Abstract
Mutations in polycystin‐2 (PC2) lead to autosomal dominant polycystic kidney disease (ADPKD). The molecular mechanism linking mutations in PC2 and the pathogenesis of ADPKD is not well understood. Therefore, understanding the functional regulation of PC2 and its interaction with other proteins under both physiological and pathogenic conditions is important for elucidating the disease mechanism and identifying potential molecular targets for treatment. Normally, PC2 functions as a calcium‐permeable channel whose activity is regulated by calcium binding to the C‐terminal domain of PC2 (PC2 Cterm). The PC2 Cterm is also involved in the PC2 channel assembly and hetero‐oligomerization with other binding partners in cells. Different functional domains of the PC2 Cterm have been studied using structural approaches. Within the PC2 Cterm, there is a calcium‐binding EF‐hand domain, crucial for the calcium‐dependent activity of the PC2 channel. Downstream of the EF‐hand domain lies a coiled‐coil region, which is involved in the assembly and hetero‐interaction of the PC2 protein. The PC2 Cterm can form an oligomer, mediated by the coiled‐coil region. Although PC2 Cterm has been extensively studied for its relationship with ADPKD and its importance in PC2 regulation, there are misunderstandings with respect to the definition of the domain topology within the PC2 Cterm and the functional role of each domain. Here, we review previous studies that connect the molecular properties of the domains of PC2 Cterm to distinct aspects of PC2 functions and regulation.

Abbreviations
- ADPKD
autosomal dominant polycystic kidney disease
- HLH
helix–loop–helix
- hPC2 Cterm
human C‐terminal tail of PC2
- hPC2 C‐EF
human PC2 C‐terminal EF‐hand
- ITC
isothermal titration calorimetry
- PC1
polycystin‐1
- PC2
polycystin‐2
- PC2 Cterm
C‐terminal tail of PC2
- RyR
ryanodine receptor
- suPC2 C‐EF
sea urchin PC2 C‐terminal EF‐hand
- TRP channels
transient receptor potential channels
Introduction
Polycystin‐2 (PC2) is a member of the transient receptor potential (TRP) family and members of this class of protein form cation‐permeable channels (Harris & Torres, 2009; Ong & Harris, 2015). As a member of a larger family of ion channels, the ability to compare and contrast the workings of different subtypes of the TRP channels will inform us how these channels work. Moreover, there is growing specific interest in understanding the relationship between structure and function of PC2, because mutations in PC2 are associated with autosomal dominant polycystic kidney disease (ADPKD) (Fig. 1). This disease is a leading cause of kidney failure. It manifests as the formation of fluid‐filled cysts in the kidney that increase in size over time, resulting in the gradual loss of kidney function and leading to the need for kidney transplantation (Fig. 1). Although the genetic cause of ADPKD is known, there is currently no cure and the molecular mechanism linking genetic mutations and cyst formation is not well understood. One of the main hypotheses proposed to explain the initiation of cyst formation is aberrant calcium signalling due to mutation‐dependent changes in PC2 function (Kuo et al. 2014 a). Many of the pathogenic mutations impair the ability of calcium to regulate PC2 (Koulen et al. 2002; Celic et al. 2012), leading to the focus on understanding the interaction between PC2 and calcium.
Figure 1. Mutations in PC1 and PC2 can lead to autosomal dominant polycystic disease .
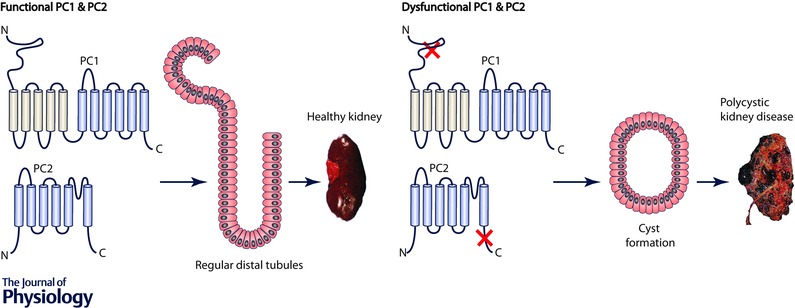
When genetic mutations affect the functionally important regions of PC1 and PC2 proteins, there will be progressive cyst growth in the kidney, leading to a dysfunctional polycystic kidney.
