Abstract
In recent years there have been significant technical and methodological advances in our ability to record the movements of the gastrointestinal tract. This has led to significant changes in our understanding of the different types of motor patterns that exist in the gastrointestinal tract (particularly the large intestine) and in our understanding of the mechanisms underlying their generation. Compared with other tubular smooth muscle organs, a rich variety of motor patterns occurs in the large intestine. This reflects a relatively autonomous nervous system in the gut wall, which has its own unique population of sensory neurons. Although the enteric nervous system can function independently of central neural inputs, under physiological conditions bowel motility is influenced by the CNS: if spinal pathways are disrupted, deficits in motility occur. The combination of high resolution manometry and video imaging has improved our knowledge of the range of motor patterns and provided some insight into the neural and mechanical factors underlying propulsion of contents. The neural circuits responsible for the generation of peristalsis and colonic migrating motor complexes have now been identified to lie within the myenteric plexus and do not require inputs from the mucosa or submucosal ganglia for their generation, but can be modified by their activity. This review will discuss the recent advances in our understanding of the different patterns of propagating motor activity in the large intestine of mammals and how latest technologies have led to major changes in our understanding of the mechanisms underlying their generation.
Abbreviations
- CMMC
colonic migrating motor complex
- DCMMC
distal colonic migrating motor complex
- HAPCs
high amplitude propagating contractions
- ICCs
interstitial cells of Cajal
Colonic motility
The large intestine in all mammalian species performs a number of functions essential for the optimal handling of ingested material. Water, ions and bile salts are also extracted from the content, and storage, formation and expulsion of faeces occurs normally in a controlled fashion suited to the physico‐chemical composition of the contents. The colonic microbiome ferments some indigestible materials, releasing nutrients (largely short chain fatty acids) that are absorbed and thus increase to a small degree the efficiency of digestion. The final common effectors of motility are longitudinally and circularly aligned smooth muscle cells. However, the key component of physiological motility is the temporal and spatial coordination of smooth muscle contractions and relaxations. These controlled movements of the intestinal muscle involve two fundamental mechanisms. One is responsible for the spontaneous ‘myogenic’ activity of the muscle and the second involves complex enteric neural circuits embedded within the gut wall that are influenced by the extrinsic innervation of the large bowel. In humans, lesions to extrinsic nerve pathways or spinal cord damage leads to abnormal colonic motility and often constipation (Anderson, 2004; Coggrave & Norton, 2010), which can be improved by stimulation of defaecation centres in the spinal cord (Ferens et al. 2011; Ellis et al. 2015). Over the past three decades significant advances have been made in revealing the bases of these mechanisms and the findings suggest strongly preserved fundamental features across all mammalian species.
The identity of enteric neurons forming simple polarized reflex pathways has been studied in detail in several species (Fig. 1 A). The major transmitter substances of excitatory and inhibitory motor neurons have been identified and the properties of intrinsic mechanosensitive enteric neurons analysed in detail (Bornstein et al. 2004; Spencer & Smith, 2004; Brookes & Costa, 2006; Costa & Brookes, 2008; Furness, 2012; Mazzuoli‐Weber & Schemann, 2015). In parallel, an early study showed that the proto‐oncogene c‐kit and the tyrosine kinase receptor that it encodes, Kit, are expressed by pacemaker cells in the gut wall and are essential for myogenic pacemaking functions (Maeda et al. 1992). This led to identification of several types of interstitial cells of Cajal (ICCs). Some of the ICCs function as the pacemakers and generate and coordinate myogenic rhythmicity. Some ICCs act as intermediaries in motor neuron to smooth muscle neurotransmission (see reviews by: Farrugia, 2008; Huizinga & Lammers, 2009; Sanders et al. 2014).
Figure 1. Originally proposed enteric neural pathway underlying distension‐evoked reflexes (A) compared with latest understanding of this neural pathway (see B) .
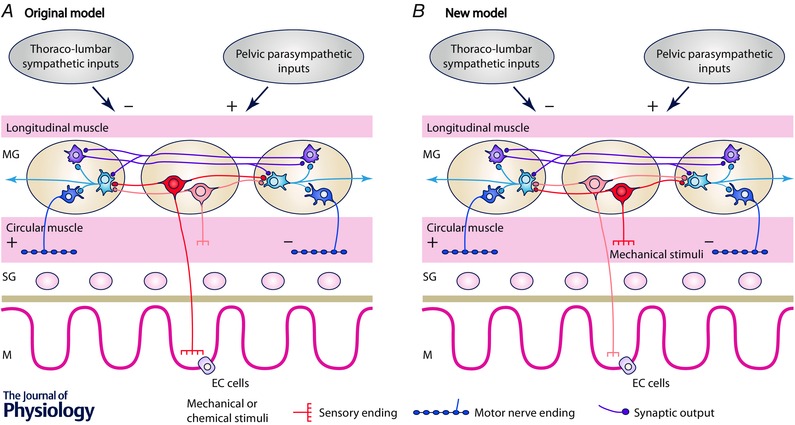
A, Originally proposed arrangement of enteric neural pathways in the intestine. Mucosally projecting sensory neurons (in red) respond to chemical and mechanical stimuli and are responsible for initiating the peristaltic reflex. Mechanosensory interneurons respond to circumferential stretch and have transduction sites in the circular muscle. They can initiate polarized neural reflexes to the circular and longitudinal muscle in the distal colon. Possible other enteric circuits of interneurons generate migrating motor complexes. Extrinsic inputs from sympathetic and parasympathetic pathways inhibit and excite motility by acting on the enteric circuits. Current knowledge of submucosal neurons and longitudinal muscle have been deliberately omitted to maintain simplicity. B, the new model describes how our understanding of these pathways has changed in recent years. We propose a refinement of the enteric circuits involved in colonic motility. The mechanosensory enteric neurons (located in the myenteric plexus) have essential mechanosensitive nerve endings located in the circular muscle (see Spencer et al. 2006) (now shown in red) which initiate polarized neural pathways that result in oral contraction and anal relaxation. These pathways do not require mucosal inputs and represent the bases of the neuromechanical loop responsible for propulsion adapted to the consistency of contents. However, stimulation of mucosal sensory nerve endings can initiate and modulate enteric neural activity. The polarized enteric circuits involved in the neuromechanical loop are modulated by underlying cyclic neural activity initiated by any maintained distension and providing the bases of the migrating motor complexes. Extrinsic excitatory inputs from both pelvic and vagal sources are involved in the greater or lesser permissive role depending on the degree of central control of colonic movements. EC cells, enterochromaffin cells, with permission.
While the cellular components of these two systems have been individually studied in detail, how they interact to coordinate motility has only recently been actively explored at the whole organ level. Contraction at any point in the bowel can either propel content, or impede its progress, depending on the mechanical state of neighbouring regions. The dynamic nature of the movements at every point along the gut renders analysis of the movements of the intestinal wall and the luminal content a significant challenge in biomechanics. Although understanding the basis of coordinated motility is in its infancy, the reality is that in all species contents in most cases appear to be propelled at a suitable rate with an appropriate degree of fluid absorption. The intrinsic neural and myogenic mechanisms operate in conjunction and extrinsic neural and hormonal influences do play critical roles.
Discoveries, techniques and methodologies that have advanced understanding of colonic motility
Two technical developments have also led to improvements in our understanding of intestinal motility. First, the description, display and analysis of complex patterns of motility recorded from isolated sections of intestine have been facilitated by the development of spatio‐temporal maps. Changes in intestinal diameter are measured from high resolution video imaging of the intestine. Maps can be constructed that detail the changes in diameter (DMaps) in real time, along the length of the isolated intestinal segments (Bouchoucha et al. 1999; Hennig et al. 1999; Bercik et al. 2000). Comparable maps can be created for longitudinal muscle contractions. These maps provide a simple, readily interpretable visual summary of motility over periods of seconds to many minutes, with automated quantitative analysis becoming increasingly feasible. Using these video‐imaging methods detailed description of motor patterns have been made on isolated colon preparations of several experimental animals (Hennig et al. 1999; D'Antona et al. 2001; Lentle et al. 2008; Huizinga et al. 2011; Costa et al. 2013 a).
A second major technical advance has been the development of high resolution manometry, for recording motor patterns in the human colon in vivo. For the most part these recordings have been made using fibre optic manometry catheters with 10 mm spacing between the 72 and 120 pressure sensors. This has increased the spatial resolution of intraluminal pressure sensing by almost an order of magnitude compared to many older catheters (Arkwright et al. 2009). Studies have shown that many previous colonic manometry studies, using more widely spaced sensors, misinterpreted a large proportion of propagating motility events (Dinning et al. 2013).
