There is no objective quantitation of toxoplasmosis disease burden in the United States. A large insurance-based dataset was used to identify clinical manifestations, residence, and associated comorbid conditions. Our analyses support the benefits of mandatory reporting and gestational screening.
Keywords: Toxoplasma gondii, toxoplasmosis, “large data”, Truven Health MarketScan Database, ICD-9 code
Abstract
Background. Toxoplasma gondii infection causes substantial morbidity and mortality in the United States, and infects approximately one-third of persons globally. Clinical manifestations vary. Seropositivity is associated with neurologic diseases and malignancies. There are few objective data concerning US incidence and distribution of toxoplasmosis.
Methods. Truven Health MarketScan Database and International Classification of Diseases, Ninth Revision (ICD-9) codes, including treatment specific to toxoplasmosis, identified patients with this disease. Spatiotemporal distribution and patterns of disease manifestation were analyzed. Comorbidities between patients and matched controls were compared.
Results. Between 2003 and 2012, 9260 patients had ICD-9 codes for toxoplasmosis. This database of patients with ICD-9 codes includes 15% of those in the United States, excluding patients with no or public insurance. Thus, assuming that demographics do not change incidence, the calculated total is 61 700 or 6856 patients per year. Disease was more prevalent in the South. Mean age at diagnosis was 37.5 ± 15.5 years; 2.4% were children aged 0–2 years, likely congenitally infected. Forty-one percent were male, and 73% of women were of reproductive age. Of identified patients, 38% had eye disease and 12% presented with other serious manifestations, including central nervous system and visceral organ damage. Toxoplasmosis was statistically associated with substantial comorbidities, including human immunodeficiency virus, autoimmune diseases, and neurologic diseases.
Conclusions. Toxoplasmosis causes morbidity and mortality in the United States. Our analysis of private insurance records missed certain at-risk populations and revealed fewer cases of retinal disease than previously estimated, suggesting undercoding, underreporting, undertreating, or differing demographics of those with eye disease. Mandatory reporting of infection to health departments and gestational screening could improve care and facilitate detection of epidemics and, thereby, public health interventions.
(See the Editorial Commentary by Wing on pages 476–7.)
Toxoplasmosis, a disease caused by infection with the Apicomplexan parasite, Toxoplasma gondii, is a major source of morbidity and mortality in the United States and globally [1]. Disease presentation is highly variable, though severe eye disease resulting in loss of sight is not uncommon, and immunocompromised patients can present with serious infections of the central nervous system (CNS) [2–7]. Acquisition of infection during pregnancy can result in vertical transmission, leading to profound disability due to untreated congenital infection; consequences for the infected, untreated infant include cognitive impairment, hydrocephalus, and disability due to loss of sight [8]. Additionally, previous studies posit a role for T. gondii infection in a variety of comorbid conditions, including epilepsy and neurologic diseases [9–13]. In these ways, toxoplasmosis continues to represent a major public health threat, as yet incompletely characterized in the United States. Thorough quantitation and characterization would facilitate targeted intervention strategies.
To our knowledge, there are no accurate, empiric data defining incidence of toxoplasmosis in the United States. According to the Centers for Disease Control and Prevention, toxoplasmosis remains one of the leading single causes of death related to foodborne illness in the United States, although numerically it is a minority. It is a neglected infection [14]. Estimates of infection prevalence are limited in scope. Seroprevalence in the United States is estimated at approximately 11% for women of childbearing age [15]. Additionally, one recent study estimates 4839 cases of symptomatic ocular toxoplasmosis per year nationally [16]. Estimates for other manifestations, including infection of the CNS and visceral organs, are lacking. Most studies of prevalence of toxoplasmic meningoencephalitis in the United States were from the 1990s, in the context of the human immunodeficiency virus (HIV)/AIDS epidemic, prior to advent of highly active antiretroviral therapy (HAART) [17, 18]. The most recent estimate, in 2003, indicated an annual risk reduction of 18% for cerebral toxoplasmosis, although these data were derived from a cohort of individuals being treated with HAART [19]. There is a paucity of estimates of noneye manifestations for the last decade.