PC2 is a widely expressed integral membrane protein that is found either on the endoplasmic reticulum (where over 99% of the protein resides) (Cai et al. 1999; Koulen et al. 2002), or in the primary cilia (where the remainder is found). In some studies a small percentage of cellular PC2 can be isolated with plasma membrane fractions of epithelial cells (Newby et al. 2002; Streets et al. 2006). In contrast, mutated forms of PC2 are often found on the plasma membrane due to the lack of the endoplasmic reticulum retention sequence (Cai et al. 1999; Koulen et al. 2002). Calcium is important for PC2 function in several ways: PC2 forms a calcium‐permeable channel in the membrane and the open probability of the channel is regulated by the cytoplasmic calcium concentration (Cai et al. 2004). The calcium‐dependent activity of the PC2 channel is also affected by direct interactions at the C‐terminal tail of PC2 (PC2 Cterm) with a number of protein partners, including proteins residing on the plasma membrane, for example, polycystin‐1 (PC1) (Qian et al. 1997; Newby et al. 2002), other cellular proteins, such as actin binding proteins (Cantero Mdel & Cantiello, 2015), and intracellular channels, for example, the inositol 1,4,5‐trisphosphate receptor (InsP 3R) (Li et al. 2005; Sammels et al. 2010) and the ryanodine receptor (RyR) (Anyatonwu et al. 2007), the major calcium release channels in virtually all cell types. Multiple pathogenic mutations of ADPKD can found to affect the C‐terminal domain of PC2 (see the Autosomal Dominant Polycystic Kidney Disease Mutation Database at http://pkdb.mayo.edu), suggesting that the PC2 C‐terminal tail is functionally related to the disease pathogenesis (Fig. 1). Because the C‐terminal cytosolic tail is so critical for both the structure and function of PC2, this review will focus on this domain of PC2.
In early reports, the domain structure of PC2 was determined using biochemical and genetic tools that identified the motifs that comprise the C‐terminal domain, most importantly a canonical calcium binding domain known as an EF‐hand domain and a coiled‐coil domain involved in mediating oligomerization (Mochizuki et al. 1996; Qian et al. 1997). But these tools generated models that were not exact. For example, the earliest models suggest that the EF‐hand and the coiled‐coil regions overlap (Qian et al. 1997; Cai et al. 1999). More recently, models have been proposed to suggest that there are coiled‐coil motifs on either side of the EF‐domain (Chebib et al. 2015), but these interpretations rely upon computational methods that provided approximations. Using more specific biophysical and structural methods we have reassessed the domain assignments (Fig. 2 A) and have monitored the structural changes that occur with calcium binding.
Figure 2. The topology of full length PC2 and the EF‐hand domain sequence alignment .

A, PC2 contains six transmembrane helices and two cytosolic termini. The C‐terminal tail of PC2 contains a calcium‐binding EF‐hand, a coiled‐coil domain, and three connecting linker regions (linker 1, linker 2 and linker 3). Helices are numbered and N and C‐termini are labelled according to the residue number of the full length PC2 sequence. B, alignment of the EF‐hand domain sequence of human (HU, upper lines) and sea urchin (SU, lower lines) PC2 homologues. Both EF‐hand domains contain two helix–loop–helix (HLH) motifs. The human PC2 EF‐hand (HU, upper lines) has a four residue truncation within the first helix–loop–helix motif, whereas the sea urchin PC2 EF‐hand (SU, lower lines) contains two intact helix–loop–helix motifs.
Domain topology of PC2 and its channel function
PC2 can oligomerize in the membrane to form a calcium‐permeable channel. Members of the TRP channel family are believed to form a channel when four monomeric units assemble together. Indeed, members of the TRP channel family, TRPV1 and TRPA1, have been shown to form a tetrameric assembly in recently solved structures using cryo‐electron microscopy (Cryo‐EM) (Liao et al. 2013; Paulsen et al. 2015). However, the oligomeric state of PC2 when it is functioning as an ion channel is unclear. Each subunit of the full length PC2 protein contains six putative transmembrane domains and two cytosolic tails (Fig. 2 A). The N‐terminal domain of PC2 is predicted to be intrinsically disordered, and because of this disordered nature, biophysical and structural characterizations of the N‐terminal tail are limited. In contrast, PC2 Cterm has canonical structural components and, as stated above, is known to be important for the function of the PC2 channel (Koulen et al. 2002; Cai et al. 2004; Celic et al. 2008).
The open probability of the PC2 channel, like the RyR, is regulated by cytoplasmic calcium levels. The open probability of the channel follows a bell‐shaped curve depending on calcium concentration (Cai et al. 2004; Celic et al. 2012; Kuo et al. 2014 b). At low calcium levels, an increase in calcium concentration results in higher open probability, whereas a further increase in calcium concentration reduces the channel open probability. It is unclear how the PC2 channel transitions between these two modes of calcium regulation. Because much of the calcium‐dependent regulation of PC2 channel function was found to be localized to the C‐terminal tail (Koulen et al. 2002; Cai et al. 2004), it was hypothesized that separate calcium‐binding sites in this region were responsible for the two different sides of the bell‐shaped calcium dependence curve (Koulen et al. 2002; Cai et al. 2004; Anyatonwu & Ehrlich, 2005; Celic et al. 2008; Petri et al. 2010). Because only one functional calcium binding site was identified in the EF‐hand domain, it was assumed that there were additional sites outside the EF‐hand domain (Petri et al. 2010; Celic et al. 2012).
