Abstract
The tubular compartment of the kidney is the primary site of a wide range of insults that can result in acute kidney injury (AKI), a condition associated with high mortality and an increased risk to develop end-stage renal disease. Nevertheless, kidney function is often quickly recovered after tubular injury. How this happens has only partially been unveiled. Indeed, although it has clearly been demonstrated that regenerated epithelial cells arise from survived intratubular cells, the true entity, as well as the cellular source of this regenerative process, remains mostly unknown. Is whichever proximal tubular epithelial cell able to dedifferentiate and divide to replace neighboring lost tubular cells, thus suggesting an extreme regenerative ability of residual tubular epithelium, or is the regenerative potential of tubular epithelium limited, and mostly related to a preexisting population of intratubular scattered progenitor cells which are more resistant to death? Gaining insights on how this process takes place is essential for developing new therapeutic strategies to prevent AKI, as well as AKI-related chronic kidney disease. The aim of this review is to discuss why the answers to these questions are still open, and how further investigations are needed to understand which is the true regenerative potential of the tubule and who are the players that allow functional recovery after AKI.
Keywords: AKI, stem cell, tubular cell dedifferentiation, tubular progenitor, tubular regeneration
INTRODUCTION
Acute kidney injury (AKI) is a multifactorial and multiphasic renal disease characterized by a rapid decline in renal function, resulting in the accumulation of metabolic waste products and toxins, with consequent complications and failure of other organs [1]. Pathologically, AKI is characterized by renal tubular damage, inflammation and vascular dysfunction. Injury and death of tubular cells are especially recognized as the precipitating factors in AKI, and as an extension, tubular repair and regeneration are considered major events in kidney recovery from AKI [2]. Traditionally, AKI was considered as fully reversible, especially after mild tubular injury episodes, because of the high regenerative capacity of the tubule. However, apparent functional recovery may occur despite persistence of significant injury and a relevant nephron loss. Consistently, epidemiological studies in various clinical settings reported an association between AKI and end-stage renal disease (ESRD) [3–5]. For example, in a cohort of patients who developed dialysis-requiring AKI during their hospital stay, the long-term risk of developing stage 4 or 5 chronic kidney disease was increased 28-fold, consistently with the massive nephron loss associated with severe AKI episodes [5]. However, more recent epidemiological studies underline that also mild AKI confers an increased risk of ESRD, going from 2.92 to >4-fold higher in patients. For example, in a cohort of patients who underwent coronary angiography and developed AKI, the risk of ESRD was >4-fold higher in patients in AKI network (AKIN) stage 1 [6] and from 3.81 to almost 12-fold higher in patients in AKIN stage 2–3, compared with patients without AKI [6, 7]. Are these observations compatible with a high regenerative potential of the tubule and a diffuse capacity of all survived tubular cells to proliferate and replace adjacent lost tubular cells?
HOW AND HOW MUCH DOES THE TUBULAR : TISSUE REALLY REGENERATE?
After AKI, the human kidney can eventually undergo a complete functional recovery, especially in mild-to-moderate injury, but the entity of true tissue regeneration occurring after tubular necrosis is unknown. Traditionally, tubular cells were considered as highly regenerative cells based on the quick functional recovery evidenced after mild-to-moderate AKI episodes, on the apparent tissue integrity observed in animal models some weeks after the injury episode and on the robust proliferative activity observed in tubular tissue after injury based on cell cycle labels [2]. However, in the kidney, functional recovery does not necessarily mirror tissue integrity, because normal renal function is observed also in patients with uninephrectomy, who are left with half of their initial nephrons [8]. In addition, apparent tissue integrity a few weeks after injury cannot give information about how many nephrons were lost and how many were already functionally injured in an irreversible manner. Finally, cell cycle labels only indicate entry into cell cycle and do not truly mirror cell division, thus suggesting that the proliferating capacity of tubular cells may be largely overestimated. Thus, what truly happens after tubular injury, and how, and to what extent, does tubular regeneration truly occur?
