Abstract
Key points
Endurance trained athletes exhibit enhanced cardiovascular function compared to non‐athletes, although it is considered that exercise training does not enhance lung structure and function.
An increased pulmonary capillary blood volume at rest is associated with a higher .
In the present study, we compared the diffusion capacity, pulmonary capillary blood volume and diffusing membrane capacity responses to exercise in endurance‐trained males compared to non‐trained males.
Exercise diffusion capacity was greater in athletes, secondary to an increased membrane diffusing capacity, and not pulmonary capillary blood volume.
Endurance‐trained athletes appear to have differences within the pulmonary membrane that facilitate the increased O2 demand needed for high‐level exercise.
Abstract
Endurance‐trained athletes exhibit enhanced cardiovascular function compared to non‐athletes, allthough it is generally accepted that exercise training does not enhance lung structure and function. Recent work has shown that an increased resting pulmonary capillary blood volume (V C) is associated with a higher maximum oxygen consumption (), although there have been no studies to date examining how aerobic fitness affects the V C response to exercise. Based on previous work, we hypothesized that endurance‐trained athletes will have greater V C compared to non‐athletes during cycling exercise. Fifteen endurance‐trained athletes (HI: 64.6 ± 1.8 ml kg−1 min−1) and 14 non‐endurance trained males (LO: 45.0 ± 1.2 ml kg−1 min−1) were matched for age and height. Haemoglobin‐corrected diffusion capacity (DLCO), V C and diffusing membrane capacity (D M) were determined using the Roughton and Forster (1957) multiple fraction of inspired O2 (FIO2)‐DLCO method at baseline and during incremental cycle exercise up to 90% of peak O2 consumption. During exercise, both groups exhibited increases in DLCO, D M and V C with exercise intensity. Athletes had a greater DLCO and greater D M at 80 and 90% of compared to non‐athletes. However, V C was not different between groups during exercise. In contrast to our hypothesis, exercise V C was not greater in endurance‐trained subjects compared to controls; rather, the increased DLCO in athletes at peak exercise was secondary to an enhanced D M. These findings suggest that endurance‐trained athletes appear to have differences within the pulmonary membrane that facilitate the increased O2 demand needed for high‐level exercise.
Key points
Endurance trained athletes exhibit enhanced cardiovascular function compared to non‐athletes, although it is considered that exercise training does not enhance lung structure and function.
An increased pulmonary capillary blood volume at rest is associated with a higher .
In the present study, we compared the diffusion capacity, pulmonary capillary blood volume and diffusing membrane capacity responses to exercise in endurance‐trained males compared to non‐trained males.
Exercise diffusion capacity was greater in athletes, secondary to an increased membrane diffusing capacity, and not pulmonary capillary blood volume.
Endurance‐trained athletes appear to have differences within the pulmonary membrane that facilitate the increased O2 demand needed for high‐level exercise.
Abbreviations
- DLCO
pulmonary diffusion capacity for carbon monoxide
- DLO2
O2 diffusion in the lung
- DM
pulmonary membrane diffusing capacity
- FIO2
fraction of inspired O2
- HI‐Fit
endurance‐trained athlete subjects
- LO‐Fit
non‐endurance trained subjects
- PAP
pulmonary arterial pressure
- PAWP
pulmonary arterial wedge pressure
partial pressure of alveolar O2
- PVR
pulmonary vascular resistance
- PWP
pulmonary wedge pressure
- Q
cardiac output
- RER
respiratory exchange ratio
- TLC
total lung capacity
- VA
alveolar volume
- VC
pulmonary capillary blood volume
carbon dioxide production
oxygen consumption
maximum oxygen consumption
Introduction
With incremental exercise, pulmonary diffusion capacity (DLCO) must increase with exercise to meet the increased O2 demand; otherwise, a diffusion limitation may occur, which may lead to an increased alveolar–arterial oxygen difference, and exercise‐induced arterial hypoxemia (Stickland et al. 2013). From rest to peak exercise, diffusion capacity, as evaluated by the diffusing capacity for carbon monoxide (DLCO), increases up to 150% (Mostyn et al. 1963; Turino 1963; Warren & Jennings, 1984; Tamhane et al. 2001; Taylor et al. 2014) as a result of increases in pulmonary capillary blood volume (V C) and diffusing membrane capacity (D M) (Hsia, 2002). As cardiac output (Q) increases to meet O2 delivery requirements during incremental exercise, the increase in right ventricular pressure results in an increase in pulmonary arterial pressure (PAP) (Reeves et al. 2005), which in turn increases V C through recruitment and distension of the pulmonary capillaries (Reeves & Taylor, 2011). Capillary recruitment results in a concurrent increase in D M because previously unperfused alveoli now receive capillary blood, thereby increasing total surface area for gas exchange. Recruitment and distension of pulmonary capillaries also serves to reduce pulmonary vascular resistance (PVR), thereby attenuating the increase in pulmonary artery pressure with exercise (Stickland et al. 2013; Laughlin et al. 2013).