The PC2 C‐terminal contains the calcium‐binding site important for functional regulation
The human PC2 (hPC2) Cterm contains two clearly defined and identified domains, a calcium‐binding EF‐hand domain and a coiled‐coil domain shown to oligomerize (Fig. 2 A). These domains have been studied separately (Celic et al. 2008; Yu et al. 2009; Petri et al. 2010; Allen et al. 2014). The EF‐hand domain (aa 717–790 of hPC2) binds to calcium and is important for the calcium‐dependent regulation of the PC2 channel (Celic et al. 2008; Petri et al. 2010; Kuo et al. 2014 b). Mutations designed to abolish its ability to bind to calcium within the EF‐hand render the PC2 channel non‐functional (Celic et al. 2012). Moreover, mutations that alter the location of the calcium‐binding site within the EF‐hand domain also shifts the calcium dependence of channel regulation (Kuo et al. 2014 b).
As part of the search for additional calcium binding sites, we examined a series of acidic residues located in the loop region (linker 2 (L2), aa 791–832 of hPC2 Cterm) connecting the EF‐hand and coiled‐coil domains which share a similar sequence to the calcium‐bowl structure found in the ‘big potassium’ (BK) channel (Yuan et al. 2010; Liu et al. 2014). The L2 region also contains several post‐translational modification phosphorylation sites that have functional significance (Streets et al. 2010, 2013; Cantero Mdel et al. 2015). Specifically, phosphorylation on Ser812 modifies the sensitivity of the PC2 channel and shifts the calcium‐dependent open probability curve so that the channel is more sensitive to calcium level changes (Cai et al. 2004). However, only one calcium‐binding site in the entire C‐terminal domain was identified, indicated both by functional and biophysical characterization as expanded below.
Studies of the hPC2 Cterm protein using isothermal titration calorimetry (ITC) verified that there are no additional calcium‐binding sites outside the EF‐hand domain (Yang et al. 2015) and there is only one calcium‐binding site identified within the EF‐hand domain (Celic et al. 2008; Keeler et al. 2013). It is important to note that most biophysical studies of the hPC2 Cterm used the recombinant proteins purified from a prokaryotic expression system, and therefore these proteins lack post‐translational modification at potentially important functional sites. Although unlikely, it is possible that the post‐translational modifications, such as the identified phosphorylation sites that would occur in eukaryotic expression systems, would uncover additional calcium‐binding regulatory sites outside the primary site identified, the EF‐hand domain in hPC2 Cterm.
Human PC2 C‐terminal EF‐hand domain properties
The C‐terminal domain sequence of PC2 is highly conserved across different species. Nevertheless, one significant difference that separates different species is the number of functional calcium‐binding sites located within the EF‐hand. Although the hPC2 C‐terminal EF‐hand contains two helix–loop–helix (HLH) motifs, structural and sequence alignments with other canonical EF‐hand motifs revealed that the first motif contains a four residue truncation in the loop region, rendering the first motif non‐functional for calcium binding (Fig. 2 B). Furthermore, phylogenetic analysis conducted on the EF‐hand domain across different species has revealed that all PC2 orthologues can be divided into two groups: evolutionarily earlier species such as non‐chordates have two intact EF‐hand motifs, and chordates, which have one intact calcium‐binding HLH motif and one truncated motif (Petri et al. 2010; Kuo et al. 2014 b). A comparison of the calcium‐binding properties between human (chordates) and sea urchin (non‐chordates) by the EF‐hand domain of PC2 verified that the sea urchin EF‐hand (suPC2 C‐EF) contains two cooperative calcium‐binding sites and human EF‐hand (hPC2 C‐EF) contains one calcium‐binding site (Fig. 2 B). With two cooperative calcium‐binding sites in the sea urchin EF‐hand, the sea urchin orthologue binds calcium with a much stronger affinity compared to the human EF‐hand domain of PC2 (Yang et al. 2015). In addition to the difference in the number of calcium‐binding sites, the sea urchin orthologue is more stable in solution. A mutagenesis study on the two calcium‐binding sites in the suPC2 C‐EF showed that the calcium‐binding site located in the 2nd helix–loop–helix motif is crucial for protein integrity. When the 2nd site is deleted, the suPC2 C‐EF protein aggregates due to a lack of structural integrity, whereas the deletion of the 1st site in the suPC2 C‐EF still results in a folded protein with only one calcium‐binding site (Keeler et al. 2013; Kuo et al. 2014 b). This result is also consistent with the observation from the phylogenetic analysis that the 2nd EF‐hand motif is conserved among all species, most likely because it is structurally crucial for the folding of the entire EF‐hand domain. The addition of the four missing residues to make a functional binding site in the first EF‐hand motif in the hPC2 EF‐hand domain does not appear to alter the structural stability, but it does alter the ability to open the full length PC2 channel (Kuo et al. 2014 b).