Combining these two technologies, high resolution manometry with spatio‐temporal mapping of diameter, in isolated sections of intestine has also provided a valuable type of analysis (Dinning et al. 2011). The separate pressure traces of high resolution recordings can be interpolated to create spatio‐temporal maps of pressure along isolated segments of intestine. These PMaps have been combined with DMaps to detail the relationships between changes in diameter and changes in intraluminal pressure, again represented as 2‐dimensional maps (DPMaps) (Costa et al. 2013 b). The application of simplified principles of muscle mechanics allows the dynamic state of the intestinal smooth muscle to be determined at each point along DPMaps (i.e. whether muscle is actively contracting or relaxing, or passively shortening or lengthening, during motility patterns (Fig. 2; Costa et al. 2013 b). All of this has added to an accurate measurement and display of the repertoire of colonic motility patterns and an understanding of how some of the patterns are generated.
Figure 2. Propulsion of luminal contents is modified by differences in the consistency of luminal contents .
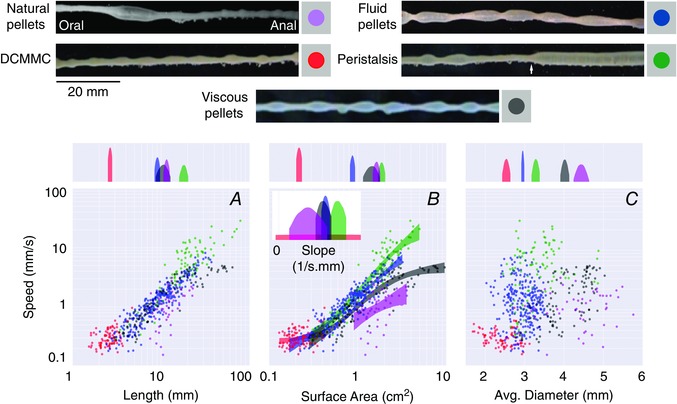
In the upper half of the figure, five different images are shown where boluses of different sizes and shapes (pellets) are present in the distal colon. Natural pellets are represented in purple, distal colonic migrating motor complexes (DCMMCs) in red, fluid pellets in blue, viscous pellets in grey, and peristaltic contractions in green. It was noted that peristaltic contractions often started approximately half‐way along the preparation (white arrow). A–C shows the relationships between speed of propulsion and the bolus size; speed correlates positively with bolus length (A); speed in relation to bolus surface area (B); speed in relation to average bolus diameter (C). In the upper quadrant of each graph (A–C) the probability density is represented on a log scale. The distributions of means are shaded within their 95% highest density interval (HDI, see text) and the gaps between shaded 95% HDIs represent significant differences between the various measures. Note that for the average diameter (C) there is a clear separation between all of the shaded 95% HDIs, indicating that the diameters of different boluses are significantly different from one another. However, a poor correlation exists between the speed of propulsion and average diameter. For surface areas (B) from about 1 cm2, the speeds of all bolus types (except DCMMCs) differ significantly, as shown by the gap between their shaded 95% HDIs (see centre square), with natural, viscous, liquid and peristaltic liquid boluses moving at increasing speeds. The inset in B depicts the distributions of the steepest slope of the sigmoid curve for each bolus type. A shows that a significant overlap exists between the length of the natural, viscous and fluid pellets. DCMMCs (shown in red) are shorter than all other bolus types and peristaltic contractions (shown in green) are significantly longer than all other bolus types. Figure reproduced from Costa et al. (2015), with permission.
Mechanisms of propulsion of colonic contents
One simple insight from this methodology was the demonstration that the propulsion of a bolus in the isolated rabbit colon was due to an area of active muscle contraction occurring at the oral end of the bolus and an active relaxation at the anal end (Dinning et al. 2014 b). These processes could be blocked by tetrodotoxin (TTX), suggesting that the active contractions and relaxations require the activity of excitatory and inhibitory enteric motor neurons, respectively. These results are a more detailed investigation of the polarized enteric pathways demonstrated by Bayliss & Starling (1899) that were shown to be responsible for propulsion of luminal contents. The existence of polarized enteric neural pathways capable of mediating ascending excitation and anal inhibition of the circular muscle has been demonstrated convincingly in several species, using localized mechanical stimuli (Crema et al. 1970; Costa & Furness, 1976; Grider, 1989; Spencer & Smith, 2001) (Fig. 1 A).
‘The Neuromechanical Loop hypothesis’
In the isolated colon of experimental animals, inserting natural or artificial faecal pellets into the oral end of the preparation evokes propulsive circular muscle contractions, after a short delay (Costa & Furness, 1976; Kadowaki et al. 1996; D'Antona et al. 2001; Spencer et al. 2011; Sia et al. 2013). This is driven by a contraction oral to the pellet by activation of polarized enteric neural pathways as described above.
Content‐dependent neural propulsion initiated by liquid or pellet distension has been reported in guinea‐pig, dog, cat, sheep and rat, and have been variously called ‘peristaltic contractions’, ‘peristaltic waves’, ‘giant contractions’ or ‘giant migrating contractions’, ‘rhythmic propulsive motor complexes’, ‘long distance contractions’, ‘antegrade propagating long distance contractions’, or ‘mass peristaltic events’ (Crema et al. 1970; Sarna et al. 1988; Bedrich & Ehrlein, 2001; D'Antona et al. 2001; Gonzalez & Sarna, 2001; Hipper & Ehrlein, 2001; Lentle et al. 2008; Dinning et al. 2012 a; Chen et al. 2013; Costa et al. 2013 a). Thus the propulsive motor patterns generated by the neuromechanical loop could be described generically as ‘neural peristalsis’ to distinguish them from migrating motor complexes or even possible myogenic peristalsis.
Importantly, in the colon of guinea‐pigs, fluid infusion generates repeated peristaltic propulsive contractions that propagate at much higher speeds than solid pellets (Costa et al. 2015). Thus, the consistency of the contents influences the rate of progression of the circular muscle contraction along the colon. This simple observation indicates that a peristaltic contraction is not a simple reflex (which would happen in a stereotyped, invariant fashion). Rather, it is a more complex mechanism that adapts to the luminal content. Bayliss & Starling (1899) proposed that polarized reflexes are the basis of propagating contractions. A bolus activates ascending excitation and descending inhibition which cause movement of the bolus aborally. From its new location, it activates another bout of polarized reflexes, causing further propulsion. We have extended this proposal into the ‘neuromechanical loop’ hypothesis (Costa et al. 2013 b; Dinning et al. 2014 b). This hypothesis suggests that distension by luminal material activates polarized reflexes (as Bayliss and Starling proposed). However, the physical consistency of the material influences how it is redistributed by the contractions and relaxations of smooth muscle in the gut wall. This, in turn, affects the pattern of distension along the gut and thus modifies the subsequent re‐activation of polarized enteric neural pathways. In this way, polarized reflex pathways and mechanical factors, including distributed distension, form a dynamic functional loop, which adapts gut motility to deal effectively with a wide range of contents (ranging from fluid to solid pellets of various dimensions).
How does the ‘neuromechanical loop’ work?
The ‘neuromechanical loop’ hypothesis predicts that the consistency, shape and size of the luminal contents should influence the speed of propulsion. This relationship has been experimentally demonstrated in the isolated guinea‐pig colon (Costa et al. 2015). Using boluses of various lengths and diameters, a clear relationship between surface area and speed of propulsion was demonstrated (Costa et al. 2015; Fig. 3). This relationship probably reflects the number of enteric mechano‐sensitive neurons activated by the bolus. The larger the surface area, the more sensory neurons are simultaneously activated and the greater activation of polarized ascending excitatory and descending inhibitory pathways. This leads to a consequential increase in the amplitude of oral contraction and anal relaxation, causing a high speed of propulsion. According to the neuromechanical loop hypothesis, increasing the fluidity of contents should increase the speed of propulsion. Indeed natural solid pellets are propelled at a slower speed than viscous material or liquid (Fig. 3). The speed of propulsion is constrained by influences that impede movement of the content. Increasing the diameter of a faecal‐shaped pellet beyond a limit, or increasing the load against which propulsion operates, slows the velocity at which contractions propagate (Costa et al. 2015). These observations support the existence of a neuromechanical loop which ensures that simple enteric neural circuitry can efficiently propel content with a wide range of physical properties. It may also explain some of the variability of colonic motor patterns studied under varying conditions in different laboratories.
Figure 3. Spatio‐temporal map of the steady states of linear and quiescent orbits .