A large body of evidence suggests that whatever the source, tubular regeneration is orchestrated within the tubule itself. Indeed, Humphreys et al. [9] ruled out the implication of murine extratubular cells in re-epithelialization following AKI due to a transgenic model in which the endogenous Six2 promoter conditionally controlled the expression of the reporter transgene LacZ. This strategy allowed them to tag only Six2+ renal epithelial precursors in the embryonic kidney. These cells give rise to adult tubular epithelial cells and can be found only in the metanephric mesenchyme cells differentiating into renal epithelium during the developmental period of active nephrogenesis [10]. The authors showed that following an ischaemia–reperfusion injury (IRI), no reporter dilution was observed and concluded that all reparative epithelial cells derived from within the tubule [9]. However, labelling Six2+ progenitors at the time of embryogenesis does not permit to distinguish between hypothetically different epithelial populations, with distinct regenerative potential, resident within the tubule, as all epithelial components of the adult tubule derive from these same tagged progenitors. In addition, cell division in this study was defined through injection of 5-bromo-2′-deoxyuridine (BrdU) after injury and labelling of cells that synthesize DNA or through Ki67 staining [9]. This strategy only permit to identify cells that have entered the cell cycle, but these cells did not necessarily complete division, as discussed in detail subsequently. Thus, what is the source of the newly formed proximal epithelial cells that allow recovery from AKI and what is the true regenerative potential of renal tubule? So far, two main hypotheses have been proposed.
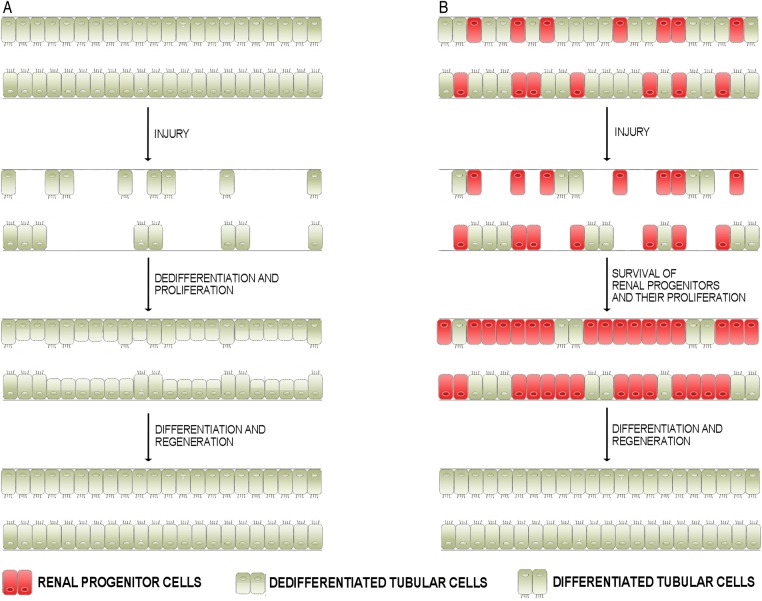
The first one is based on the concept that the low basal rate of cellular proliferation of adult proximal tubule does not require a stem cell population. This concept is supported by the observation that fully differentiated epithelial cells of the proximal tubule are poised in the G1 phase of the cell cycle, as if they were ready to progress into the cycle in the case of injury [11]. In such a scenario, fully differentiated tubular cells would transiently undergo a cycle of dedifferentiation, proliferation and, ultimately, re-differentiation (Figure 1B). The second one argues the existence of an intratubular scattered progenitor population which, poised in a quiescent state, would re-enter the cell cycle with the aim of replacing cells that are lost due to tubular damage [12–14] (Figure 1B). Indeed, a renal progenitor system consisting of a heterogeneous population of renal progenitors with different commitments has been characterized in adult human kidney in recent years [15, 16]. In particular, renal progenitors (RPC) localize at the urinary pole of Bowman's capsule and are characterized by co-expression of two progenitor markers, CD133 and CD24, in the absence of lineage markers, whereas podocyte-committed progenitors localize along Bowman's capsule close to the vascular pole and are characterized by co-expression of progenitor and podocyte markers [17, 18]. Finally, human tubular-committed progenitors, which are scattered within the proximal tubule, the thick ascending limb, the distal convoluted tubule and the connecting segment, can generate cells of all these portions of the tubule and are characterized by expression of RPC markers in the presence of low levels of tubular markers [12, 19, 20]. RPC cultures can be obtained from healthy human tissues using immunomagnetic sorting for CD133 and CD24, which distinguish them from differentiated cells of the adult kidney. The progenitor potential of these cells was functionally ascertained through in vitro and in vivo assays (Figure 2) in several studies [12, 19, 21].