The sea urchin PC2 orthologue localizes exclusively to the plasma membrane of the sperm acrosomal vesicle, suggesting that sea urchin PC2 may function as one of the cation channels mediating the sperm acrosome reaction (Neill et al. 2004). Activation of the acrosome is a very specific process and the differences in calcium‐binding sites between human and sea urchin C‐terminal domains may reflect their different calcium‐dependent channel regulation mechanisms.
The role of the coiled‐coil domain of PC2 C‐terminal
Downstream, and separate, from the EF‐hand domain, the coiled‐coil domain (aa 832–895 of hPC2) is involved in the formation of the homo‐oligomeric PC2 channel (Fig. 2 A), as well as in hetero‐oligomerization with other proteins, including PC1 (Qian et al. 1997), and other intracellular calcium channels (Qian et al. 1997; Li et al. 2005; Anyatonwu et al. 2007; Giamarchi et al. 2010; Sammels et al. 2010). The calcium‐dependent activity of the PC2 channel is also thought to be affected by direct interactions through the C‐terminal tail with a number of other interacting protein partners (Sammels et al. 2010; Wang et al. 2012; Morick et al. 2013; Streets et al. 2013). These interactions show that the coiled‐coil region is involved in many aspects of the functional regulation of the PC2 channel. The deletion of the coiled‐coil region renders the channel inactive and non‐responsive to calcium‐level changes (Yoshiba et al. 2012).
Intriguingly, the crystal structure of the isolated hPC2 coiled‐coil domain suggests that it forms a trimer, although TRP channels are generally tetramers (Yu et al. 2009). Due to the non‐spherical shape of the hPC2 Cterm in solution and formation of protein aggregates, it has proved difficult to determine the oligomeric states of the hPC2 Cterm complex (Ferreira et al. 2011; Celic et al. 2012). Nevertheless, upon the optimization of the buffer solution, the complete hPC2 Cterm was found to form a trimer in solution, measured by light scattering coupled with size‐exclusion chromatography, independent of the calcium states (Yang et al. 2015). This trimer formation of hPC2 Cterm in solution contradicts the generally believed tetrameric assembly of TRP channels. However, it is important to note that there are other structural motifs shown to be essential for channel assembly in addition to the C‐terminal domain. For example, although the N‐terminal domain of PC2 is intrinsically disordered, it also contributes to the oligomerization of the full length PC2 (Feng et al. 2008; Giamarchi et al. 2010). In addition to the N‐terminal cytosolic domain, a cysteine residue (C632) located in the extracellular loop connecting transmembrane helix 5 and helix 6 and a known ADPKD‐associated mutation, also contributes to the channel oligomerization (Giamarchi et al. 2010). Most likely, the full length channel assembly involves several portions of the protein, including the transmembrane domains, their connecting loops, and the cytosolic domains. Specifically, structural studies on other members of the TRP channel family members such as, TRPV1 and TRPA1, have revealed that the transmembrane helices form compact hydrophobic interactions that are necessary for the tetrameric assembly of the channels (Cao et al. 2013; Liao et al. 2013; Paulsen et al. 2015). These two TRP channels do not require a coiled‐coil domain to form a tetrameric assembly. When isolated in solution, the trimer complex is the preferred form of hPC2 Cterm because it is primarily driven by hydrophobic interactions throughout the coiled‐coil domain. However, in the context of the full length PC2 sequence and with the presence of the transmembrane domains, it is clear that the oligomeric state of the C‐terminal tail will reflect the combined interactions from the coiled‐coil domain, transmembrane helices, and other domains of the full length PC2 protein.
Although the N‐terminal portion of the coiled‐coil domain (aa 833–870 of hPC2) of PC2 forms a trimer complex through its hydrophobic interactions, the complex also maintains a high level of conformational flexibility in the C‐terminal portion of the coiled‐coil region (aa 871–895 of hPC2), according to both computation simulation and solution‐state studies (Zhu et al. 2011; Yang et al. 2016). This flexibility in the coiled‐coil region at the C‐terminal end allows interaction with an additional α‐helix from an adjacent PC2 molecule when they are brought together via the transmembrane domains in the lipid membrane, a circumstance where the effective local concentration of proteins would be high. With this type of interaction among subunits, the trimer formation could be a necessary intermediate on the pathway to tetramer formation. These non‐conventional tetramers generated from an α‐helix monomer and a coiled‐coil trimer have been observed in several known protein structures (Moutevelis & Woolfson, 2009).