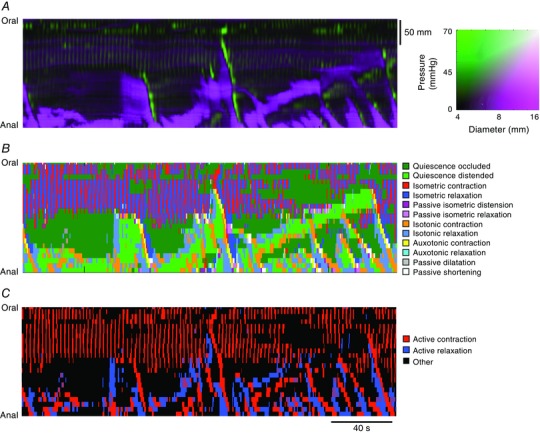
This was constructed from the composite DPMaps (shown in A) and the extracted orbits that were formed from the independent variables (diameter and pressure) (see B). In B each of the 12 possible mechanical states are mapped in different colours. The map portrays the periods of quiescence either when the intestine remains passively dilated (light green) or passively occluded (dark green). Red, orange and yellow areas represent active contractions and mark the propagating area of contraction during neural peristalsis. Active relaxation (aqua and light blue) precedes both in time and space the propagating contraction. Myogenic activity in this particular example consists of isometric relaxations and contractions. The last map (C) represents a simplification of the full composite map of states by clustering all areas undergoing active contraction (red) and active relaxation (blue). All other passive states are in black. The spatio‐temporal nature of active contractions and relaxations during neurogenic activity and myogenic activity is made very distinct. Figure reproduced from Costa et al. (2013 b).
Neurally dependent cyclic motor activity in the colon
There is considerable evidence for a neurally generated cyclical motor pattern (colonic migrating motor complex; CMMC), which is less dependent on content than neural peristalsis. CMMCs occur in the colon of many experimental animals. They have been reported in vivo in rabbits and sheep (Ehrlein et al. 1982; Bedrich & Ehrlein, 2001), dogs (Sarna, 1986), and in vitro in guinea‐pigs (Costa & Furness, 1976; D'Antona et al. 2001; Costa et al. 2015), rabbits (Lentle et al. 2008; Dinning et al. 2012 a) and mice, where the most detailed studies on the CMMC have been performed (Fida et al. 1997; Brierley et al. 2001; Powell & Bywater, 2001; Spencer & Bywater, 2002; Roberts et al. 2008; Spencer et al. 2013). These complexes can migrate either orally, aborally, or appear simultaneously along regions of colon (Sarna et al. 1984), suggesting that their direction of propagation is not hardwired into the enteric nervous system. They do not appear to be lumen‐occlusive and in the rabbit and guinea‐pig proximal colon, where they have been correlated with the actual profile of the colonic wall, they result in indentations that begin to separate soft faecal material into pellets (Lentle et al. 2008; Dinning et al. 2012 a; Costa et al. 2015).
Recent studies have challenged the idea that CMMCs occur entirely independently of content. In many studies of CMMCs, tension or length transducers were attached to the gut wall to record the complexes; the resting tension applied by the transducers may constitute an effective stimulus to drive the initiation of CMMCs. Without transducers, CMMCs actually occur infrequently and propagate over shorter distances, with slower propagation velocities (see Fig. 4) (Barnes et al. 2014). Certainly, distension can activate premature CMMCs (Zagorodnyuk & Spencer, 2011). This remains a fertile area of research that requires a combination of recording at both cellular and organ level to identify the mechanisms of initiation of this cyclical enteric neural activity.
Figure 4. The frequency of colonic migrating motor complexes (CMMCs) is significantly increased by the presence of faecal contents .
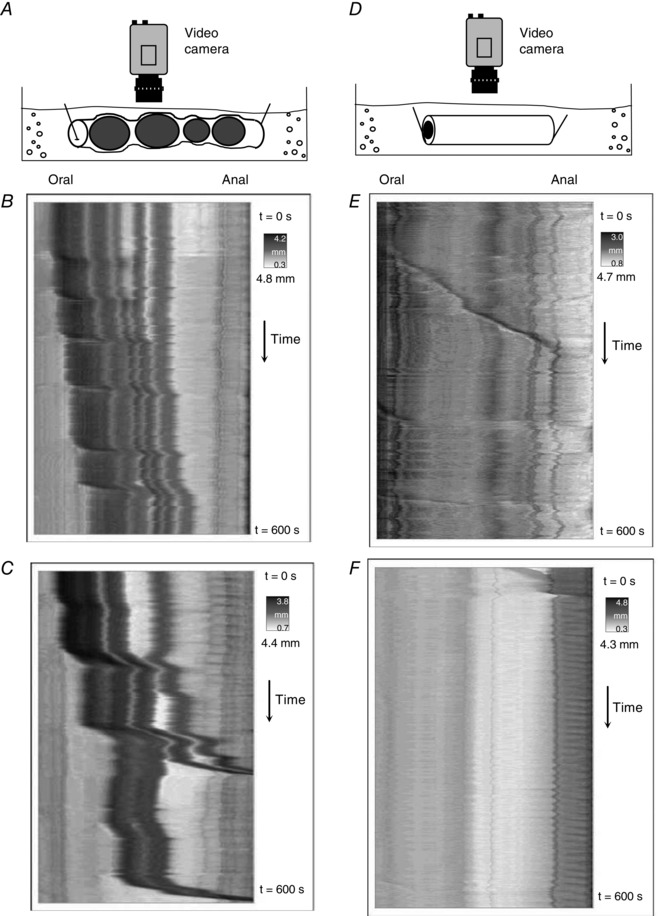
A, schematic diagram showing an isolated whole mouse colon containing multiple faecal pellets. B and C show spatio‐temporal maps from the whole colon of different mice in vivo. CMMCs occur frequently and propagate over significant lengths of colon. D and E, when the colon has expelled all contents, the same segments of colon shown in B and C, respectively, rarely generate CMMCs. When CMMCs do occur, their velocity of propagation is significantly slower. This shows that the presence of multiple pellets in the lumen enhances the velocity and frequency of CMMCs. Figure reproduced from Barnes et al. (2014).
Neural mechanisms underlying generation of peristalsis and CMMCs
Considerable work has been carried out analysing the site of initiation of content‐dependent neural peristalsis and of CMMCs. By removing layers of the gut wall of preparations of guinea‐pig (Spencer et al. 2011) and mouse large intestine, in vitro, (Keating & Spencer, 2010) it has been shown that the neural circuits responsible for both neural peristalsis and CMMCs lie in the myenteric plexus and/or muscularis externa (Keating & Spencer, 2010; Spencer et al. 2011). This suggests that the submucosal plexus and mucosa (including enterochromaffin (EC) cells) are not essential for either the initiation or propagation of these neurogenic motor patterns. Distension of the bowel increases the frequency of CMMCs; this response is also preserved after removal of the submucosa and mucosa (Zagorodnyuk & Spencer, 2011). Similar findings have been reported in the guinea‐pig colon where the frequency of peristaltic waves increased substantially in response to maintained local distension, after removal of mucosa and submucosal plexus (Spencer et al. 2011). This suggests that all of the mechanosensory elements and the neural circuits controlling CMMCs and neural peristaltic contractions must reside in the myenteric plexus and/or muscularis externa, at least in small laboratory animals. Interestingly, removal of the circular muscle layer, but not longitudinal muscle layer, has a major inhibitory effect on stretch‐activated polarized neural reflex pathways in the colon (Spencer et al. 2006) (Fig. 1 B). This is why sensory elements in the circular muscle/or connectivity between the circular muscle and myenteric plexus was determined to be critically important in activating stretch‐induced polarized reflexes (Fig. 1 B). In larger animals, such as dogs and pigs (Sanders & Smith, 1986; Furness et al. 1990; Timmermans et al. 1994; Hens et al. 2002) some motor neurons have cell bodies in the submucosal ganglia, so motility circuitry may involve the submucosal plexus in these species.
Of course given that there are nerve endings of intrinsic sensory neurons in the mucosa and that reflex responses can be elicited by mechanical and chemical stimulation of the mucosa, it is likely that neural input from the mucosa modulates the circuits underlying the neuromechanical loop.
It is likely that most of the propulsion achieved by colonic motility is mediated by the combination of CMMCs and activation of the neuromechanical loop by contents working in concert leading to propulsive movements. Both neural activities involve mechanical stimuli. While maintained distension generates cyclical enteric neural activity even in the absence of moving contents, the neuromechanical loop provides a self‐sustained propulsive mechanism. The flexibility in the speed of propulsion conferred by the neuromechanical loop may allow these patterns to propel content of a wide range of consistencies very effectively. In addition, in some conditions, extrinsic excitatory neural inputs may be required for making the enteric circuits more sensitive to content distension.
The roles of chemical factors resulting from the diet and microbiota in the modulation of these highly propulsive patterns have yet to be identified.
Role of myogenic mechanisms in colonic motility
In all species studied to date, colonic smooth muscle has rhythmic ‘myogenic’ motor activity, often with several frequency components. These are generated by networks of pacemaker ICCs (Sanders et al. 2014). We will not review here in full the role of myogenic activity, but a short summary is needed. Three main frequencies have been described in the colon of most mammalian species studied.
