Abstract
Key points
Obesity results in perilymphatic inflammation and lymphatic dysfunction.
Lymphatic dysfunction in obesity is characterized by decreased lymphatic vessel density, decreased collecting lymphatic vessel pumping frequency, decreased lymphatic trafficking of immune cells, increased lymphatic vessel leakiness and changes in the gene expression patterns of lymphatic endothelial cells.
Aerobic exercise, independent of weight loss, decreases perilymphatic inflammatory cell accumulation, improves lymphatic function and reverses pathological changes in gene expression in lymphatic endothelial cells.
Abstract
Although previous studies have shown that obesity markedly decreases lymphatic function, the cellular mechanisms that regulate this response remain unknown. In addition, it is unclear whether the pathological effects of obesity on the lymphatic system are reversible with behavioural modifications. The purpose of this study, therefore, was to analyse lymphatic vascular changes in obese mice and to determine whether these pathological effects are reversible with aerobic exercise. We randomized obese mice to either aerobic exercise (treadmill running for 30 min per day, 5 days a week, for 6 weeks) or a sedentary group that was not exercised and analysed lymphatic function using a variety of outcomes. We found that sedentary obese mice had markedly decreased collecting lymphatic vessel pumping capacity, decreased lymphatic vessel density, decreased lymphatic migration of immune cells, increased lymphatic vessel leakiness and decreased expression of lymphatic specific markers compared with lean mice (all P < 0.01). Aerobic exercise did not cause weight loss but markedly improved lymphatic function compared with sedentary obese mice. Exercise had a significant anti‐inflammatory effect, resulting in decreased perilymphatic accumulation of inflammatory cells and inducible nitric oxide synthase expression. In addition, exercise normalized isolated lymphatic endothelial cell gene expression of lymphatic specific genes, including VEGFR‐3 and Prox1. Taken together, our findings suggest that obesity impairs lymphatic function via multiple mechanisms and that these pathological changes can be reversed, in part, with aerobic exercise, independent of weight loss. In addition, our study shows that obesity‐induced lymphatic endothelial cell gene expression changes are reversible with behavioural modifications.
Key points
Obesity results in perilymphatic inflammation and lymphatic dysfunction.
Lymphatic dysfunction in obesity is characterized by decreased lymphatic vessel density, decreased collecting lymphatic vessel pumping frequency, decreased lymphatic trafficking of immune cells, increased lymphatic vessel leakiness and changes in the gene expression patterns of lymphatic endothelial cells.
Aerobic exercise, independent of weight loss, decreases perilymphatic inflammatory cell accumulation, improves lymphatic function and reverses pathological changes in gene expression in lymphatic endothelial cells.
Abbreviations
- CCL19
chemokine (C‐C motif) ligand 19
- CCL21
chemokine (C‐C motif) ligand 21
- CCR7
chemokine (C‐C motif) receptor 7
- DAPI
4',6‐Diamidino‐2‐Phenylindole
- DC
dendritic cell
- DIO
diet‐induced obesity
- ELISA
enzyme‐linked immunosorbent assay
- eNOS
endothelial nitric oxide synthase
- FITC
fluorescein isothiocyanate
- HFD
high‐fat diet
- h.p.f.
high‐power field
- ICG
indocyanine green
- IFN‐γ
interferon‐γ
- IL‐1β
interleukin‐1β
- iNOS
inducible nitric oxide synthase
- LEC
lymphatic endothelial cell
- LYVE‐1
lymphatic vessel hyaluronan receptor 1
- NIR
near‐infrared
- pSMAD3
phosphorylated SMAD3
- qRT‐PCR
quantitative real‐time PCR
- TGF‐β1
transforming growth factor‐β1
- TNF‐α
tumour necrosis factor‐α
- VEGF‐C
vascular endothelial growth factor C
- VEGFR‐3
vascular endothelial growth factor receptor 3
Introduction
Recent studies have shown that obesity markedly decreases various measures of lymphatic function. For example, clinical studies have shown that obese individuals have a decreased ability to clear macromolecules from subcutaneous tissues, are at increased risk for developing lymphoedema after lymphatic injury and, in some cases, they may develop lymphoedema spontaneously, even without an inciting injury (Helyer et al. 2010; Greene et al. 2012; Arngrim et al. 2013). Likewise, experiments in a variety of mouse models of obesity, metabolic syndrome and hypercholesterolaemia have revealed that these pathological states result in leaky lymphatics, impaired pumping of subcutaneous and mesenteric collecting vessels, and abnormal lymph node architecture (Angeli et al. 2004; Lim et al. 2009; Zawieja et al. 2012; Weitman et al. 2013; Blum et al. 2014; Savetsky et al. 2015 a). The finding that mice with congenital abnormalities of the lymphatic system develop adult‐onset obesity, as well as histological studies in patients with lymphoedema demonstrating marked accumulation of subcutaneous adipose tissues in the affected extremity, suggest that the relationship between the lymphatic system and obesity is bidirectional (Harvey et al. 2005).
More recent evidence suggests that obesity‐induced lymphatic dysfunction can increase the pathological effects of obesity on other organ systems by regulating leucocyte infiltration and expression of inflammatory cytokines. For example, our group has shown that impaired lymphatic function in obese mice markedly potentiates inflammatory responses in dermatitis (Savetsky et al. 2015 a). Others have shown that lymphatic dysfunction resulting from congenital defects decreases cholesterol transport and increases atherosclerosis in Apolipoprotein E‐deficient mice (Angeli et al. 2004). Finally, a recent study demonstrated that increased expression of vascular endothelial growth factor C (VEGF‐C) in the skin regulates macrophage differentiation and contributes to insulin resistance in obese animals (Karaman et al. 2015).
Although it is known that obesity negatively regulates lymphatic function, it remains unclear whether these changes are reversible with behavioural modifications, such as aerobic exercise or weight loss. These studies are important because they may elucidate a more detailed understanding of the cellular mechanisms that regulate lymphatic dysfunction in obesity and may uncover novel methods to decrease these pathological responses. In the present study, we analysed the effects of aerobic exercise on obesity‐induced lymphatic dysfunction based on the rationale that this intervention has previously been shown to decrease chronic inflammation, improve glucose intolerance and decrease endothelial dysfunction in obesity (Holloszy et al. 1986; Bradley et al. 2008; Song et al. 2009; Baynard et al. 2012; Samaan et al. 2014). Here, we show that aerobic exercise training of high‐fat diet (HFD)‐induced obese mice results in improved lymphatic function, independent of weight loss, as reflected by a variety of end‐point measures. In addition, we found that these changes were correlated with decreased perilymphatic inflammatory cell accumulation and normalization of the isolated lymphatic endothelial cell (LEC) gene expression profile. Taken together, our findings suggest that aerobic exercise is an effective lifestyle intervention that can improve lymphatic function in obesity.
Methods
Ethical approval and animals
All experiments were approved by the IACUC at Memorial Sloan Kettering Cancer Center, and the institution operates under the Animal Welfare Act (AWA) and Health Research Extension Act of 1985. Male C57/BL6J mice (6 weeks old) were purchased from Taconic Biosciences (Hudson, NY, USA) and maintained in a light‐ and temperature‐controlled environment. When performing experiments that required anaesthesia, mice were anaesthetized using isofluorane. To monitor depth of anaesthesia, mice were checked every 15 min throughout the procedure by performing a tail pinch and observing that there was no change in respiratory rate. At the completion of the experiment, mice were killed by carbon dioxide asphyxiation with an increasing concentration of CO2, as recommended in the Guidelines on Euthanasia by the American Veterinary Medical Association. The mice were placed under the lid and exposed to 3 l min−1 of CO2 for 3 min. At the end of the 3 min, the CO2 was turned off, but the mice were allowed to sit under the lid for an additional 15 min to ensure that they were deceased. Mice were monitored for respiration and heart beat to ensure death. The investigators of this study understand the ethical principles under which this journal operates and verify that our work meets the standards of their animal ethics checklist.
Diet‐induced obesity
Male mice (6 weeks old; n = 12) were fed high‐fat diet (60% kcal from fat; W.F. Fisher and Son, Inc., Somerville, NJ, USA) ad libitum for 10–12 weeks until reaching an average weight >45 g and were maintained on a HFD for the remainder of the experiment (Nishimura et al. 2009; Weitman et al. 2013). Control (lean) mice (n = 5) were maintained on a normal chow diet for the entire experiment (13% kcal from fat; Purina Pico Lab Rodent Diet 20; W.F. Fisher and Son, Inc.). Mice were weighed weekly using a digital scale (Satorius, Bradford, MA, USA). In addition, inguinal and reproductive fat pads were harvested after death and weighed using a digital scale (Denver Instrument Company Model 100a, Bohemia, NY, USA).