In addition to channel assembly, the coiled‐coil region also contributes to the hetero‐oligomerization of PC2 with other proteins, including PC1 (Qian et al. 1997; Newby et al. 2002; Li et al. 2005; Anyatonwu et al. 2007; Giamarchi et al. 2010; Sammels et al. 2010). Molecular dynamic simulations have suggested that the coiled‐coil trimer of the PC2 complex can further interact with the α‐helical motif from PC1, via the C‐terminal open and flexible portion of the coiled‐coil region (Zhu et al. 2011). The presence of PC2 can enhance the GPCR proteolytic site (GPS) cleavage of PC1, and PC2 acts as an essential chaperone for PC1 maturation and surface localization to the primary cilia (Gainullin et al. 2015; Su et al. 2015). The interaction between PC1 and PC2 also regulates the phosphorylation of PC2 proteins, contributing to the functional regulation of the PC2 channel (Streets et al. 2013). In addition to the computational simulation, the PC1 and PC2 interaction has also been characterized by multiple biophysical and biochemical approaches. Using surface plasma resonance (SPR) and quartz crystal microbalance technique (QCM), the binding interaction between the C‐terminal domains of PC1 and PC2 was described to be multi‐factorial and calcium dependent (Casuscelli et al. 2009; Behn et al. 2010). We propose that the different modes of these reactions are probably due to structural flexibility of the coiled‐coil region of the PC2 Cterm. When the full length PC1 and PC2 proteins are in close proximity, as they are in the primary cilia, the coiled‐coil region interaction may be more specific and will reveal how the two proteins affect each other's functions.
The coiled‐coil domain is not directly involved in calcium binding of the EF‐hand
Although the coiled‐coil domain is important for the trimer formation of the full length C‐terminal tail, such trimer formation is not directly linked thermodynamically to the calcium binding within the EF‐hand, as measured by ITC. Previous studies of hPC2 C‐EF demonstrated the existence of one weak calcium‐binding site (K D ∼461 μm), an affinity that is supramaximal in the context of the physiological range of calcium concentrations in cytoplasm (Keeler et al. 2013). However, a more recent study found that by extending the N‐terminal boundary to include the additional residues in linker 1 region (aa 704–716 of hPC2) it was sufficient to restore the calcium‐binding affinity to the level of the complete hPC2 Cterm protein (Yang et al. 2016). Furthermore, a comparison of the calcium‐binding profiles of the full length C‐terminal constructs and their isolated EF‐hand domains in both human and sea urchin orthologues shows that the inclusion of the coiled‐coil domain in the complete hPC2 Cterm constructs does not enhance the calcium‐binding affinity further (Yang et al. 2015). In addition, the NMR comparison of the isolated suPC2 C‐EF and the suPC2 Ccore protein that includes both the coiled‐coil and EF‐hand domain shows that the EF‐hand region does not interact with another EF‐hand or the coiled‐coil region in the suPC2 Ccore construct (Yang et al. 2015). Taken together, these results support the claim that the trimeric formation via the coiled‐coil region does not affect how its EF‐hand region binds to calcium.
The molecular motion of the EF‐hand and the coiled‐coil domain in the trimer complex are different from each other. The elongated coiled‐coil domain keeps the trimer together through the hydrophobic interaction and undergoes a much slower rotational movement. The L2 region connecting the coiled‐coil region and the EF‐hand region provides the structural flexibility for each individual EF‐hand in the trimer complex, so each EF‐hand domain in the trimer complex can move independent of each other. Although there is no direct structural interaction detected between the EF‐hand with the rest of the complete PC2 Cterm protein, it was also observed that the calcium‐dependent dynamics of the EF‐hand domain can be slowed down when it resides in the complete PC2 Cterm. We propose that such a decrease in the protein dynamics is due to the movement restraints applied to each EF‐hand domain from the elongated coiled‐coil domain.
The calcium‐binding affinity of hPC2 Cterm is in a calcium concentration range relevant for PC2 channel deactivation. However, this increased affinity does not explain how the channel is activated by cytoplasmic calcium concentrations near 100 nm (Cai et al. 2004; Celic et al. 2012; Kuo et al. 2014 a). It is likely that additional factors present within the full length PC2 channel expressed in eukaryotic cells contribute to a higher sensitivity to sub‐micromolar calcium concentrations. These factors include higher order structural interactions within the fully tetrameric channel and post‐translational modifications. As the molecular dynamics of the EF‐hand is greatly decreased when it is located in the C‐terminal domain, we hypothesize that its motions will be even more restrained when the C‐terminal domain is attached to the transmembrane region of the full length PC2 protein (Fig. 3). Under such circumstances, the EF‐hand domain can alter its dynamic pattern, even under a sub‐micromolar calcium concentration. The structural and conformational changes due to calcium binding provide a partial explanation for channel function and regulation by calcium. The dynamics of the conformational transition between the calcium‐bound and calcium‐unbound states of the C‐terminal domain provides another mechanism that gives insight into channel gating and regulation (Fig. 3). Based on characterization of its protein dynamics, the EF‐hand in the full length PC2 channel may experience a lower level of conformational dynamics at sub‐saturating calcium levels, compared to its isolated form. Such slow dynamics may provide the time frame necessary to relay the molecular motion to the pore‐forming loop, leading to the opening of the PC2 channel (Fig. 3). In addition to the restraints posed by the transmembrane helix, structural interactions with other domains of the full length PC2 channel, and post‐translational modifications could modify the dynamic profiles of the PC2 C‐terminal tail allosterically, providing a molecular basis for the allosteric regulation and the two different modes of calcium‐dependent activity of the PC2 channel.
