In patients with moderate but not severe Ebola virus disease (EVD), we found evidence of robust interferon responses and innate and adaptive control mechanisms. This suggests that immune modulation could be a useful therapeutic modality in the treatment of EVD.
Keywords: Ebola, biomarkers, cytokines, immunity, EVD
Abstract
Background. Ebola virus (EBOV) infection causes a severe and often fatal disease. Despite the fact that more than 30 000 individuals have acquired Ebola virus disease (EVD), the medical and scientific community still does not have a clear understanding of the mechanisms by which EBOV causes such severe disease.
Methods. In this study, 54 biomarkers in plasma samples serially collected from 7 patients with EVD were analyzed in an attempt to define the kinetics of inflammatory modulators. Two clinical disease groups were defined (moderate and severe) based on the need for clinical support. Biomarkers were evaluated for correlation with viremia and clinical disease in an effort to identify pathways that could be useful targets of therapeutic intervention.
Results. Patients with severe disease had higher viremia than those with moderate disease. Several biomarkers of immune activation and control were significantly elevated in patients with moderate disease. A series of pro-inflammatory cytokines and chemokines were significantly elevated in patients with severe disease.
Conclusions. Biomarkers that were associated with severe EVD were proinflammatory and indicative of endothelial or coagulation cascade dysfunction, as has been seen historically in patients with fatal outcomes. In contrast, biomarkers that were associated with moderate EVD were suggestive of a strong interferon response and control of both innate and adaptive responses. Therefore, clinical interventions that modulate the phenotype and magnitude of immune activation may be beneficial in treating EVD.
Ebola virus (EBOV) infection in humans causes a severe and often fatal disease. The current understanding of the pathophysiology of EBOV disease (EVD) is limited because few published studies have evaluated human samples [ 1–6 ]. These studies have used residual clinical samples that were collected as part of EVD outbreak responses, often with only 1 or 2 samples available from each patient. Collectively, the prior studies reported increased levels of both pro- and antiinflammatory cytokines in patients with EVD, indicating marked dysregulation of innate immune responses, most notably in patients who succumbed to disease. Prior studies also reported an association between lymphocyte apoptosis and fatal outcome, as well as decreased numbers of total and activated T cells in fatal cases.
Minimal healthcare infrastructure and infectious risk have limited the collection of serial blood samples from patients with EVD in Africa. In contrast, patients who acquired EVD during the 2014 West African outbreak and were repatriated to the United States or Europe for care had access to a more resource-rich level of care. Although these patients had access to more services than patients in West Africa, their clinical care was still limited by the infectious nature of EBOV [ 7 ]. Most of these patients had a central line placed for fluid and medication administration, and this allowed for regular, frequently daily, blood sampling to monitor hematologic or blood chemistry parameters. As a consequence of regular blood sampling for advanced care, residual banked samples were available for analysis.
In this study, the expression kinetics of inflammation, coagulation, endothelial function, and lymphocyte function biomarkers were examined to identify pathways associated with severe and moderate EVD. Our goal was to provide clinicians and scientific investigators insight into EVD pathophysiology for the design of targeted therapeutics.
METHODS
Patient Enrollment
Any patient admitted with EVD to Emory University Hospital, Atlanta, Georgia, or University of Nebraska Medical Center, Omaha, was eligible, and all eligible patients were included. Written informed consent to participate in local institutional review board–approved protocols (Emory University [IRB00076700], University of Nebraska Medical Center [716-14-EP], and US Centers for Disease Control and Prevention [CDC; 6643, 6774, and 1652]) was obtained from all surviving patients with EVD and from healthy control donors. Patients were randomly assigned an EVD number; in the case of EVD2, 5, 9, and 15, the number remains the same as used in a prior publication [ 8 ]. Patients were followed to time of discharge.
Biosafety
Work with infectious specimens was performed in CDC's biosafety level 4 laboratories. Plasma samples were γ-irradiated with 5 × 10 6 rads prior to processing for quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR), enzyme-linked immunosorbent assay (ELISA), and biomarker analyses.
ELISA
EBOV nucleoprotein-specific ELISA was performed as previously described [ 8 ]. Data were analyzed using Excel (Microsoft Corp, Redmond, Washington) and Prism (GraphPad Software Inc, La Jolla, California) software.
Virus-Specific qRT-PCR
RNA was isolated from plasma using MagMax Total RNA Isolation Kit (Life Technologies, Grand Island, New York). qRT-PCR of RNA was conducted using established primer/probe sets that target the EBOV nucleoprotein gene [ 9 ]. A standard curve, expressed as tissue culture infective dose 50 (TCID 50 )/mL, was generated from a passage 3 stock of EBOV isolated from patient EVD2. This stock was inoculated into human plasma and γ-irradiated with 5 × 10 6 rads to control for the effects of irradiation on the qRT-PCR reaction. The standard curve was used to convert raw C t values to relative TCID 50 /mL.