FIGURE 1:
Schematic representation of the different hypotheses proposed to explain tubular regeneration after AKI. (A) After a tubular injury, differentiated tubular cells that survived dedifferentiate and become able to proliferate, migrate and then differentiate, replacing the lost tubular cells. (B) In healthy kidneys, tubular progenitors are scattered among differentiated tubular cells. Tubular progenitors are resistant to death, so they preferentially survive following injury and proliferate, migrate and then differentiate to replace lost tubular cells.
FIGURE 2:
Schematic representation of the assays that are mandatory to characterize a putative stem/progenitor cell. The classical definition of a stem cell requires that it possesses two properties: self-renewal, which is the ability to go through numerous cycles of cell division while maintaining the undifferentiated state; differentiation potential, defined as the capacity to differentiate into specialized cell types. A progenitor cell is instead a cell that, similar to a stem cell, has a tendency to differentiate into a specific type of cell, but is already more commited than a stem cell and is pushed to differentiate into its ‘target’ cell. The most important difference between stem and progenitor cells is that stem cells can replicate indefinitely, whereas progenitor cells can divide only a limited number of times. The functional demonstration of these capacities can be achieved in vitro by isolating and cloning the putative stem or progenitor cells and analysing its capacity to generate a progeny of other stem or progenitor cells as well as one or more types of differentiated cells. In contrast, in vivo, the same properties can be evaluated by (i) injecting the putative cell into mouse models of organ or tissue injury and evaluating its capacity to engraft and reconstitute tissue integrity and (ii) labelling the putative stem or progenitor population and evaluating its capacity to generate a labelled progeny of other stem or progenitor cells as well as one or more types of differentiated cells and contribute to tissue turnover or reconstitute the integrity of the injured tissue.
Additionally, various strategies have been developed in order to understand murine tubular regeneration, and very recently, two studies that used lineage tracing [22, 23] concluded against the existence of a tubular progenitor population and favoured the hypothesis that the regenerative capacity of the tubule may be high and related to proliferation of differentiated tubular cells in a stochastic manner [24]. In this review, we will discuss some of the main points that show how data obtained from different mouse models and experimental strategies are not conclusive because different result interpretations are possible [25], not permitting to reach a univocal conclusion, such as those recently reported in this journal [26]. We propose that the evidence obtained so far for murine tubular regeneration not only does not permit to conclude whether differentiated tubular cells or progenitor cells are the main actors responsible for proximal tubular recovery, but even raises the question of whether the regenerative potential of tubular cells is as high as traditionally thought.
MARKERS THAT LABEL TUBULAR : PROGENITORS IN THE MOUSE ARE NOT YET AVAILABLE
In the last decade, transgenic animal models have been extensively used to identify stem or progenitor populations located in various organs and to characterize their role in homeostatic as well as in pathological conditions [27–30]. To this aim, various promoters can be potentially used, such as endogenous markers expressed under physiological conditions, or genes not encoded in the mouse genome.
For instance, Appel et al. [31] developed an inducible model in which LacZ expression is driven by a promoter constituted by two fused exogenous elements, namely human and rabbit podocalyxin (hPODXL1 and cPodxl1, respectively). This transgenic mouse, called PEC-rtTA, was initially created to trace parietal epithelial cells (PECs) that had already been characterized as putative renal progenitors in the adult human kidney [17]. By using this model, Appel et al. [31] demonstrated that PEC migrated onto the glomerular tuft and differentiated into podocytes in the juvenile mice. However, as in this mouse other kidney cell types were labelled, including some proximal tubular cells, in a further study, Berger et al. [22] hypothesized that the PEC-rtTA mouse may also track the mouse counterpart of CD133+ tubular progenitors and that this mouse could be used to track the fate of putative tubular progenitors in a setting of IRI-induced damage. To support their hypothesis, the authors reported that LacZ+ cells stained positive for the markers annexin A3, src-suppressed C-kinase, CD44 and KIM-1, which characterize also the CD133+ human renal progenitor cells and mouse PEC of Bowman's capsule [22]. However, these markers are also shared by other kidney cells, especially upon activation. In addition, the labelled cells were not fully phenotypically characterized, and no functional assays were performed: no in vitro clonal expansion nor differentiation assays were made; as well as no in vivo transplantation experiments, which are essential when characterizing stem or progenitor populations, were executed (Figure 2). Thus, there is no proof that the intratubular tagged population represents the mouse counterpart of CD133+ tubular progenitors. In contrast, human