Exercise protocol
After achieving HFD‐induced obesity, animals were randomly assigned to either aerobic exercise or sedentary groups (n = 6 per group). Exercised animals were trained for a total of 6 weeks with a Columbus Instruments 3/6 Treadmill (Columbus Instruments International, Columbus, OH, USA) using a modification of previously described protocols (Samaan et al. 2014). Briefly, 1 week before initiation of the exercise treatment, the experimental mice underwent treadmill acclimation by placing the animals on the treadmill and starting at a slow rate of 8 m min−1 for 8–10 min. After acclimation, experimental mice underwent exercise on the treadmill for 30 min starting at a speed of 10 m min−1, 5 days a week. The speed of the treadmill was increased each successive week by 1 m min−1, with a maximal training speed of 16 m min−1 achieved at the conclusion of the 6 week training period. Control sedentary mice were subjected to sham exercise consisting of similar handling and placement of the animals in the treadmill without activation of the platform.
Analysis of collecting lymphatic pumping frequency
All mice were subjected under general anaesthesia to baseline and postexercise lymphatic function testing using near‐infrared (NIR) lymphatic imaging with a modification of previously published techniques (Zhou et al. 2010). Briefly, 15 μl (0.15 mg ml−1) of indocyanine green (ICG; Sigma‐Aldrich, St Louis, MO, USA) was injected intradermally into the dorsal hindlimb web space, after which the mouse was allowed to ambulate for 30 min. The mouse was then placed on a heating pad, and lymphatic vessels were visualized using an EVOS EMCCD camera (Life Technologies, Carlsbad, CA, USA) with an LED light source (CoolLED, Andover, UK). A Zeiss V12 Sterolumanar microscope (Caliper Life Sciences, Hopington, MA, USA) was used to obtain video images. Using Fiji software (NIH, Bethesda, MD, USA), lymphatic vessel pulsations (i.e. pumping frequency) were calculated by finding the dominant collecting lymphatic vessel, drawing a region‐of‐interest over it, and then subtracting the background intensity of fluorescence. These pulsations were plotted over time.
Immunofluorescent staining of inflammatory cells
For analysis of immunofluorescence, cross‐sectional histological sections were harvested from the lower hindlimbs, fixed using cold 4% paraformaldehyde (PFA; Affymetrix, Cleveland, OH, USA), decalcified using 0.5 m EDTA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and embedded in paraffin. Immunofluorescent staining was performed based on our previously published methods (Avraham et al. 2010). Briefly, hindlimb tissues were rehydrated followed by antigen retrieval using boiling sodium citrate (Sigma‐Aldrich). Tissues sections were blocked using 10% blocking solution for 1 h at room temperature. Sections were incubated over night at 4°C with one or combinations of the following primary antibodies: anti‐lymphatic vessel hyaluronan receptor (LYVE‐1), anti‐CD45, anti‐CD4, anti‐CD11b (R&D Systems, Minneapolis, MN, USA), anti‐inducible nitric oxide synthase (anti‐iNOS; Abcam, Cambridge, MA, USA), anti‐CD3 (Dako, Carpinteria, CA, USA), anti‐B220 (BD Biosciences, San Jose, CA, USA) and anti‐perilipin (Fitzgerald, Acton, MA, USA). Sections were washed three times in PBS and incubated with the corresponding fluorescent conjugate secondary antibodies (all from Life Technologies, Grand Island, NY, USA) for 5 h at room temperature. Sections were then washed, and slides were mounted using Mowiol (Sigma‐Aldrich).
After staining, slides were scanned with a Mirax slide scanner (Zeiss, Munich, Germany). Analysis of tissue staining was performed using Pannoramic Viewer (3DHistech, Budapest, Hungary). LYVE‐1+ vessel counts and pSMAD3 expression were analysed using ×20 magnification by two blinded reviewers in a minimum of three high‐power fields per animal. To analyse perilymphatic inflammation and iNOS expression, the number of inflammatory cells and iNOS+ cells within a 50 μm radius of lymphatic vessels was counted by two blinded reviewers in a minimum of four quadrants per animal. Pannoramic Viewer was also used to calculate lymph node area. Lymph nodes were analysed using Metamorph software to calculate the percentage of chemokine (C‐C motif) ligand 21 (CCL21) positive cells. Lymph node size was calculated using Pannoramic software.
Inflammatory cytokine analysis in sedentary obese and exercised obese mice
Cytokine analysis was performed on hindlimb skin/subcutaneous tissue and serum harvested from mice in each group. Briefly, hindlimb skin protein was separated from the bone and muscle, flash frozen in liquid nitrogen, homogenized using a mortar and pestle, and extracted using a 100:1:1 mixture of tissue extraction protein reagent (ThermoFisher Scientific, Waltham, MA, USA), phosphatase inhibitor and protease inhibitor (Sigma‐Aldrich). Samples were centrifuged at 16,060 g for 10 min, and the supernatant was collected for protein analysis. For serum collection, under terminal general anaesthesia blood was collected by cardiac puncture into serum separator tubes (BD Microtainer, Franklin Lakes, NJ, USA) and spun at 16,060 g for 10 min. The supernatant was collected for serum analysis. Protein concentrations were measured via Bradford protein quantification. Cytokine protein analysis (n = 5 or 6 animals per group) was performed by enzyme‐linked immunosorbent assay (ELISA) according to the manufacturer's protocol (eBioscience, San Diego, CA, USA) for interferon‐γ (IFN‐γ), transforming growth factor‐β1 (TGF‐β), interleukin‐1β (IL‐1β) and tumour necrosis factor‐α (TNF‐α). All experiments were run in duplicate.
Analysis of lymphatic morphology and leakiness
Examination of initial and collecting vessel morphology and leakiness was performed under general anaesthesia using ear whole mounts after injection of Evans Blue or tomato lectin as previously described (Savetsky et al. 2015 a). Briefly, 2 μl of Evans Blue dye (1% w/v; Sigma‐Aldrich) was injected into the anterior apex of the ear, and lymphatic vessels were visualized 1 min later. In other animals, we injected 3 μl of tomato lectin (1 mg ml−1; Sigma‐Aldrich) into the apex of the ear on the anterior surface and then harvested the ears 10 min later. Ears were fixed in 4% paraformaldehyde. Non‐specific binding was blocked with 12% bovine serum albumin, followed by incubation with primary antibody and visualization with florescent secondary antibodies. The primary antibodies used were anti‐podoplanin, anti‐α‐smooth muscle actin and DAPI. Staining was visualized using a Leica SP5‐U confocal microscope (Leica Microsystems Inc., Buffalo Grove, IL, USA) with Zeiss Zen 2010 software (Carl Zeiss, Jena, Germany), and Z‐stacked confocal images were analysed using Imaris version 7.2.3 software (Bitplane, Zurich, Switzerland).
Dendritic cell trafficking to regional lymph nodes
Dendritic cell migration via the lymphatic vessels was assessed under general anaesthesia using a modification of previously reported methods (Wendland et al. 2011). Briefly, 8% fluorescein isothiocyanate (FITC, 5 mg ml−1; type I isomer; Sigma‐Aldrich) was diluted in a 1:1 mixture of acetone and dibutylphthalate (Sigma‐Aldrich). Twenty microlitres of the FITC solution was painted on both sides of the mouse's ear. After 18 h, the mice were killed and the draining cervical lymph nodes collected for analysis. Lymph nodes were digested to obtain single‐cell suspensions and migrating dendritic cells and were identified using flow cytometry (Fortesa flow cytometer; BD Biosciences, San Diego, CA, USA) gating for FITC+/CDllc+ cells.