An intermediate frequency, generated by pacemaker cells in the deeper part of the circular muscle, drives ‘slow waves’ in the circular muscle. The likely source of these intermediate slow waves is the “…ICC at the level of the submucosal border, as demonstrated in the dog colon” (Smith et al. 1987 b). These often activate shallow contractions described as ‘ripples’ in isolated preparations of colon of experimental animals (Fig. 5) (D'Antona et al. 2001; Huizinga et al. 2011; Dinning et al. 2012 a; Costa et al. 2013 a). Ripples occur at frequencies which correspond to the intrinsic frequency of slow waves in the colon of each species. For example, in the rabbit colon, under conditions where neural activity is abolished, spatio‐temporal maps revealed ripples occurring at just under 10 min−1 and propagating short distances orally and aborally (Dinning et al. 2012 a).
Figure 5. Myogenic ‘ripples’ recorded from isolated rabbit distal colon .
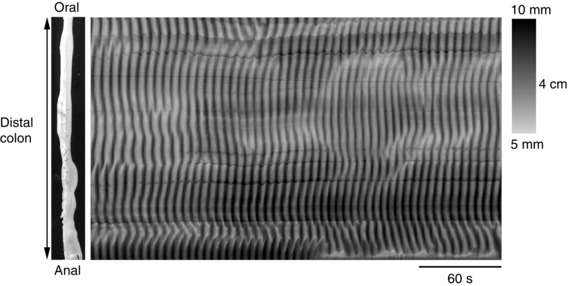
In the presence of hexamethonium and tetrodotoxin, random chaotic ripple contractions can be visualized, with initiation sites that vary and an irregular direction of propagation. Figure reproduced from Dinning et al. (2012 a).
A second faster set of oscillations is generated by pacemaker cells located near the myenteric plexus. Myogenic activity at over 30 min−1 occurs during distension of rabbit and rat colon (Lentle et al. 2008; Dinning et al. 2012 a; Costa et al. 2013 a). This probably reflects activity driven by myenteric ICCs in the colon – the so‐called ‘myenteric potential oscillations’ or ‘MPOs’ described in dog colon (Smith et al. 1987 a). These appear to play an important role in longitudinal muscle activity but may sum with intermediate frequency slow waves (Smith et al. 1987 a; Sabourin et al. 1990).
A third myogenic activity sometimes appears after blocking neural activity. It consists of slow phasic contractions, usually occurring at intervals of 1 min or more. This type of tetrodotoxin‐resistant activity has been recorded in rat colon (Pluja et al. 2001; Huizinga et al. 2011; Mane et al. 2015), rabbit colon (Dinning et al. 2012 a), dog colon (Sabourin et al. 1990) and in the guinea‐pig colonic flexure (Fujimoto et al. 2010).
In general, myogenic activity alone is not sufficient for significant propulsion of content and it is clear that a functioning enteric nervous system is essential for survival (Ro et al. 2006; Roberts et al. 2008). Interactions between the three myogenic rhythmic activities and inputs from enteric motor neurons play important roles in normal colonic motility. Distension by luminal content activates neural pathways for peristalsis or CMMCs which sum with slow‐wave‐driven ripples; this activity is readily apparent in spatio‐temporal maps of rabbit colon (Dinning et al. 2012 a). Excitatory motor neuron input to a region of muscle increases the amplitude of ripples in that same region, so that they form clusters of contractions, which can fuse into larger summated contractions (Ehrlein et al. 1982; Karaus & Sarna, 1987; Sabourin et al. 1990; Lentle et al. 2008; Dinning et al. 2012 a) although excitatory and inhibitory inputs can also alter their frequency and duration resulting in more variable rhythmic activity (Sanders & Smith, 1986).
Relation between colonic motor patterns in human and experimental animals
Studies on the cellular bases of intestinal motility indicate that humans share with other mammalian species similar fundamental mechanisms. It is therefore valuable to establish the correspondence of colonic motor patterns in humans and experimental animals. This is not a simple process; the conditions used to record human colonic motility are very different from the experimental arrangements for animal studies in vivo and in vitro.
Solely because of their amplitude, the most striking colonic motor patterns in humans are the ‘mass movements’ first described in 1909 (Holzknechtg, 1909). These are associated with powerful contractions of circular muscle which propagate down the colon; similar events have been observed in animals in vivo, including sheep (Bedrich & Ehrlein, 2001), pigs (Hipper & Ehrlein, 2001), dogs (Sarna et al. 1988) and rabbits (Ehrlein et al. 1982, 1983). Luminal infusion of a laxative induces large, single pressure waves that propagate considerable distances along the human colon (Hardcastle & Mann, 1968; Torsoli et al. 1971). These motor patterns were called ‘true peristalsis’ or ‘colonic peristalsis’, and were associated with ‘mass movements’ (Torsoli et al. 1971). These peristaltic motor patterns were neurally mediated because their initiation by bisacodyl could be blocked by prior application of the local anaesthetic lidocaine (lignocaine) (Hardcastle & Mann, 1968).
Narducci et al. recorded similar motor patterns occurring spontaneously and coined the name ‘high amplitude propagating contractions’ (HAPCs) to describe them (Narducci et al. 1987) (Fig. 6 A). These high amplitude events were infrequent, occurring between 6 and 20 times per 24 hours in normal human subjects, but were often prevalent after morning waking (Bampton et al. 2001). They often appeared shortly after a high calorie meal (Bassotti & Gaburri, 1988; Bassotti et al. 1989, 1990) and were often followed by defaecation (Kamm et al. 1992; Bampton et al. 2000). These large propagating contractile events stand out in manometric recordings of colonic motility and have received more attention than any other pattern.
Figure 6. Colonic manometry recordings made using high resolution fibre optic technology taken from healthy adult human colon .
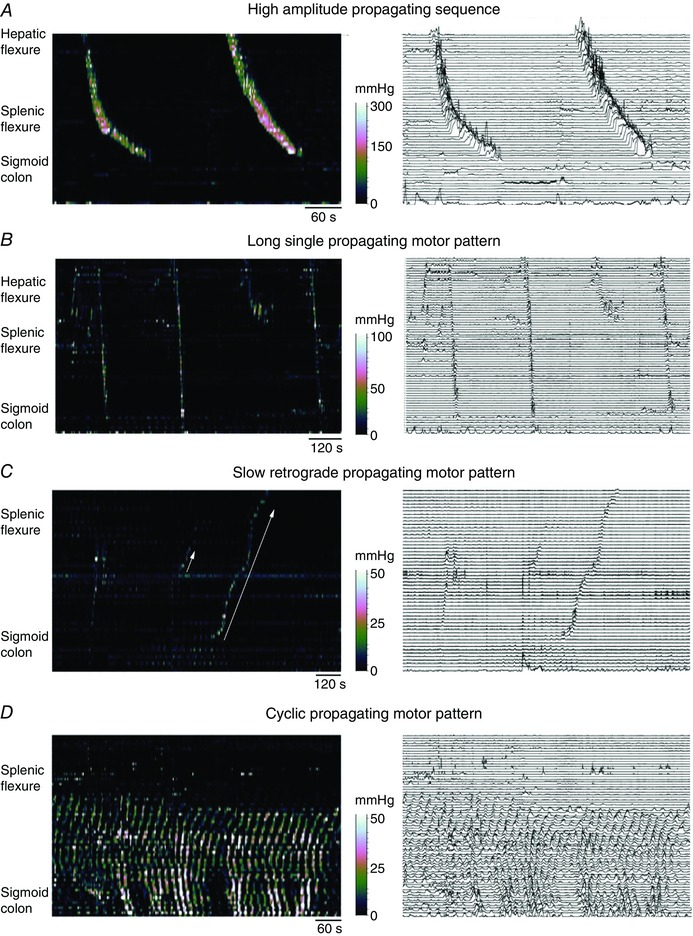
The left‐hand images are displayed as spatio‐temporal colour plots and on the right is the same image displayed as a conventional line plot. A shows the well‐described high amplitude propagating sequences. In B, three long single propagating motor patterns can be seen (black arrows). These motor patterns rapidly propagate across the transverse and descending colon and the component pressure waves have a lower amplitude than the pressure wave shown in A. In C, an example of a slowly propagating retrograde motor pattern is shown (solid white arrow). These originate in the sigmoid colon and over several minutes propagate into the transverse colon. These motor patterns appear during an unstimulated period of recording (i.e. before a meal). Preceding the slow retrograde motor patterns is a short single motor pattern (short white arrow). In D, the cyclic propagating motor pattern is shown. This is the most common postprandial propagating motor pattern. It is largely confined to the distal regions of the colon and propagates predominantly in a retrograde direction, although in this instance both antegrade and retrograde propagation can be seen. The cyclic propagating motor patterns occur at the colonic slow wave frequency of 2–4 cycles min−1. Figure constructed from data published in Dinning et al. (2014 a).
