Prior studies have suggested that marijuana use may accelerate liver fibrosis progression. In this prospective longitudinal study, we found no evidence that tetrahydrocannabinol influences liver fibrosis progression in human immunodeficiency virus/hepatitis C virus–coinfected women, even among daily users or with prolonged use.
Keywords: liver fibrosis, marijuana, HIV, HCV, women
Abstract
Background. Marijuana (hereafter “tetrahydrocannabinol [THC]”) use has been associated with liver fibrosis progression in retrospective analyses of patients with chronic hepatitis C (HCV). We studied long-term effects of THC on fibrosis progression in women coinfected with human immunodeficiency virus (HIV)/HCV enrolled in the Women's Interagency HIV Study (WIHS).
Methods. Liver fibrosis was categorized according to FIB-4 scores as none, moderate, or significant. THC and alcohol use were quantified as average exposure per week. Associations between THC use and progression to significant fibrosis were assessed using Cox proportional hazards regression.
Results. Among 575 HIV/HCV-coinfected women followed for a median of 11 (interquartile range, 6–17) years, 324 (56%) reported no THC use, 141 (25%) less than weekly use, 70 (12%) weekly use, and 40 (7%) daily use at WIHS entry. In univariable analysis, entry FIB-4 score (hazard ratio [HR], 2.26 [95% confidence interval {CI}, 1.88–2.73], P < .001), log HCV RNA (HR, 1.19 [95% CI, 1.02–1.38], P = .02), tobacco use (HR, 1.37 [95% CI, 1.02–1.85], P = .04), CD4+ count (risk per 100-cell increase: HR, 0.90 [95% CI, .86–.95], P < .001), and log HIV RNA (HR, 1.18 [95% CI, 1.05–1.32], P = .005) were associated with progression to significant fibrosis, as was cumulative alcohol use in follow-up (HR, 1.03 [95% CI, 1.02–1.04], P < .001). In multivariable analysis, entry FIB-4, entry CD4+ count, and cumulative alcohol use remained significant. Cumulative THC use was not associated with fibrosis progression (HR, 1.01 [95% CI, .92–1.10], P = .83).
Conclusions. In this large cohort of HIV/HCV-coinfected women, THC was not associated with progression to significant liver fibrosis. Alcohol use was independently associated with liver fibrosis, and may better predict fibrosis progression in HIV/HCV-coinfected women.
The cannabinoid system has an important impact on liver-related fibrosis, steatosis, regeneration, and portal hypertension [1–5]. Endocannabinoid ligands are ubiquitous and interact with cannabinoid receptors 1 and 2 (CB1 and CB2) [6], which have high affinity for tetrahydrocannabinol (THC), the psychoactive mediator of marijuana. Under physiologic conditions, hepatic expression of CB1 and CB2 is weak or absent [7]. However, both receptors are upregulated in a variety of liver diseases, including alcoholic and nonalcoholic liver disease, liver fibrosis, chronic hepatitis C, primary biliary cirrhosis, and hepatocellular carcinoma [8–13].
Limited studies have examined the impact of THC use and liver fibrosis progression, with conflicting results. Some cross-sectional studies have shown evidence of increased fibrosis [14, 15], and other longitudinal studies of relatively short duration suggest no impact on fibrosis progression [16, 17].
Data are lacking on the influence of long-term THC use on liver-related outcomes such as fibrosis in human immunodeficiency virus (HIV)/hepatitis C virus (HCV)–coinfected individuals. For this reason, we sought to examine the impact of THC use on liver fibrosis progression in a well-characterized cohort of women coinfected with HIV and HCV.
METHODS
Population
The Women's Interagency HIV Study (WIHS) is a prospective, multicenter, longitudinal observational cohort of adult women infected with HIV or at high risk of acquiring HIV, described in detail elsewhere [18, 19]. Approximately 30% of HIV-infected women enrolled were coinfected with HCV prior to enrollment in WIHS. A total of 4137 women have been enrolled in the first 3 enrollment cohorts (1 = 1994–1995; 2 = 2001–2002; 3 = 2011–2012). All women gave informed consent at entry, and the study was approved by each center's institutional review board. Patients are seen in follow-up every 6 months, where detailed sociodemographic, medical, and behavioral data are collected through structured interviews, physical examination, and biologic specimen collection. As of visit 37 (conducted between October 2012 and April 2013), a total of 790 women with HIV/HCV coinfection at baseline had enrolled in the WIHS into cohorts 1 and 2, and were included in our analysis. HIV infection was confirmed by positive HIV enzyme-linked immunosorbent assay and a confirmatory Western blot, and HCV infection was defined as a positive result for serum HCV RNA at entry into the study. THC use is prevalent in the WIHS; 14% of participants regularly use marijuana, and 6% report daily use [20]. Liver biopsy is not performed as part of the WIHS observational study. However, noninvasive markers of liver fibrosis, aspartate aminotransferase to platelet ratio index (APRI), and FIB-4 have been validated in this cohort [21].