Biomarker Assays
Assays were obtained from Affymetrix (Santa Clara, California) and performed according to manufacturer's instructions; a 34-plex assay for B-cell activating factor, fractalkine, granulocyte colony stimulating factor, granulocyte monocyte colony stimulating factor, granzyme B, interferon (IFN)-α, IFN-β, IFN-γ, IFN-γ–induced protein 10, interleukin (IL) 1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-29, IL-1 receptor antagonist (IL-1RA), monocyte colony stimulating factor (MCSF), monocyte chemoattractant protein (MCP)-1, MCP-2, plasminogen activator inhibitor–1, platelet endothelial cell adhesion molecule (PECAM)-1, tumor necrosis factor (TNF)-α, TNF-related apoptosis-inducing ligand, thrombopoietin, soluble CD40 ligand, sE-selectin, soluble Fas cell surface death receptor ligand (sFas-L), sIL-2 receptor, sL-selectin, sP-selectin, tissue plasminogen activator, and MCP-3; a 7-plex assay for apoptosis antigen-Fas (APO-Fas), melanoma growth stimulation activity alpha (Gro-alpha) Gro-α, IL-1α, macrophage inflammatory protein (MIP)-1α, MIP-1β, TNF Receptor-I (TNFR-I), and TNFR-II; a 3-plex assay for D-dimer, soluble intracellular adhesion molecule (sICAM)-1, and soluble vascular cell adhesion molecule (sVCAM)-1; and a single-plex assay for regulated on activation normal T-cell–expressed and –secreted (RANTES). Assays were obtained from Millipore (Billerica, Maryland) and performed according to manufacturer's instructions: a 2-plex assay for tissue factor (TF) and thrombomodulin; a 3-plex assay for fibrinogen, platelet factor-4, and von Willebrand factor (vWF); and single-plex assays for ferritin, complement factor H, anti-thrombin-III, and a disintegrin and metalloproteinase with a thrombospondin type 1 motif 13. If samples had values outside of the standard curve range, additional dilutions were made to obtain accurate values for all analytes. Data were collected on a Luminex 200. Samples from 10 healthy human donors were analyzed in each assay since many of these assays do not have an established normal range.
Statistical Analysis
For each patient, 2 acute illness phase cutoffs were defined: the last day of fever and the last day of measurable viremia. For each of the 54 biomarkers, a T test was conducted in order to compare values measured during the acute phase in patients with severe EVD against the values of moderately ill patients. The false discovery rate method [ 10 ] was used to determine the statistical significance of each biomarker with an overall α = 0.05, taking into account the multiple tests run. For sensitivity analysis, individual patients (EVD 2, 5, 10, 11, or 15) were removed from the dataset, and the analysis was repeated to discover if this impacted the significance of specific biomarkers. Only associations that remained significant after the sensitivity analysis were included. The correlation of each biomarker with level of viremia was analyzed using the nonparametric Spearman coefficient of rank correlation.
RESULTS
Clinical Diversity
Seven EVD patients treated at Emory University Hospital or University of Nebraska Medical Center in 2014 were included in this study. The patients were aged 29–59 years; 5 were male and 2 were female. Their clinical histories have been reported [ 11–15 ]. Table 1 provides a brief description and clinical classification for all patients. Severity of disease was defined by the degree of clinical support required; the 2 patients who required extracorporeal support with dialysis and ventilation were classified as having severe disease, and the 5 who did not were classified as having moderate disease. It is not known if extracorporeal support could have influenced the levels of the biomarkers independent of the disease process; however, the 2 patients with severe disease also had the highest detected viremia. The patients received a variety of experimental therapeutics that could have influenced their clinical course or immune response. Neither platelet count nor aspartate aminotransferase levels correlated with disease severity. Six patients survived EBOV infection and 1 succumbed to disease; EVD9, who was classified as severe but survived, would have succumbed to disease without medical intervention.
Table 1.
Clinical Details of Seven Patients With Ebola Virus Disease Cared for in the United States
| Patient | Extracorporeal Support | Experimental Drug Therapy | Day of Fever Resolution | Peak Aspartate Aminotransferase a | Platelets b | Severity of Disease c |
|---|---|---|---|---|---|---|
| EVD2 | ZMapp (days 9, 12, 15) | 15 | 723 | <100 | Moderate | |
| EVD5 | ZMapp (days 10, 13, 17) | 14 | 107 | <100 | Moderate | |
| EVD9 | Ventilated, day 9; dialysis, day 11 | TKM-100802 (days 3–8); convalescent plasma (days 8, 9, 11, 12, 14, 15) | 12 | >2000 | <100 | Severe |
| EVD10 | TKM-100802 (days 8–13); convalescent plasma (days 9, 10) | 11 | 1158 | <100 | Moderate | |
| EVD11 | Brincidofovir (days 6, 9, 13, 16); convalescent plasma (day 8) | 17 | 1828 | <100 | Moderate | |
| EVD12 | Ventilated, day 14; dialysis, day 14 |
Convalescent whole blood (days 9, 13); convalescent
plasma (day 14) ZMapp (day 14) |
n/a | 1579 | <150 | Severe d |
| EVD15 | Brincidofovir (days 1 and 4); convalescent plasma (days 2, 3) | 2 | 491 | <150 | Moderate |
All patients have been reported previously in the literature (references [ 6–10 ]). The patients' care began at various times after self-reported onset of symptoms: EVD2, day 12; EVD5, day 15; EVD9, day 5; EVD10, day 8; EVD11, day 6; EVD12, day 14; EVD15, day 2.