HAPCs are strikingly similar to the neurogenic peristaltic contractions evoked by fluid or pellet distension of the colon of experimental animals, in vitro and described above. Large peristaltic contractions in the human colon, as in experimental animals, are usually initiated by luminal distension, and probably modulated by mucosal stimuli. In conscious human patients, HAPCs are almost absent in the empty ‘prepared’ bowel (Dinning et al. 2014 a) suggesting that they require distension to be initiated. Further, they are likely to require extrinsic autonomic input because they can be triggered by a meal (see above) or by stress. This suggests an involvement of vagal and sacral parasympathetic pathways to the bowel (Bharucha, 2012). The distinctive nature of the HAPCs/peristaltic contractions is supported by discriminant and multivariate analysis applied to pressure waves (duration, gradient and amplitude) from high‐resolution recordings from human colon. This showed that these neurally mediated, high amplitude propagating peristaltic sequences formed a category that was clearly distinguishable from all other propagating motor patterns in the human colon (Dinning et al. 2014 a).
As described above, activation of enteric ascending excitatory and descending inhibitory neural pathways, linked in neuromechanical loops, can explain this powerful pattern of highly propulsive motility in the human colon. Consistent with this, polarized reflexes can be activated by local luminal distension in isolated specimens of human colon (Spencer et al. 2012).
Colonic migrating motor complexes in humans
In humans CMMCs have been recorded less often, but there are a few reports suggesting they can occur (Adler et al. 1941; Cook et al. 2000; Hagger et al. 2002). Hagger et al. (2002) showed repetitive small amplitude contractile complexes in ambulant studies of normal subjects. Furthermore, a recent study of human colon in vitro reported motor complexes that travelled in both directions, measured with force transducers (Spencer et al. 2012). The paucity of recordings of CMMCs in human colon may be because the associated contractions are of small amplitude, or because widely spaced sensors used in traditional manometry were unable to detect them. Some evidence of motor complexes is apparent in high resolution colonic recordings where sensors are spaced at 1 cm intervals (Fig. 7).
Figure 7. Colonic motor patterns recorded with a fibre optic manometry catheter in the sigmoid colon and rectum .
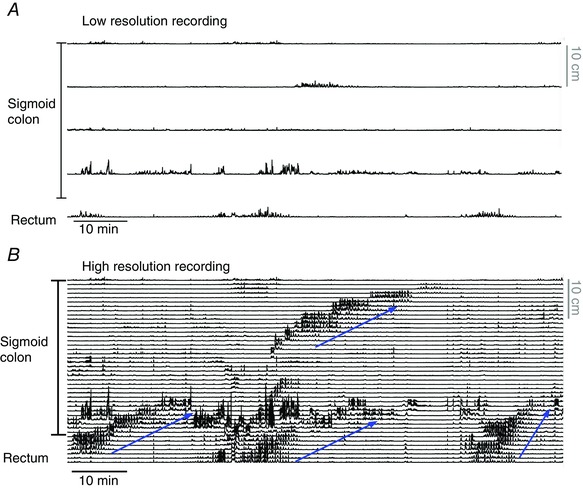
A shows the low‐resolution recording (10 cm spaced sensors) used for most colonic manometry studies. In B, the complete data set is shown. With the high resolution recording (1 cm spaced sensors) four propagating motor patterns can be seen (blue arrows indicate the actual direction of propagation). These propagating contractions may represent the colonic motor complex. Figure constructed from data published in Dinning et al. (2013).
Myogenic colonic activity in humans
As in experimental animals, in humans extensive nets of pacemaker cells generate at least three independent frequencies of myogenic activity. Cyclic contractions at an intermediate frequency of 2–8 min−1 (Narducci et al. 1987; Rao & Welcher, 1996; Dinning et al. 2014 a) are frequently recorded in human colon or rectum . These are probably due to the myogenic slow waves which have a similar frequency in human colonic tissue in vitro (Rae et al. 1998; Auli et al. 2008) and correspond to the intermediate slow wave‐generated ripples in experimental animals.
An early study in the human descending and sigmoid colons distinguished several types of pressure waves (Adler et al. 1941) which usually occurred asynchronously in adjacent balloon sensors. It was concluded that many colonic contractions lack close coordination along the colon (Adler et al. 1941). Segmental, non‐propagating, uncoordinated motor activity was believed to be the major pattern of activity between HAPCs in the healthy human colon. This description was widely accepted for the next 70 years (Narducci et al. 1987; Soffer et al. 1989; Bampton et al. 2001; Rao et al. 2001). However, in the rectum, several reports identified regular, rhythmic pressure waves (Kumar et al. 1989; Rao et al. 2001), with similar frequency (2–8 min−1) to those described by Adler (Adler et al. 1941). They proposed that this activity was stimulated by the arrival of stool or gas, and might act to retard the flow of colonic contents into the rectum (Rao & Welcher, 1996). Other studies showed that pressure waves with a similar frequency could be occasionally recorded in other regions of the colon (Kerlin et al. 1983; Soffer et al. 1989; Bampton et al. 2001). Hagger et al. (2002) provided evidence that the events can migrate either orally or aborally (Rao & Welcher, 1996).
In recent years, high resolution catheters have greatly improved the ability to detect small amplitude propagating motor patterns in the healthy adult colon. It has become apparent that earlier studies using balloons and other widely spaced sensors mis‐categorized much of the contractile activity of the human colon (Dinning et al. 2013) (Fig. 7). Indeed when colonic motor patterns are recorded with sensors spaced at ≥7 cm intervals, approximately half of all propagating motor patterns are either missed or mislabelled compared to recordings with sensor spacing of 1 cm (Dinning et al. 2013).
High resolution recordings have now shown that these intermediate frequency cyclic contractions (2–6 min−1) are the most common motor pattern in the human colon. These ripples propagate antegradely or retrogradely, or sometimes occur synchronously over several centimetres of bowel (Dinning et al. 2013, 2014 a), just as in other species (see above). As noted previously, these short‐extent, small amplitude contractions occur in all regions of the colon but are most often evident in the distal colon, sigmoid and rectum (Fig. 6 D). The predominant retrograde direction of these complexes in the sigmoid colon and rectum supports the suggestion that they resist anally directed flow and assist in the maintenance of continence and control of defaecation (Dinning et al. 2014 a).
Ingestion of a meal (Dinning et al. 2014 a) or electrical stimulation of sacral nerves (Dinning et al. 2012 b; Patton et al. 2013) rapidly increases the occurrence and amplitude of this cyclic activity, suggesting that extrinsic nerves can modulate it.
Other colonic motility patterns in humans
Three other patterns of colonic motility have been distinguished in human colon by high resolution manometry:
Short single motor pattern. As their name suggests, these are isolated patterns that propagate antegradely or retrogradely and occur in the proximal or distal colon. Their rate of propagation overlapped with cyclic myogenic contractions, as did their amplitude and duration (Fig. 6 C).
Long single motor patterns. These consist of a single pressure event which propagates along the colon at rate slightly higher than cyclic myogenic contractions (1.8 ± 1.2 cm s−1). They had similar durations to the myogenic cyclic contractions, but propagated over much longer distances. These occurred more than 1 min apart when occurring repetitively, and generally originated proximal to the mid‐descending colon (Fig. 6 B).
Retrograde slow propagating motor patterns. These were recorded in only 2 out of 10 subjects, but were a distinctive pattern, travelling slowly at less than 0.5 cm s−1 over distances exceeding 40 cm, starting in the sigmoid colon and extending into the transverse colon (Fig. 6 C).
Discriminant analysis suggested that all three of these patterns were more similar to cyclic myogenic contractions than they were to HAPCs/peristaltic contractions in terms of amplitude and duration (Dinning et al. 2014 a). This raises the possibility that the time course of the contractions may be influenced by myogenic mechanisms. However, the distinctive velocities, directions, extents and intermittency of these contractions suggest involvement of neural coordination. Exactly how neural activity and myogenic activity interact to drive these patterns is currently unclear.
In the human colon, as in other species, there are two other frequencies of myogenic activity. A faster pacemaker system with higher frequency than slow waves has been demonstrated both electrophysiologically (Rae et al. 1998) and mechanically (Carbone et al. 2013). A third type of slower myogenic activity has also been observed in human colon preparations, when neural activity is blocked. It consists of contractions of longer duration and larger amplitude (Mane et al. 2015). Isolated specimens of human colon in vitro show slow spontaneous contractions, at intervals of 0.5–5 min, called ‘slow phasic contractions’ (Carbone et al. 2013). They could be initiated prematurely by electrical activation of enteric neural pathways, which then re‐set their rhythmicity. After tetrodotoxin similar slow phasic contractions persisted, confirming that also in the human colon, as in other species, all three myogenic frequencies exist. These slow phasic contractions continued after the submucosa and mucosa had been removed, suggesting that, as in other species they were initiated by myenteric or intramuscular pacemakers. In fact, this rhythm appears to be seen most commonly in colonic preparations that lack submucosal ICCs, suggesting that it may normally be suppressed when the gut wall is intact.