Active infection with T. gondii is treated successfully with pyrimethamine and sulfadiazine [6–8, 20]. These are the most effective medicines for treating toxoplasmosis, including ocular and CNS manifestations, and are not indicated in other clinical contexts. Additionally, there has been increasing use of trimethoprim-sulfamethoxazole (TMP-SMX) to treat ocular toxoplasmosis, with some authors suggesting similar clinical outcomes [21, 22], although TMP-SMX is 10-fold less active, with suboptimal ratios of constituent medications with similar risks of hypersensitivity.
Given gaps in knowledge of incidence in the United States and uncertainties about frequency of different disease manifestations and other potential comorbid conditions, we use a novel approach to understand epidemiology of toxoplasmosis, namely, use of large insurance-based datasets. The Truven Health MarketScan Commercial Claims and Encounter Database was queried to answer questions related to epidemiology of this infection. This database contains insurance claims from privately insured patients, not including individuals with access only to Medicare or Medicaid or without any health insurance. This database represents approximately 15% of the total US population. The MarketScan Databases have been used many times in the context of infectious diseases epidemiology and cost-related studies, although we did not find such reports for toxoplasmosis [23–27].
Use of large datasets to characterize epidemiology of infectious disease presents a unique opportunity to assess prevalence and incidence of infection with minimal cost, while allowing for characterization of occurrence across time and space. Additionally, this approach facilitates identification of patterns of infection with respect to patient age, gender, and common comorbidities. Herein, we present a novel approach to understanding epidemiology of toxoplasmosis through use of “large data.”
METHODS
Database Information
The Truven Health MarketScan Commercial Claims and Encounter Database includes privately insured patients, and was used to identify individuals with toxoplasmosis from 2003 to 2012 (see Supplementary Data).
Identification of Patients and Assessment of Demographic Information
The 130.x series of International Classification of Diseases, Ninth Revision (ICD-9) codes (Supplementary Table 1) are used to specifically indicate infection with T. gondii, including diverse manifestations, such as CNS infection and eye disease.
To identify cases of toxoplasmosis not specifically coded for by ICD-9 codes, numbers of claims for medicines specific to treatment of toxoplasmosis also were assessed.
The database identified patients who received these medicines, even in absence of an ICD-9 code indicating presence of this specific infection. Claims were considered to represent toxoplasmosis if they included a relevant ICD-9 code and a medicine use claim within 7 days of each other.
Once patients with disease due to T. gondii were identified, patients were evaluated for age at first diagnosis, gender, and US census region.
Estimates of Annual Rates of Toxoplasmosis
Making an assumption that rates of infection are identical between our study population and the general population regardless of type of insurance, which might not be accurate, and recognizing that the database represents 15% of the total population, and only those with indemnity insurance, the number of cases for the entire population from 2003 to 2012 was calculated. Then, we subdivided by 10 years studied to give a predicted/estimated annual incidence of infection.
Comorbidity in T. gondii Infection
Patients who have 130.x toxoplasmosis-specific ICD-9 codes were compared to controls matched for age, geography, and health, with fewer years in the database indicating improved health. After matching cases and controls, we constructed contingency tables for all possible toxoplasmosis-by-all-disease pairs. Using these contingency tables, we then computed the following statistics for each pair: odds ratio (OR) of disease comorbidity (Supplementary Table 2) with toxoplasmosis codes of interest, conditional maximum likelihood estimate of disease OR (with 95% confidence interval), and P value for a null model in which 2 diseases occur independent of one another. We considered an association significant if it passed Benjamin-Hochberg correction with a very conservative false discovery rate (FDR) threshold of 0.1%:
where r is rank of a disease ordered by increasing P values, Pi is P value for disease with rank i, and N is total number of diseases tested [28].
RESULTS
Study Population
ICD-9 codes specifically indicating infection with T. gondii identified 9260 unique patients from 2003 to 2012, out of a total of 151 million patients.
Use of medicine codes for pyrimethamine or sulfadiazine, and TMP-SMX in context of T. gondii infection or eye disease, identified 2305 and 7690 cases, respectively. There was an overlap of 225 cases between those with toxoplasmosis-specific drugs and cases where TMP-SMX was used.