Figure 3. Schematic hypothesis for how calcium binding within the PC2 Cterm can regulate PC2 channel gating .

We hypothesize that calcium binding to the EF‐hand domain of PC2 can alter the conformation and dynamics of the Cterm. The conformational changes can be propagated to the transmembrane helices due to the decreased protein dynamics in sub‐micromolar calcium levels and cause the channel to be opened.
Summary
Full length PC2 contains two cytosolic tails, the N‐terminal and C‐terminal domains (Fig. 2 A). Structural studies of the regulation of PC2 channel function have focused on the C‐terminal domain because the N‐terminal domain is intrinsically disordered and because the PC2 Cterm has structural components known to play regulatory roles in many channels. PC2 Cterm contains an EF‐hand domain and a coiled‐coil domain that are separated by a flexible linker 2. The coiled‐coil domain contributes to oligomerization of the channel subunits, but is not necessary for calcium binding. The structure and dynamic properties of the EF‐hand domain are essential for the calcium‐dependent activity of the PC2 channel. Truncations or mutations to PC2 in either Cterm domain leads to loss of channel function. Although the precise sequence of events between loss of PC2 channel activity and altered kidney cell function still need to be identified, it is clear that mutations in hPC2 Cterm decrease intracellular calcium signalling and are associated with cyst formation in the kidney which eventually leads to ADPKD. As more and more molecular knowledge of the PC2 Cterm indicates its involvement in both the physiology and pathophysiology of PC2, structural and biophysical studies of full length PC2 in its native form will be able to provide valuable information regarding how all the domains of PC2 respond to the conformational and dynamical changes at different calcium concentrations. Additionally, as different protein partners have been shown to interact with PC2 in cells, and cause differentiated functional effects on PC2, future biophysical and structural studies characterizing the different modes of interactions between PC2 and its binding partners would provide insight to the structural basis of these different functional effects. In the future such structural and functional studies may provide the link between the mechanism of PC2 activity and kidney function in normal physiology and in ADPKD.
Additional information
Competing interests
None declared.
Funding
This work was funded by NIH grants R01 DK087844, DK057751 and DK090744 to Barbara Ehrlich. Yifei Yang was supported by a pre‐doctoral fellowship from the CSC‐Yale World Scholars program. The authors used the resources provided by the Yale Liver Center (DK34989).
Acknowledgements
The authors thank Dr Ivana Kuo for a critical reading of the manuscript and helpful suggestions.
Biographies
Yifei Yang recently defended her PhD thesis, focused on studying the molecular mechanism of PC2 function regulation, in the Department of Pharmacology at Yale University. She is now a Clinical Chemistry Fellow at University of Chicago Medical Center, applying chemical biology tools in clinical diagnostics.

Barbara E. Ehrlich is Professor and Director of the Molecular Hermeneutics group in the Department of Pharmacology, Yale University. She has a long‐standing interest in intracellular calcium signalling and ion channel function. She obtained her PhD at UCLA with Dr Jared Diamond, before doing postdoctoral studies with Dr Alan Finkelstein at Albert Einstein College of Medicine.
This review was presented at the symposium “Non‐selective cationic channels in chemical and physical stress?”, which took place at Physiology 2015 in Cardiff, UK, 6–8 July 2015.