The existence of the slower myogenic activity in vivo in an intact colon has not been clearly demonstrated. However, in recent high‐resolution manometry recordings a ‘pan‐colonic pressurization’ has been described (Corsetti et al. 2015). This motor pattern consists of a pressure increase of 15 ± 3 mmHg amplitude and 24 ± 4 s duration, which occurs simultaneously in all colonic sensors (Corsetti et al. 2015). These motor patterns are recorded in both healthy controls and patients with constipation and can occur in isolation or in a rhythmic pattern ranging from 3 min−1 to ∼1 min−1 (Fig. 8). The physiological role of this motor pattern in the human colon remains unknown, but it has been associated with relaxation of the internal anal sphincter (Corsetti et al. 2015).
Figure 8. Examples of pan‐colonic pressurizations recorded from descending colon of a patient with slow transit constipation, using a fibre optic manometry catheter .
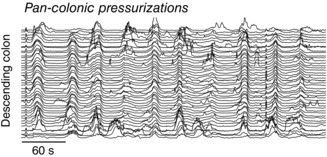
Figure constructed from data published in Dinning et al. (2015).
Complex interaction between neural inputs and myogenic mechanisms
The relationship between enteric neural circuitry and ICCs is complex. There is considerable evidence that neurotransmission from excitatory and nitrergic inhibitory enteric motor neurons to gut smooth muscle is mediated via ICCs (Sanders et al. 2010), although this has been disputed (Goyal & Chaudhury, 2010). More recent evidence, based on inducible knockdown of kit expression, has confirmed that ICCs play a critical role in motor neurotransmission, including in the colon (Klein et al. 2013). ICCs also play an important role in the stretch sensitivity of smooth muscle excitability (Won et al. 2005), another potent contributor to smooth muscle excitability (Bulbring, 1955). Enteric neurons are also capable of phase shifting or altering the frequency of myogenic activity (Smith et al. 1989; Beckett et al. 2003; Bayguinov et al. 2010).
Important challenges for future studies
The relation between enteric neural inputs and the slow phasic myogenic contractions remains one important issue to be resolved. In fact, the role of the myogenic slow phasic contractions is an area of uncertainty. Certainly, they can mimic some of the propulsive, content‐dependent contractions, but generally they show considerably less spatial coordination. As these slow large phasic myogenic contractions appear only after blocking neural activity it is possible that they are normally suppressed, presumably by enteric inhibitory circuits, and that they may emerge either in experimental conditions, or in colonic dysfunction. The contributions of the fast myogenic events is also uncertain. While they play a role in patterning longitudinal muscle contractions, their role in shaping circular muscle activity and interactions with motor neuronal input remain uncertain. The muscularis mucosa is the Cinderella of the muscle layers with little or no investigations addressing the role of this muscle layer in motility. Furthermore, how the enteric nervous system coordinates its activity to give rise to short and long‐extent contractions is another area lacking a good understanding (Fig. 9). Working out the details of the interactions between enteric neural pathway and local myogenic mechanisms promises to be a difficult but fruitful target for future study. The other major challenge for the future remains to determine how these patterns are modified in gastrointestinal disease and which mechanisms can be selectively targeted.
Figure 9. Schematic interpretation of the neuro‐mechanical loop mechanisms for propulsion of intestinal content initiated and sustained by a bolus .
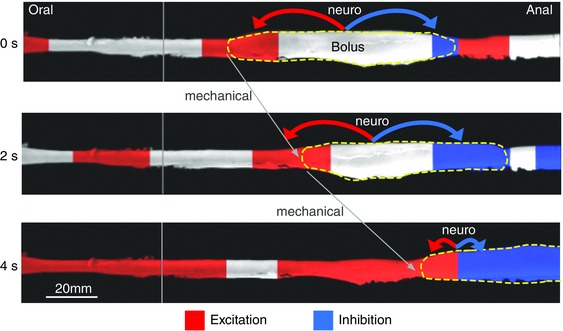
Three separate images have been taken of a bolus (yellow dashed line) moving along the rabbit distal colon at 2 s intervals. The inferred state of enteric motor neuron activity was determined from the relationships that exist between changes in diameter (video image) and the corresponding changes in intraluminal pressure (manometry). These relationships allow for the calculation of the mechanical state of the muscle (Costa et al. 2013 b; Dinning et al. 2014 b). The red regions indicate activation of excitatory motor neurons and the blue regions indicate the activation of enteric inhibitory motor neurons. The liquid bolus is propelled by oral excitation and anal inhibition, as proposed by Bayliss & Starling (1899), and predicted by the neuro‐mechanical loop mechanisms. The bolus distends the gut and activates the polarized ascending excitatory reflex pathways resulting in oral active contraction of the muscle (red) and anal active relaxation (blue). This results in a mechanical event of propulsion of the bolus in the anal direction distending a new area of intestine, then initiating neural activity of polarized enteric pathways, resulting in further propulsion of the bolus, which becomes self‐sustained.
Additional information
Competing interests
None declared.
Funding
The experiments carried out in this study were funded by grants from the National Health and Medical Research Council (NHMRC) of Australia to N.J.S. (grant nos 1067335 and 1067317) and to P.G.D. (1064835).
Biographies
Nick Spencer completed his PhD in 1998 at Monash University, Australia in the field of enteric neurogastroenterology. After his PhD he spent 10 years at the University of Nevada School of Medicine, investigating neuronal mechanisms underlying gastrointestinal motility, where he obtained independent NIH funding. Nick has published >80 peer‐reviewed research publications and in 2015 was promoted to Professor. Nick has had continuous NH&MRC funding since returning to Australia in 2008.

Phil Dinning is senior clinical scientist in the Department of Gastroenterology and Surgery at Flinders Medical Centre, Adelaide, South Australia. He has over 20 years’ experience related to the physiological investigation of human lower gastrointestinal disorders.
Simon Brookes studied insect nervous system for his PhD at Bristol University before moving to the London Hospital Medical College, then Flinders University where he has studied the nerves and motility of the gastrointestinal tract. He has studied primarily the enteric nervous system and the sensory innervation of upper and lower gut. He is currently the Head of Department of Human Physiology at Flinders University.
Marcello Costa was born in Italy where he obtained his medical degree. He moved to Australia in 1970 to a postdoc position at the Department of Zoology in Melbourne, then a foundation lecturer in the School of Medicine at Flinders in 1975, where he established one of the first multidisciplinary laboratories in neurogastroenterology.
References
- Adler HF, Atkinson AJ & Ivy AC (1941). A study of the motility of the human colon: an explanation of dysynergia of the colon, or of the ‘unstable colon’. Am J Dig Dis 8, 197–202. [Google Scholar]
- Anderson KD (2004). Targeting recovery: priorities of the spinal cord‐injured population. J Neurotrauma 21, 1371–1383. [DOI] [PubMed] [Google Scholar]
- Arkwright JW, Underhill ID, Maunder SA, Blenman N, Szczesniak MM, Wiklendt L, Cook IJ, Lubowski DZ & Dinning PG (2009). Design of a high‐sensor count fibre optic manometry catheter for in‐vivo colonic diagnostics. Opt Express 17, 22423–22431. [DOI] [PubMed] [Google Scholar]
- Auli M, Martínez E, Gallego D, Opazo A, Espín F, Marti‐Gallostra M, Jiménez M & Clavé P (2008). Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro . Br J Pharmacol 155, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ & Cook IJ (2001). Prolonged multi‐point recording of colonic manometry in the unprepared human colon: Providing insight into potentially relevant pressure wave parameters. Am J Gastroenterol 96, 1838–1848. [DOI] [PubMed] [Google Scholar]
- Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, deCarle D & Cook IJ (2000). Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am J Gastroenterol 95, 1027–1035. [DOI] [PubMed] [Google Scholar]
- Barnes KJ, Beckett EA, Brookes SJ, Sia TC & Spencer NJ (2014). Control of intrinsic pacemaker frequency and velocity of colonic migrating motor complexes in mouse. Front Neurosci 8, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassotti G, Betti C, Imbimbo BP, Pelli MA & Morelli A (1989). Colonic motor response to eating: a manometric investigation in proximal and distal portions of the viscus in man. Am J Gastroenterol 84, 118–122. [PubMed] [Google Scholar]
- Bassotti G, Bucaneve G, Betti C & Morelli A (1990). Sudden awakening from sleep: effects on proximal and distal colonic contractile activity in man. Eur J Gastroenterol Hepatol 2, 475–478. [Google Scholar]
- Bassotti G & Gaburri M (1988). Manometric investigation of high‐amplitude propagated contractile activity of the human colon. Am J Physiol 255, G660–G664. [DOI] [PubMed] [Google Scholar]
- Bayguinov PO, Hennig GW & Smith TK (2010). Ca2+ imaging of activity in ICC‐MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol 588, 4453–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM & Starling EH (1899). The movements and innervation of the small intestine. J Physiol 24, 99–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett EA, McGeough CA, Sanders KM & Ward SM (2003). Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation. J Physiol 553, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrich M & Ehrlein H (2001). Motor function of the large intestine and flow of digesta in sheep. Small Ruminant Res 42, 141–155. [Google Scholar]