Endurance‐trained athletes exhibit enhanced cardiovascular function compared to non‐athletes (Stickland et al. 2006); however, it is generally accepted that exercise training does not affect lung structure and function (Hagberg et al. 1988; Womack et al. 2000; Green et al. 2008). Recent work has shown that individuals with a higher maximum oxygen consumption () have greater resting pulmonary V C (Lalande et al. 2012). Combined with data demonstrating that resting pulmonary artery and pulmonary wedge pressures (PAWP) are probably lower in athletes (Levine et al. 1991; Stickland et al. 2006), this suggests that more fit individuals may have a more distensible pulmonary circulation (Laughlin et al. 2013). These findings would suggest that there might be differences in the pulmonary vasculature between endurance trained vs. untrained subjects that may be related to . Because endurance‐trained athletes have a greater , they would require a greater ability to increase diffusion capacity with exercise to prevent or limit a diffusion limitation and gas exchange impairment. Although it has traditionally been assumed that the pulmonary vasculature is insensitive to exercise training (Johnson et al. 1960; Stickland et al. 2006; Green et al. 2008), and that pulmonary capillary recruitment plateaus at a critical exercise intensity and/or recruitment is not affect by training (Warren et al. 1991), recent work conducted at rest suggests that the exercise response may be different between trained and untrained individuals (Lalande et al. 2012).
There are a number of studies examining diffusion capacity during exercise (Mostyn et al. 1963; Warren & Jennings, 1984; Tamhane et al. 2001; Taylor et al. 2014). However, none of these studies have looked at exercise above 80% of , and thus it is not fully understood exactly how V C and D M contribute to the increased DLCO during exercise, or how aerobic fitness may modulate this response. We hypothesized that endurance‐trained athletes would have an increased DLCO, V C, and D M compared to non‐athletes during exercise. To test this, we adapted the Roughton and Forster (1957) multiple fraction of inspired O2 (FIO2)–DLCO technique (Roughton & Forster, 1957) to determine the effect of aerobic fitness on DLCO, V C, and D M during incremental exercise. If DLCO, and thus V C and/or D M, were found to be higher during exercise in highly fit athletes, this would suggest that an element of pulmonary diffusion is enhanced in these individuals, facilitating the increased O2 demand needed for high‐level exercise.
Methods
Ethical approval
All subjects provided their written, informed consent to the study, which was approved by the Human Research Ethics Board of the University of Alberta. The study conformed with the standards set by latest revision of the Declaration of Helsinki.
Fourteen non‐endurance‐trained males (LO: mean ± SD: 45.0 ± 4.4 ml kg−1 min−1) and 15 endurance‐trained males (HI: : 64.6 ± 6.9 ml kg−1 min−1) volunteered for the present study. All subjects were non‐smokers, had normal pulmonary function and had no history of pulmonary or cardiovascular disease. Subject characteristics and pulmonary function data are presented in Table 1.
Table 1.
Subject characteristics and pulmonary function
| LO‐Fit | HI‐Fit | |||
|---|---|---|---|---|
| Mean | % Predicted | Mean | % Predicted | |
| N | 14 | 15 | ||
| Age (years) | 26.1 ± 1.8 | 26.7 ± 6.8 | ||
| Height (m) | 1.77 ± 0.02 | 1.77 ± 0.02 | ||
| Mass (kg) | 85.5 ± 4.9 | 77.9 ± 2.0 | ||
| (l min−1) | 3.81 ± 0.18 | 113.4 ± 5.6 | 5.04 ± 0.20* | 152.4 ± 7.4* |
| (ml kg−1 min−1) | 45.0 ± 1.2 | 99.0 ± 3.1 | 64.6 ± 1.8* | 144.5 ± 5.4* |
| TLC (litres) | 6.99 ± 0.24 | 105.3 ± 2.6 | 7.69 ± 0.25 | 113.4 ± 2.8 |
| FEV1 (litres) | 4.38 ± 0.14 | 96.6 ± 2.8 | 5.04 ± 0.18* | 109.6 ± 3.3* |
| FVC (litres) | 5.56 ± 0.17 | 102.0 ± 2.7 | 6.28 ± 0.20* | 112.4 ± 2.5* |
| FEV1/FVC (%) | 78.9 ± 1.4 | 95.4 ± 1.6 | 80.1 ± 1.4 | 97.2 ± 1.8 |
Values are expressed as the mean ± SE. FEV1, forced expired volume in 1 s; FVC, forced vital capacity. *Significantly greater than the LO‐Fit group (P < 0.05).