Data Collection
Sociodemographic data including age, ethnicity, race, and past substance use were collected at entry into WIHS. Biologic specimens were collected at visits every 6 months with testing for liver enzymes, renal function, complete blood count, CD4 count, and HIV RNA load. HIV RNA was measured using the isothermal nucleic acid sequence–based amplification (NASBA/Nuclisens) method (bioMérieux, Durham, North Carolina) with a lower limit of detection of 80 copies/mL. Highly active antiretroviral therapy was defined by the contemporaneous US Department of Health and Human Services guidelines [22]. Second- or third-generation enzyme immunoassays were utilized for HCV antibody detection (Ortho-Diagnostic Systems, Rochester, New York) and confirmed by documenting HCV RNA seropositivity (Quantiplex 2.0 branched chain DNA-enhanced label amplification assay; Bayer-Versant Diagnostics, Emeryville, California) and by reverse transcription polymerase chain reaction (COBAS Amplicor HCV Detection Kit, Roche Diagnostic Systems, Pleasanton, California). Hepatitis B was tested within the first year of enrollment and was defined as hepatitis B surface antigenemia. Data including body mass index, history of hypertension, diabetes, and insulin resistance were collected. Diabetes was defined as any fasting glucose measurement of >126 mg/dL, hemoglobin A1c measured at ≥6.5%, or self-report of taking antidiabetic medication.
Drug and Alcohol Use
THC use was assessed at the entry and follow-up visits. THC users were asked to quantify the amount consumed over the preceding 6 months (less than once per month, at least once per month but less than once a week, once a week, 2–3 times per week, 4–6 times a week, or daily [1 or more times per day]). The number of uses per 6 months was cumulated to estimate the mean number of uses per week during the entire observation period. Women were then categorized based on average in-WIHS THC use (abstinent throughout WIHS; <1 use per week; 2–3 uses per week; 4–7 uses per week). Similarly, participants reported the number of alcoholic drinks consumed over the preceding 6 months. Drinks per week were cumulated to estimate the mean number of drinks per week during the observation period. For both THC and alcohol use, exposure was tallied until the event of interest (severe fibrosis) or last observation for patients without the event. Women were considered heavy drinkers if they consumed >7 drinks per week.
Fibrosis Measurements
Noninvasive fibrosis measurements including FIB-4 and APRI have been validated in patients with HIV/HCV coinfection [23, 24], including this cohort [21]. WIHS subjects had FIB-4 measured at every visit. Standard cutoffs of FIB-4 were used to define no fibrosis (<1.5) and significant fibrosis (>3.25) for each measure. FIB-4 values were considered invalid in patients if aspartate aminotransferase or alanine aminotransferase was >10 times the upper limit of normal or if platelet counts were <25 000 × 106 cells/L, as these extreme values are unlikely to be due to chronic liver fibrosis, and more likely to be caused by acute hepatitis or another disease process.
Statistical Analysis
Discrete variables were summarized using frequency and percentages; continuous variables were summarized using mean and standard deviation for normally distributed data, and median and interquartile range (IQR) for nonnormally distributed data. Comparisons between THC use groups were made using the χ2 or Fisher exact tests for categorical data and analysis of variance or Kruskal–Wallis tests for continuous data. The cumulative probability of progression to advanced fibrosis was estimated using the Kaplan–Meier method and compared by THC use category with the log-rank test. All analyses were 2 tailed, with P < .05 considered statistically significant.