Abbreviations: EVD, Ebola virus disease; n/a, not applicable; TKM, Tekmira.
a Expressed in international units per liter.
b Expressed in billion per liter.
c Severity of disease was defined by degree of clinical support required.
d Fatal at day 16 post symptom onset and complicated by Escherichia coli sepsis.
Viremia
All surviving patients had detectable viremia that declined over time (Figure 1A ). Since each patient was received for care at different time points in the disease course, only later time points were available for analysis for some patients. Because of this, peak viremia is only known for EVD9, in whom EBOV peaked at more than 10 7 relative TCID 50 /mL. Notably, the viremia of the patient who died (EVD12) increased in serial samples late in the disease course at the time when viremia in all surviving patients was declining. This phenomenon has been historically reported in patients with fatal outcomes [ 16 ].
Figure 1.
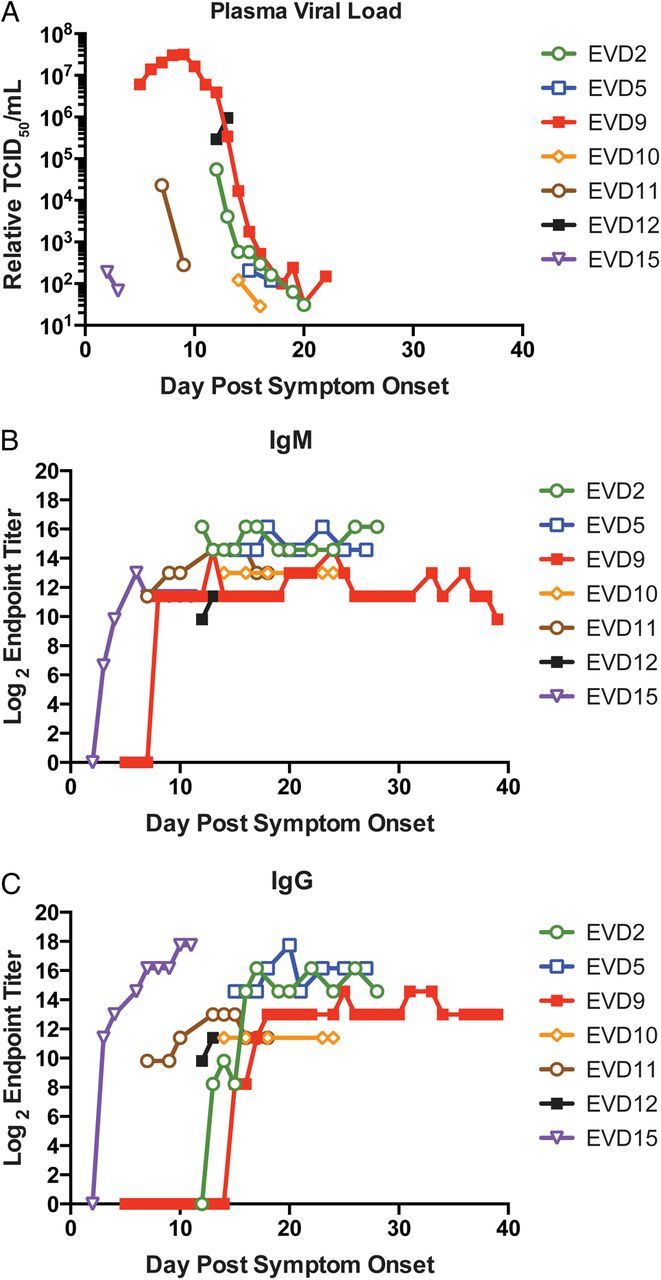
Viremia and humoral immune responses during Ebola virus disease (EVD). Patient plasma samples were assayed by quantitative reverse-transcriptase polymerase chain reaction, and relative viremia ( A ) was determined as tissue culture infective dose 50 (TCID 50 )/mL by comparison to an Ebola virus standard curve. Antinucleocapsid immunoglobulin M (IgM) ( B ) and immunoglobulin G (IgG) ( C ) titers were determined by enzyme-linked immunosorbent assay. Each patient's data are indicated by the specified color and symbol. The x -axis shows days after onset of self-reported symptoms. Of note, data for EVD2, 5, 9, and 15 have been previously reported [ 8 ].
