaxis should be strongly considered as a standard for early detection of toxoplasma reactivation after SCT.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work is supported by the MSK Cancer Center Support Grant/Core Grant (P30 CA008748) National Institutes of Health (NIH) T32-2T32AI007613-16 (To F. I. and K. S.), NIH/National Center for Advancing Translational Sciences KL2TR000458 (F. I.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol 2009; 39:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derouin F, Pelloux H; Parasitology ESGoC. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect 2008; 14:1089–101. [DOI] [PubMed] [Google Scholar]
- 3.Meers S, Lagrou K, Theunissen K et al. Myeloablative conditioning predisposes patients for Toxoplasma gondii reactivation after allogeneic stem cell transplantation. Clin Infect Dis 2010; 50:1127–34. [DOI] [PubMed] [Google Scholar]
- 4.Martino R, Bretagne S, Einsele H et al. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin Infect Dis 2005; 40:67–78. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt M, Sonneville R, Schnell D et al. Clinical features and outcomes in patients with disseminated toxoplasmosis admitted to intensive care: a multicenter study. Clin Infect Dis 2013; 57:1535–41. [DOI] [PubMed] [Google Scholar]
- 6.Mele A, Paterson PJ, Prentice HG, Leoni P, Kibbler CC. Toxoplasmosis in bone marrow transplantation: a report of two cases and systematic review of the literature. Bone Marrow Transplant 2002; 29:691–8. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs GS, Hamdi A, Hilden PD et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant 2015; 50:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small TN, Leung L, Stiles J et al. Disseminated toxoplasmosis following T cell-depleted related and unrelated bone marrow transplantation. Bone Marrow Transplant 2000; 25:969–73. [DOI] [PubMed] [Google Scholar]
- 9.Gea-Banacloche J, Masur H, Arns da Cunha C et al. Regionally limited or rare infections: prevention after hematopoietic cell transplantation. Bone Marrow Transplant 2009; 44:489–94. [DOI] [PubMed] [Google Scholar]
- 10.Fricker-Hidalgo H, Bulabois CE, Brenier-Pinchart MP et al. Diagnosis of toxoplasmosis after allogeneic stem cell transplantation: results of DNA detection and serological techniques. Clin Infect Dis 2009; 48:e9–15. [DOI] [PubMed] [Google Scholar]
- 11.Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis 2012; 44:805–14. [DOI] [PubMed] [Google Scholar]
- 12.Gajurel K, Dhakal R, Montoya JG. Toxoplasma prophylaxis in haematopoietic cell transplant recipients: a review of the literature and recommendations. Curr Opin Infect Dis 2015; 28:283–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.