Risk of progression to advanced fibrosis was evaluated separately for FIB-4 and APRI outcomes using Cox proportional hazards regression. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated from the models. To account for the fact that patients must remain alive to enter the study, age at study entry was treated as a left-truncated variable with age used as the time scale. Patients were followed until their first visit with significant fibrosis (>3.25 for FIB-4 and >1.5 for APRI) or were censored at the date of last fibrosis measurement for those not progressing to the event. Factors with a univariable P < .1 and THC use were evaluated in the multivariable model. The model was selected by backward elimination with P > .05 for removal from the model. The final model included 2 additional variables: (1) biologically relevant HCV RNA and (2) THC use to assess the independent association of its use with risk of fibrosis progression. Subanalyses were conducted among patients with a minimum of 2 years of follow-up to evaluate whether long-term THC exposure impacted fibrosis progression. We also conducted subanalyses in participants with at least mild fibrosis (FIB-4 score >1.5) to evaluate whether the presence of some fibrosis is a prerequisite for THC to modulate fibrosis progression. Data were analyzed using SAS software version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Study Population
Characteristics at WIHS entry for the 575 HIV/HCV-coinfected women included in this analysis are summarized in Table 1. Median follow-up in WIHS was 11 (IQR, 6–17) years. The majority of women were African American (63%) and had HCV genotype 1 (88%); the mean HCV viral load was 6.1 log10 IU/mL. Mean log10 HIV RNA was 4.1 IU/mL, and median CD4 count at entry was 375 cells/µL. Six percent of women were on antiretroviral therapy (ART) at entry. Median FIB-4 score at entry was 1.3 (IQR, 0.9–1.8). Only 2% (n = 11) of women had evidence of significant fibrosis (FIB-4 > 3.25) at entry, and these women were therefore excluded from the time to significant fibrosis analysis. Within the entire cohort, a total of 15% (n = 88) received interferon-based HCV treatment (11 achieved sustained virologic response, 73 did not clear HCV, and 4 had unknown outcomes). Metabolic risk factors were common in this cohort (Table 1). Overall, alcohol use at entry was low in this cohort (median, 0.5 [IQR, 0–4] drinks per week), but 19% of women had heavy alcohol use (>7 drinks per week) at entry and 9% (n = 53) reported heavy alcohol use at last follow-up.
Table 1.
Characteristics of Participants at Women's Interagency Human Immunodeficiency Virus Study Entry and at Last Visit
| Variable | WIHS Entry (n = 575) | Last Visit (n = 575) |
|---|---|---|
| Age, y, mean (SD) | 40 (6) | 51 (7) |
| Race/ethnicity | ||
| White, non-Hispanic | 16% (94) | |
| African American, non-Hispanic | 63% (363) | |
| Hispanic | 19% (108) | |
| HCV genotype 1 | 88% (418) | |
| HCV VL, log IU/mL, mean (SD) | 6.1 (0.91) | 6.3 (0.81) |
| CD4 count, cells/µL, median (IQR) | 375 (218–569) | 330 (109–583) |
| HIV VL, log IU/mL, mean (SD) | 4.1 (1.1) | 3.1 (1.62) |
| % HIV undetectable | 7% (41) | 33% (189) |
| ART use | 6% (32) | 63% (360) |
| FIB-4 score, median (IQR) | 1.3 (0.9–1.8) | 2.1 (1.4–3.8) |
| BMI, kg/m2, mean (SD) | 26 (6) | 26 (7) |
| Hypertension | 30% (171) | 49% (283) |
| Diabetes | 18% (105) | 18% (105) |
| eGFR, mL/min/1.73 m2, median (IQR) | 88 (73–102) | 83 (57–101) |
| Current cigarette use | 80% (460) | 66% (381) |
| Current intravenous drug use | 23% (130) | 8% (48) |
| Alcohol use, drinks/wk, median (IQR) | 0.5 (0–4) | 0 (0–1) |
| Heavy alcohol use (>7 drinks/wk) | 19% (106) | 9% (53) |
Data are presented as % (No.) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis 4; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation; VL, viral load; WIHS, Women's Interagency HIV Study.
