Abstract
It is important to realize that guidelines cannot always account for individual variation among patients. They are not intended to supplant physician judgment with respect to particular patients or special clinical situations. IDSA considers adherence to these guidelines to be voluntary, with the ultimate determination regarding their application to be made by the physician in the light of each patient's individual circumstances.
Keywords: aspergillosis, invasive aspergillosis, allergic aspergillosis, chronic aspergillosis, fungal diagnostics, azoles, echniocandins, amphotericin
EXECUTIVE SUMMARY
Background
Aspergillus species continue to be an important cause of life-threatening infection in immunocompromised patients. This at-risk population is comprised of patients with prolonged neutropenia, allogeneic hematopoietic stem cell transplant (HSCT), solid organ transplant (SOT), inherited or acquired immunodeficiencies, corticosteroid use, and others. This document constitutes the guidelines of the Infectious Diseases Society of America (IDSA) for treatment of aspergillosis and replaces the practice guidelines for Aspergillus published in 2008. Since that publication, clinical studies evaluating new and existing therapies including combination therapy for the management of Aspergillus infection have been conducted and the data on use of non-culture-based biomarkers for diagnosing infection have been expanded. The objective of these guidelines is to summarize the current evidence for treatment of different forms of aspergillosis. This document reviews guidelines for management of the 3 major forms of aspergillosis: invasive aspergillosis (IA); chronic (and saprophytic) forms of aspergillosis; and allergic forms of aspergillosis. Given the clinical importance of IA, emphasis is placed upon the diagnosis, treatment, and prevention of the different forms of IA, including invasive pulmonary aspergillosis (IPA), Aspergillus sinusitis, disseminated aspergillosis, and several types of single-organ IA.
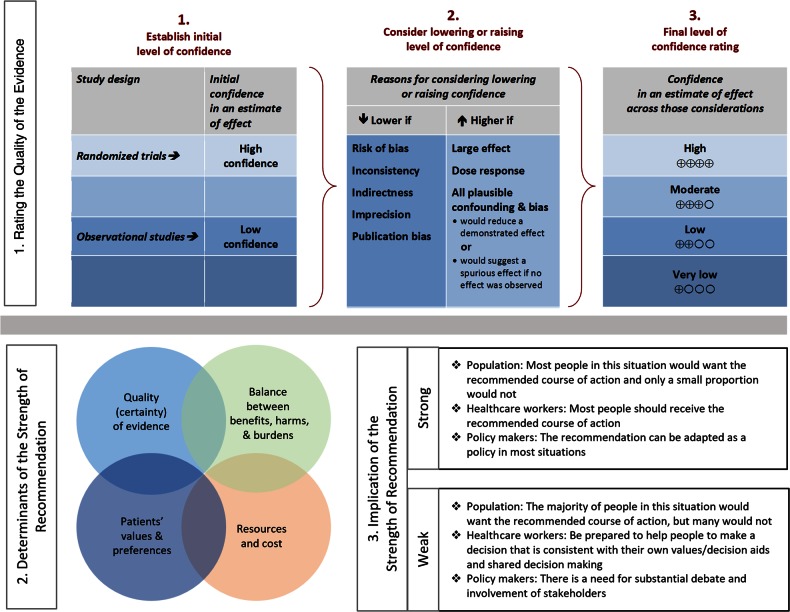
Summarized below are the 2016 recommendations for the management of aspergillosis. Due to the guidelines’ relevance to pediatrics, the guideline has been reviewed and endorsed by the Pediatric Infectious Diseases Society (PIDS). The panel followed a guideline development process that has been adopted by IDSA, which includes use of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system, a systematic method of grading both the strength of the recommendation (weak or strong) and the quality of evidence (very low, low, moderate, and high) (Figure 1). The guidelines are not intended to replace clinical judgment in the management of individual patients. A detailed description of the methods, background, and evidence summaries that support each recommendation can be found in the full text of the guideline.
Figure 1.
Approach and implications to rating the quality of evidence and strength of recommendations using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology (unrestricted use of the figure granted by the US GRADE Network) [1].
EPIDEMIOLOGY AND RISK FACTORS FOR INFECTION
I. How Can the Most Susceptible Patients Be Protected From Aspergillosis, and Which Patients Are Most Susceptible?
What Are Sources of Exposure to Aspergillus, and How Can Exposure Be Decreased? Is Environmental Surveillance Useful?
Recommendations
1. Hospitalized allogeneic HSCT recipients should be placed in a protected environment to reduce mold exposure (strong recommendation; low-quality evidence).
2. These precautions can be reasonably applied to other highly immunocompromised patients at increased risk for IA, such as patients receiving induction/reinduction regimens for acute leukemia (strong recommendation; low-quality evidence).
3. In hospitals in which a protected environment is not available, we recommend admission to a private room, no connection to construction sites, and not allowing plants or cut flowers to be brought into the patient's room (strong recommendation; low-quality evidence).
4. We recommend reasonable precautions to reduce mold exposure among outpatients at high risk for IA, including avoidance of gardening, spreading mulch (compost), or close exposure to construction or renovation (strong recommendation; low-quality evidence).