Lymphatic endothelial cell isolation and gene expression analysis with qRT‐PCR
Lymphatic endothelial cells were isolated from lean, sedentary obese and exercised obese animals as previously reported (Vigl et al. 2011). Briefly, mice underwent depilation and 2 cm × 3 cm pieces of abdominal and back skin were harvested post‐mortem, finely diced into small pieces, and digested for 45 min using a 0.4% collagenase IV digestion buffer (collagenase IV; MP Biomedicals, Solon, OH, USA). The digested tissue was then filtered through a 100 μm nylon cell strainer, resuspended with digestion buffer, and then filtered through a 70 μm nylon cell strainer to produce a single‐cell suspension. Lymphatic endothelial cell isolation was performed using flow cytometric cell sorting (BD Biosciences, Aria 5, San Jose, CA, USA) with fluorophore‐conjugated monoclonal antibodies (EBioscience, San Diego, CA, USA). Lymphatic endothelial cells were gated as CD45 negative, CD31 positive, podoplanin positive and were collected into TRIzol (Invitrogen, Life Technologies, Carlsbad, CA, USA).
RNA extraction from the sorted LECs was performed according to the TRIzol manufacturer's recommendations. The isolated RNA was converted into cDNA using a TaqMan reverse transcriptase kit (Roche, Branchburg, NJ, USA). Quantitative real‐time PCR (qRT‐PCR; ViiA7; Life Technologies, Carlsbad, CA, USA) analysis was performed using LEC specific primers, vascular endothelial growth factor receptor 3 (VEGFR‐3), Prox1 and CCL21 (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). All samples were run in triplicate, and gene expression was normalized relative to GAPDH.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA). Values are presented as means ± SD (unless otherwise noted), and P < 0.05 was considered significant. We compared differences between two groups using Student's paired t test. One‐way ANOVA with post hoc Tukey–Kramer tests were used to compare multiple groups and analyse differences within each comparison.
Results
Aerobic treadmill exercise does not result in weight loss
Obese mice subjected to the exercise protocol tolerated training well, with no adverse events. In addition, consistent with previous studies (Samaan et al. 2014), we found that exercised obese animals continued to gain weight in a similar manner to the sedentary group, presumably as a result of increased food intake (n = 5 or 6; Fig. 1 A). Exercise had no effect on subcutaneous (inguinal fat pad) or visceral (epididymal fat pad) adipose tissue deposition either as a change in tissue weight or as a function of individual fat pad weight divided by the total body weight (n = 5 or 6, n.s.; Fig. 1 B–D). Similar findings were observed in histological sections of the ear (a tissue that was used in the remainder of the study to analyse structural changes in the lymphatic system), with nearly identical patterns of subcutaneous tissue adipose deposition in exercised and sedentary obese mice (n = 5 or 6; Fig. 1 E).
Figure 1. Treadmill training results in aerobic exercise independent of weight loss .
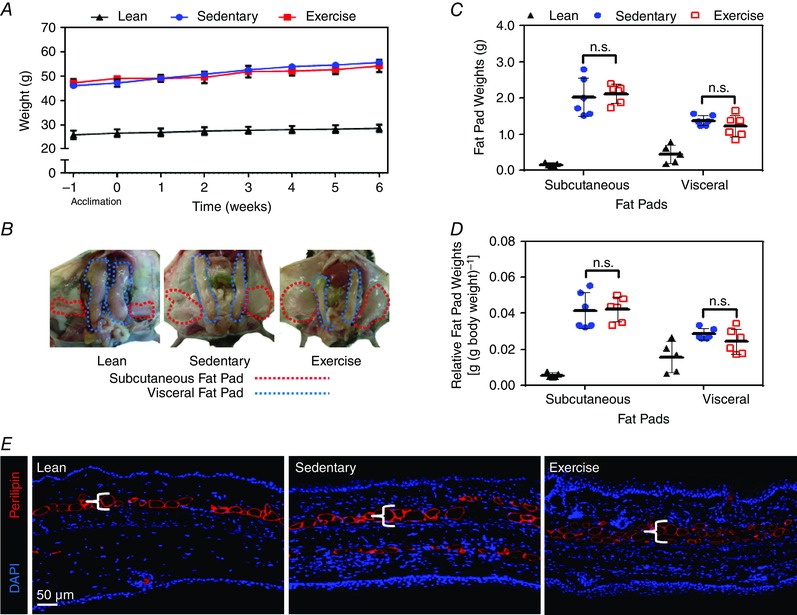
A, weight changes over time in lean mice (fed a normal chow diet throughout the course of the study), sedentary obese (sham exercise) and exercised obese animals (both fed a high‐fat diet throughout the course of the study; n = 5 or 6 animals per group). B, representative gross photograph of subcutaneous (inguinal) and visceral (epididimal) fat pads in lean, sedentary obese and exercised obese mice (n = 5 or 6 animals per group). C, quantification of subcutaneous and visceral fat pad weights in lean, sedentary obese and exercised obese mice (n = 5 or 6 animals per group; Student's paired t test: n.s.). D, quantification of subcutaneous and visceral fat pad weights in relationship to total body weight in lean, sedentary obese and exercised obese mice (n = 5 or 6 animals per group; Student's paired t test: n.s.). E, representative photomicrograph of ear tissues from lean, sedentary obese and exercised obese mice stained with perilipin (red) and DAPI (blue).
Aerobic exercise improves collecting lymphatic vessel pumping in obesity
Sedentary obese mice had a marked decrease (3.1‐fold) in collecting lymphatic vessel pumping frequency (n = 5 or 6, P < 0.001; Fig. 2 A and B) compared with lean control animals. In contrast, exercised obese mice had significantly improved lymphatic function (more than twofold increase in lymphatic pumping frequency) compared with sedentary obese mice (P < 0.05; Fig. 2 A and B).
Figure 2. Aerobic exercise improves collecting lymphatic vessel pumping in obesity .
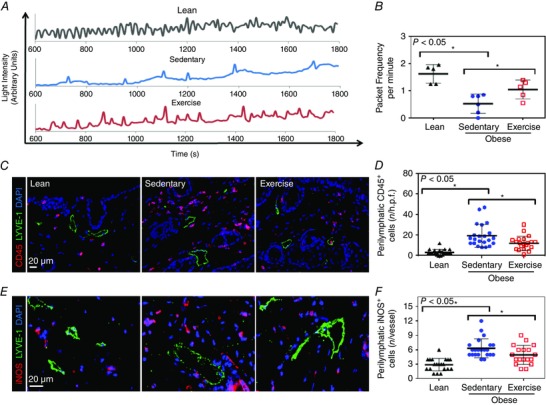
A, representative line graphs demonstrating changes in light intensity (in arbitrary units), which corresponds to hindlimb collecting lymphatic packet frequency (pumping) in lean, sedentary obese and exercised obese groups (n = 5 or 6 animals per group). B, quantification of hindlimb collecting lymphatic packet frequency (pumping) in experimental groups (n = 5 or 6 animals per group; Student's paired t test: lean vs. sedentary P = 0.0006, sedentary vs. exercise P = 0.0365). C, representative photomicrographs of hindlimb skin immunofluorescent staining localizing leucocytes (CD45+ cells; red), lymphatic vessels (LYVE‐1+; green) and nuclei (DAPI; blue). Note perilymphatic accumulation of CD45+ cells in sedentary obese mice and partial resolution in exercised obese mice. D, quantification of perilymphatic CD45+ cells (located within a 50 μm radius of lymphatic vessels) in various experimental groups [n = 5 or 6 animals, with 4 high‐power fields (h.p.f.) per animal; Student's paired t test: lean vs. sedentary P = < 0.0001, sedentary vs. exercise P = 0.0159]. E, representative photomicrographs of hindlimb skin immunofluorescent staining localizing iNOS+ cells (red), lymphatic vessels (LYVE‐1+; green) and nuclei (DAPI; blue). Note perilymphatic accumulation of iNOS+ cells in sedentary obese mice and partial resolution in exercised obese mice. F, quantification of perilymphatic iNOS+ cells (located within a 50 μm radius of lymphatic vessels) in various experimental groups (n = 5 or 6 animals with 4 h.p.f. per animal; Student's paired t test: lean vs. sedentary P = < 0.0001, sedentary vs. exercise P = 0.0346).
We next analysed changes in inflammatory cell accumulation within a 50 μm radius of lymphatic vessels by co‐localizing lymphatics (LYVE‐1+) with a pan‐leucocyte marker (CD45+). This analysis demonstrated a marked increase (5.8‐fold) in the number of perilymphatic inflammatory cells in sedentary obese mice compared with lean mice (n = 20–24, P < 0.0001; Fig. 2 C and D). Exercise significantly decreased (but did not completely normalize) this effect, reducing the number of perilymphatic inflammatory cells by 1.6‐fold, as compared with sedentary obese mice (P < 0.05; Fig. 2 C and D).