Study design overview
At a preliminary testing session, subjects underwent an incremental test of on a cycle ergometer to determine their aerobic fitness. At least 48 h later, subjects returned to the laboratory for the exercise DLCO sessions. Subjects performed multiple‐FIO2 DLCO manoeuvres when exercising at power outputs corresponding to 30%, 50%, 70%, 80% and 90% of their previously determined . With three FIO2 and five exercise intensities, each subject performed a minimum of 15 DLCO manoeuvres. The DLCO sessions were spread out over 3 days, with the order of workloads and the FIO2 randomized.
Preliminary testing
Subjects reported to the laboratory and their completed physical activity readiness questionnaires were screened for any cardiopulmonary disorders and/or medications, and then the subjects completed resting pulmonary function testing. Subjects then performed a test for (Encore229 Vmax; SensorMedics, Yorba Linda, CA, USA) using an incremental test to volitional fatigue on a cycle ergometer (Ergoselect II 1200; Ergoline, Blitz, Germany). Initial power output was set to 50 W and the power output was increased by 25 W every 2 min until ventilatory threshold, and each stage above ventilatory threshold was characterized by increments of 25 W every 1 min. Confirmation of required that three of four conditions were satisfied: volitional exhaustion; a respiratory exchange ratio (RER) greater than 1.1; increases in oxygen consumption <100 ml min−1 with further increase in power output; and reaching age‐predicted maximum heart rate. Additionally, Q was evaluated using impedance cardiography during the incremental exercise test (PhysioFlow; Manatec Biomedical LLC, Ebersviller, France).
Exercise DLCO sessions
No less than 48 h after preliminary testing, subjects returned to the laboratory for further testing. Lung diffusing capacity for carbon monoxide (DLCO) was determined using the single‐breath breath‐hold technique (MacIntyre, 2005) at baseline and during exercise (Encore V62J Autobox; SensorMedics, Yorba Linda, CA, USA). Haemoglobin concentration ([Hb]) was measured at the beginning of each session (HemoCue 201+; HemoCue AB, Angelholm, Sweden) to correct DLCO for [Hb] using the equation (Marrades et al. 2011): DLCOadj = DLCO × . To calculate V C and D M, DLCO breath holds at three different FIO2 values (0.21, 0.40, 0.60) were performed during steady‐state at each exercise intensity, with 2 min of washout time between trials. Methane (0.3%) was used in each gas mixture to determine alveolar volume (V A) and gas equilibration.
The order of the FIO2 and exercise intensity was randomized, and completed over three different days, separated by at least 48 h, to minimize COHb build up and fatigue. Prior to data collection, subjects were coached in the proper breath hold manoeuvre. Immediately prior to each DLCO manoeuvre, each subject pre‐breathed five breaths of gas from a Douglas bag at the respective FIO2 to the specific DLCO gas to be used to ensure alveolar PO2 was stable. After pre‐breathing, subjects were instructed to inhale to total lung capacity (TLC), and to perform a breath hold for 6 s, avoiding Valsalva or Müllerian manoeuvres. During the exhalation, the methane tracing was monitored to ensure that the slope was horizontal, indicating that the test gas was well equilibrated in the lung. The trial was repeated if V A for a trial and/or breath hold time was not within 5% of previous trials. The V A for each individual trial was similar at baseline, submaximal exercise and peak exercise (Table 2).
Table 2.
Physiological responses at baseline and during exercise at 70% and 90% of
| Baseline | 70% | 90% | ||||
|---|---|---|---|---|---|---|
| LO | HI | LO | HI | LO | HI | |
| PO (W) | 185 ± 10 | 263 ± 13* | 254 ± 12 | 347 ± 17* | ||
| (l min−1 ) | 0.49 ± 0.04 | 0.55 ± 0.05 | 2.62 ± 0.16 | 3.51 ± 0.19* | 3.43 ± 0.20 | 4.51 ± 0.23* |