Disease Manifestations
Toxoplasmic chorioretinitis accounted for 3492 (37.7%) identified patients. Meningoencephalitis occurred in 472 (5.1%) patients. Less common manifestations of infection include myocarditis (113), pneumonitis (113), and hepatitis (188). These conditions comprised 1.2% of total cases each for myocarditis and pneumonitis, and 2.0% of total cases for hepatitis. Disseminated toxoplasmosis occurred in 120 patients. Codes indicating unspecified toxoplasmosis were assigned to 4194 (45.3%) patients.
Demographics
Patients ranged in age from 0 to 70 years. Two hundred eighteen cases (2.4%) occurred in children 2 years of age and younger, indicating likely congenital infection (Figure 1A). Mean age at diagnosis was 37.5 ± 15.5 years. Approximately 41% (3776) of cases occurred in males, while the remaining 5484 occurred in females. One demographic of particular interest is women of reproductive age, here defined as aged 13–51 years, who have potential for vertical transmission to a fetus. In this cohort, 73% (4022) were of reproductive age.
Figure 1.
Demographic statistics of cohort, 2003–2012. A, Age at first diagnosis with toxoplasmosis. Increasing numbers of patients were diagnosed with toxoplasmosis as a function of age, indicating that there is an increasing disease burden among older individuals, who are potentially more capable of moving through the environment and engaging in risky behaviors, including the consumption of undercooked food, which pose the threat of exposure to infectious oocysts and ingestion of contaminated materials. More females than males were identified with toxoplasmosis in this cohort (approximately 59% vs 41% of the total number of cases). The ratio of male to female was most similar early on in the patient's lifetime, whereas there was an increasing disparity skewing toward increased incidence in females. This could be explained by screening of women of reproductive age, or could potentially indicate a difference in risk of exposure to the pathogen. B, Map of toxoplasmosis prevalence by county in the United States. Darker regions indicate increased prevalence of the infection. States with highest prevalence include Texas, California, New York, Illinois, Georgia, Florida, Maryland, and South Carolina. All these states had >400 patients with toxoplasmosis over the study period. There are pockets of increased prevalence across the southern United States. This is consistent with previous studies indicating increased prevalence among rural populations, with increased risk of environmental exposure.
Geographic Distribution of Toxoplasmosis Cases
A total of 9260 identified cases were divided up based on US census region. South, including South Atlantic and East and West South Central census regions, encompassed most identified cases, with 4614 of 9260, or almost 50% of cases. West, including the Mountain and Pacific regions, contained only 1205 of 9260 cases (13%). A χ2 analysis revealed that distribution of cases was not consistent with distribution based on population (P < .001). See Figure 1B and Supplementary Figure 1 for distribution of cases across census regions and a map of disease prevalence across the country.
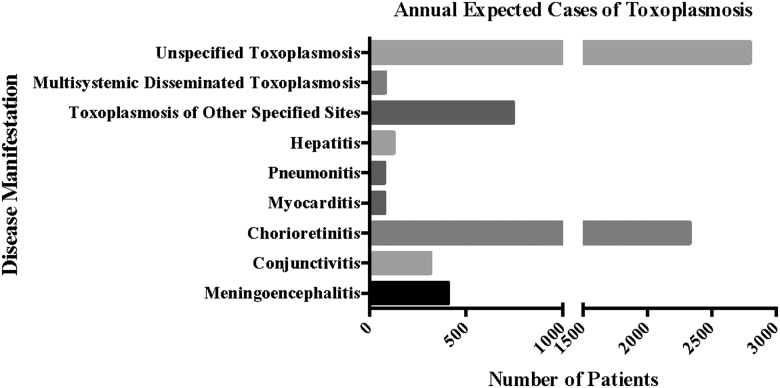
Estimated Annual Case Burden by Disease Manifestation in the United States
This study identified 9260 cases of toxoplasmosis over the period from 2003 to 2012, corresponding to 61 373 cases over the study period, or a rate of 6137 cases per year for the whole population of the United States. Estimated annual incidence by disease manifestation is listed in Table 1 and Figure 2.
Table 1.