We next examined whether aerobic exercise improves collecting lymphatic pumping frequency by decreasing perilymphatic expression of iNOS, an important regulator of lymphatic pumping (Liao et al. 2011). Indeed, consistent with our findings demonstrating an accumulation of perilymphatic inflammatory cells, we also noted a marked increase in the number of perilymphatic iNOS+ cells (2.2‐fold) in sedentary obese mice compared with lean control animals (n = 20–24, P < 0.0001; Fig. 2 E and F). This effect was partly reversed in exercised obese mice (1.3‐fold decrease in the number of perilymphatic iNOS+ cells compared with sedentary obese control animals, P < 0.05; Fig. 2 E and F).
In order to characterize perilymphatic inflammatory changes in obesity better, we co‐localized T cells (CD3+) and macrophages (CD11b+) with lymphatic vessels (LYVE‐1+) in mouse hindlimb tissues. This analysis demonstrated that sedentary obese mice had markedly increased numbers of perilymphatic T cells (53‐fold) and, to a lesser degree, macrophages (2.3‐fold) compared with lean mice (n = 20–24, P < 0.0001 and P < 0.0001, respectively; Fig. 3 A and B). Interestingly, we also noted a modest though significant decrease in cutaneous lymphatic LYVE‐1+ vessel density in sedentary obese mice (1.3‐fold) compared with lean animals (n = 20–24, P < 0.05; Fig. 3 C). Aerobic exercise markedly decreased these responses, reducing the number of perilymphatic T cells (2.2‐fold decrease) and macrophages (1.6‐fold decrease) and increasing lymphatic vessel density (1.2‐fold increase) when compared with sedentary obese control animals (P < 0.0001, P < 0.05 and P < 0.05, respectively; Fig. 3 A–C).
Figure 3. Aerobic exercise decreases perilymphatic accumulation of T cells and macrophages in obesity .
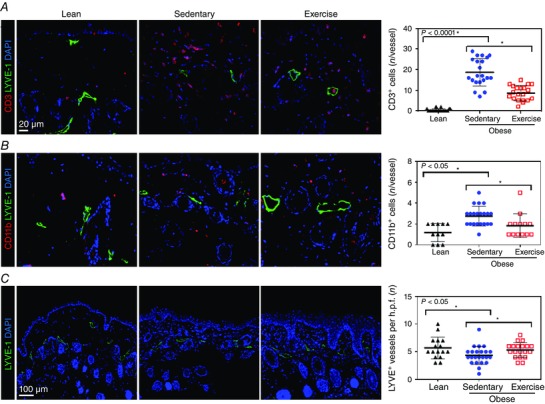
A, left three panels, representative photomicrographs of hindlimb skin immunofluorescent staining localizing T cells (CD3+; red), lymphatic vessels (LYVE‐1+; green) and nuclei (DAPI; blue). Note perilymphatic accumulation of CD3+ cells in sedentary obese mice and partial resolution in exercise odbese mice. A, right panel, quantification of perilymphatic CD3+ cells in various experimental groups (n = 5 or 6 animals with 4 h.p.f. per animal; Student's paired t test: lean vs. sedentary P = < 0.0001, sedentary vs. exercise P = < 0.0001). B, left three panels, representative photomicrographs of hindlimb skin immunofluorescent staining localizing macrophages (CD11b+; red), lymphatic vessels (LYVE‐1+; green) and nuclei (DAPI; blue). B, right panel, quantification of perilymphatic CD11b+ cells in various experimental groups (n = 5 or 6 animals with 4 h.p.f. per animal; Student's paired t test: lean vs. sedentary P = < 0.0001, sedentary vs. exercise P = 0.0163). C, left three panels, representative photomicrographs of hindlimb skin with localization of lymphatic vessels (LYVE‐1+; green) and nuclei (DAPI) in various experimental groups. C, right panel, quantification of lymphatic vessel density (LYVE‐1+ vessels per h.p.f. (×20 magnification) in hindlimb skin sections from various experimental groups (n = 5 or 6 animals with 4 h.p.f. per animal; Student's paired t test: lean vs. sedentary P = 0.0249, sedentary vs. exercise P = 0.0431).
Aerobic exercise decreases expression of anti‐lymphangiogenic molecules
We next sought to determine whether the obesity‐associated reduction in lymphatic vessel density was correlated with increased expression of anti‐lymphangiogenic molecules. Consistent with this hypothesis, we found significant increases in the expression of TNF‐α (6.3‐fold), INF‐γ (2.7‐fold), nd interleukin‐1β (threefold) in skin/subcutaneous tissues harvested from sedentary obese mice compared with lean control animals (n = 5 or 6, P < 0.05, P < 0.01 and P < 0.05, respectively; Fig. 4 A–C). Likewise, sedentary obese mice had markedly increased expression of TGF‐β1 and its downstream mediator, phosphorylated SMAD3 (pSMAD3), in tissues harvested from the hindlimb (n = 20–24, P < 0.0001; Fig. 4 D and E and not shown). Consistent with these findings, we noted a significant increase in serum TGF‐β1 expression (3.2‐fold) in sedentary obese mice compared with lean control animals (n = 5 or 6, P < 0.0001; Fig. 4 F). More importantly, we found that, compared with sedentary obese mice, exercised obese mice had markedly decreased skin and subcutaneous tissue expression of TNF‐α, IFN‐γ and pSMAD3, as well as decreased serum levels of TGF‐β, suggesting that improvements in lymphatic function and normalization in lymphatic vessel density in aerobic exercise mice are regulated, at least in part, by decreased expression of anti‐lymphangiogenic forces (n.s., n.s., P < 0.0001 and P < 0.005, respectively; Fig. 4 A–F).
Figure 4. Aerobic exercise decreases expression of anti‐lymphangiogenic molecules .
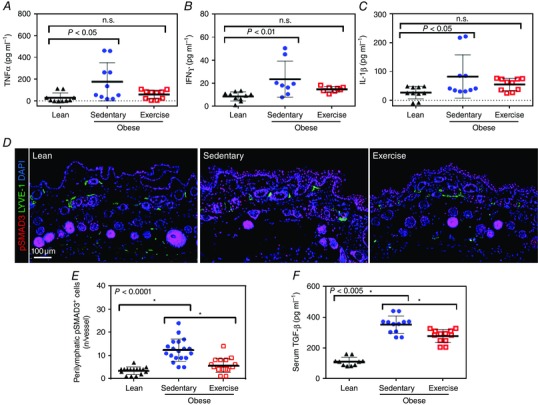
A, enzyme‐linked immunosorbent assay (ELISA) for tumour necrosis factor‐α (TNF‐α) expression in hindlimb tissues from lean, sedentary obese and exercised obese mice (n = 5 or 6 animals per group; one‐way ANOVA: lean vs. sedentary P = 0.0116, lean vs. exercise n.s.). B, ELISA for interferon‐γ (IFN‐γ) expression in hindlimb tissues of mice from various experimental groups (n = 5 or 6 animals per group; one‐way ANOVA: lean vs. sedentary P = 0.0078, lean vs. exercise n.s.). C, ELISA for interleukin‐1β (IL‐1β) expression in hindlimb tissues of mice from various experimental groups (n = 5 or 6 animals per group; one‐way ANOVA: lean vs. sedentary P = 0.0427 and lean vs. exercise n.s.). D, representative photomicrographs of immunofluorescent staining for phosphorylated SMAD3 (pSMAD+; red), lymphatic vessels (LYVE‐1+; green) and nuclei (DAPI; blue) in various experimental groups. E, quantification of perilymphatic pSMAD3+ cells in various experimental groups (n = 5 or 6 animals, with 4 h.p.f. per animal; Student's paired t test: lean vs. sedentary P < 0.0001, sedentary vs. exercise P < 0.0001). F, ELISA for serum transforming growth factor‐β1 (TGF‐β1) in various experimental groups (n = 5 or 6 animals per group; Student's paired t test: lean vs. sedentary P < 0.0001, sedentary vs. exercise P = 0.0015).
Exercise decreases lymphatic leakiness and improves migration of dendritic cells in obesity
We next analysed lymphatic vessel leakiness in the ear with Evans Blue lymphangiography and found that sedentary obese mice had markedly abnormal Evans Blue lymphatic uptake by ear lymphatics compared with lean control animals (n = 3; Fig. 5 A). These changes were characterized by impaired uptake of dye by the lymphatics, as well as punctate areas of dye leakage (marked by red arrowheads) after uptake distal to the site of injection (white dotted circle). Aerobic exercise in obese mice resulted in marked improvements in lymphatic uptake of Evans Blue, with expansion of the functional lymphatic vessel network and visibly reduced punctate areas of dye leakage compared with sedentary obese mice (Fig. 5 A).