- Bercik P, Bouley L, Dutoit P, Blum AL & Kucera P (2000). Quantitative analysis of intestinal motor patterns: spatiotemporal organization of nonneural pacemaker sites in the rat ileum. Gastroenterology 119, 386–394. [DOI] [PubMed] [Google Scholar]
- Bharucha AE (2012). High amplitude propagated contractions. Neurogastroenterol Motil 24, 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Costa M & Grider JR (2004). Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil 16, Suppl. 1, 34–38. [DOI] [PubMed] [Google Scholar]
- Bouchoucha M, Benard T & Dupres M (1999). Temporal and spatial rhythmicity of jejunal wall motion in rats. Neurogastroenterol Motil 11, 339–346. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Nichols K, Grasby DJ & Waterman SA (2001). Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br J Pharmacol 132, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ & Costa M (2006). Functional histoanatomy of the enteric nervous system In Physiology of the Gastrointestinal Tract, 4th edn, ed. Johnson LR, pp. 577–602. Elsevier. [Google Scholar]
- Bulbring E (1955). Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol 128, 200–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone SE, Dinning PG, Costa M, Spencer NJ, Brookes SJ & Wattchow DA (2013). Ascending excitatory neural pathways modulate slow phasic myogenic contractions in the isolated human colon. Neurogastroenterol Motil 25, 670–676. [DOI] [PubMed] [Google Scholar]
- Chen JH, Zhang Q, Yu Y, Li K, Liao H, Jiang L, Hong L, Du X, Hu X, Chen S, Yin S, Gao Q, Yin X, Luo H & Huizinga JD (2013). Neurogenic and myogenic properties of pan‐colonic motor patterns and their spatiotemporal organization in rats. PLoS One 8, e60474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggrave MJ & Norton C (2010). The need for manual evacuation and oral laxatives in the management of neurogenic bowel dysfunction after spinal cord injury: a randomized controlled trial of a stepwise protocol. Spinal Cord 48, 504–510. [DOI] [PubMed] [Google Scholar]
- Cook IJ, Furukawa Y, Panagopoulos V, Collins PJ & Dent J (2000). Relationships between spatial patterns of colonic pressure and individual movements of content. Am J Physiol Gastrointest Liver Physiol 278, G329–G341. [DOI] [PubMed] [Google Scholar]
- Corsetti M, Pagliaro G, Demedts I, Scheerens C, Rommel N, Tack J & Deloose E (2015). Pan‐colonic pressurizations associated with relaxation of the anal sphincter in man: A highly prevalent colonic motor event identified using high‐resolution manometry and associated with feeling and desire to evacuate gas. Gastroenterology 148, Suppl. 1, S‐192. [DOI] [PubMed] [Google Scholar]
- Costa M & Brookes S (2008). Architecture of enteric neural circuits involved in intestinal motility. Eur Rev Med Pharmacol Sci 12, 3–19. [PubMed] [Google Scholar]
- Costa M, Dodds KN, Wiklendt L, Spencer NJ, Brookes SJ & Dinning PG (2013. a). Neurogenic and myogenic motor activity in the colon of the guinea‐pig, mouse, rabbit and rat. Am J Physiol Gastrointest Liver Physiol 305, G749–G759. [DOI] [PubMed] [Google Scholar]
- Costa M & Furness JB (1976). The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol 294, 47–60. [DOI] [PubMed] [Google Scholar]
- Costa M, Wiklendt L, Arkwright JW, Spencer NJ, Omari T, Brookes SJ & Dinning PG (2013. b). An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Front Syst Neurosci 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Wiklendt L, Simpson P, Spencer NJ, Brookes SJ & Dinning PG (2015). Neuromechanical factors involved in the formation and propulsion of fecal pellets in the guinea‐pig colon. Neurogastroenterol Motil 27, 1466–1477. [DOI] [PubMed] [Google Scholar]
- Crema A, Frigo GM & Lecchini S (1970). A pharmacological analysis of the peristaltic reflex in the isolated colon of the guinea‐pig or cat. Br J Pharmacol 39, 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G, Hennig GW, Costa M, Humphreys CM & Brookes SJ (2001). Analysis of motor patterns in the isolated guinea‐pig large intestine by spatio‐temporal maps. Neurogastroenterol Motil 13, 483–492. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Arkwright JW, Costa M, Wiklendt L, Hennig G, Brookes SJ & Spencer NJ (2011). Temporal relationships between intraluminal manometry and gut wall movement in the isolated rabbit small intestine. Am J Physiol Gastrointest Liver Physiol 300, G577–G585. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Costa M, Brookes SJ & Spencer NJ (2012. a). Neurogenic and myogenic motor patterns of rabbit proximal, mid and distal colon. Am J Physiol Gastrointest Liver Physiol 303, G83–G92. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Hunt L, Arkwright JW, Patton V, Szczesniak MM, Wiklendt L, Davidson JB, Lubowski DZ & Cook IJ (2012. b). Pancolonic motor response to subsensory and suprasensory sacral nerve stimulation in patients with slow‐transit constipation. Br J Surg 99, 1002–1010. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Wiklendt L, Gibbins I, Patton V, Bampton PA, Lubowski DZ, Cook IJ & Arkwright JW (2013). Low‐resolution colonic manometry leads to a gross mis‐interpretation of the frequency and polarity of propagating sequences: Initial results from fibre‐optic high‐resolution manometry studies. Neurogastroenterol Motil 25, e640–e649. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Wiklendt L, Maslen L, Gibbins I, Patton V, Arkwright JW, Lubowski DZ, O'Grady G, Bampton PA, Brookes SJ & Costa M (2014. a). Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high‐resolution fiber‐optic manometry. Neurogastroenterol Motil 26, 1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinning PG, Wiklendt L, Maslen L, Patton V, Lewis H, Arkwright JW, Wattchow DA, Lubowski DZ, Costa M & Bampton PA (2015). Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre‐optic manometry. Neurogastroenterol Motil 27, 379–388. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Wiklendt L, Omari T, Arkwright JW, Spencer NJ, Brookes SJ & Costa M (2014. b). Neural mechanisms of peristalsis in the isolated rabbit colon: a neuromechanical loop hypothesis. Front Neurosci 8, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlein HJ, Reich H & Schwinger M (1982). Physiological significance of the contractions of the rabbit proximal colon. Q J Exp Physiol 67, 407–417. [DOI] [PubMed] [Google Scholar]
- Ehrlein HJ, Reich H & Schwinger M (1983). Colonic motility and transit of digesta during hard and soft faeces formation in rabbits. J Physiol 338, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis AG, Zeglinski PT, Brown DJ, Frauman AG, Millard M & Furness JB (2015). Pharmacokinetics of the ghrelin agonist capromorelin in a single ascending dose Phase‐I safety trial in spinal cord‐injured and able‐bodied volunteers. Spinal Cord 53, 103–108. [DOI] [PubMed] [Google Scholar]
- Farrugia G (2008). Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 20, Suppl. 1, 54–63. [DOI] [PubMed] [Google Scholar]
- Ferens DM, Habgood MD, Saunders NR, Tan YH, Brown DJ, Brock JA & Furness JB (2011). Stimulation of defecation in spinal cord‐injured rats by a centrally acting ghrelin receptor agonist. Spinal Cord 49, 1036–1041. [DOI] [PubMed] [Google Scholar]
- Fida R, Lyster DJ, Bywater RA & Taylor GS (1997). Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil 9, 99–107. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Shigemasa Y & Suzuki H (2010). Properties of spontaneous contractions and their modulation by transmural nerve stimulation in circular smooth muscle isolated from the pacemaker area in the flexure region of the guinea‐pig colon. J Smooth Muscle Res 46, 293–308. [DOI] [PubMed] [Google Scholar]
- Furness JB (2012). The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9, 286–294. [DOI] [PubMed] [Google Scholar]
- Furness JB, Lloyd KC, Sternini C & Walsh JH (1990). Projections of substance P, vasoactive intestinal peptide and tyrosine hydroxylase immunoreactive nerve fibres in the canine intestine, with special reference to the innervation of the circular muscle. Arch Histol Cytol 53, 129–140. [DOI] [PubMed] [Google Scholar]
- Gonzalez A & Sarna SK (2001). Neural regulation of in vitro giant contractions in the rat colon. Am J Physiol Gastrointest Liver Physiol 281, G275–G282. [DOI] [PubMed] [Google Scholar]
- Goyal RK & Chaudhury A (2010). Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol 298, G10–G13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR (1989). Identification of neurotransmitters regulating intestinal peristaltic reflex in humans. Gastroenterology 97, 1414–1419. [DOI] [PubMed] [Google Scholar]
- Hagger R, Kumar D, Benson M & Grunday A (2002). Periodic colonic motor activity identified by 24‐h pancolonic ambulatory manometry in humans. Neurogastroenterol Motil 14, 271–278. [DOI] [PubMed] [Google Scholar]