tubular progenitor cells were phenotypically characterized through arrays and functionally studied for their self-renewal and differentiation capacity through in vitro assays and transplantation in in vivo models of acute tubular injury [12]. Moreover, tagged tubular cell distribution in the PEC-rtTa mouse varies from that described for CD133+ human tubular progenitors, which are principally located into the S3 proximal tubule segment as well as in the distal tubule [12]; on the contrary, LacZ+ cells evidenced in the PEC-rtTA mouse were distributed into S1 and S2 segments, in addition to the S3 segment [22]. Interestingly, Berger et al. [22] failed to demonstrate, following IRI-induced damage, the amplification of the tagged tubular cells, denying their role in proximal tubule regeneration. However, as no proof was provided that these cells truly represent the mouse counterpart of human CD133+ tubular progenitors, the lack of amplification in response to injury of tagged tubular cells in the PECrtTA mouse does not permit to draw any definitive conclusion about their existence and role [25]. As a general concept, if lineage tracing strategies demonstrate self-renewal and differentiation capacity of the tagged cell population, one can conclude positively on the existence of a progenitor population. However, if the cell type marked by the promoter is unknown, negative results do permit to conclude that the chosen promoter does not label progenitors or that the population labelled does not mediate regeneration, but not that progenitors do not exist [25].
LINEAGE TRACING USING MARKERS OF : DIFFERENTIATED TUBULAR CELLS CANNOT EXCLUDE TUBULAR PROGENITOR EXISTENCE
To clarify the mechanisms of tubular response to injury, Kusaba et al. [23] developed an inducible transgenic mouse model in which the sodium-dependent inorganic phosphate transporter (SLC34a1) promoter was used to tag proximal tubule epithelial cells. The authors hypothesized that, if differentiated proximal tubular epithelial cells are responsible for tubular recovery after IRI, there would be no reporter dilution despite intense proliferation, thus excluding any role of putative tubular progenitors in the regenerative phase. On the contrary, if untagged progenitors contribute to tubular regeneration, a dilution of labelled cells would have been observed due to differentiated cell death and progenitor amplification [23]. To dissect this point, the authors performed a unilateral IRI on the SLC34a1 transgenic model and concluded that, as no fate marker dilution was detectable, differentiated tubular cells are responsible for proximal tubule regeneration [23].
However, this study did not take into consideration that putative tubular progenitors might express, to some extent, markers of terminally differentiated cells, as already demonstrated in other tissues in addition to the kidney. Indeed, for example, during mouse prostatic post-natal development, clonal analysis on basal cells not only revealed the existence of both unipotent and bipotent basal progenitors but also evidenced the presence of cells already committed toward the luminal lineage and co-expressing basal as well as luminal markers [32]. Analogously, clonal fate and proliferation dynamic analysis revealed, in mouse skin interfollicular epidermis, the presence of progenitors committed to undergo terminal differentiation that already express differentiation markers associated with suprabasal layers of terminally differentiated cells [33]. Similar findings have also been reported for the haematopoietic system, the central nervous system and the hair follicle compartments [34–36]. Committed progenitors usually represent not only indispensable integrators of stem cell niche, but also the compartment which is first activated in early tissue regeneration [36]. Thus, the evidence obtained from different organs and organ systems demonstrates that progenitor populations can exhibit, to some degree, the expression of markers typically associated with fully differentiated cells.
More importantly, several studies have proved the existence of scattered tubular-committed progenitors that co-express CD133, CD24 as well as markers of differentiated tubular cells in adult human kidney [12, 19, 20]. Such tubular progenitors would also have been tagged in a mouse model driven by a promoter constituted by a tubule differentiation marker as in [23], thus explaining why no label dilution is observed by the authors.
Moreover, Kusaba et al. [23] were able to tag proximal epithelial tubular cells with high efficiency only in the S1 and S2 segments of the proximal tubule, but to a lower extent (about 55%) in the cortical S3 segment, which is the primary site of IRI damage [2], as well as of localization of tubular progenitors in humans [12]. Finally, the authors reported an upregulation of CD133 and CD24 mRNA, but these markers are not homologs among humans and mice and thus cannot be used to detect the mouse counterpart of CD133+ human tubular progenitors [25, 37]. Indeed, most progenitor markers are species-specific, as in the case of human CD133, which is currently employed as a marker to identify various stem and progenitor compartments in adult human tissues, but is instead also frequently expressed by differentiated epithelia in mice [37]. Thus, in such an experimental setting, the existence of tubular progenitors cannot be ruled out, and their possible role in tubular regeneration after injury remains to be established.