5. Leukemia and transplant centers should perform regular surveillance of cases of invasive mold infection. An increase in incidence over baseline or the occurrence of invasive mold infections in patients who are not at high risk for such infections should prompt evaluation for a hospital source (strong recommendation; low-quality evidence).
DIAGNOSIS OF ASPERGILLOSIS
II. How Can a Diagnosis of Invasive Aspergillosis Be Established?
How Should Aspergillus Be Identified, and How Does This Influence Management?
Recommendation
6. Until molecular tools are more widely used in clinical laboratories, we recommend that tissue and fluid specimens be submitted in adequate quantities for simultaneous histopathologic/cytologic and culture examination. In the case of isolates with atypical growth or concerns for resistance, species identification by molecular methods should be employed (strong recommendation; high-quality evidence).
What Is the Diagnostic Value of Nucleic Acid Testing in Clinical Specimens?
Recommendations
7. There was debate among the committee members regarding the clinical utility of blood-based polymerase chain reaction (PCR) in diagnosing IA, and experts were not in agreement. One group favored recommendations for PCR testing, based on publications validating its role when used in conjunction with other tests such as antigen detection assays to diagnose IA and/or reduce preemptive antifungal usage. The other group thought that PCR assays are promising but could not be recommended for routine use in clinical practice at present due to the lack of conclusive validation for commercially available assays, the variety of methodologies in the literature, and questions about the extent to which results assisted diagnosis.
8. As research in the area continues, we recommend that clinicians choosing to use PCR assays employ them carefully in the management of individual patients on a case-by-case basis. Clinicians should be aware of the methodologies and performance characteristics of the specific assay used, and interpret results accordingly. When PCR assays are used, results should be considered in conjunction with other diagnostic tests and the clinical context (strong recommendation; moderate-quality evidence).
How Should Galactomannan and (1 → 3)-β-D-Glucan Be Used for the Diagnosis of Aspergillosis?
Recommendations
9. Serum and BAL galactomannan (GM) is recommended as an accurate marker for the diagnosis of IA in adult and pediatric patients when used in certain patient subpopulations (hematologic malignancy, HSCT) (strong recommendation; high-quality evidence).
10. GM is not recommended for routine blood screening in patients receiving mold-active antifungal therapy or prophylaxis, but can be applied to bronchoscopy specimens from those patients (strong recommendation; high-quality evidence).
11. GM is not recommended for screening in SOT recipients or patients with chronic granulomatous disease (CGD) (strong recommendation; high-quality evidence).
12. Serum assays for (1 → 3)-β-D-glucan are recommended for diagnosing IA in high-risk patients (hematologic malignancy, allogeneic HSCT), but are not specific for Aspergillus (strong recommendation; moderate-quality evidence).
What Is the Approach to the Radiographic Diagnosis of Invasive Pulmonary Aspergillosis?
Recommendations
13. We recommend performing a chest computed tomographic (CT) scan whenever there is a clinical suspicion for IPA regardless of chest radiograph results (strong recommendation; high-quality evidence).
14. Routine use of contrast during a chest CT scan for a suspicion of IPA is not recommended (strong recommendation; moderate-quality evidence). Contrast is recommended when a nodule or a mass is close to a large vessel (strong recommendation; moderate-quality evidence).
15. We suggest a follow-up chest CT scan to assess the response of IPA to treatment after a minimum of 2 weeks of treatment; earlier assessment is indicated if the patient clinically deteriorates (weak recommendation; low-quality evidence). When a nodule is close to a large vessel, more frequent monitoring may be required (weak recommendation; low-quality evidence).
What Is the Role of Bronchoscopy in the Diagnosis of Invasive Pulmonary Aspergillosis?
Recommendation
16. We recommend performing a bronchoscopy with bronchoalveolar lavage (BAL) in patients with a suspicion of IPA (strong recommendation; moderate-quality evidence). Significant comorbidities such as severe hypoxemia, bleeding, and platelet transfusion-refractory thrombocytopenia may preclude BAL. The yield of BAL is low for peripheral nodular lesions, so percutaneous or endobronchial lung biopsy should be considered. We recommend the use of a standardized BAL procedure and sending the BAL sample for routine culture and cytology as well as non-culture-based methods (eg, GM) (strong recommendation; moderate-quality evidence).
III. What Antifungal Agents Are Available for the Treatment and Prophylaxis of Invasive Aspergillosis, Including Pharmacologic Considerations, and What Is the Role for Susceptibility Testing?
Amphotericin B
Recommendations
17. Amphotericin B (AmB) deoxycholate and its lipid derivatives are appropriate options for initial and salvage therapy of Aspergillus infections when voriconazole cannot be administered. However, AmB deoxycholate should be reserved for use in resource-limited settings in which no alternative agents are available. Lipid formulations of AmB should be considered in settings in which azoles are contraindicated or not tolerated (strong recommendation; moderate-quality evidence).
18. Aerosolized formulations of AmB may be considered as prophylaxis in patients with prolonged neutropenia (patients receiving induction/reinduction therapy for acute leukemia and allogeneic HSCT recipients following conditioning or during treatment of graft-vs-host disease [GVHD]) and in lung transplant recipients (weak recommendation; low-quality evidence).