| (l min−1) | 0.42 ± 0.04 | 0.46 ± 0.04 | 2.63 ± 0.17 | 3.37 ± 0.15* | 3.80 ± 0.21 | 4.80 ± 0.23* |
| RER | 0.88 ± 0.08 | 0.88 ± 0.08 | 1.00 ± 0.02 | 0.96 ± 0.03 | 1.11 ± 0.02 | 1.07 ± 0.03 |
| V E (l min−1) | 14.8 ± 1.2 | 15.2 ± 1.2 | 67.7 ± 4.8 | 91.1 ± 4.8* | 109.0 ± 8.0 | 146.1 ± 9.1* |
| V T (litres) | 0.82 ± 0.04 | 0.95 ± 0.06* | 2.24 ± 0.15 | 3.03 ± 0.21* | 2.82 ± 0.18 | 3.40 ± 0.17* |
| RR (breaths min−1) | 17.2 ± 2.2 | 16.4 ± 1.6 | 28.8 ± 3.1 | 30.8 ± 1.8 | 36.8 ± 4.3 | 43.3 ± 2.3 |
| V A (litres) | 6.68 ± 0.26 | 7.55 ± 0.31* | 6.67 ± 0.20 | 7.49 ± 0.26* | 6.87 ± 0.22 | 7.64 ± 0.29* |
| Q (l min−1) | 7.6 ± 0.4 | 7.2 ± 0.4 | 17.5 ± 1.2 | 20.0 ± 1.3* | 19.9 ± 1.2 | 22.2 ± 1.7* |
| SV (ml) | 99 ± 5 | 108 ± 8 | 119 ± 7 | 126 ± 9 | 117 ± 8 | 123 ± 9 |
| HR (breaths min−1) | 77 ± 3 | 68 ± 4* | 147 ± 5 | 159 ± 4* | 173 ± 6 | 179 ± 3 |
| DLCO/V A | 5.1 ± 0.3 | 5.0 ± 0.2 | 7.4 ± 0.4 | 7.2 ± 0.3 | 7.4 ± 0.3 | 7.7 ± 0.3 |
| V C/V A | 12.9 ± 0.8 | 13.5 ± 0.6 | 18.8 ± 1.5 | 18.9 ± 0.9 | 20.8 ± 1.6 | 20.3 ± 1.2 |
| D M/V A | 11.4 ± 1.0 | 10.3 ± 0.9 | 16.6 ± 1.3 | 16.7 ± 1.8 | 14.8 ± 1.4 | 18.2 ± 1.8* |
| DLCO/Q | 4.6 ± 0.3 | 5.4 ± 0.3* | 2.9 ± 0.2 | 2.9 ± 0.1 | 2.6 ± 0.2 | 2.7 ± 0.2 |
| V C/Q | 11.4 ± 0.9 | 14.4 ± 0.9* | 7.21 ± 0.5 | 7.36 ± 0.6 | 7.3 ± 0.6 | 6.9 ± 0.4 |
| D M/Q | 10.3 ± 1.2 | 10.9 ± 0.9 | 6.8 ± 0.5 | 6.6 ± 0.6 | 5.1 ± 0.4 | 6.6 ± 0.9* |
Values are the mean ± SE. PO, power output; V E, minute ventilation; V T, tidal volume; RR, respiratory rate; SV, stroke volume; HR, heart rate. *Significantly greater than the LO‐Fit group (P < 0.05).
Pulmonary V C and D M were determined using the Roughton and Forster (1957) multiple‐FIO2 DLCO breath‐hold technique with the equation: 1/DLCO = + and θCO (i.e. the reaction rate of CO with haemoglobin) was calculated using the equation: = 0.0058 × + 0.73 (Roughton & Forster, 1957). was calculated from the equation: = FIO2(P Bar – ) – × .
As with previous studies (Sansores et al. 1995; Smith et al. 2015), we did not correct for carboxyhaemoglobin because subjects were non‐smokers (Sansores et al. 1995; West, 2012) and 2 min between DLCO breath‐hold tests was shown to be sufficient to clear CO from the lungs (Blakemore et al. 1957). Also, there is evidence that multiple short breath hold manoeuvres (∼5 s) do not appreciably decrease DLCO until COHb is greater than 6% (Zavorsky, 2013). Finally, exercise promotes clearance of CO from the lungs and blood (Zavorsky et al. 2012).
Partial pressure of arterial CO2 () was estimated from end‐tidal CO2 values. For each workload, the relationship between 1/DLCO and 1/θ for the three FIO2 values were plotted, and a regression equation was calculated. The minimum acceptable r 2 value was set to 0.95, and DLCO manoeuvres were repeated when r 2 values were outside of this range. Values for 1/V C (slope) and 1/D M (y‐intercept) were then determined (Roughton & Forster, 1957).
Statistical analysis
For all inferential analyses, the probability of a type I error was set at 0.05. Group data for each variable are expressed as the mean ± SE unless otherwise indicated. Statistical analysis was performed using two‐way repeated measures ANOVA (SigmaPlot, version 11.1; Systat Software, Inc., San Jose, CA, USA) to evaluate the effect of aerobic fitness (HI‐Fit vs. LO‐Fit), on the diffusion capacity response (dependent variables: DLCO, D M, V C) to exercise (six levels of exercise: baseline, 30%, 50%, 70%, 80% and 90% of ). Where there were main effects of exercise intensity, Fisher's least significant difference was performed to determine whether there was a plateau effect. Finally, three pre‐planned comparison t tests were performed as post hoc tests to determine differences between HI‐ and LO‐Fit subjects in DLCO, D Mand V C at baseline, 70% and 90% of .
Results
All subjects tolerated the study phases and procedures well. Descriptive characteristics of all participants are provided in Table 1.