Humoral Responses
All patients had detectable IgM (Figure 1B ) and IgG (Figure 1C ) antibody responses against EBOV. ZMapp treatment (EVD2, EVD5, and EVD12) would not have affected these results, but convalescent plasma administration may have. This is clearly seen in EVD15, who had detectable anti-EBOV IgG after administration of convalescent plasma and prior to developing anti-EBOV IgM titers. Convalescent plasma administration appeared to have no detectable effect on ELISA titer for EVD9, but no conclusions can be made regarding plasma administration to EVD10 or EVD11 since plasma was administered prior to the first available sample. Previous studies have shown that patients often succumb to EVD without developing detectable virus-specific antibody responses [ 3 , 16 ]. EVD12, the patient who died, received convalescent whole blood and plasma, so it is unclear if IgG titers in samples from EVD12 are the donor's or the recipient's antibody response. However, the presence of detectable IgM suggests that EVD12 may have developed EBOV-specific IgM prior to death, since convalescent plasma would not have significant amounts of EBOV-specific IgM.
Biomarker Measurements
Fifty-four biomarkers were selected based on their roles in innate immunity, adaptive immunity, endothelial function, or coagulation/fibrinolysis. For all biomarkers tested, at least 1 EVD patient had levels outside of the reference range, which was based on samples collected from 10 healthy humans. Convalescent plasma administration did not appear to alter biomarker levels in the recipients based on examination of before and after data from EVD9, the patient for whom there was the most kinetic data. The kinetic data for all biomarkers that are not otherwise discussed below are contained in the Supplementary Material .
To determine which biomarkers were associated with moderate vs severe EVD, biomarker values from samples collected during the acute phase (while patients had detectable viremia) from the 2 patients with severe disease were compared with those from the 5 patients with moderate disease. Six biomarkers had a statistically significant association with moderate disease and 17 with severe disease (Table 2 ). Patients with severe disease had higher viremia than those with moderate disease ( P = .0003). Therefore, an additional analysis was performed to determine which biomarkers correlated with viremia. Sixteen biomarkers correlated with viremia ( Supplementary Table 1 ) and, not surprisingly, 11 of those were also associated with severe disease (Table 2 ).
Table 2.
Statistically Significant Associations Between Biomarkers and Disease Severity During the Acute Phase of Ebola Virus Disease
| Biomarker | Disease Association | P Value |
|---|---|---|
| APO-Fas | Moderate | .0001880 |
| Interferon -β | Moderate | .0045833 |
| IL-29 | Moderate | .0000235 |
| IL-5 | Moderate | .0000058 |
| sFas-L | Moderate | .0154270 |
| TNFR-II | Moderate | .0044500 |
| D-dimer* | Severe | .0000182 |
| Fractalkine | Severe | .0000733 |
| Granzyme B* | Severe | .0001579 |
| IL-10 | Severe | .0007791 |
| IL-1RA* | Severe | .0010439 |
| IL-8 | Severe | .0056168 |
| MCP-1* | Severe | .0000063 |
| MCP-2* | Severe | .0033463 |
| Monocyte colony stimulating factor | Severe | .0004575 |
| Macrophage inflammatory protein-1β* | Severe | .0000180 |
| Platelet endothelial cell adhesion molecule-1* | Severe | 6.8 × 10 −11 |
| P-selectin | Severe | .0000122 |
| Soluble intracellular adhesion molecule* | Severe | 9.99 × 10 −9 |
| Thrombomodulin* | Severe | .0000812 |
| Tissue factor | Severe | .0008097 |
| TNFR-I* | Severe | .0055870 |
| von Willebrand factor* | Severe | .0000527 |
| IL-6 | Severe | N/A a |
For each patient, the acute phase of disease was determined as the time prior to the cessation of viremia. The 2 patients classified as having severe disease were grouped together, and the 5 remaining patients formed the moderate disease group. The groups were compared with one another to identify biomarkers associated with disease severity. Asterisk (*) indicates a biomarker that also correlated with viremia.
Abbreviations: APO-Fas, apoptosis antigen-Fas; IL, interleukin; MCP, monocyte chemoattractant protein; N/A, not applicable; sFas-L, soluble Fas ligand; TNFR, tumor necrosis factor receptor.
a The lack of significant amounts of IL-6 in patients with moderate disease prevented a statistical comparison, but levels were clearly elevated in patients with severe disease.
Biomarkers Associated With Moderate Disease
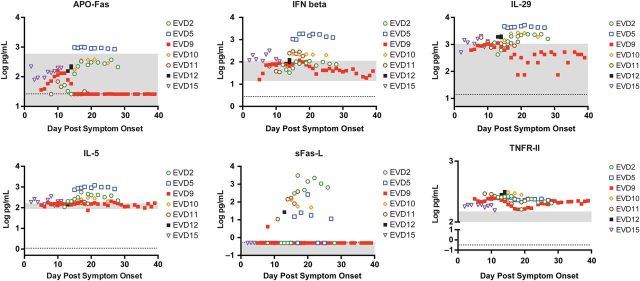
APO-Fas, IFN-β, IL-29, IL-5, sFas-L, and TNFR-II were elevated in patients with moderate disease (Figure 2 ). EVD5 had the highest levels of APO-Fas, IFN-β, IL-29, and IL-5; in sensitivity analysis, the association of these markers with moderate disease was still observed if EVD5 was excluded from the dataset. The physiologic significance of the association between APO-Fas and moderate disease is unclear, since most EVD patients had APO-Fas levels that fell within the reference range. Elevated levels of IFN-β in moderately ill patients were consistent with the observation that administering IFN-β to EBOV-infected macaques prolonged survival [ 17 ]. IL-29 (also known as IFN-λ) has never been examined in the context of EVD, and higher levels in patients with moderate disease could simply reflect its antiviral function similar to IFN-β. Elevated IL-5 in patients with moderate disease could indicate skewing of the adaptive response toward a Th2 phenotype, although this was not statistically significant for the main Th2 cytokine, IL-4 ( Supplementary data ). Elevated sFas-L and TNFR-II in patients with moderate EVD is suggestive of timely and appropriate control of the innate and adaptive immune responses.