Echinocandins
Recommendation
19. Echinocandins are effective in salvage therapy (either alone or in combination) against IA, but we do not recommend their routine use as monotherapy for the primary treatment of IA (strong recommendation; moderate-quality evidence).
Triazoles
Recommendations
20. Triazoles are preferred agents for treatment and prevention of IA in most patients (strong recommendation; high-quality evidence).
21. For patients receiving triazole-based therapy for IA, prolonged azole prophylaxis, or other therapies for which drug interactions with azoles are anticipated, the committee recommends therapeutic drug monitoring (TDM) once the steady state has been reached. A moderate amount of data for itraconazole, voriconazole, and posaconazole suspension suggests this approach may be valuable in enhancing therapeutic efficacy, in evaluating therapeutic failures attributable to suboptimal drug exposures, and to minimize toxicities potentially attributable to the azoles (strong recommendation; moderate-quality evidence). Further studies are needed to address whether TDM is helpful or necessary with the extended-release or intravenous formulations of posaconazole or for isavuconazole.
22. Clinicians should obtain serum trough drug levels for azole antifungal agents (itraconazole, voriconazole, posaconazole, and possibly isavuconazole) and for potentially interacting drugs such as cyclosporine, tacrolimus, and sirolimus (and other CYP3A4 substrates such as tyrosine kinase inhibitors) to optimize therapeutic efficacy and to avoid potential toxicities of both groups of agents (strong recommendation; moderate-quality evidence).
Preclinical and Laboratory Assessment of Combination Antifungal Therapy
23. Combinations of polyenes or azoles with echinocandins suggest additive or synergistic effects in some preclinical studies. However, variable test designs and conflicting results of preclinical and in vitro testing have led to uncertainty as to how to interpret the findings (weak recommendation; low-quality evidence).
When Should Antifungal Susceptibility Testing Be Performed, and How Should Results Be Interpreted and Affect Management?
Recommendation
24. Routine antifungal susceptibility testing (AFST) of isolates recovered during initial infection is not recommended. AFST of Aspergillus isolates using a reference method is reserved for patients suspected to have an azole-resistant isolate or who are unresponsive to antifungal agents, or for epidemiological purposes (strong recommendation; moderate-quality evidence).
INVASIVE SYNDROMES OF ASPERGILLUS
IV. What Are the Recommended Treatment Regimens and Adjunctive Treatment Measures for the Various Clinical Presentation of Invasive Aspergillosis?
How Should IPA Be Treated?
Recommendations
25. We recommend primary treatment with voriconazole (strong recommendation; high-quality evidence).
26. Early initiation of antifungal therapy in patients with strongly suspected IPA is warranted while a diagnostic evaluation is conducted (strong recommendation; high-quality evidence).
27. Alternative therapies include liposomal AmB (strong recommendation; moderate-quality evidence), isavuconazole (strong recommendation; moderate-quality evidence), or other lipid formulations of AmB (weak recommendation; low-quality evidence).
28. Combination antifungal therapy with voriconazole and an echinocandin may be considered in select patients with documented IPA (weak recommendation; moderate-quality evidence).
29. Primary therapy with an echinocandin is not recommended (strong recommendation; moderate-quality evidence). Echinocandins (micafungin or caspofungin) can be used in settings in which azole and polyene antifungals are contraindicated (weak recommendation; moderate-quality evidence).
30. We recommend that treatment of IPA be continued for a minimum of 6–12 weeks, largely dependent on the degree and duration of immunosuppression, site of disease, and evidence of disease improvement (strong recommendation; low-quality evidence).
31. For patients with successfully treated IPA who require subsequent immunosuppression, secondary prophylaxis should be initiated to prevent recurrence (strong recommendation; moderate-quality evidence).
Adjunctive Measures and Immunomodulation: When Should Withdrawal of Immunosuppressive Agents, or Addition of Colony-Stimulating Factors or Granulocyte Transfusions, Be Considered in the Treatment of Invasive Aspergillosis?
Recommendations
32. Reducing doses of, or eliminating altogether, immunosuppressive agents, when feasible, is advised as a component of anti-Aspergillus therapy (strong recommendation; low-quality evidence).
33. Colony-stimulating factors may be considered in neutropenic patients with diagnosed or suspected IA (weak recommendation; low-quality evidence). There is insufficient evidence regarding the value of granulocyte colony-stimulating factor vs granulocyte macrophage colony-stimulating factor (GM-CSF) in this setting.
34. Granulocyte transfusions can be considered for neutropenic patients with IA that is refractory or unlikely to respond to standard therapy, and for an anticipated duration of more than one week (weak recommendation; low-quality evidence).
35. Recombinant interferon-γ is recommended as prophylaxis in CGD patients (strong recommendation; high-quality evidence). Its benefit as adjunctive therapy for IA is unknown.