Effect of fitness on exercise DLCO
At baseline, DLCO was higher in the HI‐Fit group compared to the LO‐Fit group (P = 0.047). With incremental exercise, both LO‐ and HI‐Fit subjects exhibited an increased DLCO with increasing oxygen consumption (P < 0.001). DLCO was higher in HI‐Fit subjects compared to LO‐Fit subjects at 70% (P = 0.028) and 90% (P = 0.013) of (Fig. 1).
Figure 1. Diffusing capacity response to exercise .
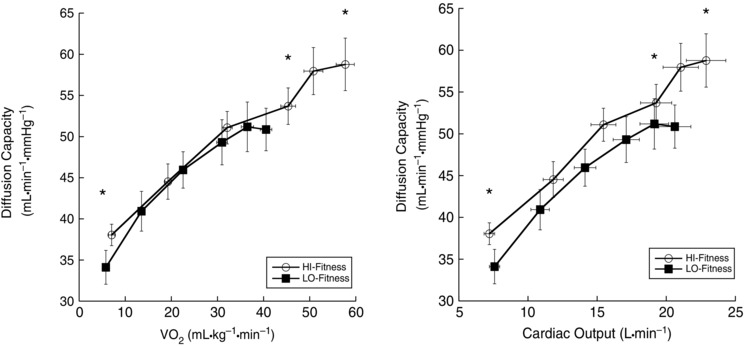
*DLCO was greater in HI‐Fit subjects at baseline (P = 0.047), at 70% (P = 0.028) and at 90% of (P = 0.013) compared to LO‐Fit subjects.
Although TLC was not statistically different between groups (P = 0.052), V A was significantly larger in HI‐Fit (mean ± SE: HI‐Fit 7.55 ± 0.31; LO‐Fit 6.68 ± 0.26 l, P = 0.013). When DLCO was corrected for V A, both HI‐ and LO‐Fit groups increased DLCO/V A with exercise intensity, although there were no differences between HI‐ and LO‐Fit groups (Table 2). When DLCO was expressed relative to Q, DLCO/Q was greater in HI‐Fit at baseline (P = 0.019) but not at 70% (P = 0.335) or 90% (P = 0.459) of (Table 2).
Pulmonary V C
At baseline, V C was higher in the HI‐Fit group compared to the LO‐Fit group (P = 0.005). With incremental exercise (Fig. 2), both LO‐ and HI‐Fit subjects increased V C with increasing oxygen consumption (P < 0.001), although there was no statistical difference between groups during exercise (P = 0.498). When V C was expressed relative to Q, VC/Q was greater in HI‐Fit at baseline (P = 0.002) but not at 70% (P = 0.403) or 90% (P = 0.229) of (Table 2).
Figure 2. Pulmonary VC response to exercise .
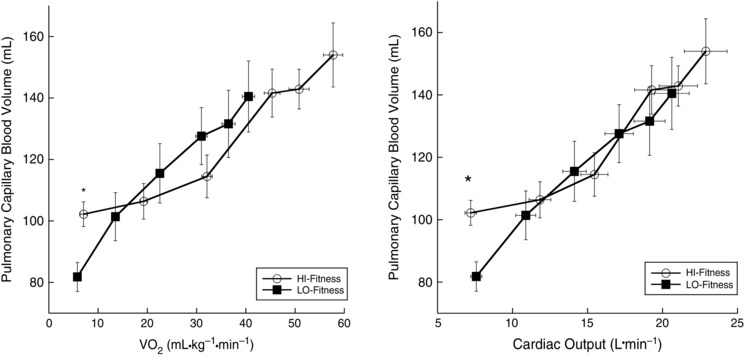
*V C was significantly higher in HI‐Fit subjects compared to LO‐Fit subjects only at baseline (P = 0.005).
There was a significant, positive correlation between baseline pulmonary V C and individual (r = 0.509, P = 0.004) (Fig. 3 A), although pulmonary V C at the 90% workload was not correlated with individual (r = 0.012, P = 0.949) (Fig. 3 B). When plotted against peak Q, both baseline V C (r = 0.573, P = 0.001) (Fig. 4 A) and peak V C were significantly correlated with peak Q (r = 0.368, P = 0.049) (Fig. 4 B).
Figure 3. Correlations of individual VC and DM to .
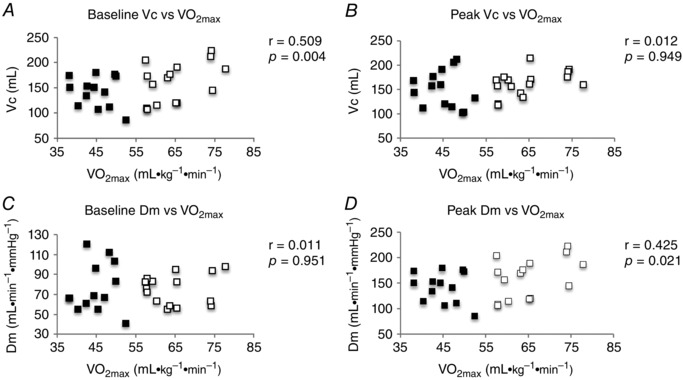
A, baseline V C. B, V C at 90% . C, Baseline D M. D, D M at 90% . Black squares, LO‐Fit group; white squares, HI‐Fit group.