Figure 2.
Biomarkers that were associated with moderate Ebola virus disease (EVD). Expression of each biomarker was assayed in all patient plasma samples as described. Each patient's data are indicated by the specified color and symbol. The x -axis shows days after onset of self-reported symptoms. The gray area represents the reference values of each biomarker as measured in samples from 10 healthy humans. The dotted line is the limit of the assay. For any sample that had a biomarker level that was below the limit of detection or undetectable, a non-zero value below the limit of detection was assigned to allow visualization of the data on the graph. This applies to apoptosis antigen-Fas (APO-Fas) and soluble Fas ligand (s-FasL). Abbreviations: IFN, interferon; IL, interleukin; TNFR, tumor necrosis factor receptor.
Biomarkers Associated With Severe Disease
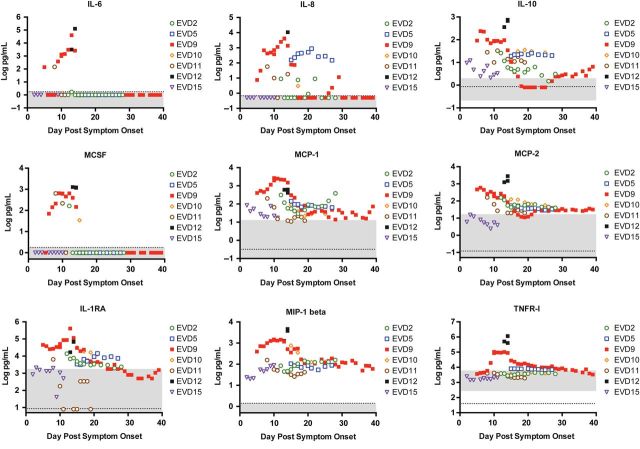
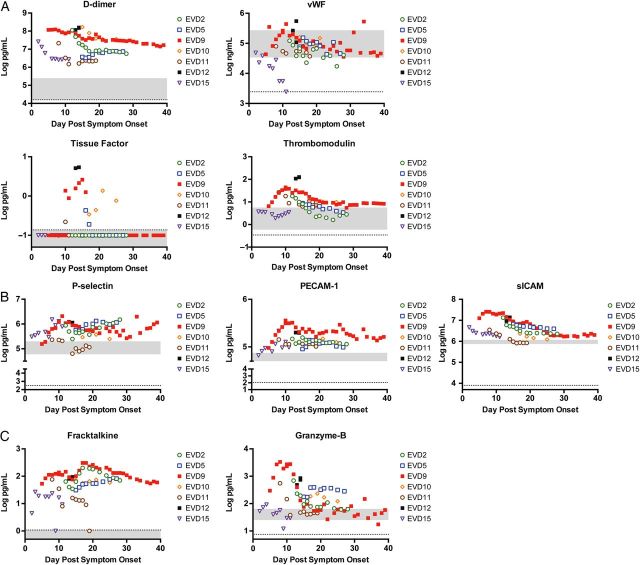
Severe EVD was associated with biomarkers of innate immune function, coagulation, endothelial function, lymphocyte activity, and inflammation. IL-6, IL-8, IL-10, MCSF, MCP-1, MCP-2, IL-1RA, MIP-1β, and TNFR-I were elevated in patients with severe disease, indicating innate immune dysregulation (Figure 3 ), as previously described [ 1 ]. IL-6 differences between moderate and severe disease were not statistically significant due to the small numbers of samples in the moderate group that had detectable levels. However, it is clear that there is a direct association between severity and IL-6 (Figure 3 ). Biomarkers suggesting coagulation pathway imbalance (D-dimer, vWF, TF, and thrombomodulin; Figure 4A ) and biomarkers indicating endothelial dysfunction (P-selectin, PECAM-1, and sICAM; Figure 4B ) were associated with severe EVD. Fractalkine, a marker of lymphocyte homing, and granzyme B, a marker of lymphocyte cytotoxicity, were also associated with severe EVD (Figure 4C ).
Figure 3.
Cytokines and chemokines that were associated with severe Ebola virus disease (EVD). Expression of each biomarker was assayed in all patient plasma samples as described. Each patient's data points are indicated by the specified color and symbol. The x -axis shows days after onset of self-reported symptoms. The gray area represents the reference values of each biomarker as measured in samples from 10 healthy humans. The dotted line is the limit of the assay. For any sample that had a biomarker level that was below the limit of detection or undetectable, a non-zero value below the limit of detection was assigned to allow visualization of the data on the graph. This applies to interleukin (IL)-6, IL-8, IL-10, monocyte colony stimulating factor (MCSF), and IL-1 receptor antagonist (RA). Abbreviations: MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TNFR, tumor necrosis factor receptor.