36. Surgery for aspergillosis should be considered for localized disease that is easily accessible to debridement (eg, invasive fungal sinusitis or localized cutaneous disease) (strong recommendation; low-quality evidence). The benefit for IA in other settings such as in the treatment of endocarditis, osteomyelitis, or focal central nervous system (CNS) disease appears rational. Other indications are less clear and require consideration of the patient's immune status, comorbidities, confirmation of a single focus, and the risks of surgery.
When Is It Safe to Proceed With Chemotherapy or Transplantation in a Patient With Invasive Aspergillosis?
Recommendations
37. IA is not an absolute contraindication to additional chemotherapy or HSCT (strong recommendation; moderate-quality evidence).
38. Decisions about when to proceed with additional chemotherapy or HSCT following the diagnosis of aspergillosis should involve both infectious diseases specialists and hematologists/oncologists. These decisions must consider the risk of progressive aspergillosis during periods of subsequent antineoplastic treatment vs the risk of death from the underlying malignancy if this treatment is delayed (strong recommendation; low-quality evidence).
What Approaches Are Needed for Refractory or Progressive Aspergillosis (Salvage Therapy)?
Recommendations
39. We recommend an individualized approach that takes into consideration the rapidity, severity, and extent of infection, patient comorbidities, and to exclude the emergence of a new pathogen (strong recommendation; low-quality evidence). The general strategies for salvage therapy typically include (i) changing the class of antifungal, (ii) tapering or reversal of underlying immunosuppression when feasible, and (iii) surgical resection of necrotic lesions in selected cases.
40. In the context of salvage therapy, an additional antifungal agent may be added to current therapy, or combination antifungal drugs from different classes other than those in the initial regimen may be used (weak recommendation; moderate-quality evidence).
41. In patients currently receiving an antifungal and exhibiting an adverse event attributable to this agent, we recommend changing to an alternative class of antifungal, or the use of an alternative agent with a nonoverlapping side-effect profile (strong recommendation; low-quality evidence).
42. For salvage therapy, agents include lipid formulations of AmB, micafungin, caspofungin, posaconazole, or itraconazole. The use of a triazole as salvage therapy should take into account prior antifungal therapy, host factors, pharmacokinetic considerations, and possible antifungal resistance (strong recommendation; moderate-quality evidence).
How Can Biomarkers Be Used to Assess Patient Response to Therapy?
Recommendations
43. Serial monitoring of serum GM can be used in the appropriate patient subpopulations (hematologic malignancy, HSCT) who have an elevated GM at baseline to monitor disease progression and therapeutic response, and predict outcome (strong recommendation; moderate-quality evidence).
44. (1 → 3)-β-D-glucan has not been extensively studied in IA to predict outcome (weak recommendation; low-quality evidence).
What Are the Recommended Treatments for Pediatric Patients With Aspergillosis?
Recommendation
45. Treatment of aspergillosis in children uses the same recommended therapies as in adult patients; however, the dosing is different and for some antifungals is unknown (strong recommendation; high-quality evidence).
What Are Treatment Options for Aspergillosis of the Airways in Transplant and Nontransplant Recipients, and How Does It Differ From Invasive Pulmonary Aspergillosis?
Recommendations
46. Saprophytic forms of tracheobronchial aspergillosis (TBA) do not require antifungal treatment except for symptomatic or immunosuppressed patients. Treatment includes bronchoscopic removal of mucoid impaction. Mold-active triazole agents are recommended for immunocompromised patients in whom the possibility of invasive disease cannot be eliminated (strong recommendation; moderate-quality evidence).
47. Bronchocentric granulomatosis is treated in the same fashion as allergic bronchopulmonary aspergillosis (ABPA) (strong recommendation; low-quality evidence).
48. Invasive forms of TBA are treated with a mold-active triazole or intravenous lipid formulations of AmB (strong recommendation; moderate-quality evidence). We also recommend minimization or reversal of underlying immunosuppression when feasible, and bronchoscopic debridement of airway lesions in selected cases (strong recommendation; low-quality evidence).
49. In lung transplant recipients, we recommend treatment with a systemic antimold antifungal for TBA, including saprophytic forms. We also recommend adjunctive inhaled AmB in the setting of TBA associated with anastomotic endobronchial ischemia or ischemic reperfusion injury due to airway ischemia associated with lung transplant (strong recommendation; moderate-quality evidence). Duration of antifungal therapy is at least 3 months or until TBA is completely resolved, whichever is longer.
MANAGEMENT OF EXTRAPULMONARY ASPERGILLOSIS
What Are the Treatment Considerations for Central Nervous System Aspergillosis?
Recommendation
50. We recommend voriconazole as primary therapy for CNS aspergillosis (strong recommendation; moderate-quality evidence). Lipid formulations of AmB are reserved for those intolerant or refractory to voriconazole (strong recommendation; moderate-quality evidence).
How Is Aspergillus Endophthalmitis Treated?
Recommendation
51. We recommend that Aspergillus endophthalmitis be treated with systemic oral or intravenous voriconazole plus intravitreal voriconazole or intravitreal AmB deoxycholate (strong recommendation; weak-quality evidence).
What Is the Role of Surgery in Aspergillosis of the Paranasal Sinuses?