References
- Allen MD, Qamar S, Vadivelu MK, Sandford RN & Bycroft M (2014). A high‐resolution structure of the EF‐hand domain of human polycystin‐2. Protein Sci 23, 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyatonwu GI & Ehrlich BE (2005). Organic cation permeation through the channel formed by polycystin‐2. J Biol Chem 280, 29488–29493. [DOI] [PubMed] [Google Scholar]
- Anyatonwu GI, Estrada M, Tian X, Somlo S & Ehrlich BE (2007). Regulation of ryanodine receptor‐dependent calcium signaling by polycystin‐2. Proc Natl Acad Sci USA 104, 6454–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behn D, Bosk S, Hoffmeister H, Janshoff A, Witzgall R & Steinem C (2010). Quantifying the interaction of the C‐terminal regions of polycystin‐2 and polycystin‐1 attached to a lipid bilayer by means of QCM. Biophys Chem 150, 47–53. [DOI] [PubMed] [Google Scholar]
- Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE & Somlo S (2004). Calcium dependence of polycystin‐2 channel activity is modulated by phosphorylation at Ser812 . J Biol Chem 279, 19987–19995. [DOI] [PubMed] [Google Scholar]
- Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R & Somlo S (1999). Identification and characterization of polycystin‐2, the PKD2 gene product. J Biol Chem 274, 28557–28565. [DOI] [PubMed] [Google Scholar]
- Cantero Mdel R & Cantiello HF (2015). Polycystin‐2 (TRPP2) regulation by Ca2+ is effected and diversified by actin‐binding proteins. Biophys J 108, 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero Mdel R, Velázquez IF, Streets AJ, Ong AC & Cantiello HF (2015). The cAMP signaling pathway and direct protein kinase A phosphorylation regulate polycystin‐2 (TRPP2) channel function. J Biol Chem 290, 23888–23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Liao M, Cheng Y & Julius D (2013). TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casuscelli J, Schmidt S, DeGrey B, Petri ET, Celic A, Folta‐Stogniew E, Ehrlich BE & Boggon TJ (2009). Analysis of the cytoplasmic interaction between polycystin‐1 and polycystin‐2. Am J Physiol Regul Integr Comp Physiol 297, F1310–F1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celic A, Petri ET, Demeler B, Ehrlich BE & Boggon TJ (2008). Domain mapping of the polycystin‐2 C‐terminal tail using de novo molecular modeling and biophysical analysis. J Biol Chem 283, 28305–28312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celic AS, Petri ET, Benbow J, Hodsdon ME, Ehrlich BE & Boggon TJ (2012). Calcium‐induced conformational changes in C‐terminal tail of polycystin‐2 are necessary for channel gating. J Biol Chem 287, 17232–17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib FT, Sussman CR, Wang X, Harris PC & Torres VE (2015). Vasopressin and disruption of calcium signaling in polycystic kidney disease. Nat Rev Nephrol 11, 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Okenka GM, Bai CX, Streets AJ, Newby LJ, DeChant BT, Tsiokas L, Obara T & Ong AC (2008). Identification and functional characterization of an N‐terminal oligomerization domain for polycystin‐2. J Biol Chem 283, 28471–28479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira FM, Oliveira LC, Germino GG, Onuchic JN & Onuchic LF (2011). Macromolecular assembly of polycystin‐2 intracytosolic C‐terminal domain. Proc Natl Acad Sci USA 108, 9833–9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainullin VG, Hopp K, Ward CJ, Hommerding CJ & Harris PC (2015). Polycystin‐1 maturation requires polycystin‐2 in a dose‐dependent manner. J Clin Invest 125, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarchi A, Feng S, Rodat‐Despoix L, Xu Y, Bubenshchikova E, Newby LJ, Hao J, Gaudioso C, Crest M, Lupas AN, Honore E, Williamson MP, Obara T, Ong AC & Delmas P (2010). A polycystin‐2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J 29, 1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PC & Torres VE (2009). Polycystic kidney disease. Annu Rev Med 60, 321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler C, Poon G, Kuo IY, Ehrlich BE & Hodsdon ME (2013). An explicit formulation approach for the analysis of calcium binding to EF‐hand proteins using isothermal titration calorimetry. Biophys J 105, 2843–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE & Somlo S (2002). Polycystin‐2 is an intracellular calcium release channel. Nat Cell Biol 4, 191–197. [DOI] [PubMed] [Google Scholar]
- Kuo IY, DesRochers TM, Kimmerling EP, Nguyen L, Ehrlich BE & Kaplan DL (2014. a). Cyst formation following disruption of intracellular calcium signalling. Proc Natl Acad Sci USA 111, 14283–14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo IY, Keeler C, Corbin R, Celic A, Petri ET, Hodsdon ME & Ehrlich BE (2014. b). The number and location of EF hand motifs dictates the calcium dependence of polycystin‐2 function. FASEB J 28, 2332–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wright JM, Qian F, Germino GG & Guggino WB (2005). Polycystin 2 interacts with type I inositol 1,4,5‐trisphosphate receptor to modulate intracellular Ca2+ signalling. J Biol Chem 280, 41298–41306. [DOI] [PubMed] [Google Scholar]