Figure 4. Correlations of individual DM and VC to peak Q .
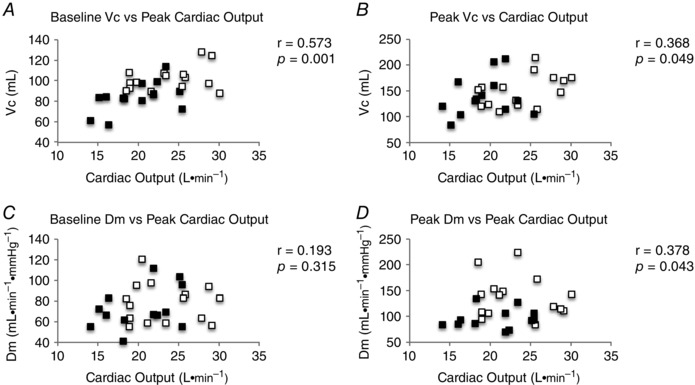
A, baseline V C. B, V C at 90% . C, Baseline D M. D, D M at 90% . Black squares, LO‐Fit group; white squares, HI‐Fit group.
Membrane D M
At baseline, D M was not different between HI‐Fit and LO‐Fit groups (P = 0.83). With incremental exercise, both LO‐ and HI‐Fit subjects increased D M with increasing oxygen consumption (P < 0.001). During exercise at 70% and 90% of , HI‐Fit subjects had a greater D M compared to LO‐Fit subjects (Fig. 5). Likewise, D M/VA was greater at peak exercise in HI‐Fit as compared to LO‐Fit subjects. Both groups exhibited a plateau in their respective D M responses to exercise, with HI‐Fit showing no significant increase in D M above 50% of (P = 0.582) and, in LO‐Fit, after 30% of (P = 0.655). When D M was expressed relative to Q, D M/Q was not different at baseline (P = 0.320) or 70% (P = 0.401), although D M/Q was greater in the HI‐Fit group at 90% (P = 0.048) of (Table 2).
Figure 5. DM during exercise .
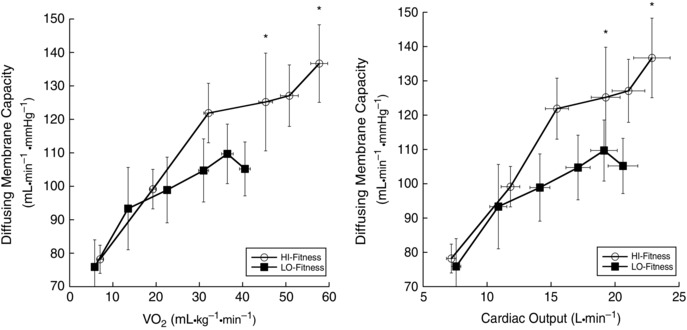
*D M was significantly greater in HI‐Fit subjects compared to LO‐Fit subjects during exercise at 70% (P = 0.035) and at 90% of (P = 0.006).
Unlike V C, baseline D M was not correlated with (r = 0.011, P = 0.951) (Fig. 3 C); however, D M at the 90% workload was correlated with (r = 0.425, P = 0.021) (Fig. 3 D). Baseline D M was not correlated with peak Q (r = 0.193, P = 0.315) (Fig. 4 C); however, peak D M was significantly correlated with peak Q (r = 0.378, P = 0.043) (Fig. 4 D).
Discussion
The present study examined the effect of aerobic fitness on DLCO, pulmonary V C, and D M response during exercise. Consistent with previous work, the pulmonary V C in athletes was higher than in non‐athletes at baseline, and V C was related to (Lalande et al. 2012). However, in contrast to our hypothesis, we found that the greater exercise DLCO in endurance‐trained athletes is not secondary to an increased pulmonary V C but rather to an increased D M. These data suggest that endurance‐trained athletes appear to have differences within the alveolar‐capillary membrane that facilitate the increased O2 delivery needed at peak exercise.