Recommendation
52. We recommend that both surgery and either systemic voriconazole or a lipid formulation of AmB be used in invasive Aspergillus fungal sinusitis but that surgical removal alone can be used to treat Aspergillus fungal ball of the paranasal sinus. Enlargement of the sinus ostomy may be needed to improve drainage and prevent recurrence (strong recommendation; moderate-quality evidence).
What Are the Treatment Recommendations for Aspergillus Endocarditis, Pericarditis, and Myocarditis?
Recommendation
53. In Aspergillus endocarditis, we recommend early surgical intervention combined with antifungal therapy in attempts to prevent embolic complications and valvular decompensation (strong recommendation; moderate-quality evidence). Voriconazole or a lipid formulation of AmB is recommended as initial therapy (strong recommendation; low-quality evidence). Following surgical replacement of an infected valve, lifelong antifungal therapy should be considered (strong recommendation; low-quality evidence).
What Are the Treatment Recommendations for Aspergillus Osteomyelitis and Septic Arthritis?
Recommendation
54. Surgical intervention is recommended, where feasible, for management of Aspergillus osteomyelitis and arthritis, combined with voriconazole (strong recommendation; moderate-quality evidence).
What Are the Treatment Recommendations for Cutaneous Aspergillosis?
Recommendations
55. As cutaneous lesions may reflect disseminated infection, we recommend treatment with voriconazole in addition to evaluation for a primary focus of infection (strong recommendation; low-quality evidence).
56. In cases of aspergillosis in burns or massive soft tissue wounds, surgical debridement is recommended, in addition to antifungal therapy (strong recommendation; moderate-quality evidence).
What Are the Treatment Recommendations for Aspergillus Peritonitis?
Recommendation
57. We recommend prompt peritoneal dialysis catheter removal accompanied by systemic antifungal therapy with voriconazole (strong recommendation; low-quality evidence).
What Are the Treatment Recommendations for Esophageal, Gastrointestinal, and Hepatic Aspergillosis?
Recommendations
58. We suggest voriconazole and surgical consultation in attempts to prevent complications of hemorrhage, perforation, obstruction, or infarction (weak recommendation; low-quality evidence).
59. We suggest antifungal therapy with voriconazole or a lipid formulation of AmB as initial therapy for hepatic aspergillosis. For extrahepatic or perihepatic biliary obstruction, or localized lesions that are refractory to medical therapy, surgical intervention should be considered (weak recommendation; low-quality evidence).
What Are the Treatment Recommendations for Renal Aspergillosis?
Recommendation
60. We suggest a combined approach of medical and urologic management for renal aspergillosis. Obstruction of one or both ureters should be managed with decompression if possible and local instillation of AmB deoxycholate. Parenchymal disease is best treated with voriconazole (weak recommendation; low-quality evidence).
What Are the Treatment Regimens for Aspergillus Ear Infections?
Recommendations
61. Noninvasive Aspergillus otitis externa, also called otomycosis, is treated by thorough mechanical cleansing of the external auditory canal followed by topical antifungals or boric acid (strong recommendation; moderate-quality evidence).
62. We recommend that clinicians treat IA of the ear with a prolonged course of systemic voriconazole, usually combined with surgery (strong recommendation; low-quality evidence).
What Are the Treatment Recommendations for Aspergillus Keratitis?
Recommendation
63. We recommend that clinicians treat Aspergillus keratitis with topical natamycin 5% ophthalmic suspension or topical voriconazole (strong recommendation; moderate-quality evidence).
How Should Aspergillus Bronchitis Be Diagnosed and Treated in the Nontransplant Population?
Recommendations
64. We suggest the diagnosis of Aspergillus bronchitis in nontransplant patients be confirmed by detection of Aspergillus spp in respiratory secretions, usually sputum, with both PCR and GM on respiratory samples being much more sensitive than culture (weak recommendation; low-quality evidence).
65. We suggest treatment with oral itraconazole or voriconazole with TDM (weak recommendation; low-quality evidence).
PROPHYLAXIS OF INVASIVE ASPERGILLOSIS
V. What Are the Recommended Prophylactic Regimens, Who Should Receive Them, and How Should Breakthrough Infection Be Managed?
In Which Patients Should Antifungal Prophylaxis Against Aspergillosis Be Used?
Recommendation
66. We recommend prophylaxis with posaconazole (strong recommendation; high-quality evidence), voriconazole (strong recommendation; moderate-quality evidence), and/or micafungin (weak recommendation; low-quality evidence) during prolonged neutropenia for those who are at high risk for IA (strong recommendation; high-quality evidence). Prophylaxis with caspofungin is also probably effective (weak recommendation; low-quality evidence). Prophylaxis with itraconazole is effective, but therapy may be limited by absorption and tolerability (strong recommendation; moderate-quality evidence). Triazoles should not be coadministered with other agents known to have potentially toxic levels with concurrent triazole coadministration (eg, vinca alkaloids, and others) (strong recommendation; moderate-quality evidence).
What Are the Recommended Prophylactic Regimens for Patients With Graft-Versus-Host Disease?