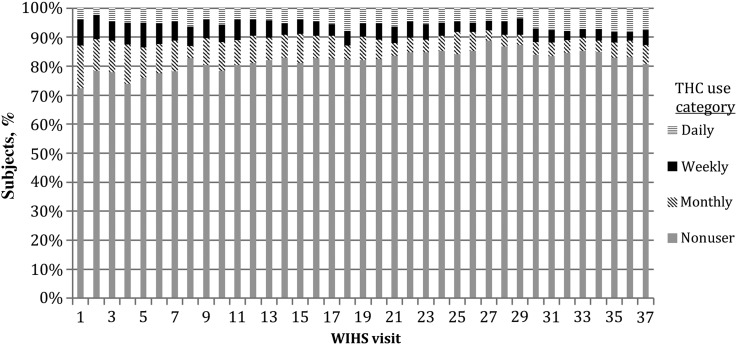
Table 2 describes cohort characteristics by entry THC use. The groups were similar overall in terms of entry characteristics. THC users were more likely to smoke cigarettes, use intravenous drugs, and consume alcohol. Eighty-three percent of participants had used THC prior to entry into WIHS, and 44% of women had used THC in the 6 months prior to enrollment, 19% with at least weekly use. Median follow-up in WIHS was similar between THC use groups (abstinent group, 10 [IQR, 6–17] years; monthly users, 10 [IQR, 5–17] years; weekly users, 11 [IQR, 6–17] years; and daily users, 11 [IQR, 7–14] years). Eleven percent of women reported at least weekly THC use in the preceding 6 months, and 6% (n = 35) reported daily use. Forty-six percent (n = 262) remained THC nonusers throughout follow-up. Figure 1 describes the distribution of THC use in WIHS over time.
Table 2.
Women's Interagency Human Immunodeficiency Virus Study Entry Characteristics by Entry Tetrahydrocannabinol Use Category (n = 575)
| Variable | WIHS Entry THC Use |
||||
|---|---|---|---|---|---|
| None (n = 324) | Monthly Use (n = 141) | Weekly Use (n = 70) | Daily Use (n = 40) | P Value | |
| Age, y, mean (SD) | 40 (6) | 40 (7) | 39 (6) | 40 (6) | .61 |
| Race/ethnicity | |||||
| White, non-Hispanic | 14% (45) | 21% (29) | 19% (13) | 18% (7) | .76 |
| African American, non-Hispanic | 66% (214) | 60% (85) | 57% (40) | 60% (24) | |
| Hispanic | 19% (61) | 17% (24) | 21% (15) | 20% (8) | |
| HCV genotype 1 | 88% (239) | 88% (102) | 89% (50) | 87% (27) | .99 |
| HCV VL, log IU/mL, mean (SD) | 4.2 (1.1) | 6.1 (0.9) | 6.2 (0.8) | 6.1 (1.0) | .72 |
| CD4 count, cells/µL, median (IQR) | 354 (195–568) | 388 (257–564) | 378 (235–556) | 391 (236–664) | .25 |
| HIV VL, log IU/mL, mean (SD) | 4.2 (1.1) | 3.9 (1.1) | 4.0 (1.1) | 3.8 (1.3) | .03 |
| FIB-4 score, median (IQR) | 1.3 (1.0–1.9) | 1.2 (0.8–1.8) | 1.3 (1.0–1.9) | 1.2 (1.0–1.7) | .35 |
| BMI, kg/m2, mean (SD) | 26 (6) | 26 (6) | 26 (7) | 25 (5) | .71 |
| Hypertension | 30% (98) | 28% (39) | 31% (22) | 30% (12) | .93 |
| Diabetes | 18% (59) | 19% (27) | 16% (11) | 20% (8) | .93 |
| eGFR, mL/min/1.73 m2, median (IQR) | 87 (72–102) | 88 (72–101) | 87 (77–103) | 93 (77–112) | .42 |
| Cigarette use | 74% (241) | 89% (126) | 86% (60) | 83% (33) | .001 |
| Intravenous drug use | 19% (63) | 28% (39) | 31% (22) | 15% (6) | <.001 |
| Alcohol use, drinks/wk, median (IQR) | 0 (0–3) | 1 (0–9) | 1 (0–8) | 0.5 (0–4.5) | <.001 |
| Heavy alcohol use (>7 drinks/wk) | 12% (40) | 28% (39) | 26% (18) | 23% (9) | <.001 |
Data are presented as % (No.) unless otherwise indicated.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis 4; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation; THC, tetrahydrocannabinol; VL, viral load; WIHS, Women's Interagency HIV Study.
Figure 1.
Tetrahydrocannabinol (THC) use during the Women's Interagency Human Immunodeficiency Virus Study (WIHS) from visit 1 (1997) to visit 37 (2012). Use was daily, weekly, monthly, or nonusers.