Diffusion capacity during exercise
In the present study, we found that athletes have a greater DLCO at baseline and during exercise compared to non‐athletes. Aside from pulmonary V C and D M, diffusion capacity is affected by [Hb] and V A (Roughton & Forster, 1957; Hlastala et al. 1976; Rose et al. 1979). To determine the difference in V C and D M between athletes and non‐athletes, we aimed to minimize these confounding factors by correcting DLCO for [Hb] (Marrades et al. 2011) and V A. Interestingly, despite being matched for height, the HI‐Fit group had greater V A, and the between‐group differences in DLCO disappeared when expressed relative to V A, suggesting that the enhanced DLCO in endurance‐trained athletes stems from a larger alveolar volume (Table 1). This is consistent with work in high‐level swimmers showing an increased V A compared to control subjects (Armour et al. 1993). Importantly, however, peak D M remains greater in the HI‐Fit subjects when correcting for V A (Table 2), indicating that the differences in membrane diffusion are independent of alveolar volume.
Pulmonary V C
The increase in diffusion capacity with exercise results from increases in gas exchange surface area, with increasing D M and pulmonary V C, secondary to recruitment and distension of capillaries (Johnson et al. 1960). It was previously theorized that pulmonary V C may plateau despite an increasing Q because of a morphological limitation of pulmonary V C expansion, resulting in a decreased pulmonary capillary transit time with increasing exercise intensity, which may predispose athletes to a diffusion limitation (Warren et al. 1991). In the present study, V C was not significantly different between HI‐ or LO‐Fit subjects with incremental exercise, and there was no apparent plateau in V C in either group. The lack of plateau in V C is consistent with previous work in humans (Mostyn et al. 1963; Warren et al. 1991; Johnson & Hsia, 1994), and animals (Wagner & Latham, 1975; Hsia et al. 1993, 1994) showing a lack of plateau in DLCO up to 80% of , and suggests that reserves for diffusion and V C are substantial and not completely exhausted with maximal exercise (Johnson et al. 2010).
D M
Fick's first Law of Diffusion (1855) states that diffusion of gas across a membrane is determined by: (1) surface area; (2) thickness of the membrane; (3) difference in partial pressure across the membrane; and (4) the diffusing property of the gas (Fick, 1855). In the present study, we observed an increase in D M in both HI‐ and LO‐Fit groups during incremental exercise. D M reflects the available alveolar‐capillary surface area for gas exchange (Hsia, 2002; Laughlin et al. 2013) and is increased by unfolding and distension of alveolar septa during lung inflation, and recruitment of capillaries associated with previously un‐perfused alveoli (Hsia, 2002). D M was greater during exercise in HI‐Fit participants compared to LO‐Fit participants, with the greatest difference seen at maximal exercise. There are a number of possible reasons that may explain why HI‐Fit participants are able to increase their D M more than LO‐Fit participants at high levels of exercise. Alveolar volume can affect D M (Gehr et al. 1978; Colebatch & Ng, 1992); however, D M/V A at peak exercise was still greater in HI‐Fit participants (Table 2), suggesting that there may be a morphological difference in the lungs of HI‐Fit athletes beyond having larger lungs that contributes to the increased D M. Because D M cannot be measured in un‐perfused alveoli, this may suggest that D M represents pulmonary capillary recruitment (Lewis et al. 1958; Johnson & Hsia, 1994; Lalande et al. 2012; Bartesaghi et al. 2014) and that capillary recruitment may be greater in endurance‐trained athletes.
As previously mentioned, the increase in pulmonary artery pressure and Q during exercise recruits previously unperfused capillaries, thereby increasing membrane conductance (i.e. D M). Computational modelling would suggest that the increase in Q with exercise also improves regional erythrocyte spacing (haematocrit), which may account for 30–50% of the increase in D M from rest to peak exercise (Hsia et al. 1999; Hsia, 2002). Therefore, Q may play a role in the increased D M in HI‐Fit subjects. As conceptualized by Hsia et al. (1992), the effectiveness of pulmonary vascular recruitment and diffusion during exercise can be evaluated by the ratio of DLCO/Q. The DLCO/Q ratio progressively declines with incremental exercise, and would contribute to gas exchange impairment once DLCO/Q drops below a critical value (Hsia, 2002). By extension, evaluating D M and V C relative to Q would normalize these variables for the greater Q in HI‐Fit subjects. The current data show that DLCO/Q and V C/Q are similar between HI‐ vs. LO‐Fit subjects during exercise; however, D M/Q was greater in HI‐Fit subjects at peak exercise (Table 2). These results demonstrate that the increased D M observed in HI‐Fit at peak exercise is not explained by a greater Q.
It is accepted that in healthy subjects is limited by O2 availability (Wagner, 1996 a). Although Q is predominant in O2 delivery, a theoretical analysis of the factors that determine found that O2 diffusion in the lung (DLO2) was as influential as Q in determining (Wagner, 1996 a, 1996 b). Based on the principle of mass‐balance for O2 exchange, highly fit individuals with a high require a greater DLO2 compared to less fit individuals (Wagner, 1996 a). A failure to increase DLO2 relative to the increased O2 demand during exercise would result in a gas exchange impairment (Stickland et al. 2013). In this context, the finding that highly fit athletes have an increased pulmonary membrane diffusing capacity compared to less fit subjects (Fig. 5), and that D M at peak exercise is correlated with (Fig. 3 D), is entirely consistent with the importance of O2 supply relative to O2 delivery in the determination of (Wagner, 1996 a).