Figure 5. Exercise decreases lymphatic leakiness and improves migration of dendritic cells (DCs) in obesity .
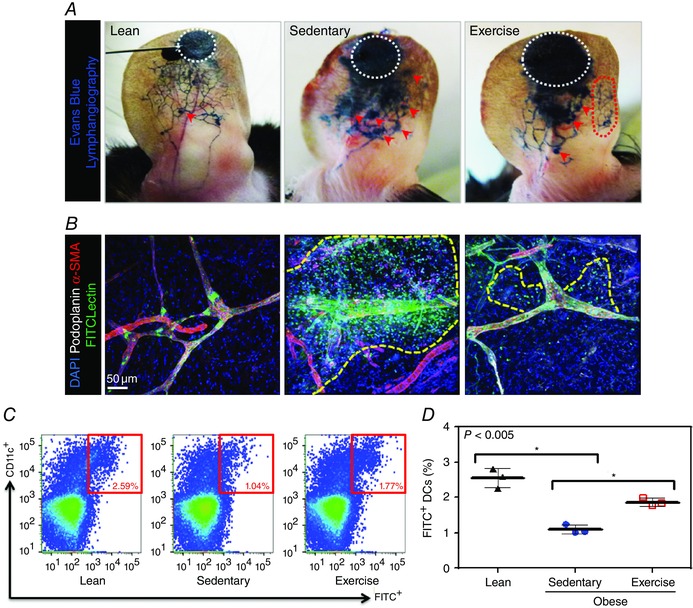
A, representative photographs of Evans Blue microlymphangiography in ears from lean, sedentary obese and exercised obese mice. White dotted circles correspond to the site of injection; red arrowheads show areas of lymphatic leakage; and red dotted circle represents possible expansion of lymphatic filling in exercise obese mice (n = 3 ears per group). B, representative photomicrograph of whole mount images obtained from mouse ears following fluorescein isothiocyanate (FITC) lectin injection and localizing lymphatic vessels (podoplanin+; green), α‐smooth muscle actin (α‐SMA+; red), FITC‐labelled injected lectin (green) and nuclei (DAPI). Note massive extravasation of dye in sedentary obese mice (yellow dotted line) and improvement in exercised obese mice (n = 3 ears per group). C, representative flow plots of FITC‐labelled dendritic cells (CD11c+) in cervical lymph nodes after FITC painting in various experimental groups. Red boxes show the percentage of CD11c/FITC double‐positive cells. D, quantification of migrated FITC+ dendritic cells present in cervical lymph nodes harvested from animals in various experimental groups (n = 3 animals per group; Student's paired t test: lean vs. sedentary P = 0.0011, sedentary vs. exercise P = 0.0016).
Our observations with Evans Blue lymphangiography were confirmed with lymphatic uptake of FITC‐conjugated tomato lectin in whole mounts of the ear (n = 3; Fig. 5 B). In this analysis, lean mice exhibited rapid uptake of dye within lymphatic vessels, with no visible leakage of dye into the interstitial space. In contrast, sedentary obese mice showed several leaky spots, with marked extravasation of dye in the interstitial space surrounding the lymphatics. Interestingly, this effect was significantly decreased in exercised obese mice (Fig. 5 B).
In order to measure migration of dendritic cells (DCs) from peripheral tissue to regional draining lymph nodes quantitatively, we performed FITC painting assays on the ear skin of mice and quantified the number of FITC+ DCs that migrated to the draining deep cervical lymph nodes using flow cytometry. We found that sedentary obese mice displayed markedly impaired migration of DCs (2.4‐fold decrease) from the ear to the cervical lymph nodes (n = 3, P < 0.005; Fig. 5 C and D). Furthermore, aerobic exercise significantly improved these deficits in obese mice and increased DC migration to the cervical lymph nodes (1.8‐fold) compared with sedentary obese mice (P < 0.005; Fig. 5 C and D).
Analysis of subcutaneous lymph nodes demonstrated that sedentary obese mice had significantly smaller popliteal lymph nodes compared with both lean and exercised obese mice. Additionally, sedentary obese mice, but not exercised obese mice, had loss of B cell follicular structure and distortion of T cell zones in the paracortex (n = 8, P < 0.05 and P < 0.0001, respectively; Fig. 6 A upper panel and B). Consistent with these results, we also noted that the expression of CCL21 within lymph node paracortex was markedly downregulated in sedentary obese mice compared with lean control animals (n = 4, P < 0.005; Fig. 6 A lower panel and C). In contrast, exercised obese mice had preserved expression of CCL21 within the lymph node paracortex, similar to the popliteal lymph nodes in lean mice (Fig. 6 A lower panel and C).
Figure 6. Obesity regulates lymphatic endothelial cell gene expression .
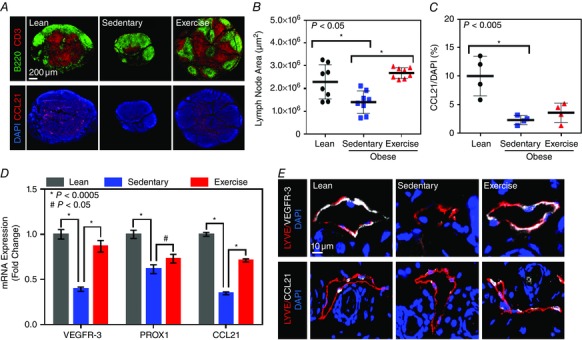
A, upper panels, representative photomicrographs of popliteal lymph nodes localizing B cells (B220+; green) and T cells (CD3+; red) in lean, obese sedentary and obese exercise mice. Note the smaller size of the lymph node of obese sedentary mice, with loss of follicular pattern and T cell zone. A, lower panels, representative photomicrographs of popliteal lymph nodes from various experimental groups stained for nuclei (DAPI; blue) and CCL21 (red). Note the decreased expression of CCL21 in obese sedentary mice and restoration to lean levels in exercise obese mice. B, quantification of popliteal lymph node area (in square micrometres) in various experimental groups (n = 8 lymph nodes per group; Student's paired t test: lean vs. sedentary P = 0.0140, sedentary vs. exercise P = < 0.0001). C, quantification of CCL21 expression (expressed as the percentage of CCL21+ cells as a function of DAPI+ cells in the central‐most section of the lymph node) in popliteal lymph nodes harvested from mice in various experimental groups (n = 4 lymph nodes per group; Student's paired t test: lean vs. sedentary P = 0.0049, sedentary vs. exercise n.s.). D, semi‐quantitative PCR in cell‐sorted, isolated lymphatic endothelial cells harvested from the back and hindlimb skin of mice from various experimental groups. Note the significant decrease in expression of VEGFR‐3, Prox1 and CCL21 in sedentary obese mice compared with lean control animals and the significant improvement in exercised obese mice (n = 5 or 6 animals per group; *P < 0.0005 and # P < 0.05). E, representative high‐powered photomicrographs co‐localizing nuclei (DAPI), VEGFR‐3 (white; upper panels) or CCL21 (white; lower panels) and LYVE‐1 (red) in hindlimb skin harvested from mice in various experimental groups (n = 5 or 6 animals per group).
Obesity regulates lymphatic endothelial cell gene expression
In order to determine how obesity and exercise modify LEC gene expression, we performed qRT‐PCR on RNA extracted from cell‐sorted LECs. This analysis revealed a markedly decreased expression of VEGFR‐3 (2.5‐fold), Prox1 (1.6‐fold) and CCL21 (threefold) in sedentary obese mouse LECs compared with those from lean control animals (n = 5 or 6, P < 0.005 for all groups; Fig. 6 D). More interestingly, we found that these changes were reversed in exercised obese animals, resulting in a significantly increased expression of VEGFR‐3 (2.2‐fold), Prox1 (1.2‐fold) and CCL21 (2.1‐fold), compared with LECs harvested from sedentary obese mice (P < 0.005, P < 0.05 and P < 0.005, respectively; Fig. 6 D). These gene expression changes were also reflected at the protein level in histological sections of hindlimb skin tissues, in which we noted markedly decreased VEGFR‐3 and CCL21 expression in lymphatic vessels of sedentary obese but not exercised obese mice (n = 5 or 6; Fig. 6 E).