PUTATIVE TUBULAR PROGENITORS ARE : MORE RESISTANT TO DEATH THAN OTHER TUBULAR CELLS
The existence of a tubular progenitor population is suggested also by several studies that evidenced that CD133+ tubular cells exhibit unique ultrastructural characteristics and are highly resistant to death. Indeed, CD133+ tubular progenitors are small and flask-shaped with no, or less pronounced, brush border [20, 38]. Furthermore, these cells have smaller and darker nuclei than the surrounding cells, indicative of condensed chromatin. The basal compartment of the cells displays a filamentous mat consisting of vimentin and COL7A1, which confers increased adherence to the basement membrane. Also, these cells demonstrate increased BCL-2 expression, contributing to anti-apoptotic features and very few mitochondria when compared with the surrounding epithelium, which may explain their characteristics of robustness [20, 38]. Indeed, Angelotti et al. [12] evidenced how CD133+ progenitors exhibit an increased resistance to death in vitro in comparison to other tubular cells, following exposure to potentially nephrotoxic agents such as haemoglobin. This evidence was further corroborated by Hansson et al. [38], who developed an ex vivo explant culture system in which the vascular clamping of human kidneys undergoing nephrectomy was used to simulate acute tubular necrosis. After a defined exposure to ischaemia in conjunction with nephrectomy, renal cortical tissue was allowed to re-oxygenate in a cell culture environment. The procedure produced massive tubular necrosis leaving, however, CD133+ tubular progenitors intact in the tubular cross-sections, thus providing further functional evidence that these cells are more resistant to insult than bulk epithelium. This hypothesis is further sustained by a study in rodents by Langworthy et al. [39]. Indeed, by developing a model in which β-galactosidase labelling is driven by the NFATc1 (nuclear factor of activated T cells, cytoplasmic 1) promoter, the authors demonstrated the existence of a tubular subpopulation that displays a high resistance to death and amplifies following mercuric chloride injury [39].
Taken together, these studies suggest that tubular cells are heterogeneous and that two distinct tubular populations, exhibiting different sensitivities to injury, exist in healthy adult kidneys. The presence of these putative tubular progenitors at all ages, even in biopsies from healthy donors, as well as in pigs and monkeys, excludes that they represent the result of dedifferentiation and further suggests that they instead represent a distinct tubular subpopulation that is conserved across evolution [40]. In addition, even if in healthy human kidneys CD133+ tubular progenitors represent only 2–6% of all tubular cells, immediately after injury, due to their survival capacity, they become the dominant existing population, as adjacent differentiated tubular cells preferentially die [12, 19]. This relative enrichment may explain why CD133+ tubular progenitor markers increase immediately after injury and why the increased prevalence of such an undifferentiated population may be wrongly interpreted as a result of dedifferentiation.