Recommendations
67. We recommend prophylaxis with posaconazole for allogeneic HSCT recipients with GVHD who are at high risk for IA (strong recommendation; high-quality evidence). Prophylaxis with other mold-active azoles is also effective. Voriconazole is commonly used for prophylaxis against IA in high-risk patients but did not show improved survival in clinical trials (strong recommendation; moderate-quality evidence). Prophylaxis with itraconazole is limited by tolerability and absorption (strong recommendation; high-quality evidence).
68. We recommend continuation of antifungal prophylaxis throughout the duration of immunosuppression in patients with chronic immunosuppression associated with GVHD (corticosteroid equivalent of >1 mg/kg/day of prednisone for >2 weeks and/or the use of other anti-GVHD therapies, such as lymphocyte-depleting agents, or tumor necrosis factor α (TNF-α) inhibition, for refractory GVHD) (strong recommendation; high-quality evidence).
What Are the Recommendations for Antifungal Prophylaxis in Lung Transplant Patients?
Recommendations
69. We recommend antifungal prophylaxis with either a systemic triazole such as voriconazole or itraconazole or an inhaled AmB product for 3 to 4 months after lung transplant (strong recommendation; moderate-quality evidence).
70. Systemic voriconazole or itraconazole is suggested over inhaled AmB for lung transplant recipients with mold colonization pre- or post–lung transplant, mold infections found in explanted lungs, fungal infections of the sinus, and single-lung transplant recipients (weak recommendation; low-quality evidence).
71. We recommend reinitiating antifungal prophylaxis for lung transplant recipients receiving immunosuppression augmentation with either thymoglobulin, alemtuzumab, or high-dose corticosteroids (strong recommendation; moderate-quality evidence).
What Are the Recommendations for Antifungal Prophylaxis in Nonlung Solid Organ Transplant Recipients?
Recommendation
72. We recommend prophylactic strategies in SOT recipients based on the institutional epidemiology of infection and assessment of individual risk factors (strong recommendation; low-quality evidence). Prospective trials are lacking to address the need for routine anti-Aspergillus prophylaxis other than for lung transplant recipients. Individual risk factors have been identified in cardiac (pretransplant colonization, reoperation, cytomegalovirus [CMV] infection, renal dysfunction, institutional outbreak), liver (fulminant hepatic failure, reoperation, retransplantation, or renal failure), and others with institutional outbreaks or prolonged or high-dose corticosteroid use. In such patients, the optimal duration of prophylaxis is not known.
MANAGEMENT OF BREAKTHROUGH INFECTION
How Should Breakthrough Aspergillosis Be Managed?
Recommendation
73. We suggest an individualized approach that takes into consideration the rapidity and severity of infection and local epidemiology. As principles, we recommend an aggressive and prompt attempt to establish a specific diagnosis with bronchoscopy and/or CT-guided biopsy for peripheral lung lesions. Documentation of serum azole levels should be verified if TDM is available for patients receiving mold-active triazoles. Antifungal therapy should be empirically changed to an alternative class of antifungal with Aspergillus activity. Other considerations include reduction of underlying immunosuppression if feasible, and susceptibility testing of any Aspergillus isolates recovered from the patient (weak recommendation; moderate-quality evidence).
VI. When Should Patients Be Treated Empirically?
What Strategies Are Recommended for Empiric and Preemptive Strategies in Allogeneic Hematopoietic Stem Cell Transplant Recipients and Patients Treated for Acute Myelogenous Leukemia?
Recommendations
74. Empiric antifungal therapy is recommended for high-risk patients with prolonged neutropenia who remain persistently febrile despite broad-spectrum antibiotic therapy. Antifungal options include a lipid formulation of AmB (strong recommendation; high-quality evidence), an echinocandin (caspofungin or micafungin) (strong recommendation; high-quality evidence), or voriconazole (strong recommendation; moderate-quality evidence).
75. Empiric antifungal therapy is not recommended for patients who are anticipated to have short durations of neutropenia (duration of neutropenia <10 days), unless other findings indicate a suspected invasive fungal infection (IFI) (strong recommendation; moderate-quality evidence).
76. The use of serum or BAL fungal biomarkers such as GM or (1 → 3)-β-D-glucan to guide antifungal therapy in asymptomatic or febrile high-risk patients (often referred to as preemptive or biomarker-driven antifungal therapy) can reduce unnecessary antifungal therapy. The preemptive approach can result in more documented cases of IA without compromise in survival and can be used as an alternative to empiric antifungal therapy (strong recommendation; moderate-quality evidence).
77. Early initiation of antifungal therapy in patients with strongly suspected IPA is warranted while a diagnostic evaluation is conducted (strong recommendation; moderate-quality evidence).
78. Management of suspected or documented breakthrough IPA in the context of mold-active azole prophylaxis or empiric suppressive therapy is not defined by clinical trial data, but a switch to another drug class is suggested (weak recommendation; low-quality evidence).
How Do Lung Transplant Recipients Differ From Other Immunosuppressed Patients in Management of Suspected Invasive Pulmonary Aspergillosis?