- Hardcastle JD & Mann CV (1968). Study of large bowel peristalsis. Gut 9, 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN & Brookes SJH (1999). Quantitative analysis of peristalsis in the guinea‐pig small intestine using spatio‐temporal maps. J Physiol 517, 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens J, Gajda M, Scheuermann DW, Adriaensen D & Timmermans JP (2002). The longitudinal smooth muscle layer of the pig small intestine is innervated by both myenteric and submucous neurons. Histochem Cell Biol 117, 481–492. [DOI] [PubMed] [Google Scholar]
- Hipper K & Ehrlein HJ (2001). Motility of the large intestine and flow of digesta in pigs. Res Vet Sci 71, 93–100. [DOI] [PubMed] [Google Scholar]
- Holzknechtg G (1909). Die normale Persistatlik des Kolon. Muench Med Wochenschr 47, 2401–2403. [Google Scholar]
- Huizinga JD & Lammers WJEP (2009). Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol 296, G1–G8. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M & Parsons S (2011). Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci 5, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Wade PR & Gershon MD (1996). Participation of 5‐HT3, 5‐HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 34, G849–G857. [DOI] [PubMed] [Google Scholar]
- Kamm MA, van der Sijp JR & Lennard‐Jones JE (1992). Observations on the characteristics of stimulated defaecation in severe idiopathic constipation. Int J Colorectal Dis 7, 197–201. [DOI] [PubMed] [Google Scholar]
- Karaus M & Sarna SK (1987). Giant migrating contractions during defecation in the dog colon. Gastroenterology 92, 925–933. [DOI] [PubMed] [Google Scholar]
- Keating DJ & Spencer NJ (2010). Release of 5‐hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138, 659–670.e2. [DOI] [PubMed] [Google Scholar]
- Kerlin P, Zinsmeister A & Phillips S (1983). Motor responses to food of the ileum proximal colon, and distal colon of healthy humans. Gastroenterology 84, 762–770. [PubMed] [Google Scholar]
- Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G & Saur D (2013). Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow‐wave activity. Nat Commun 4, 1630. [DOI] [PubMed] [Google Scholar]
- Kumar D, Williams NS, Waldron D & Wingate DL (1989). Prolonged manometric recording of anorectal motor activity in ambulant human subjects: evidence of periodic activity. Gut 30, 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentle RG, Janssen PW, Asvarujanon P, Chambers P, Stafford KJ & Hemar Y (2008). High‐definition spatiotemporal mapping of contractile activity in the isolated proximal colon of the rabbit. J Comp Physiol B 178, 257–268. [DOI] [PubMed] [Google Scholar]
- Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K & Nishikawa S (1992). Requirement of c‐kit for development of intestinal pacemaker system. Development 116, 369–375. [DOI] [PubMed] [Google Scholar]
- Mane N, Martinez‐Cutillas M, Gallego D & Jimenez M (2015). Enteric motor pattern generators involve both myogenic and neurogenic mechanisms in the human colon. Front Physiol 6, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuoli‐Weber G & Schemann M (2015). Mechanosensitivity in the enteric nervous system. Front Cell Neurosci 9, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narducci F, Bassotti G, Gaburri M & Morelli A (1987). Twenty four hour manometric recording of colonic motor activity in healthy man. Gut 28, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton V, Wiklendt L, Arkwright JW, Lubowski DZ & Dinning PG (2013). The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br J Surg 100, 959–968. [DOI] [PubMed] [Google Scholar]
- Pluja L, Alberti E, Fernandez E, Mikkelsen HB, Thuneberg L & Jimenez M (2001). Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol 281, G255–G266. [DOI] [PubMed] [Google Scholar]
- Powell AK & Bywater RA (2001). Endogenous nitric oxide release modulates the direction and frequency of colonic migrating motor complexes in the isolated mouse colon. Neurogastroenterol Motil 13, 221–228. [DOI] [PubMed] [Google Scholar]
- Rae MG, Fleming N, McGregor DB, Sanders KM & Keef KD (1998). Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol 510, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Sadeghi P, Beaty J, Kavlock R & Ackerson K (2001). Ambulatory 24‐h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol 280, G629–G639. [DOI] [PubMed] [Google Scholar]
- Rao SS & Welcher K (1996). Periodic rectal motor activity: the intrinsic colonic gatekeeper? Am J Gastroenterol 91, 890–897. [PubMed] [Google Scholar]
- Ro S, Hwang SJ, Muto M, Jewett WK & Spencer NJ (2006). Anatomic modifications in the enteric nervous system of piebald mice and physiological consequences to colonic motor activity. Am J Physiol Gastrointest Liver Physiol 290, G710–G718. [DOI] [PubMed] [Google Scholar]
- Roberts RR, Bornstein JC, Bergner AJ & Young HM (2008). Disturbances of colonic motility in mouse models of Hirschsprung's disease. Am J Physiol Gastrointest Liver Physiol 294, G996–G1008. [DOI] [PubMed] [Google Scholar]
- Sabourin PJ, Kingma YJ & Bowes KL (1990). Electrical and mechanical interactions between the muscle layers of canine proximal colon. Am J Physiol 258, G484–G491. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Hwang SJ & Ward SM (2010). Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588, 4621–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM & Smith TK (1986). Motoneurones of the submucous plexus regulate electrical activity of the circular muscle of canine proximal colon. J Physiol 380, 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ward SM & Koh SD (2014). Interstitial cells: regulators of smooth muscle function. Physiol Rev 94, 859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna SK (1986). Myoelectric correlates of colonic motor complexes and contractile activity. Am J Physiol 250, G213–G220. [DOI] [PubMed] [Google Scholar]
- Sarna SK, Condon RE & Cowles V (1984). Colonic migrating and non‐migrating motor complexes in dogs. Am J Physiol 9, G355–G360. [DOI] [PubMed] [Google Scholar]
- Sarna SK, Prasad KR & Lang IM (1988). Giant migrating contractions of the canine cecum. Am J Physiol 254, G595–G601. [DOI] [PubMed] [Google Scholar]
- Sia TC, Flack N, Robinson L, Kyloh M, Nicholas SJ, Brookes SJ, Wattchow DA, Dinning P, Oliver J & Spencer NJ (2013). Is serotonin in enteric nerves required for distension‐evoked peristalsis and propulsion of content in guinea‐pig distal colon? Neuroscience 240, 325–335. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB & Sanders KM (1987. a). Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol 252, C290–C299. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB & Sanders KM (1987. b). Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol 252, C215–C224. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB & Sanders KM (1989). Electrical pacemakers of canine proximal colon are functionally innervated by inhibitory motor neurons. Am J Physiol 256, C466–C477. [DOI] [PubMed] [Google Scholar]
- Soffer EE, Scalabrini P & Wingate DL (1989). Prolonged ambulant monitoring of human colonic motility. Am J Physiol 257, G601–G606. [DOI] [PubMed] [Google Scholar]
- Spencer NJ & Bywater RA (2002). Enteric nerve stimulation evokes a premature colonic migrating motor complex in mouse. Neurogastroenterol Motil 14, 657–665. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Dickson EJ, Hennig GW & Smith TK (2006). Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea‐pig distal colon. J Physiol 576, 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Kyloh M, Wattchow DA, Thomas A, Sia TC, Brookes SJ & Nicholas SJ (2012). Characterization of motor patterns in isolated human colon: are there differences in patients with slow‐transit constipation? Am J Physiol Gastrointest Liver Physiol 302, G34–G43. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk VP & Keating DJ (2011). Mechanisms underlying distension‐evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol 301, G519–G527. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Nicholas SJ, Sia TC, Staikopoulos V, Kyloh M & Beckett EA (2013). By what mechanism does ondansetron inhibit colonic migrating motor complexes: does it require endogenous serotonin in the gut wall? Neurogastroenterol Motil 25, 677–685. [DOI] [PubMed] [Google Scholar]
- Spencer NJ & Smith TK (2001). Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea‐pig distal colon. J Physiol 533, 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ & Smith TK (2004). Mechanosensory S‐neurons rather than AH‐neurons appear to generate a rhythmic motor pattern in guinea‐pig distal colon. J Physiol 558, 577–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans JP, Barbiers M, Scheuermann DW, Stach W, Adriaensen D, Mayer B & Degroodtlasseel M (1994). Distribution pattern, neurochemical features and projections of nitrergic neurons in the pig small intestine. Ann Anat 176, 515–525. [DOI] [PubMed] [Google Scholar]
- Torsoli A, Ramorino ML, Ammaturo MV, Capurso L, Paoluzi P & Anzini F (1971). Mass movements and intracolonic pressures. Am J Dig Dis 16, 693–696. [DOI] [PubMed] [Google Scholar]
- Won KJ, Sanders KM & Ward SM (2005). Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci USA 102, 14913–14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP & Spencer NJ (2011). Localization of the sensory neurons and mechanoreceptors required for stretch‐evoked colonic migrating motor complexes in mouse colon. Front Physiol 2, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]