Discussion
In this study, we have shown that obesity markedly impairs lymphatic function by decreasing the pumping frequency of collecting lymphatics, decreasing lymphatic vessel density, increasing lymphatic leakiness and altering LEC gene expression of lymphatic markers. More importantly, we have shown that this phenotype is partly reversible by aerobic exercise, independent of weight loss, suggesting that behavioural modifications may represent a viable means of treating obesity‐induced lymphatic dysfunction clinically. Identifying treatments that decrease obesity‐induced lymphatic dysfunction is important because recent studies have shown that abnormalities in the lymphatic system can amplify the pathology of obesity in a variety of settings. For example, our group has recently shown that obesity‐induced lymphatic dysfunction significantly contributes to contact hypersensitivity and dermatitis in obesity (Savetsky et al. 2015 a). Others have shown that lymphatic defects increase atherosclerosis and plaque deposition in hypercholesterolaemic mice and that the lymphatic system plays a key role in the regulation of reverse cholesterol transport (Randolph & Miller, 2014; Vuorio et al. 2014). Finally, changes in expression of VEGF‐C have been shown to modulate macrophage differentiation and, in turn, regulate glucose sensitivity (Karaman et al. 2015).
Consistent with previously reported studies (Samaan et al. 2014), we found that a modified aerobic exercise protocol resulted in aerobic conditioning without concomitant weight loss. This experimental approach enabled us to study the effects of exercise, independent of dietary changes or alterations in subcutaneous or visceral adipose tissue deposition. Our exercised obese mice demonstrated clear improvements in lymphatic function despite continued weight gain, as well as no appreciable architectural changes in adipose tissue deposition throughout the course of the study. This suggests that obesity‐induced lymphatic dysfunction results from autocrine or paracrine responses to obesity, rather than direct injury to the lymphatic system from dietary toxins or compression of lymphatic vessels by increasing adipose deposition. This hypothesis is supported by previous studies demonstrating that exercised animals have decreased inflammatory responses without concomitant weight loss and despite increased caloric intake (Samaan et al. 2014). Our hypothesis is additionally supported by other groups who demonstrated that chronic inflammation and adipokines impair lymphatic function even in lean animals (Liao et al. 2011; Cromer et al. 2014).
Using NIR lymphangiography, we found that obesity significantly decreases lymphatic collecting vessel pumping frequency and that this response is partly reversed by exercise. Our study also suggests that this response is related to perilymphatic accumulation of inflammatory cells and increased expression of iNOS, because exercise markedly decreased these responses in obese mice. Our findings are consistent with previous studies demonstrating that obesity results in chronic, low‐grade inflammation in a variety of tissues, including subcutaneous and mesenteric fat, as well as perivascular accumulation of leucocytes in medium and large blood vessels (Nieman et al. 1999; Womack et al. 2007). Accumulation of inflammatory cells around blood vessels causes vascular endothelial cell dysfunction and coronary artery disease by release of reactive oxygen species, changes in expression patterns of adipokines and alterations in nitric oxide signalling (Greenstein et al. 2009; Ketonen et al. 2010; Cao, 2014). Thus, perilymphatic inflammation may likewise impair lymphatic function by activating a variety of pathological mechanisms, including direct injury to LECs and changes in NO levels. This hypothesis is supported by our PCR findings demonstrating significant changes in LEC gene expression of lymphatic markers, including VEGFR‐3, Prox1 and CCL21. In addition, our theory is corroborated by previous studies demonstrating that expression of iNOS by inflammatory cells impairs collecting vessel pumping capacity by producing large amounts of NO, which disrupt endogenous gradients (Aktan 2004; Liao et al. 2011). Likewise, other reports using NIR lymphangiography have shown that inflammatory cytokines, including IL‐1β, decrease lymphatic pumping by activating iNOS (Aldrich & Sevick‐Muraca, 2013). Our findings are also in agreement with previous studies demonstrating increased expression of iNOS in skeletal muscle and adipose tissues of HFD‐fed mice and genetically obese (ob/ob) mice (Perreault & Marette, 2001; Sugita et al. 2005). Taken together, our data suggest that perilymphatic accumulation of inflammatory cells and expression of iNOS play a key role in the regulation of obesity‐related lymphatic dysfunction and that interventions designed to alter this response may improve lymphatic pumping capacity.
A key finding of our study was that aerobic exercise markedly decreases perilymphatic accumulation inflammatory cells (T cells and macrophages) and decreases local and systemic expression of inflammatory cytokines. These findings are in line with previous clinical and experimental studies that used a variety of models of obesity to demonstrate that exercise training significantly decreases white adipose macrophage infiltration, inflammation in skeletal muscles and release of inflammatory cytokines (Bruun et al. 2006; Baynard et al. 2012; Samaan et al. 2014). Other studies have shown that aerobic exercise decreases expression of TNF‐α and inhibitor of nuclear factor κβ in pregonadal and mesenteric fat pads, as well as iNOS in skeletal muscle (Bradley et al. 2008; Song et al. 2009). Taken together, our findings suggest that anti‐inflammatory responses in exercise play a key role in diminishing obesity‐induced lymphatic dysfunction. This hypothesis is supported by our previous studies demonstrating that obese mice with genetic absence of T and B cells (Rag mice) have improved lymphatic transport of macromolecules, normal lymphatic vessel density and increased dendritic cell migration to regional lymph nodes, in comparison to obese wild‐type mice (Weitman et al. 2013).
We found that obesity significantly decreases the number of dermal lymphatics and that this response is ameliorated by aerobic exercise. Consistent with this observation, we noted that LECs isolated from sedentary obese mice have significantly decreased expression of VEGFR‐3 and Prox1. These findings are important because VEGFR‐3 stimulation and Prox1 expression are necessary for maintenance of lymphatic phenotype, cellular proliferation and protection from apoptosis (Dumont et al. 1998; Wigle et al. 2002; Coso et al. 2014). Our study also suggests that changes in lymphatic vessel density are not simply related to alterations in adipose tissue architecture, because our exercise protocol had no effect on adipose tissue deposition but restored lymphatic vascular density to lean levels. Finally, recent studies have shown that lymphangiogenesis is regulated by a co‐ordinated expression of pro‐ and anti‐lymphangiogenic cytokines (Kataru et al. 2011; Zampell et al. 2012 a). Therefore, our findings, when taken in the context of recent reports demonstrating that the expression of VEGF‐C is markedly increased in obese animals and patients (Karaman et al. 2015; Savetsky et al. 2015 a), suggest that deficits in lymphatic vascular density are not related to decreased levels of lymphangiogenic cytokines. Instead, changes in lymphatic vascular density in sedentary obese mice may be related to increased expression of T cell‐derived anti‐lymphangiogenic cytokines, including TNF‐α, IFN‐γ and TGF‐β1 (Shao & Liu, 2006; Clavin et al. 2008; Oka et al. 2008; Kataru et al. 2011). This hypothesis is supported by recent studies demonstrating that anti‐lymphangiogenic cytokine expression is increased in chronic inflammatory responses and that these forces can modulate the effects of lymphangiogenic growth factors, including VEGF‐C, VEGF‐A and hepatocyte growth factor (Zampell et al. 2012 b; Shin et al. 2015; Savetsky et al. 2015 b). These effects may be compounded in sedentary obese mice by decreased LEC expression of VEGFR‐3, thus making lymphatic vessels of obese mice less responsive to lymphangiogenic signals. Clearly, these questions require additional study but will be an important step in understanding obesity‐induced lymphatic pathology.
Using Evans Blue microlymphangiography, as well as whole mount staining after injection of tomato lectin, we found that sedentary obese mice had markedly increased lymphatic leakiness and that exercise partly restored this phenotype. This observation is reinforced by previous studies demonstrating that obesity and hypercholesterolaemia increase lymphatic vessel leakiness (Lim et al. 2009; Kuan et al. 2015; Savetsky et al. 2015 a), as well as studies reporting that dietary fatty acids increase LEC permeability (Sawane et al. 2013). Although the specific cellular mechanisms that regulate lymphatic leakiness in obesity remain unclear, our findings suggest that perilymphatic accumulation of inflammatory cells, increased production of inflammatory cytokines (e.g. IFN‐γ and TNF‐α) and increased expression of iNOS contribute to this phenotype. This hypothesis is supported by previous studies demonstrating that inflammatory cytokines, including IL‐1β, TNF‐α, interleukin‐6 and INF‐γ, as well as increased production of NO, enhance permeability of intact murine lymphatic collectors and monolayers of LECs and decrease VE‐cadherin expression in vitro (Cromer et al. 2014; Scallan et al. 2015). In addition, other studies have shown that NO can be converted to peroxynitrite, a reactive oxygen species, leading to lipoperoxidation and a breakdown of cell membranes (Thangaswamy et al. 2012). This pathological effect may be particularly important to LECs, because these cells have been shown to be highly susceptible to oxidative stress (Kasuya et al. 2014). Based on the aforementioned data, it is likely that obesity‐induced lymphatic defects result from multiple insults and that inflammation plays a central role in this process.