CELL CYCLE LABELS DO NOT FAITHFULLY : REPRESENT CELL DIVISION AND REGENERATION
Various works demonstrated that injured tubular cells enter the cell cycle and that this event can easily be visualized and quantified by BrdU nuclear labelling [14, 41]. Analogous observations have also been made with the most widely accepted and historically used cell cycle markers, such as Ki-67 which is expressed from G1 to the mitotic phase, or phosphohistone H3 which marks the G2 to M transition, or proliferating cell nuclear antigen, expressed from late G1 to early mitotic phase [42–44] (Figure 3). These labels showed that injured tubular cells upregulate cell cycle markers following IRI damage, thus enabling visualization of cellular activation [45, 46]. Although the entry of numerous tubular cells in the cell cycle after injury was taken as proof of their mitosis, neither BrdU nor cell cycle markers can ensure that mitotic division has effectively happened. Using such labels, Humphreys et al. [24] demonstrated that IRI induces tubular epithelial cells to step into the cell cycle. In addition, in this work, the authors were able to track sequential cycles of epithelial cell proliferation and to discriminate cells that are rapidly cycling from slow-cycling ones by performing proliferation analysis with two thymidine analogs 5-chloro-2-deoxyuridine (CldU) and 5-iodo-2-deoxyuridine (IdU). In order to discriminate rapidly cycling from slow-cycling cells, unilateral IRI mice were injected with a single dose of CldU 24 h after injury, followed by the administration of IdU 45 h after the damage [24]. This experimental procedure demonstrated that only rare cells were double-positive for the two compounds, whereas the vast majority of proximal tubular epithelial cells had incorporated only one or the other analog. This finding induced the authors to assume that, in survived differentiated tubular cells, proliferation happens in a stochastic fashion and that these cells, upon injury, dedifferentiate to regenerate the tubular structure, but may also suggest that these cells mainly undergo a single round of cell cycle entry. Indeed, single-labelling evidence suggests that cells have entered into the S-phase of the cell cycle, but it does not permit to conclude that they underwent a complete mitosis [24]. On the contrary, only double-positive CldU and IdU cells (about 5% of all the cells) [24] have certainly completed at least one cell cycle division. In contrast, no conclusion can be drawn for all other cells that exhibited only single labelling. Indeed, these cells may have undergone cell division, but also growth arrest or even death [47]. Further studies are needed to verify this point. Interestingly, in other organs such as the liver, in which although most hepatocytes enter into the cell cycle after 70% hepatectomy, most of them do not undergo effective cellular division, as demonstrated by the presence of binucleated hepatocytes, or hepatocytes with nuclei of increased dimension and DNA content, but ineffective mitosis [48]. Taken together, these considerations suggest that the true division potential of intratubular cells after injury is unknown and may be considerably lower than previously thought. Interestingly, old pathological studies that evaluated injured tubular tissue for the occurrence of mitosis, the only proof of effective cell division, reported that they are rare [49]. More limited regenerative potential may better explain the high incidence of ESRD observed in patients with previous AKI episodes, and, therefore, this possibility deserves to be carefully analysed in future studies.
FIGURE 3:
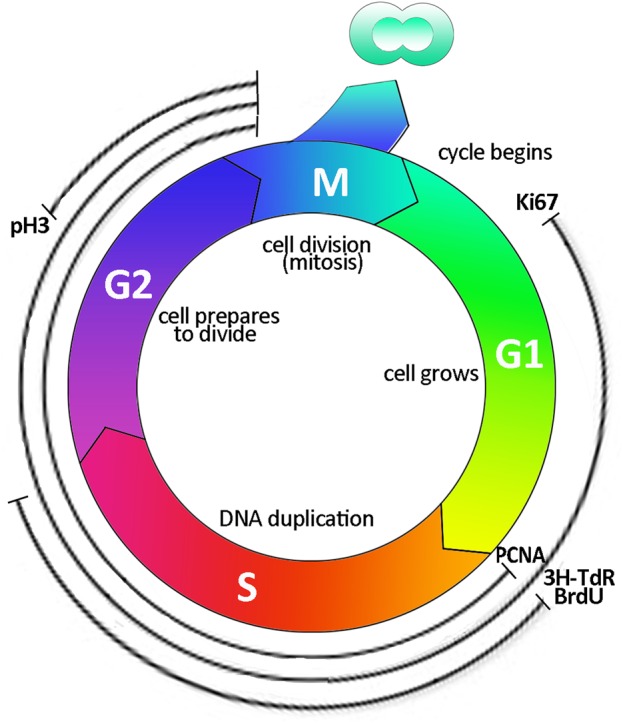
Cell cycle labelling. Schematic representation of the cell cycle and indication of the principal markers that are traditionally used to label the different phases. 3-TdR, tritiated thymidine; BrdU, 5-bromo-2′-deoxyuridine; PCNA, proliferating cell nuclear antigen; pH3, phosphohistone H3; G1, gap 1 phase; S, synthesis; G2, gap 2 phase; M, mitosis.