Number of Cases of Toxoplasmosis in the United States, by Disease Manifestation, and Estimated Annual Incidence
| Disease Manifestation | No. of Cases Identified by MarketScan Database, 2003–2012 | Estimated No. of Cases in United States, 2003–2012 | Estimated Annual Incidence |
|---|---|---|---|
| Meningoencephalitis | 610 | 4067 | 407 |
| Conjunctivitisa | 472 | 3147 | 315 |
| Chorioretinitis | 3492 | 23 280 | 2328 |
| Myocarditis | 113 | 753 | 75 |
| Pneumonitis | 113 | 753 | 75 |
| Hepatitis | 188 | 1253 | 125 |
| Toxoplasmosis of other specified sites | 1114 | 7427 | 743 |
| Multisystemic disseminated toxoplasmosis | 120 | 800 | 80 |
| Unspecified toxoplasmosis | 4194 | 27 960 | 2796 |
| Total No. of cases | 9206 | 61 373 | 6137 |
a It is important to note that toxoplasmic conjunctivitis is not a known clinical condition, and it is likely that this was simply coded for toxoplasmic eye disease, more generally.
Figure 2.
Clinical manifestations of toxoplasmosis, 2003–2012. Expected annual incidence of toxoplasmosis by disease manifestation in the United States. Using the number of identified cases of toxoplasmosis over the 10-year study period, recognizing that a previous estimate indicated the database accounted for 15% of the total population of the United States, and dividing this by the total number of years of the study, an estimated annual incidence of toxoplasmosis was found as a function of disease manifestation. These values represent the first quantitation of some of these manifestations for the United States in more than a decade.
Comorbidity in T. gondii Infection Based on Specific Toxoplasma ICD-9 Code
ORs for statistically significant comorbidities of interest in patients with codes indicating toxoplasmosis, compared with matched controls without these codes, can be found in Table 2.
Table 2.
Comorbidity Odds Ratios
| Comorbidity | OR Mean (95% CI) | |
|---|---|---|
| Comorbidity odds ratios with toxoplasmosis ICD-9 codes | ||
| HIV | 17.57 (14.61–21.13) | |
| Malignant brain neoplasm | 8.69 (4.60–16.41) | |
| Unspecified encephalopathy (hydrocephalus) | 5.55 (4.75–6.48) | |
| Epilepsy | 3.51 (3.00–4.12) | |
| Thrombocytopenia | 3.17 (2.64–3.80) | |
| Benign brain neoplasm | 2.80 (2.08–3.74) | |
| Visual loss (acquired visual disturbances) | 2.55 (2.39–2.73) | |
| Systemic lupus erythematosus | 2.37 (1.88–2.99) | |
| Schizophrenia | 2.21 (1.80–2.72) | |
| Multiple sclerosis | 2.05 (1.64–2.58) | |
| Gestational and pregnancy-related disorder | 1.92 (1.80–2.05) | |
| IBS and Crohn disease (regional enteritis, Crohn disease) | 1.49 (1.33–1.67) | |
| Bipolar disorder | 1.38 (1.17–1.62) | |
| Substance abuse | 1.24 (1.14–1.36) | |
| Anxiety | 1.24 (1.16–1.33) | |
| OR Mean (95% CI), TMP-SMX | OR Mean (95% CI), Sulfadiazine-Pyrimethamine | |
| Comorbidity odds ratios with NDC | ||
| HIV | 27.97 (22.35–35.00) | 62.78 (44.64–88.28) |
| Malignant brain neoplasm | 4.04 (3.05–5.34) | 15.9 (9.81–25.78) |
| Unspecified encephalopathy (hydrocephalus) | 2.77 (2.48–3.09) | 10.02 (8.14–12.34) |
| Epilepsy | 2.35 (2.02–2.72) | 6.27 (4.88–8.08) |
| Thrombocytopenia | 2.94 (2.61–3.32) | 4.37 (3.44–5.55) |
| Benign brain neoplasm | 1.62 (1.28–2.06) | 4.25 (2.65–6.83) |
| Visual loss (acquired visual disturbances) | 2.91 (2.74–3.07) | 2.14 (1.88–2.43 |
| Systemic lupus erythematosus | 2.37 (1.88–2.99) | 4.64 (3.166.79) |
| Schizophrenia | 2.03 (1.78–2.31) | 2.77 (2.09–3.69) |
| Multiple sclerosis | 3.10 (2.54–3.78) | 3.73 (2.55–5.45) |
| IBS and Crohn disease (regional enteritis, Crohn disease) | 1.58 (1.44–1.74) | 3.26 (2.75–3.87) |
| Bipolar disorder | 1.48 (1.27–1.73) | NS |
| Substance abuse | 1.29 (1.20–1.40) | NS |
| Anxiety | 1.52 (1.43–1.62) | NS |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IBS, irritable bowel syndrome; ICD-9, International Classification of Diseases, Ninth Revision; NDC, National Drug Code; NS, not significant; OR, odds ratio; TMP-SMX, trimethoprim-sulfamethoxazole.