In addition to fluid uptake and transport, another major function of the lymphatic system is to transport immune cells from peripheral tissues to regional lymph nodes. This is an active process that necessitates expression of gradients of chemokines on lymphatic endothelial cells [chemokine (C‐C motif) ligand 19 and 21; CCL21 and CCL19] and cognate receptors [chemokine (C‐C motif) receptor 7; CCR7] on DCs (Gunn et al. 1998; Förster et al. 1999; MartIn‐Fontecha et al. 2003). Mirroring our previous reports, we found that sedentary but not exercised obese mice had markedly impaired migration of DCs to regional lymph nodes, decreased expression of CCL21 in LECs and lymph nodes, and marked abnormalities in lymph node architecture (Weitman et al. 2013). Our findings are supported by other studies, which showed that HFD‐fed mice have decreased T cell populations and smaller mesenteric lymph nodes in comparison to lean mice maintained on a low‐fat diet (Kim et al. 2008), as well as other studies demonstrating that obese mice have impaired T cell recall responses (Savetsky et al. 2015 a). In addition, it is known that expression of CCL21 in the lymph node paracortex is regulated by lymphatic flow and that these expression patterns, in turn, regulate T cell distribution within the lymph node (Tomei et al. 2009; Thomas et al. 2012; Denton et al. 2014). These findings are also supported by experiments on K14‐VEGFR3‐Ig mice demonstrating that decreased lymphatic transport attributable to hypoplastic dermal lymphatics markedly decreases lymph node size, causes loss of B cell follicular patterns and alters expression patterns of CCL21 (Thomas et al. 2012). Indeed, it is likely that changes in CCL21 expression also regulate the abnormalities in DC trafficking noted in our study, given the central role of this chemokine in regulating immune cell trafficking. These studies have shown that high‐level expression of CCL21 by LECs guides DCs to regional lymphatics, followed by migration to regional lymph nodes (Ohl et al. 2004; Johnson & Jackson, 2010). Thus, it is possible that changes in gradients of CCL21 expression in obese mice cause ‘trapping’ of DCs in tissues. This hypothesis is supported by our previous data demonstrating that the expression of CCL21 in tissues isolated from sedentary obese mice is increased and the observation from the present study that the expression of CCL21 by isolated LECs is decreased.
In conclusion, in the present study we showed that aerobic exercise, independent of weight loss, can reverse obesity‐related lymphatic dysfunction. Following exercise, we observed markedly increased lymphatic vessel packet frequency, decreased vessel permeability, increased migration of dendritic cells and increased expression of lymphatic endothelial cell markers. These results were correlated with a decrease in perilymphatic inflammation, decreased local and systemic levels of inflammatory cytokines and decreased perilymphatic iNOS. We also observed improved lymph node architecture, enhanced CCL21 expression and an increase in lymph node size back to baseline. This study suggests that implementation of aerobic exercise in the setting of obesity can improve lymphatic function and may limit some of the pathological consequences of obesity.
Additional information
Competing interests
None declared.
Author contributions
G.E.H, R.P.K., L.W.J. and B.J.M. conceived and designed the work; G.E.H, R.P.K., I.L.S., G.D.G.N., J.S.T., M.D.N., J.C.G., J.Z. and J.Z.Y conducted acquisition and analysis of data; G.E.H, R.P.K., I.L.S., G.D.G.N., J.S.T., M.D.N., J.C.G., J.Z., J.Z.Y., L.W.J. and B.J.M. performed interpretation of data for the work; G.E.H, R.P.K., I.L.S., G.D.G.N., J.S.T., M.D.N., J.C.G., J.Z., J.Z.Y., L.W.J. and B.J.M. drafted the work or revised it critically for important intellectual content.
Funding
This study was supported by the National Institutes of Health (R01 HL111130‐01 awarded to B.J.M.) and National Institutes of Health/NCI Cancer Center Support Grant P30 CA008748.
Translational perspective
In this study, we show that aerobic exercise markedly improves obesity‐induced lymphatic dysfunction, resulting in increased clearance of interstitial fluid and improved migration of antigen‐presenting cells to regional lymph nodes. We show that aerobic exercise, independent of weight loss, increases lymphatic vessel density, enhances collecting lymphatic pumping capacity and decreases lymphatic leakiness. These improvements are associated with markedly decreased accumulation of inflammatory cells and expression of pro‐inflammatory cytokines, both locally and systemically. The translational significance of these findings is that obesity‐induced lymphatic injury is reversible with behavioural modifications. In addition, our findings suggest that interventions that improve lymphatic function can decrease accumulation of inflammatory cells. This is important because previous studies have shown a key role for chronic tissue inflammation in the regulation of pathological effects of obesity in a number of settings, including metabolic syndrome and cancer metastasis.
Supporting information
Supplemental Video S1. Lymphatic collecting vessel contractions in lean control mice
Representative time‐lapse video of NIR imaging using ICG in the mouse hindlimb. Videos are condensed into 8 frames s−1 following 30 min of recorded video. Lymphatic contractions were quantified during the final 20 min of the video.
Supplemental Video S2. Lymphatic collecting vessel contractions in sedentary obese mice
Representative time‐lapse video of NIR imaging using ICG in the mouse hindlimb. Videos are condensed into 8 frames s−1 following 30 min of recorded video. Lymphatic contractions were quantified during the final 20 min of the video. Note the decreased frequency in contractions, in comparison to the lean control mouse.
Supplemental Video S3. Lymphatic collecting vessel contractions in exercised obese mice
Representative time‐lapse video of NIR imaging using ICG in the mouse hindlimb. Videos are condensed into 8 frames s−1 following 30 min of recorded video. Lymphatic contractions were quantified during the final 20 min of the video. Note the increased frequency in contractions, in comparison to the sedentary obese mouse.
Acknowledgements
The authors are grateful to Navid Paknejad, Ning Fan, Mesruh Turkekul, Sho Fujisawa and Yevgeniy Romin of the Molecular Cytology Core at Memorial Sloan Kettering Cancer Center for assistance with both histology and tissue imaging [Core Grant (P30 CA008748)].
This is an Editor's Choice article from the 1 August 2016 issue.