Figure 4.
Other biomarkers that were associated with severe Ebola virus disease (EVD). Expression of each biomarker was assayed in all patient plasma samples as described; markers of coagulation ( A ), markers of endothelial function ( B ), and markers of T-cell activity ( C ) were examined. Each patient's data points are indicated by the specified color and symbol. The x -axis shows days after onset of self-reported symptoms. The gray area represents the reference values of each biomarker as measured in samples from 10 healthy humans. The dotted line is the limit of the assay. For any sample that had a biomarker level that was below the limit of detection or undetectable, a non-zero value below the limit of detection was assigned to allow visualization of the data on the graph. This applies to von Willebrand factor (vWF), tissue factor, and fractalkine. Abbreviations: PECAM, platelet endothelial cell adhesion molecule; sICAM, soluble intracellular adhesion molecule.
DISCUSSION
Endothelial Function and EVD
Previous studies have suggested endothelial dysfunction in EVD: third-spacing in patients [ 12 , 18 ], detection of viral antigen in endothelial cells in fatal cases [ 19 ], and association of multiple markers of endothelial dysfunction with disease and sometimes fatal outcome [ 20 ]. Selectins are upregulated on the surface of activated leukocytes (L-selectin), activated endothelium (P-selectin or E-selectin), or activated platelets (P-selectin) and subsequently shed into the endovascular space [ 21 ]. The levels of L-selectin in all patients studied were essentially normal, while P-selectin and E-selectin were elevated in all patients, indicating endothelial and platelet activation during EVD (Figure 4B and Supplementary data ). Activated endothelia upregulate expression of ICAM and VCAM on the surface and shed soluble forms into plasma. sICAM levels were elevated in all patients, while sVCAM levels were within normal range in most patients. PECAM-1, which maintains tight junctions between endothelial cells [ 22 ], was markedly elevated in all patients. Furthermore, P-selectin, sICAM, and PECAM-1 correlated with disease severity in a statistically significant manner and remained elevated throughout early convalescence, supporting the role of endothelial activation in the EBOV disease process and highlighting the need to consider targeted endothelial therapies for EVD treatment. Many existing therapeutic strategies could be evaluated for efficacy against EVD [ 23 ], particularly statins, which downregulate ICAM and P-selectin, both of which are associated with severe EVD.
Given the overlap between viremia and outcome [ 16 ], it is difficult to derive conclusions about biomarkers associated with both severe EVD and viremia. Fractalkine, IL-10, IL-8, MCSF, IL-6, P-selectin, and TF correlated only with severe EVD, and not viremia. The last 2 could be related to endothelial activation and subsequent release of endothelium-derived procoagulant molecules, further highlighting the need for therapeutics that target the endothelium.
Coagulation and EVD
EVD was originally called Ebola hemorrhagic fever because of the hemorrhagic manifestations observed during the first 2 outbreaks in 1976 [ 24 , 25 ]. However, most studies from the current outbreak reported hemorrhagic manifestations (any form of bleeding) in fewer than 5% of patients [ 26–28 ]. Despite the lack of frank bleeding, there is an intimate relationship between coagulation and inflammation. An activated endothelium is both proinflammatory and procoagulant. The patients in this study have clear evidence of endothelial activation, which could trigger some aspects of the coagulation and fibrinolysis pathways. This battle to balance coagulation and fibrinolysis is clearly exemplified by the simultaneous upregulation of biomarkers of coagulation, anticoagulation, and fibrinolysis observed in these 7 patients; however, only thrombomodulin, TF, vWF, and D-dimer were associated with severe disease. All of these proteins are released from activated or damaged endothelial cells, and increased expression could reflect the endothelial dysfunction seen in EVD patients. Elevations in D-dimer have been associated with fatal EVD and were also associated with severe EVD in this cohort. One study on TF levels in human plasma during EVD reported no association with fatal outcomes [ 20 ], as had been suggested by nonhuman primate data [ 29 ]. However, in this study, the patients with severe disease had higher TF levels and both exhibited clinical coagulopathy (EVD9, oozing from venipuncture sites, and EVD12, elevated PTT and INR) during their clinical course.
Cellular Immunity and EVD
In a previous study of human immune responses to EVD, lymphocyte markers of activation and cytotoxicity were measured; expression of CX3CR1 (the fractalkine receptor) and intracellular granzyme B on CD8 cells indicated that these cells were homing to sites of inflammation and functionally cytotoxic [ 8 ]. Consistent with those data, both free plasma fractalkine and granzyme B were elevated in all patients studied, and both biomarkers correlated with disease severity. These markers of T-cell activity may reflect the higher viremia in the severely affected patients, indicating more T-cell–stimulating antigen. Consistent with this, granzyme B correlated with both viremia and disease severity. Additionally, these effector CD8 T cells may cause direct tissue damage and contribute to the disease process.