CONCLUSIONS
All the evidenced concepts underline how a final response to the questions of what is the effective regenerative potential of the tubule and how it occurs is still lacking. Clearly, novel experimental strategies are needed to provide definite answers to these crucial questions. In particular, what would be necessary to identify and study tubular progenitors is their direct and specific labelling, as exploited by Barker et al. [29], that identified a stem cell pool within the developing kidney characterized by the expression of the stem-cell-associated gene Lgr5, which is, however, silenced in the adult mouse kidney. A desirable transgenic model for fate mapping of the mouse counterpart of a human progenitor population in the adult mouse kidney will require a promoter constituted by a marker specifically expressed by that progenitor population and that can be activated in a temporal-controlled manner. Another essential criterion to fulfill is that the chosen promoter must be homologous between humans and mice. Unfortunately, a marker that specifically allows progenitors tagging in mouse adult kidneys remains unknown. In the absence of a known candidate gene that can serve as specific marker to identify putative tubular progenitors, a solution can be to infer the characteristics of putative stem cell compartments by ‘indirect’ analysis, that is, tagging all kidney cells in an unbiased manner and reconstructing backwards regeneration dynamics. In this regard, of note is the recent publication by Rinkevich et al. [50], in which the authors demonstrated that adult damaged mouse kidneys can undergo tubulogenesis through expansions of clonal precursors with segment-specific borders. Indeed, by using an inducible mouse model driven by the unbiased Actin promoter, the authors performed a clonal analysis on IRI kidneys and highlighted how intratubular fate-restricted cells repair only the specific tubule segment in which the clone is located [50]. This evidence closely resembles and supports the hypothesis of the presence of tubular-committed progenitors, each responsible for the turnover and repair of the belonging segment. In this study, the authors also applied an innovative strategy that allows to analyse progenitor population at the single cell level, by enabling clonal analysis and thus to evaluate effective cell division using the Confetti reporter [50]. This reporter enables the expression of one out of four fluorescent proteins in a stochastic manner, thus permitting to examine individual behavior of multiple cells and to visualize clonal expansion of single cells, which will appear as continuous clusters of cells of the same colour [30]. In this study, a quantitation of the frequency of effective cell division in comparison to cell cycle labelling was not provided, but such a strategy has the potential to clarify this point. Future studies using an inducible transgenic mouse model, in which the Confetti reporter is activated in a time-controlled manner, specifically in tubular progenitors, are finally needed to provide definite answers to all these questions.
CONFLICT OF INTEREST STATEMENT
The results presented in this review have not been published previously in whole or part, except in the abstract format. All authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This article is supported by funding from the European Research Council under the Consolidator Grant RENOIR (ERC-2014-CoG grant number 648274 to P.R.).
REFERENCES
- 1.Uchino S, Kellum JA, Bellomo R et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818 [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2012; 2: 1303–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James MT, Ghali WA, Tonelli M et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int 2010; 78: 803–809 [DOI] [PubMed] [Google Scholar]
- 4.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012; 81: 442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo LJ, Go AS, Chertow GM et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 2009; 76: 893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rydén L, Sartipy U, Evans M et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130: 2005–2011 [DOI] [PubMed] [Google Scholar]
- 7.James MT, Ghali WA, Knudtson ML et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 2011; 123: 409–416 [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim HN, Foley R, Tan L et al. Long-term consequences of kidney donation. N Engl J Med 2009; 360: 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys BD, Valerius MT, Kobayashi A et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008; 2: 284–291 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi A, Valerius MT, Mugford JW et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 2008; 3: 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogetseder A, Picard N, Gaspert A et al. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 2008; 294: C22–C28 [DOI] [PubMed] [Google Scholar]
- 12.Angelotti ML, Ronconi E, Ballerini L et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 2012; 30: 1714–1725 [DOI] [PubMed] [Google Scholar]
- 13.Kitamura S, Yamasaki Y, Kinomura M et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J 2005; 19: 1789–1797 [DOI] [PubMed] [Google Scholar]
- 14.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 2003; 14: 3138–3146 [DOI] [PubMed] [Google Scholar]
- 15.Romagnani P, Remuzzi G. Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol Metab 2013; 24: 13–20 [DOI] [PubMed] [Google Scholar]