Predictors of Fibrosis Progression
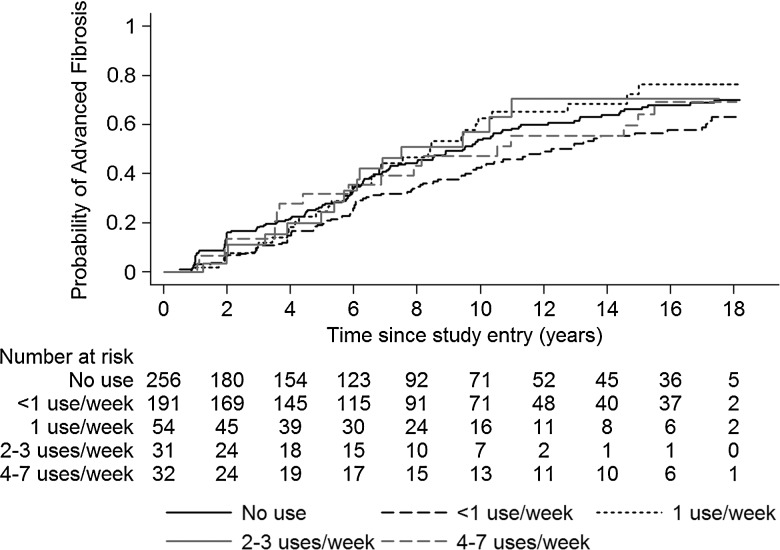
During follow-up, 51% (n = 291) of women developed significant fibrosis (FIB-4 score >3.25). Fibrosis progression was similar between categories of THC use (Figure 2). On univariate analysis, entry FIB-4, HIV RNA load, lower CD4 count, log HCV RNA, alcohol use, and smoking status were associated with progression to significant fibrosis during follow-up, whereas African American race was found to be protective (Table 3). These variables were also found to be predictive of fibrosis progression in the subgroup with baseline fibrosis (FIB-4 score ≥1.5 at WIHS entry). Entry FIB-4, lower entry CD4 count, and alcohol use in follow-up remained significant in multivariable analysis (Table 3). Mean THC use per week during the observation period was not independently associated with a greater risk of progression to significant fibrosis in multivariable analysis when evaluated as a continuous variable (HR, 1.01 per 1 use per week increase [95% CI, .92–1.10]). Similarly, no association between fibrosis progression and THC use was detected when mean THC use per week was treated as a categorical variable (<1 use per week: HR, 0.84 [95% CI, .64–1.12], P = .23; 1 use per week: HR, 1.40 [95% CI, .95–2.07], P = .09; 2–3 uses per week: HR, 1.11 [95% CI, .65–1.91], P = .70; 4–7 uses per week: HR, 1.01 [95% CI, .61–1.66], P = .97, compared with no reported THC use). There was no association between THC use and fibrosis progression among those women with fibrosis at entry (HR, 1.01 per 1 use per week increase [95% CI, .88–1.16]).
Figure 2.
Probability of developing advanced fibrosis (FIB-4 score >3.25) over time by average weekly tetrahydrocannabinol use.
Table 3.
Selected Univariable and Multivariable Predictors of Significant Fibrosis (FIB-4 > 3.25)a on Follow-up in Women Without Significant Fibrosis at Women's Interagency Human Immunodeficiency Virus Study Entry (n = 564)
| Variable | Univariable Analysis |
Multivariable Analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Entry FIB-4 score | 2.26 (1.88–2.73) | <.001 | 1.98 (1.62–2.42) | <.001 |
| Black (Hispanic or African American) vs non-black race | 0.78 (.61–.99) | .04b | ||
| Entry BMI | 0.98 (.96–1.00) | .09 | ||
| Entry log10 HCV RNA | 1.19 (1.02–1.38) | .02 | 1.07 (.92–1.24) | .37 |
| Baseline log10 HIV RNA load | 1.18 (1.05–1.32) | .005b | ||
| Entry CD4 | 0.90 (.86–.95) | <.001 | 0.93 (.89–0.98) | .006 |
| Entry tobacco use | 1.37 (1.02–1.85) | .04b | ||
| Heavy entry alcohol use (>7 drinks/wk) vs abstainers | 1.26 (.94–1.70) | .12 | ||
| Risk per 1-drink increase in the weekly average | 1.03 (1.02–1.04) | <.001 | 1.02 (1.01–1.03) | <.001 |
| Risk per 1-use increase in average THC use per week | 1.00 (.92–1.09) | 1.00 | 1.01 (.92–1.10) | .83 |
Abbreviations: BMI, body mass index; CI, confidence interval; FIB-4, fibrosis 4; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; THC, tetrahydrocannabinol.
a Cox proportional hazards regression, accounting for left truncation.
b Not significant on multivariable analysis.