Immune Modulation and EVD
The human immune response to a viral infection generally allows for clearance of the virus via antibody-mediated neutralization and destruction of infected cells via cell-mediated immunity. However, this response can be destructive to the host if it continues unchecked. A dysregulated innate immune signaling system, with prolonged elevation of various cytokines, has been noted in patients with fatal EVD, suggesting, at least in part, a potentially harmful failure to downregulate the immune response in fatal cases. The results of the present study show that immune activity associated with moderate EVD includes antiviral interferons (IFN-β and IL-29/IFN-λ) and timely and appropriate contraction of the adaptive immune response (sFas-L and TNFR-II). It is possible that immune control mediated via sFas-L contributes to the moderate disease phenotype by preventing immunopathology. Supporting this hypothesis, administering sFas-L improved clinical outcome in herpes simplex virus–mediated keratitis, presumably by activating apoptosis of inflammatory cells [ 30 ]. The finding that TNFR-II is released in soluble form by T-regulatory cells and can control IL-6–mediated inflammation [ 31 ] adds further credence to the idea of immune control playing a critical role in controlling EBOV clinical severity. Additionally, in collagen-induced arthritis, TNFR-II was associated with T-regulatory activity, while TNFR-I was proinflammatory [ 32 ]. These data complement our findings of elevated TNFR-I in patients with severe disease and an association of TNFR-II with moderate disease and begin to distinguish between beneficial vs harmful host immune responses.
The findings in this study suggest a number of potential targets for clinical intervention. General downregulation of immune signaling pathways via administration of prednisone, specific administration of anti-IL-6R antibody [ 33 ] to block proinflammatory signaling by IL-6, or downregulation of the adaptive response by inhibition of lymphocyte proliferation using anti-IL-2R antibody [ 34 ] are possible ways to inhibit an overactive immune response. Additionally, activating IFN signaling by direct IFN administration could augment other therapeutic modalities. Not only will using the correct therapies be important, but also the timing of administration will be critical. Immune inhibition, if initiated at an inappropriate time, could be detrimental to the host. Our biomarker data argue for expanded evaluation of potential immunomodulatory therapies for EVD treatment using available animal models.
The limitations of our study include the small sample size; late medical attention received by some patients, preventing our ability to see the entire time course of viremia; and different forms of experimental therapeutics received by the patients. Despite these limitations, this extensive kinetic biomarker study can be useful for determining which molecular and cellular pathways are significant in the pathophysiology of the disease. The results from this study illuminate the beneficial vs harmful host immune responses in EVD. The next step is to generate testable hypotheses about these physiologic processes and evaluate them using relevant animal models.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org . Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the patients for agreeing to participate in the study and the staff of both the Emory University Serious Communicable Diseases Unit and the Nebraska Biocontainment Unit for their hard work and dedication in caring for patients with Ebola virus disease. We thank Tim Uyeki for providing coordination between clinical and research staff and Tatyana Klimova for editing the manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the CDC, the National Institutes of Health Atlanta Pediatric Scholars program (K12HD072245 to A. K. M.), the Defense Advanced Research Projects Agency (W31P4Q-14-1-0010 to R. A.), and the Burroughs Wellcome Fund (1013362.01 to A. K. M.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McElroy AK , Spiropoulou CF . Biomarkers for understanding Ebola virus disease . Biomark Med 2014. ; 8 : 1053 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baize S , Leroy EM , Georges AJ et al. . Inflammatory responses in Ebola virus-infected patients . Clin Exp Immunol 2002. ; 128 : 163 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baize S , Leroy EM , Georges-Courbot MC et al. . Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients . Nat Med 1999. ; 5 : 423 – 6 . [DOI] [PubMed] [Google Scholar]
- 4. Wauquier N , Becquart P , Padilla C , Baize S , Leroy EM . Human fatal Zaire Ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis . PLoS Negl Trop Dis 2010. ; 4 : e837 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rollin PE , Bausch DG , Sanchez A . Blood chemistry measurements and D-dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus . J Infect Dis 2007. ; 196 ( suppl 2 ): S364 – 71 . [DOI] [PubMed] [Google Scholar]
- 6. Sanchez A , Lukwiya M , Bausch D et al. . Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels . J Virol 2004. ; 78 : 10370 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hogardt M , Wolf T , Kann G et al. . Management of microbiological samples in a confirmed case of Ebola virus disease: constraints and limitations . J Clin Microbiol 2015. ; 53 : 3396 – 400 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McElroy AK , Akondy RS , Davis CW et al. . Human Ebola virus infection results in substantial immune activation . Proc Natl Acad Sci U S A 2015. ; 112 : 4719 – 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Towner JS , Sealy TK , Ksiazek TG , Nichol ST . High-throughput molecular detection of hemorrhagic fever virus threats with applications for outbreak settings . J Infect Dis 2007. ; 196 ( suppl 2 ): S205 – 12 . [DOI] [PubMed] [Google Scholar]