National Drug Code Use in the Identification of Toxoplasmosis Cases
In addition to patients with a diagnosis of toxoplasmosis coded, analyzed as above, patients were identified with National Drug Codes (NDCs), considered separately because diagnosis was less certain (Figure 3). Pyrimethamine and sulfadiazine are specific to treatment of infection with this parasite, indicating potential utility in identifying patients with active toxoplasmosis. However, treatment could have been presumptive and then discontinued when another disease was diagnosed. TMP-SMX sometimes has been used for treating retinal disease due to Toxoplasma, although it is a suboptimal treatment. Therefore, presence of eye disease and TMP-SMX treatment might suggest, but not confirm, this diagnosis. Other illnesses causing eye disease, and even different diseases requiring treatment with TMP-SMX, could confound this surrogate marker for active toxoplasmosis. As shown in Figure 3, there were 2080 patients with only pyrimethamine and/or sulfadiazine (2305 – parts b [152] + d [73]); 7465 patients with only TMP-SMX plus chorioretinitis (7690 – parts b [152] + d [73]). In addition, 225 patients took both, not necessarily simultaneously (Figure 3; parts b [152] + d [73]). Of the total 9260 patients with a toxoplasmosis diagnostic code, 339 also had a pyrimethamine and/or sulfadiazine code; 690 also had a TMP-SMX code; and 152 had both. NDC cohort demographics (Supplementary Table 3) and a comparison between the 2 medicine treatment groups were similar to the toxoplasmosis ICD-9 code group, except age was younger ( years [ICD-9 toxoplasmosis] vs 51 years [NDC]; Supplementary Table 4 and Figure 2). Also, there were more patients in middle and south Atlantic states in the toxoplasmosis code group. ORs for comorbidities comparing treatment with pyrimethamine-sulfadiazine vs TMP-SMX–chorioretinitis group are shown in Table 2 and are similar to those with ICD-9 codes for toxoplasmosis.
Figure 3.

Venn diagram showing numbers of patients who were diagnosed by International Classification of Diseases, Ninth Revision (ICD-9) codes for toxoplasmosis with and without codes for medicines. Abbreviation: TMP-SMX, trimethoprim-sulfamethoxazole.
DISCUSSION
Use of the Truven Health MarketScan Commercial Claims and Encounter Database presented a novel opportunity to assess prevalence of medical diagnosis of toxoplasmosis in the US population with indemnity insurance, confirmed some trends highlighted in earlier literature, and yielded some unexpected results.
Almost half of identified patients had codes indicating “unspecified toxoplasmosis,” making it impossible to accurately quantitate disease manifestations. Of cases of toxoplasmosis with specified clinical manifestation, toxoplasmic chorioretinitis represented the highest proportion. This indicates a comparatively high frequency of eye disease relative to other manifestations, that eye disease due to T. gondii is more often diagnosed, or that it drives patients to seek care more than occurs for patients with other clinical manifestations. Our data do not provide support for other explanations for this observation.
Almost 500 patients presented with one of the most lethal complications of toxoplasmosis, meningoencephalitis. This emphasizes that immunocompromised patients remain at risk for life-threatening manifestations. Our estimate indicates an annual disease burden of 488 cases of CNS disease due to T. gondii. Other, less common, manifestations of toxoplasmosis, including myocarditis, pneumonitis, and hepatitis, cause morbidity and mortality in the United States. It is critical that the index of suspicion for these rarer manifestations remain high, especially in immunocompromised patients, and that appropriate therapy is initiated when toxoplasmosis is suspected.
Numbers of cases of toxoplasmosis, identified by ICD-9 code, were higher in the southern United States than would be expected based on population. The environment is more conducive to extended viability of the environmentally resistant, highly infective oocyst stage in this region [29]. Further, behavioral factors such as agricultural activity may represent an additional explanation for this comparatively high prevalence. Moreover, the Western census region demonstrated fewer cases than expected. An environment that is dryer and more hostile to oocysts, or differences in parasite or vector distribution or in recognition and reporting, could explain this.