- Liao M, Cao E, Julius D & Cheng Y (2013). Structure of the TRPV1 ion channel determined by electron cryo‐microscopy. Nature 504, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HW, Hou PP, Guo XY, Zhao ZW, Hu B, Li X, Wang LY, Ding JP & Wang S (2014). Structural basis for calcium and magnesium regulation of a large conductance calcium‐activated potassium channel with β1 subunits. J Biol Chem 289, 16914–16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Wu GQ, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai YQ, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJM & Somlo S (1996). PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342. [DOI] [PubMed] [Google Scholar]
- Morick D, Schatz M, Hubrich R, Hoffmeister H, Krefft A, Witzgall R & Steinem C (2013). Phosphorylation of C‐terminal polycystin‐2 influences the interaction with PIGEA14: a QCM study based on solid supported membranes. Biochem Biophys Res Commun 437, 532–537. [DOI] [PubMed] [Google Scholar]
- Moutevelis E & Woolfson DN (2009). A periodic table of coiled‐coil protein structures. J Mol Biol 385, 726–732. [DOI] [PubMed] [Google Scholar]
- Neill AT, Moy GW & Vacquier VD (2004). Polycystin‐2 associates with the polycystin‐1 homolog, suREJ3, and localizes to the acrosomal region of sea urchin spermatozoa. Mol Reprod Dev 67, 472–477. [DOI] [PubMed] [Google Scholar]
- Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ & Ong AC (2002). Identification, characterization, and localization of a novel kidney polycystin‐1‐polycystin‐2 complex. J Biol Chem 277, 20763–20773. [DOI] [PubMed] [Google Scholar]
- Ong AC & Harris PC (2015). A polycystin‐centric view of cyst formation and disease: the polycystins revisited. Kidney Int 88, 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Armache JP, Gao Y, Cheng Y & Julius D (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri ET, Celic A, Kennedy SD, Ehrlich BE, Boggon TJ & Hodsdon ME (2010). Structure of the EF‐hand domain of polycystin‐2 suggests a mechanism for Ca2+‐dependent regulation of polycystin‐2 channel activity. Proc Natl Acad Sci USA 107, 9176–9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Germino FJ, Cai Y, Zhang X, Somlo S & Germino GG (1997). PKD1 interacts with PKD2 through a probable coiled‐coil domain. Nat Genet 16, 179–183. [DOI] [PubMed] [Google Scholar]
- Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, Cai Y, Somlo S & De Smedt H (2010). Polycystin‐2 activation by inositol 1,4,5‐trisphosphate‐induced Ca2+ release requires its direct association with the inositol 1,4,5‐trisphosphate receptor in a signalling microdomain. J Biol Chem 285, 18794–18805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streets AJ, Moon DJ, Kane ME, Obara T & Ong AC (2006). Identification of an N‐terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin‐2 in vivo and in vitro . Hum Mol Genet 15, 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streets AJ, Needham AJ, Gill SK & Ong AC (2010). Protein kinase D‐mediated phosphorylation of polycystin‐2 (TRPP2) is essential for its effects on cell growth and calcium channel activity. Mol Biol Cell 21, 3853–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streets AJ, Wessely O, Peters DJ & Ong AC (2013). Hyperphosphorylation of polycystin‐2 at a critical residue in disease reveals an essential role for polycystin‐1‐regulated dephosphorylation. Hum Mol Genet 22, 1924–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Wu M, Yao G, El‐Jouni W, Luo C, Tabari A & Zhou J (2015). Regulation of polycystin‐1 ciliary trafficking by motifs at its C‐terminus and polycystin‐2 but not by cleavage at the GPS site. J Cell Sci 128, 4063–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Dai XQ, Li Q, Wang Z, Cantero Mdel R, Li S, Shen J, Tu JC, Cantiello H & Chen XZ (2012). Structural interaction and functional regulation of polycystin‐2 by filamin. PLoS One 7, e40448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hodsdon ME, Lolis EJ & Ehrlich BE (2016). Conformational dynamics of Ca2+‐dependent responses in the polycystin‐2 C‐terminal tail. Biochem J 473, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Keeler C, Kuo IY, Lolis EJ, Ehrlich BE & Hodsdon ME (2015). Oligomerization of the polycystin‐2 C‐terminal tail and effects on its Ca2+‐binding properties. J Biol Chem 290, 10544–10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, Nakai J, Dworniczak B, Ehrlich BE, Pennekamp P & Hamada H (2012). Cilia at the node of mouse embryos sense fluid flow for left‐right determination via Pkd2. Science 338, 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, Tong L, Isacoff EY & Yang J (2009). Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci USA 106, 11558–11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y & MacKinnon R (2010). Structure of the human BK channel Ca2+‐activation apparatus at 3.0 Å resolution. Science 329, 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yu Y, Ulbrich MH, Li MH, Isacoff EY, Honig B & Yang J (2011). Structural model of the TRPP2/PKD1 C‐terminal coiled‐coil complex produced by a combined computational and experimental approach. Proc Natl Acad Sci USA 108, 10133–10138. [DOI] [PMC free article] [PubMed] [Google Scholar]