- 10. Hochberg BYaY . Controlling the false discovery rate: a practical and powerful approach to multiple testing . J R Stat Soc 1995. ; 57 : 289 – 300 . [Google Scholar]
- 11. Kraft CS , Hewlett AL , Koepsell S et al. . The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States . Clin Infect Dis 2015. ; 61 : 496 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyon GM , Mehta AK , Varkey JB et al. . Clinical care of two patients with Ebola virus disease in the United States . N Engl J Med 2014. ; 371 : 2402 – 9 . [DOI] [PubMed] [Google Scholar]
- 13. Liddell AM , Davey RT Jr , Mehta AK et al. . Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States . Ann Intern Med 2015. ; 163 : 81 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sueblinvong V , Johnson DW , Weinstein GL et al. . Critical care for multiple organ failure secondary to Ebola virus disease in the United States . Crit Care Med 2015. ; 43 : 2066 – 75 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Florescu DF , Kalil AC , Hewlett AL et al. . Administration of brincidofovir and convalescent plasma in a patient with Ebola virus disease . Clin Infect Dis 2015. ; 61 : 969 – 73 . [DOI] [PubMed] [Google Scholar]
- 16. Towner JS , Rollin PE , Bausch DG et al. . Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome . J Virol 2004. ; 78 : 4330 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith LM , Hensley LE , Geisbert TW et al. . Interferon-beta therapy prolongs survival in rhesus macaque models of Ebola and Marburg hemorrhagic fever . J Infect Dis 2013. ; 208 : 310 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolf T , Kann G , Becker S et al. . Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care . Lancet 2015. ; 385 : 1428 – 35 . [DOI] [PubMed] [Google Scholar]
- 19. Martines RB , Ng DL , Greer PW , Rollin PE , Zaki SR . Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses . J Pathol 2015. ; 235 : 153 – 74 . [DOI] [PubMed] [Google Scholar]
- 20. McElroy AK , Erickson BR , Flietstra TD et al. . Ebola hemorrhagic fever: novel biomarker correlates of clinical outcome . J Infect Dis 2014. ; 210 : 558 – 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McEver RP . Selectins: initiators of leucocyte adhesion and signalling at the vascular wall . Cardiovasc Res 2015. ; 107 : 331 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Privratsky JR , Newman PJ . PECAM-1: regulator of endothelial junctional integrity . Cell Tissue Res 2014. ; 355 : 607 – 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darwish I , Liles WC . Emerging therapeutic strategies to prevent infection-related microvascular endothelial activation and dysfunction . Virulence 2013. ; 4 : 572 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebola haemorrhagic fever in Zaire, 1976 . Bull World Health Organ 1978. ; 56 : 271 – 93 . [PMC free article] [PubMed] [Google Scholar]
- 25. Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team . Bull World Health Organ 1978. ; 56 : 247 – 70 . [PMC free article] [PubMed] [Google Scholar]
- 26. Yan T , Mu J , Qin E et al. . Clinical characteristics of 154 patients suspected of having Ebola virus disease in the Ebola holding center of Jui Government Hospital in Sierra Leone during the 2014 Ebola outbreak . Eur J Clin Microbiol Infect Dis 2015. ; 34 : 2089 – 95 . [DOI] [PubMed] [Google Scholar]
- 27. Schieffelin JS , Shaffer JG , Goba A et al. . Clinical illness and outcomes in patients with Ebola in Sierra Leone . N Engl J Med 2014. ; 371 : 2092 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lado M , Walker NF , Baker P et al. . Clinical features of patients isolated for suspected Ebola virus disease at Connaught Hospital, Freetown, Sierra Leone: a retrospective cohort study . Lancet Infect Dis 2015. ; 15 : 1024 – 33 . [DOI] [PubMed] [Google Scholar]
- 29. Geisbert TW , Young HA , Jahrling PB , Davis KJ , Kagan E , Hensley LE . Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event . J Infect Dis 2003. ; 188 : 1618 – 29 . [DOI] [PubMed] [Google Scholar]
- 30. Rogge M , Yin XT , Godfrey L et al. . Therapeutic use of soluble fas ligand ameliorates acute and recurrent herpetic stromal keratitis in mice . Invest Ophthalmol Vis Sci 2015. ; 56 : 6377 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Mierlo GJ , Scherer HU , Hameetman M et al. . Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators . J Immunol 2008. ; 180 : 2747 – 51 . [DOI] [PubMed] [Google Scholar]
- 32. McCann FE , Perocheau DP , Ruspi G et al. . Selective tumor necrosis factor receptor I blockade is antiinflammatory and reveals immunoregulatory role of tumor necrosis factor receptor II in collagen-induced arthritis . Arthritis Rheumatol 2014. ; 66 : 2728 – 38 . [DOI] [PubMed] [Google Scholar]
- 33. Mihara M , Kasutani K , Okazaki M et al. . Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family . Int Immunopharmacol 2005. ; 5 : 1731 – 40 . [DOI] [PubMed] [Google Scholar]
- 34. Pascual J , Marcen R , Ortuno J . Anti-interleukin-2 receptor antibodies: basiliximab and daclizumab . Nephrol Dial Transplant 2001. ; 16 : 1756 – 60 . [DOI] [PubMed] [Google Scholar]