References
- Aktan F (2004). iNOS‐mediated nitric oxide production and its regulation. Life Sci 75, 639–653. [DOI] [PubMed] [Google Scholar]
- Aldrich MB & Sevick‐Muraca EM (2013). Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine 64, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA & Randolph GJ (2004). Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574. [DOI] [PubMed] [Google Scholar]
- Arngrim N, Simonsen L, Holst JJ & Bulow J (2013). Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond) 37, 748–750. [DOI] [PubMed] [Google Scholar]
- Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG & Mehrara BJ (2010). Blockade of transforming growth factor‐β1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177, 3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynard T, Vieira‐Potter VJ, Valentine RJ & Woods JA (2012). Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators Inflamm 2012, 767953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C & Detmar M (2014). Chronic high‐fat diet impairs collecting lymphatic vessel function in mice. PLoS ONE 9, e94713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RL, Jeon JY, Liu FF & Maratos‐Flier E (2008). Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet‐induced obese mice. Am J Physiol Endocrinol Metab 295, E586–E594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun JM, Helge JW, Richelsen B & Stallknecht B (2006). Diet and exercise reduce low‐grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290, E961–E967. [DOI] [PubMed] [Google Scholar]
- Cao H (2014). Adipocytokines in obesity and metabolic disease. J Endocrinol 220, T47–T59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A & Mehrara BJ (2008). TGF‐β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295, H2113–H2127. [DOI] [PubMed] [Google Scholar]
- Coso S, Bovay E & Petrova TV (2014). Pressing the right buttons: signaling in lymphangiogenesis. Blood 123, 2614–2624. [DOI] [PubMed] [Google Scholar]
- Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK & Zawieja DC (2014). The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis 17, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton AE, Roberts EW, Linterman MA & Fearon DT (2014). Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proc Natl Acad Sci USA 111, 12139–12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M & Alitalo K (1998). Cardiovascular failure in mouse embryos deficient in VEGF receptor‐3. Science 282, 946–949. [DOI] [PubMed] [Google Scholar]
- Förster R, Schubel A, Breitfeld D, Kremmer E, Renner‐Müller I, Wolf E & Lipp M (1999). CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33. [DOI] [PubMed] [Google Scholar]
- Greene AK, Grant FD & Slavin SA (2012). Lower‐extremity lymphedema and elevated body‐mass index. N Engl J Med 366, 2136–2137. [DOI] [PubMed] [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA & Heagerty AM (2009). Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119, 1661–1670. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD & Williams LT (1998). A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 95, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW & Oliver G (2005). Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult‐onset obesity. Nat Genet 37, 1072–1081. [DOI] [PubMed] [Google Scholar]
- Helyer LK, Varnic M, Le LW, Leong W & McCready D (2010). Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 16, 48–54. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Schultz J, Kusnierkiewicz J, Hagberg JM & Ehsani AA (1986). Effects of exercise on glucose tolerance and insulin resistance. Brief review and some preliminary results. Acta Med Scand Suppl 711, 55–65. [DOI] [PubMed] [Google Scholar]
- Johnson LA & Jackson DG (2010). Inflammation‐induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin‐mediated dendritic cell transmigration. Int Immunol 22, 839–849. [DOI] [PubMed] [Google Scholar]
- Karaman S, Hollmén M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C & Detmar M (2015). Blockade of VEGF‐C and VEGF‐D modulates adipose tissue inflammation and improves metabolic parameters under high‐fat diet. Mol Metab 4, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya A, Sakabe J & Tokura Y (2014). Potential application of in vivo imaging of impaired lymphatic duct to evaluate the severity of pressure ulcer in mouse model. Sci Rep 4, 4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH & Koh GY (2011). T Lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34, 96–107. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Shi J, Martonen E & Mervaala E (2010). Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet‐induced obese C57Bl/6 mice. Circ J 74, 1479–1487. [DOI] [PubMed] [Google Scholar]
- Kim CS, Lee SC, Kim YM, Kim BS, Choi HS, Kawada T, Kwon BS & Yu R (2008). Visceral fat accumulation induced by a high‐fat diet causes the atrophy of mesenteric lymph nodes in obese mice. Obesity (Silver Spring) 16, 1261–1269. [DOI] [PubMed] [Google Scholar]
- Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC & Randolph GJ (2015). Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node‐homing adipose tissue dendritic cells. J Immunol 194, 5200–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D & Padera TP (2011). Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108, 18784–18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ & Angeli V (2009). Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol 175, 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A & Sallusto F (2003). Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med 198, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Nehlsen‐Cannarella SL, Ekkens M, Utter AC, Butterworth DE & Fagoaga OR (1999). Influence of obesity on immune function. J Am Diet Assoc 99, 294–299. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T & Nagai R (2009). CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15, 914–920. [DOI] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G & Forster R (2004). CCR7 governs skin dendritic cell migration under inflammatory and steady‐state conditions. Immunity 21, 279–288. [DOI] [PubMed] [Google Scholar]
- Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR & Miyazono K (2008). Inhibition of endogenous TGF‐β signaling enhances lymphangiogenesis. Blood 111, 4571–4579. [DOI] [PubMed] [Google Scholar]
- Perreault M & Marette A (2001). Targeted disruption of inducible nitric oxide synthase protects against obesity‐linked insulin resistance in muscle. Nat Med 7, 1138–1143. [DOI] [PubMed] [Google Scholar]
- Randolph GJ & Miller NE (2014). Lymphatic transport of high‐density lipoproteins and chylomicrons. J Clin Invest 124, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaan MC, Marcinko K, Sikkema S, Fullerton MD, Ziafazeli T, Khan MI & Steinberg GR (2014). Endurance interval training in obese mice reduces muscle inflammation and macrophage content independently of weight loss. Physiol Rep 2, e12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savetsky IL, Albano NJ, Cuzzone DA, Gardenier JC, Torrisi JS, García Nores GD, Nitti MD, Hespe GE, Nelson TS, Kataru RP, Dixon JB & Mehrara BJ (2015. a). Lymphatic function regulates contact hypersensitivity dermatitis in obesity. J Invest Dermatol 135, 2742–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, García Nores GD, Hespe GE, Nitti MD, Kataru RP & Mehrara BJ (2015. b). Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 10, e0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawane M, Kajiya K, Kidoya H, Takagi M, Muramatsu F & Takakura N (2013). Apelin inhibits diet‐induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes 62, 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Hill MA & Davis MJ (2015). Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res 107, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X & Liu C (2006). Influence of IFN‐α and IFN‐γ on lymphangiogenesis. J Interferon Cytokine Res 26, 568–574. [DOI] [PubMed] [Google Scholar]
- Shin K, Kataru RP, Park HJ, Kwon BI, Kim TW, Hong YK & Lee SH (2015). TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Commun 6, 6196. [DOI] [PubMed] [Google Scholar]
- Song W, Kwak HB, Kim JH & Lawler JM (2009). Exercise training modulates the nitric oxide synthase profile in skeletal muscle from old rats. J Gerontol A Biol Sci Med Sci 64, 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, Martyn JA & Kaneki M (2005). Inducible nitric‐oxide synthase and NO donor induce insulin receptor substrate‐1 degradation in skeletal muscle cells. J Biol Chem 280, 14203–14211. [DOI] [PubMed] [Google Scholar]
- Thangaswamy S, Bridenbaugh EA & Gashev AA (2012). Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol 10, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ & Swartz MA (2012). Impaired humoral immunity and tolerance in K14‐VEGFR‐3‐Ig mice that lack dermal lymphatic drainage. J Immunol 189, 2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomei AA, Siegert S, Britschgi MR, Luther SA & Swartz MA (2009). Fluid flow regulates stromal cell organization and CCL21 expression in a tissue‐engineered lymph node microenvironment. J Immunol 183, 4273–4283. [DOI] [PubMed] [Google Scholar]
- Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O & Halin C (2011). Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus‐dependent manner. Blood 118, 205–215. [DOI] [PubMed] [Google Scholar]
- Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K & Ylä‐Herttuala S (2014). Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol 34, 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman ES, Aschen SZ, Farias‐Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D & Mehrara BJ (2013). Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS ONE 8, e70703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M, Willenzon S, Kocks J, Davalos‐Misslitz AC, Hammerschmidt SI, Schumann K, Kremmer E, Sixt M, Hoffmeyer A, Pabst O & Forster R (2011). Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity 35, 945–957. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG & Oliver G (2002). An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21, 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack J, Tien PC, Feldman J, Shin JH, Fennie K, Anastos K, Cohen MH, Bacon MC & Minkoff H (2007). Obesity and immune cell counts in women. Metabolism 56, 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampell JC, Avraham T, Yoder N, Fort N, Yan A, Weitman ES & Mehrara BJ (2012. a). Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti‐lymphangiogenic cytokines. Am J Physiol Cell Physiol 302, C392–C404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E & Mehrara BJ (2012. b). CD4+ cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 7, e49940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC & Muthuchamy M (2012). Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 302, H643–H653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wood R, Schwarz EM, Wang YJ & Xing L (2010). Near‐infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis Rheum 62, 1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video S1. Lymphatic collecting vessel contractions in lean control mice
Representative time‐lapse video of NIR imaging using ICG in the mouse hindlimb. Videos are condensed into 8 frames s−1 following 30 min of recorded video. Lymphatic contractions were quantified during the final 20 min of the video.
Supplemental Video S2. Lymphatic collecting vessel contractions in sedentary obese mice
Representative time‐lapse video of NIR imaging using ICG in the mouse hindlimb. Videos are condensed into 8 frames s−1 following 30 min of recorded video. Lymphatic contractions were quantified during the final 20 min of the video. Note the decreased frequency in contractions, in comparison to the lean control mouse.
Supplemental Video S3. Lymphatic collecting vessel contractions in exercised obese mice
Representative time‐lapse video of NIR imaging using ICG in the mouse hindlimb. Videos are condensed into 8 frames s−1 following 30 min of recorded video. Lymphatic contractions were quantified during the final 20 min of the video. Note the increased frequency in contractions, in comparison to the sedentary obese mouse.