Of the 489 participants with at least 2 years (4 intervals) of follow-up, 15% (n = 74) reported weekly or greater THC use and 6% (n = 29) daily use for ≥2 years. Among these women, neither duration nor frequency of THC use was found to be predictive of significant fibrosis (HR, 1.26 [95% CI, .86–1.83], P = .23 for weekly or greater THC use vs no use). Similarly, no association was detected between the number of intervals with weekly or greater THC use and significant fibrosis compared with abstainers with a similar length of follow-up (HR, 1.23 [95% CI, .87–1.73], P = .58). Similar results were seen in the subgroup of women with baseline fibrosis at entry (FIB-4 score >1.5) and when APRI was used instead of FIB-4 as a marker for liver fibrosis progression (results not shown).
DISCUSSION
Currently marijuana has been legalized for medicinal and/or recreational use in 23 US states, as well as the District of Columbia [25, 26], and its use in HIV/HCV-coinfected patients is common. Given that it is becoming more widely available and more regularly consumed, it is critical to assess its clinical effects, including any negative impact of THC use on liver fibrosis progression.
We found no evidence that THC use increases the risk of progression of liver fibrosis in women with HIV/HCV coinfection on univariable or multivariable analyses. We examined the impact of THC use in a few key subgroups including women with fibrosis at WIHS entry (FIB-4 score >1.5 or APRI >0.5), daily THC users, and women with regular THC use throughout WIHS follow-up, and found no evidence of an association with accelerated liver fibrosis (compared with nondaily use or abstinence). The development of liver fibrosis can take years, and prolonged THC use may be required to change rates of liver fibrosis. Similar to other published studies, we found that presence of baseline fibrosis, entry CD4 count and HCV RNA level, and ongoing alcohol consumption were predictive of significant fibrosis in WIHS follow-up [27, 28], whereas African American race was found to be protective [29, 30].
Chronic liver disease modulates hepatic cannabinoid (CB) receptor expression [3, 13, 31]. CB1 is upregulated in chronic liver disease including HCV infection [12]. CB2 is also upregulated in chronic liver disease and prevents fibrosis progression [3, 32, 33], possibly through inhibition of hepatic myofibroblasts, or by increasing hepatocyte survival [11], downregulation of interleukin 17 [34], and modulation of Kupffer cells [35]. Thus, the balance between CB1 vs CB2 activation may modulate fibrosis progression in patients with liver disease. If overexpression of both receptors is balanced, there is no change in liver fibrosis.
Previous cross-sectional analyses suggested that marijuana use was associated with accelerated fibrosis progression. In one study of HCV-monoinfected patients, daily THC use was associated with higher fibrosis progression rate than was occasional use or nonuse, and remained significant on multivariable analysis [14]. In another study, daily THC use was correlated with advanced fibrosis on liver biopsy in HCV-monoinfected and HIV/HCV-coinfected participants [15]. Although THC users appeared to have more advanced medical disease, daily THC use was predictive of significant fibrosis on multivariable analysis.
To our knowledge, there is only one other large longitudinal study that explores the relationship between THC use and fibrosis progression in HIV/HCV-coinfected individuals. In this study, participants were predominantly male (73%) and had minimal fibrosis at entry and at follow-up (median APRI, 0.52 [IQR, 0.37–0.79] and 0.55 [IQR, 0.37–0.90], respectively). This study also failed to demonstrate an increased risk of fibrosis progression with THC use. Importantly, median follow-up was only 2.7 years, perhaps insufficient time to demonstrate fibrosis progression in subjects with minimal fibrosis. In our cohort, the median follow-up time was 11 years, with 25% having >13 years of follow-up. Despite long follow-up, we still were unable to demonstrate a relationship between THC use and fibrosis progression.