- 16.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol 2013; 9: 137–146 [DOI] [PubMed] [Google Scholar]
- 17.Sagrinati C, Netti GS, Mazzinghi B et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. Am Soc Nephrol 2006; 17: 2443–2456 [DOI] [PubMed] [Google Scholar]
- 18.Ronconi E, Sagrinati C, Angelotti ML et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 2009; 20: 322–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindgren D, Boström AK, Nilsson K et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol 2011; 178: 828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeets B, Boor P, Dijkman H et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 2013; 229: 645–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussolati B, Bruno S, Grange C et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol 2005; 166: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger K, Bangen JM, Hammerich L et al. Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci USA 2014; 111: 1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusaba T, Lalli M, Kramann R et al. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 2014; 111: 1527–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys BD, Czerniak S, DiRocco DP et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 2011; 108: 9226–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romagnani P, Rinkevich Y, Dekel B. The use of lineage tracing to study kidney injury and regeneration. Nat Rev Nephrol 2015. doi:10.1038/nrneph.2015.67 [DOI] [PubMed] [Google Scholar]
- 26.Kramann R, Kusaba T, Humphreys BD. Who regenerates the kidney tubule? Nephrol Dial Transplant 2016; 30: 903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker N, van Es JH, Kuipers J et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- 28.Rios AC, Fu NY, Lindeman GJ et al. In situ identification of bipotent stem cells in the mammary gland. Nature 2014; 506: 322–327 [DOI] [PubMed] [Google Scholar]
- 29.Barker N, Rookmaaker MB, Kujala P et al. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep 2012; 2: 540–552 [DOI] [PubMed] [Google Scholar]
- 30.Lombardi D, Lasagni L. Transgenic strategies to study podocyte loss and regeneration. Stem Cells Int 2015; doi:10.1155/2015/678347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appel D, Kershaw DB, Smeets B et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 2009; 20: 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ousset M, Van Keymeulen A, Bouvencourt G et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol 2012; 14: 1131–1138 [DOI] [PubMed] [Google Scholar]
- 33.Mascré G, Dekoninck S, Drogat B et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012; 489: 257–262 [DOI] [PubMed] [Google Scholar]
- 34.Pina C, Fugazza C, Tipping AJ et al. Inferring rules of lineage commitment in haematopoiesis. Nat Cell Biol 2012; 14: 287–294 [DOI] [PubMed] [Google Scholar]
- 35.Jensen P, Farago AF, Awatramani RB et al. Redefining the serotonergic system by genetic lineage. Nat Neurosci 2008; 11: 417–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 2014; 157: 935–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grosse-Gehling P, Fargeas CA, Dittfeld C et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol 2013; 229: 355–378 [DOI] [PubMed] [Google Scholar]
- 38.Hansson J, Hultenby K, Cramnert C et al. Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Hum Pathol 2014; 45: 382–393 [DOI] [PubMed] [Google Scholar]
- 39.Langworthy M, Zhou B, de Caestecker M et al. NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol 2009; 20: 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Axelson H, Johansson ME. Renal stem cells and their implications for kidney cancer. Semin Cancer Biol 2013; 23: 56–61 [DOI] [PubMed] [Google Scholar]
- 41.Nonclercq D, Wrona S, Toubeau G et al. Tubular injury and regeneration in the rat kidney following acute exposure to gentamicin: a time-course study. Ren Fail 1992; 14: 507–521 [DOI] [PubMed] [Google Scholar]
- 42.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000; 182: 311–322 [DOI] [PubMed] [Google Scholar]
- 43.Wang F, Higgins JM. Histone modifications and mitosis: countermarks, landmarks, and bookmarks. Trends Cell Biol 2013; 23: 175–184 [DOI] [PubMed] [Google Scholar]
- 44.Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol 1996; 48: 175–181 [DOI] [PubMed] [Google Scholar]
- 45.Nguan CY, Guan Q, Gleave ME et al. Promotion of cell proliferation by clusterin in the renal tissue repair phase after ischemia–reperfusion injury. Am J Physiol Renal Physiol 2014; 306: F724–F733 [DOI] [PubMed] [Google Scholar]
- 46.Ye Y, Wang B, Jiang X et al. Proliferative capacity of stem/progenitor-like cells in the kidney may associate with the outcome of patients with acute tubular necrosis. Hum Pathol 2011; 42: 1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomasova D, Anders HJ. Cell cycle control in the kidney. Nephrol Dial Transplant 2016; 30: 1622–1630 [DOI] [PubMed] [Google Scholar]
- 48.Miyaoka Y, Ebato K, Kato H et al. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol 2012; 22: 1166–1175 [DOI] [PubMed] [Google Scholar]
- 49.Cuppage FE, Tate A. Repair of the nephron following injury with mercuric chloride. Am J Pathol 1967; 51: 405–429 [PMC free article] [PubMed] [Google Scholar]
- 50.Rinkevich Y, Montoro DT, Contreras-Trujillo H et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 2014; 7: 1270–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]