Prevalence of comorbidities associated with toxoplasmosis also was assessed; affording insight into how commonly individuals with this infection suffer from other conditions. Compared to controls matched for age, geography, and health, patients with codes for toxoplasmosis had greater odds of suffering from conditions including HIV, benign and malignant brain neoplasm, epilepsy, autoimmune diseases including lupus and multiple sclerosis, and psychiatric conditions including substance abuse, anxiety, bipolar disorder, and schizophrenia. Directionality and causality of these relationships is not clear. However, immunosuppression with HIV infection, lupus or malignancy, or immunosuppressive therapies predisposes to severe infection. Chronic inflammation produced by presence of organisms in the brain may promote neoplastic transformation and epilepsy, as could focal lesions or changes in neurotransmitters as occurs in animal models [30]. Associations between seroprevalence of T. gondii and neuropsychiatric illness are demonstrated in the literature without directionality or causality. Ascertainment bias could contribute to ORs, with neurologic signs and symptoms precipitating testing for toxoplasmosis. There are certainly billions of infected persons without neurobehavioral disease, so if there is a real association, other factors such as host or parasite genetics or timing of infection must be at play. Furthermore, as this cohort is predominantly <65 years of age and without public insurance, it remains of interest to study the effect of this possible source of chronic inflammation in the brain on neurodegenerative diseases in geriatric populations.
While this analysis has offered potentially valuable insight into toxoplasmosis in the United States, use of insurance databases for epidemiology has obvious limitations that must be addressed. Disease is complicated, with many factors influencing its prevalence and distribution. Host behavior can influence rates of parasite transmission and risk of acquisition. This database has privately insured patients, and does not include Medicaid or Medicare patients, or those without insurance. This introduces sampling bias. While T. gondii is an equal-opportunity parasite, capable of infecting people irrespective of socioeconomic status, there are risk factors that make infection more likely in certain populations. This is especially among agricultural laborers in the South, where a lack of private health insurance is not uncommon. In calculations presented, we assume that the population in the database is reflective of the population as a whole. It is likely this is not the case, and thus our analysis underestimates actual rates of infection and disease due to T. gondii. Anecdotally, of 8 patients seen by our group in a private hospital setting with toxoplasmosis in the last month, 6 did not have private insurance, and therefore would not have been identified by this approach. If this is reflective of the general population, as current payer mix for pyrimethamine prescriptions suggests, with only 23% having private insurance (approximate N = 1026, 3 August 2015 to 27 November 2015; J. Casoy, N. Retzlaff, E. Salinas, personal communication, 2016), there is substantial underestimation using this private insurance–only approach. The corrected annual rate would be considerably higher. Thus, true incidence estimates would require a more aggressive public health approach to reporting toxoplasmosis or another method for estimating prevalence.
Additionally, ways in which physicians use ICD-9 codes in contexts of toxoplasmosis present a limitation to assessment. Most patients whose claims contain references directly to toxoplasmosis have codes indicating “unspecified toxoplasmosis,” which offers no information about symptoms. As such, this approach to characterizing toxoplasmosis on a population level is unlikely to be reflective of true diversity of disease manifestations, which can range from lymphadenopathy, which is less likely to drive a patient to seek care when self-limited, to meningoencephalitis, a potentially fatal form of infection. Of note, more individuals had codes indicating treatment for infection with T. gondii than had claims indicating infection. A substantial number of patients did not have ICD-9 codes for infection, even though they received medication used to treat this infection. There is the possibility that a considerable number of patients are not being coded appropriately when symptomatic. Thus, more people are receiving medications than would be detected by ICD-9 codes, indicating a limitation of using ICD-9 codes for epidemiological studies.
If previous estimates of disease burden are correct, this method underestimates prevalence. Annual burden of symptomatic eye disease due to infection with T. gondii has been estimated at 4839 cases annually [16]. Our analysis conservatively estimates 2643 cases per year. Thus, this analysis underestimates cases by almost half, if previous reports are accurate. Other manifestations of toxoplasmosis, including meningoencephalitis, have not been estimated in the years since development of HAART, limiting comparative analyses with the MarketScan Database for CNS infection.