This is the first study describing liver fibrosis outcomes by THC use in HIV/HCV-coinfected women and one of the few longitudinal studies of the influence of THC on fibrosis progression. Strengths of this study include the long follow-up and prospective collection of relevant data. However, there are limitations. THC and alcohol use may have been underreported, which may impact results. Liver biopsy was not performed in this observational study, and therefore we determined fibrosis stage using noninvasive tests (FIB-4 and APRI). Although these measures may be less accurate than liver biopsy, FIB-4 was specifically developed for use in HIV/HCV-coinfected patients and accurately predicts liver fibrosis in this group, with areas under the receiver operating characteristic curve of 0.73–0.79 for predicting significant liver fibrosis [24]. Although FIB-4 increases over time as age is incorporated into the calculation, it is an accepted noninvasive measure of liver fibrosis. Moreover, we repeated our calculations using APRI, which does not include age in its calculation, and similar results were obtained. Additionally, both APRI and FIB-4 have been validated in WIHS and shown to be independently associated with all-cause mortality in HCV/HIV-coinfected women [21].
Overall THC use in this cohort was low, with 67% of women abstaining from THC or using less than weekly for the entire duration of follow-up. The relatively small number of heavy THC users in this cohort may have impacted our ability to detect an association between daily THC use and fibrosis progression. Nonetheless, the long duration of follow-up and size of the study allowed us to conclude that occasional THC use is unlikely to impact fibrosis progression. Current and ever THC use was recorded at entry without an estimate of duration or frequency of use prior to WIHS entry. Therefore, we are unable to assess the impact of THC use prior to WIHS or lifetime cumulative use on clinical outcomes. The majority of women in this cohort are African American, with well-described lower rates of fibrosis progression; therefore, our findings may not be generalizable to males or white individuals. This study was a cohort study recruited from the community, not a treatment study. Our data found that HIV status did predict liver fibrosis, shown by both CD4 and HIV RNA in univariate analyses. While baseline CD4 count remained significant in multivariable analysis, HIV RNA load was not predictive of fibrosis progression in multivariable analysis. This longitudinal cohort began in an era with more limited treatment options for HIV and HCV. Few women received interferon-based antiviral therapy, with low rates of sustained virologic response. Most women were not receiving ART at study entry; therefore, entry FIB-4 may be influenced by HIV-associated thrombocytopenia or elevations in liver enzymes, leading to overestimates of baseline fibrosis, which may underestimate fibrosis progression. We safeguarded for this by censoring extreme laboratory values that were unlikely to be related to HCV fibrosis. It is also possible that viral factors played a more important role in the development and progression of fibrosis in this group of participants. Nonetheless, we did not identify a cohort effect when cohort 1 (1994–1995 entry) and cohort 2 (2001–2002 entry) were compared (data not shown).
CONCLUSIONS
In this longitudinal analysis of HIV/HCV-coinfected women, we found no association between THC use and liver fibrosis progression. Although few women had heavy prolonged THC exposure, the robust follow-up data among light users in this study supports that occasional THC does not impact fibrosis progression. Fibrosis progression was associated with poor HIV control and alcohol use. Clinicians should therefore counsel patients on limiting or excluding alcohol intake, in addition to optimizing medical treatment for HIV and HCV, as these factors are more important than THC use in modulating liver disease.
Notes
Disclaimer. Data in this manuscript were collected by the Women's Interagency Human Immunodeficiency Virus Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (award numbers U01-AI-103401, U01-AI-103408, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, U01-AI-103397, U01-AI-103390, UO1-AI-34989, and UO1-AI-42590), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and Other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by the University of California, San Francisco Clinical and Translational Science Award program (UCSF CTSA) (award number UL1-TR000004) and Atlanta CTSA (award number UL1-TR000454). The study was also supported by the UCSF Liver Center National Institute of Health (award number P30 DK026743) and by the NIAID (award numbers R21 AI088351 to M. G. P., K24 AI 108516 and R01 AI 087176 to P. C. T.), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. WIHS (Principal Investigators): U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I–WIHS IV). The WIHS is funded primarily by the NIAID, with additional co-funding from the NICHD, NCI, NIDA, and NIMH. Targeted supplemental funding for specific projects is also provided by NIDCR, NIAAA, NIDCD, and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA).