Recommendations
79. In lung transplant recipients not on antimold prophylaxis, we suggest preemptive therapy with an antimold antifungal for asymptomatic patients with Aspergillus colonization of the airways within 6 months of lung transplant or within 3 months of receiving immunosuppression augmentation for rejection (weak recommendation; moderate-quality evidence).
80. Six months after lung transplant and in the absence of recent immunosuppression augmentation for rejection, it may be prudent to withhold antifungal therapy for Aspergillus airway colonization (ie, Aspergillus respiratory cultures in the absence of clinical features that suggest disease, such as compatible symptoms, or bronchoscopic, histopathologic, and/or radiographic findings) (weak recommendation; low-quality evidence).
CHRONIC AND SAPROPHYTIC SYNDROMES OF ASPERGILLUS
VII. How Should Chronic Aspergillosis, Allergic Syndromes, or Noninvasive Syndromes Be Managed?
How Can Chronic Cavitary Pulmonary Aspergillosis Be Diagnosed and Treated?
Recommendations
81. The diagnosis of chronic cavitary pulmonary aspergillosis (CCPA) requires: (i) 3 months of chronic pulmonary symptoms or chronic illness or progressive radiographic abnormalities, with cavitation, pleural thickening, pericavitary infiltrates, and sometimes a fungal ball; (ii) Aspergillus IgG antibody elevated or other microbiological data; and (iii) no or minimal immunocompromise, usually with one or more underlying pulmonary disorders. The Aspergillus IgG antibody test is the most sensitive microbiological test (strong recommendation; moderate-quality evidence). Sputum Aspergillus PCR testing is more sensitive than culture (weak recommendation; moderate-quality evidence).
82. Patients with CCPA without pulmonary symptoms, weight loss, or significant fatigue, and those without major impairment of pulmonary function or gradual loss of pulmonary function may be observed without antifungal therapy and followed every 3–6 months (weak recommendation; low-quality evidence).
83. Patients with CCPA and either pulmonary or general symptoms or progressive loss of lung function or radiographic progression should be treated with a minimum of 6 months of antifungal therapy (strong recommendation; low-quality evidence).
84. Oral itraconazole and voriconazole are the preferred oral antifungal agents (strong recommendation; high-quality evidence); posaconazole is a useful third-line agent for those with adverse events or clinical failure (strong recommendation; moderate-quality evidence).
85. Hemoptysis may be managed with oral tranexamic acid (weak recommendation; low-quality evidence), bronchial artery embolization (strong recommendation; moderate-quality evidence), or antifungal therapy to prevent recurrence (strong recommendation; low-quality evidence). Patients failing these measures may require surgical resection (weak recommendation; moderate-quality evidence).
86. In those who fail therapy, develop triazole resistance, and/or have adverse events, intravenous micafungin (weak recommendation; low-quality evidence), caspofungin (weak recommendation; low-quality evidence), or AmB (weak recommendation; low-quality evidence) yield some responses. Treatment may need to be prolonged.
87. Surgical resection is an option for some patients with localized disease, unresponsive to medical therapy, including those with pan-azole-resistant Aspergillus fumigatus infection or persistent hemoptysis despite bronchial artery embolization (strong recommendation; moderate-quality evidence). The outcomes from surgery are less favorable than those with single aspergilloma, and a careful risk assessment prior to surgical intervention is required.
88. In those with progressive disease, long-term, even lifelong antifungal therapy may be required to control disease (weak recommendation; low-quality evidence), with continual monitoring for toxicity and resistance.
What Are the Management Options for an Aspergillus Fungal Ball of the Lung (Aspergilloma)?
Recommendations
89. Asymptomatic patients with a single aspergilloma and no progression of the cavity size over 6–24 months should continue to be observed (strong recommendation; moderate-quality evidence).
90. Patients with symptoms, especially significant hemoptysis, with a single aspergilloma, should have it resected, assuming that there are no contraindications (strong recommendation; moderate-quality evidence).
91. Peri-/postoperative antifungal therapy is not routinely required, but if the risk of surgical spillage of the aspergilloma is moderate (related to location and morphology of the cavity), antifungal therapy with voriconazole (or another mold-active azole) or an echinocandin is suggested to prevent Aspergillus empyema (weak recommendation; low-quality evidence).
ALLERGIC SYNDROMES OF ASPERGILLUS
How Is Allergic Bronchopulmonary Aspergillosis Identified and Managed in Patients With Asthma and Cystic Fibrosis?
Recommendations
92. Elevated Aspergillus immunoglobulin E (IgE) and total IgE are recommended to establish the diagnosis and are useful for screening (strong recommendation; high-quality evidence).
93. We suggest treating symptomatic asthmatic patients with bronchiectasis or mucoid impaction, despite oral or inhaled corticosteroid therapy, with oral itraconazole therapy with TDM (weak recommendation; low-quality evidence).
94. In CF patients with frequent exacerbations and/or falling forced expiratory volume 1 (FEV1), we suggest treating with oral itraconazole to minimize corticosteroid use with TDM, and consideration of other mold-active azole therapy if therapeutic levels cannot be achieved (weak recommendation; low-quality evidence).
What Is the Medical Management of Allergic Fungal Rhinosinusitis Caused by Aspergillus Species?