Congenital infection remains a substantial cause of significant human suffering in association with vertical transmission from mother to fetus. Of the identified patients, 218 were between the ages of 0 and 2 at diagnosis. Detection of this disease at birth identifies significantly symptomatic patients, which would be expected to represent only a small proportion of these infections. Most would be mild and not come to medical attention in absence of systematic screening. Forty-four percent of patients were women of reproductive age, indicating that this infection likely poses considerable medical burden during fetal life. A number of countries, including France, have implemented mandatory screening during gestation for infection with T. gondii. This approach has been demonstrated to be efficacious in reducing the frequency and severity of congenital infection by facilitating early diagnosis and treatment, and to be cost-beneficial [31–34]. Screening would facilitate appropriate treatment of acutely infected mothers, which has been demonstrated to reduce disease severity in and frequency of infected infants.
Our observations of inability of analyses of large databases such as the Truven MarketScan Commercial Claims and Encounter Database [23–27, 35] to identify all cases of toxoplasmosis in the United States presents a different type of opportunity for intervention. The fact that physicians are not coding for toxoplasmosis, due to an inability to recognize it, a lack of time to properly code, or some other barrier, in addition to the fact that this parasite presents a health threat to the American population and globally, suggests that it may be useful to make reporting of the disease to health departments mandatory. This would facilitate more accurate assessment of prevalence and severity of toxoplasmosis in the United States. This then could enable public health interventions that will ultimately reduce human suffering and mortality. Toxoplasmosis is a treatable condition, but an inability to appreciate the magnitude of the problem in this country presents a barrier to appropriate management of disease due to this parasite.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Sarah Dovgin for her help and work during the initial phase of this research. We thank J. Casoy, N. Retzlaff, and E. Salinas for data and information concerning payer mix associated with recent pyrimethamine prescriptions.
Financial support. This work was supported by the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (grant number R01 AI27530); the Conte Center; and the National Institutes of Health (grant numbers 1P50MH094267 and U01HL108634-01). We thank the Mann Cornwell family, Engel family (and “Taking Out Toxo”), Morel family, and Rooney family for their support of this work.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Furtado JM, Smith JR, Belfort R, Gattey D, Winthrop KL. Toxoplasmosis: a global threat. J Glob Infect Dis 2011; 3:281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latkany P. Ocular disease due to Toxoplasma gondii. In: Weiss LM, Kim K, eds. Toxoplasma gondii. London: Academic Press, 2007:101–5. Available at: http://www.sciencedirect.com/science/article/pii/B9780123695420500076 Accessed 19 June 2014. [Google Scholar]
- 3.Arevalo JF, Belfort R, Muccioli C, Espinoza JV. Ocular toxoplasmosis in the developing world. Int Ophthalmol Clin 2010; 50:57–69. [DOI] [PubMed] [Google Scholar]
- 4.Wong B, Gold JWM, Brown AE et al. Central-nervous-system toxoplasmosis in homosexual men and parenteral drug abusers. Ann Intern Med 1984; 100:36–42. [DOI] [PubMed] [Google Scholar]
- 5.Grant IH, Gold JW, Rosenblum M, Niedzwiecki D, Armstrong D. Toxoplasma gondii serology in HIV-infected patients: the development of central nervous system toxoplasmosis in AIDS. AIDS 1990; 4:519–21. [PubMed] [Google Scholar]
- 6.Dedicoat M, Livesley N. Management of toxoplasmic encephalitis in HIV-infected adults (with an emphasis on resource-poor settings). Cochrane Database Syst Rev 2006; 3:CD005420. [DOI] [PubMed] [Google Scholar]
- 7.Yan J, Huang B, Liu G et al. Meta-analysis of prevention and treatment of toxoplasmic encephalitis in HIV-infected patients. Acta Trop 2013; 127:236–44. [DOI] [PubMed] [Google Scholar]
- 8.McLeod R, Lykins J, Noble AG et al. Management of congenital toxoplasmosis. Curr Pediatr Rep 2014; 2:166–94. [Google Scholar]
- 9.Akyol A, Bicerol B, Ertug S, Ertabaklar H, Kiylioglu N. Epilepsy and seropositivity rates of Toxocara canis and Toxoplasma gondii. Seizure 2007; 16:233–7. [DOI] [PubMed] [Google Scholar]
- 10.Brown AS, Schaefer CA, Quesenberry J et al. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry 2005; 162:767–73. [DOI] [PubMed] [Google Scholar]
- 11.Torrey EF, Bartko JJ, Lun Z-R, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 2007; 33:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley RA, Taber KH. Latent Toxoplasmosis gondii: emerging evidence for influences on neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 2012; 24:376–83. [DOI] [PubMed] [Google Scholar]
- 13.Miman O, Kusbeci OY, Aktepe OC, Cetinkaya Z. The probable relation between Toxoplasma gondii and Parkinson's disease. Neurosci Lett 2010; 475:129–31. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Toxoplasmosis. Available at: http://www.cdc.gov/parasites/toxoplasmosis/ Accessed 22 August 2014.