Potential conflicts of interest. M. J. G. has received payments for lectures from the International Antiviral Society–USA, manuscript preparation from Clinical Care Options, and royalties from UpToDate. M. P. is employed by Hoffman La Roche, and has received consultancy fees from Abbott, Merck, Roche, Johnson & Johnson, Gilead, and Biotron. P. C. T. has received payment from Brystol Myers Squibb and AbbVie for board membership. M. A. has received institutional grant funding from State University of New York Downstate. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Siegmund SV, Schwabe RF. Endocannabinoids and liver disease. II. Endocannabinoids in the pathogenesis and treatment of liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2008; 294:G357–62. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira-Clerc F, Julien B, Grenard P et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 2006; 12:671–6. [DOI] [PubMed] [Google Scholar]
- 3.Julien B, Grenard P, Teixeira-Clerc F et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005; 128:742–55. [DOI] [PubMed] [Google Scholar]
- 4.Batkai S, Jarai Z, Wagner JA et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 2001; 7:827–32. [DOI] [PubMed] [Google Scholar]
- 5.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut 2008; 57:1140–55. [DOI] [PubMed] [Google Scholar]
- 6.Mallat A, Teixeira-Clerc F, Lotersztajn S. Cannabinoid signaling and liver therapeutics. J Hepatol 2013; 59:891–6. [DOI] [PubMed] [Google Scholar]
- 7.Baldassarre M, Giannone FA, Napoli L et al. The endocannabinoid system in advanced liver cirrhosis: pathophysiological implication and future perspectives. Liver Int 2013; 33:1298–308. [DOI] [PubMed] [Google Scholar]
- 8.Siegmund SV. Role of the endocannabinoid system in alcoholic liver disease. Dig Dis 2010; 28:751–5. [DOI] [PubMed] [Google Scholar]
- 9.Patsenker E, Stoll M, Millonig G et al. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol Med 2011; 17:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol 2013; 28(suppl 1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teixeira-Clerc F, Belot MP, Manin S et al. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology 2010; 52:1046–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Poorten D, Shahidi M, Tay E et al. Hepatitis C virus induces the cannabinoid receptor 1. PLoS One 2010; 5:e12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology 2011; 53:346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida JH, Peters MG, Jin C et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol 2008; 6:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hezode C, Roudot-Thoraval F, Nguyen S et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 2005; 42:63–71. [DOI] [PubMed] [Google Scholar]
- 16.Brunet L, Moodie EE, Rollet K et al. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: a longitudinal cohort analysis. Clin Infect Dis 2013; 57:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol 2014; 28:381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkan SE, Melnick SL, Preston-Martin S et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 19.Bacon MC, von Wyl V, Alden C et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Souza G, Matson PA, Grady CD et al. Medicinal and recreational marijuana use among HIV-infected women in the Women's Interagency HIV Study (WIHS) cohort, 1994-2010. J Acquir Immune Defic Syndr 2012; 61:618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bambha K, Pierce C, Cox C et al. Assessing mortality in women with hepatitis C virus and HIV using indirect markers of fibrosis. AIDS 2012; 26:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, 2008. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 23.Wai CT, Greenson JK, Fontana RJ et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–26. [DOI] [PubMed] [Google Scholar]
- 24.Sterling RK, Lissen E, Clumeck N et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 25.Marijuana Policy Project. The twenty three states and one federal district with effective medical marijuana laws. Available at: https://www.mpp.org/wp-content/plugins/download-attachments/includes/download.php?id=5624. Accessed 1 May 2016.
- 26.Ammerman S, Ryan S, Adelman WP. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics 2015; 135:e769–85. [DOI] [PubMed] [Google Scholar]
- 27.Benhamou Y, Bochet M, Di Martino V et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30:1054–8. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Sierra C, Arizcorreta A, Diaz F et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis 2003; 36:491–8. [DOI] [PubMed] [Google Scholar]
- 29.Silver D, Karnik G, Osinusi A et al. Effect of HIV on liver fibrosis among HCV-infected African Americans. Clin Infect Dis 2013; 56:1280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar M, Bacchetti P, Tien P et al. Racial/ethnic differences in spontaneous HCV clearance in HIV infected and uninfected women. Dig Dis Sci 2013; 58:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallat A, Teixeira-Clerc F, Deveaux V, Manin S, Lotersztajn S. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol 2011; 163:1432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Luque J, Ros J, Fernandez-Varo G et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther 2008; 324:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avraham Y, Amer J, Doron S et al. The direct profibrotic and indirect immune antifibrotic balance of blocking the cannabinoid 2 receptor. Am J Physiol Gastrointest Liver Physiol 2012; 302:G1364–72. [DOI] [PubMed] [Google Scholar]
- 34.Guillot A, Hamdaoui N, Bizy A et al. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology 2014; 59:296–306. [DOI] [PubMed] [Google Scholar]
- 35.Louvet A, Teixeira-Clerc F, Chobert MN et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011; 54:1217–26. [DOI] [PubMed] [Google Scholar]