Recommendations
95. We recommend establishing the diagnosis of allergic fungal rhinosinusitis in patients with nasal polyposis and thick eosinophilic mucin by visualizing hyphae in mucus, which is supported by a positive anti-Aspergillus IgE serum antibody assay or skin-prick test (where available) (strong recommendation; moderate-quality evidence).
96. We recommend polypectomy and sinus washout as the optimal means of symptom control and inducing remission; however, relapse is frequent (strong recommendation; moderate-quality evidence).
97. We recommend the use of topical nasal steroids to reduce symptoms and increase time to relapse, especially if given after surgery (strong recommendation; moderate-quality evidence).
98. We suggest oral antifungal therapy using mold-active triazoles for refractory infection and/or rapidly relapsing disease, although this approach is only partially effective (weak recommendation; low-quality evidence).
Notes
Dedication. The panel dedicates these guidelines to the memory of our dear friend Susan Hadley, MD, a core member of the Mycoses Study Group, caring physician, and wonderful colleague.
Acknowledgments. The Expert Panel expresses its gratitude for thoughtful reviews of an earlier version by Sanjay Revankar and Samuel Lee. The Panel also greatly appreciates the work of Charles B. Wessels and Michele Klein Fedyshin of the Health Sciences Library System of the University of Pittsburgh for the development and execution of the systematic literature searches for this guideline. We give special thanks to Genet Demisashi of the Infectious Diseases Society of America (IDSA) staff for her support in the development of this guideline.
Financial support. Support for this guideline was provided by the IDSA.
Potential conflicts of interest. The following list is a reflection of what has been reported to IDSA. To provide thorough transparency, IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential conflicts of interest (COI) is determined by a review process that includes assessment by the Standards and Practice Guideline Committee (SPGC) Chair, the SPGC liaison to the development panel, the Board of Directors liaison to the SPGC, and, if necessary, the COI Task Force of the Board. This assessment of disclosed relationships for possible COI will be based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. For activities outside the submitted work, T. F. P. received research grant support to the University of Texas Health Science Center San Antonio from Astellas, Merck, and Revolution Medicines and has been a consultant for or served on advisory boards to Amplyx, Astellas, Durata, Cidara Therapeutics, Gilead, Merck, Pfizer, Revolution Medicines, Scynexis, Toyama, Vical, and Viamet. For activities outside of the submitted work, G. R. T. received research support to the University of California, Davis from Astellas, Merck, Pfizer, and Scynexis, and has been a consultant for Astellas. For activities outside the submitted work, D. W. D. holds Founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company and in Novocyt, which markets the Myconostica real-time molecular assays; has current grant support from the National Institute of Health Research, Medical Research Council, Global Action Fund for Fungal Infections, and the Fungal Infection Trust; serves as a consultant to Astellas, Sigma Tau, Basilea, and Pulmocide; and has received honoraria from Astellas, Dynamiker, Gilead, Merck, and Pfizer. For activities outside the submitted work, J. A. F. served on scientific advisory boards for Revolution Medicines. For activities outside the submitted work, S. H. served as a consultant to Merck. For activities outside the submitted work, R. H. served on advisory boards for Astellas, Basilea, Gilead, and Pfizer and received research grants from Alsace contre le Cancer and Pfizer. For activities outside the submitted work, D. P. K. served as a consultant to Astellas, Merck, and Pfizer; received research support from Astellas, Merck, Pfizer, and T2 Biosystems; and received honoraria from Astellas, Merck, Pfizer, T2 Biosystems, Gilead, and F2G, Inc. For activities outside the submitted work, K. A. M. received honoraria from Amplyx, Astellas, Cidara, F2G, Merck, Pfizer, Revolutions Medicine, and Vical, and has a patent US No. 13/511 264 licensed. For activities outside the submitted work, V. A. M. served as a consultant for Celgene, Amgen, GSK, Merck, and Astellas, and served on the speaker's bureaus for Genentech and Celgene. For activities outside the submitted work, M. H. N. received research grants from Astellas, Pfizer, Merck, ViraCor, and the National Institutes of Health (National Institute of Allergy and Infectious Diseases). For activities outside the submitted work, B. H. S. served on advisory boards for Merck and Astellas, and has contracts for laboratory research from Astellas and Assembly Biosciences. For activities outside the submitted work, W. J. S. served on scientific advisory boards from Merck and received research grants to Duke University from Merck and Astellas. For activities outside the submitted work, T. J. W. served as a consultant or scientific advisor for Astellas, Novartis, Pfizer, and Methlygene and received research grants to Weill Cornell Medical Center from Astellas, Merck, and Novartis. For activities outside the submitted work, J. R. W. served as consultant/scientific advisor for Gilead, Astellas, Pfizer, Merck, and Vical. For activities outside the submitted work, J. H. Y. received research support to the University of Minnesota from Astellas, Merck, and Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Reference
- 1.US GRADE Network. Approach and implications to rating the quality of evidence and strength of recommendations using the GRADE methodology. Available at: http://www.gradeworkinggroup.org/ Accessed 1 April 2016.