- 15.Jones JL, Kruszon-Moran D, Rivera HN, Price C, Wilkins PP. Toxoplasma gondii seroprevalence in the United States 2009–2010 and comparison with the past two decades. Am J Trop Med Hyg 2014; 90:1135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the United States. Am J Trop Med Hyg 2010; 82:464–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 1992; 327:1643–8. [DOI] [PubMed] [Google Scholar]
- 18.Jones JL, Hanson DL, Chu SY et al. Toxoplasmic encephalitis in HIV-infected persons: risk factors and trends. The Adult/Adolescent Spectrum of Disease Group. AIDS 1996; 10:1393–9. [DOI] [PubMed] [Google Scholar]
- 19.San-Andrés F-J, Rubio R, Castilla J et al. Incidence of acquired immunodeficiency syndrome-associated opportunistic diseases and the effect of treatment on a cohort of 1115 patients infected with human immunodeficiency virus, 1989–1997. Clin Infect Dis 2003; 36:1177–85. [DOI] [PubMed] [Google Scholar]
- 20.Hotop A, Hlobil H, Groß U. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2012; 54:1545–52. [DOI] [PubMed] [Google Scholar]
- 21.Torre D, Casari S, Speranza F et al. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Antimicrob Agents Chemother 1998; 42:1346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soheilian M, Sadoughi M-M, Ghajarnia M et al. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 2005; 112:1876–82. [DOI] [PubMed] [Google Scholar]
- 23.Owusu-Edusei K, Chesson HW, Gift TL. The economic burden of pediculosis pubis and scabies infections treated on an outpatient basis in the United States: evidence from private insurance claims data, 2001–2005. Sex Transm Dis 2009; 36:297–9. [DOI] [PubMed] [Google Scholar]
- 24.Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J 2010; 29:489–94. [DOI] [PubMed] [Google Scholar]
- 25.Owusu-Edusei K, Gift TL, Chesson HW. Treatment cost of acute gonococcal infections: estimates from employer-sponsored private insurance claims data in the United States, 2003–2007. Sex Transm Dis 2010; 37:316–8. [DOI] [PubMed] [Google Scholar]
- 26.Hellinger DFJ, Encinosa WE. The cost and incidence of prescribing errors among privately insured HIV patients. PharmacoEconomics 2012; 28:23–34. [DOI] [PubMed] [Google Scholar]
- 27.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013; 103:1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser A 1995; 57:289–300. [Google Scholar]
- 29.Lélu M, Villena I, Dardé M-L et al. Quantitative estimation of the viability of Toxoplasma gondii oocysts in soil. Appl Environ Microbiol 2012; 78:5127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe 2014; 15:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roos T, Maritus J, Gross U, Schrod L. Systematic serologic screening for toxoplasmosis in pregnancy. Obstet Gynecol 1993; 81:243–50. [PubMed] [Google Scholar]
- 32.Stillwaggon E, Carrier CS, Sautter M, McLeod R. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis 2011; 5:e1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagel U, Krämer A, Mikolajczyk RT. “Blind periods” in screening for toxoplasmosis in pregnancy in Austria—a debate. BMC Infect Dis 2012; 12:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallon M, Peyron F, Cornu C et al. Congenital toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis 2013; 56:1223–31. [DOI] [PubMed] [Google Scholar]
- 35.Gastañaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting—United States, 2001–2009. J Infect Dis 2013; 207:1058–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.