Pulmonary vascular adaptation in athletes
The initial increase in pulmonary artery pressure with exercise is considered to recruit and distend pulmonary capillaries previously under‐perfused at rest (Johnson & Hsia, 1994; Hsia, 2002; Reeves & Taylor, 2011), increasing the cross‐sectional area of the pulmonary capillary network, reducing pulmonary vascular resistance and increasing V C (Short et al. 1996; La Gerche et al. 2010; Reeves & Taylor, 2011; Lalande et al. 2012). Although the individual contribution of capillary recruitment or distension to the overall increase in V C has yet to be determined (Staub et al. 1962; Reeves et al. 2005), a greater resting V C has been attributed to an enhanced pulmonary vascular distensibility and, subsequently, a higher (Lalande et al. 2012). There is evidence that athletes have the same or lower left arterial pressure (pulmonary wedge pressure; PWP) compared to non‐athletes at rest and during exercise, as well as similar or lower PAP (Levine et al. 1991; Stickland et al. 2006). Pressure within the pulmonary capillaries is uncertain, although the best estimation is approximately half the difference between PAP and PWP (West, 2012). Thus, an elevated resting V C at similar Q, but lower pressure within the pulmonary capillaries would suggest that athletes have a more compliant pulmonary vasculature compared to non‐athletes, and that this enhanced pulmonary compliance may be related to increased (Fig. 3 A). Work by La Gerche et al. (2010) demonstrated that pulmonary vascular compliance is important in decreasing PVR, preventing excessive right ventricular afterload, and thereby enhancing right ventricular function and Q during exercise. Thus, the response of the pulmonary vasculature to increasing pulmonary artery pressures during exercise will directly affect Q and, ultimately, . Taken together, these studies suggest that endurance‐trained athletes may have beneficial adaptations within the pulmonary vasculature that enable greater Q and .
Recent work by Brown et al. (2015) shows that lung density is positively correlated to diffusion capacity, and lung size is negatively correlated to lung density. This suggests that the number of alveolar units are very similar between large and small lungs in healthy humans (Brown et al. 2015) and, thus, when normalized for the larger alveolar size (and presumably surface area), it would be expected that the athlete lung would have a decreased diffusion capacity, and thus decreased D M. However, in the present study, DLCO and D M were greater in HI‐Fit athletes during exercise. It is possible that endurance‐trained athletes may have thinner alveolar‐capillary membranes to compensate for a decreased lung density; however, this remains speculative without histological evaluation of membrane thickness.
The results of the present study are consistent with other evidence that endurance‐trained athletes have greater diffusion capacity at rest (Armour et al. 1993; Degens et al. 2013) and during exercise (Mostyn et al. 1963; Armour et al. 1993; Degens et al. 2013). However, none of these studies have examined DLCO responses to exercise above 80% of , and only one has measured V C and D M during exercise (Mostyn et al. 1963). Mostyn et al. (1963) studied DLCO, V C, and D M in athletes at rest and during steady‐state treadmill exercise at a mean of 2.0 l min−1, finding that athletes have an increased DLCO during submaximal exercise secondary to an increased D M, and not an increased V C (Mostyn et al. 1963). However, this previous investigation pre‐breathed gas containing FIO2 of 0.6 and 1.0 for 5 min before performing the respective DLCO breath hold (Mostyn et al. 1963), which may affect V C because exposure to high O2 has been shown to alter the distribution of pulmonary blood (Ley et al. 2007). Although the results of the present study are consistent with the findings of this landmark study (Mostyn et al. 1963), our methodology provides greater detail of the DLCO, D M and V C responses from baseline to near‐maximal exercise.
Study limitations
It is assumed that θco does not change with exercise (Johnson et al. 1960) because there is evidence showing that θco is relatively insensitive to changes in pH (Roughton & Forster, 1957). When Roughton and Forster (1957) first introduced their method of estimating V C and D M, the recommended values for α (temperature‐ and pH‐dependent coefficient linked to kinetic reactions of CO with Hb) and β (i.e. ratio of red‐cell membrane to red cell interior permeability) were not explicitly given for the calculation of θco. As a result, several studies have used different variants of these constants (Hsia, 2002; Ceridon et al. 2010; Smith et al. 2015), ultimately affecting their respective V C and D M calculations. The assumption of a pH of 8.0 was addressed by Forster (1987) in a subsequent study, providing a correction for α that accounts for a pH of 7.4. More recently, Ceridon et al. (2010) employed Forster's corrected α, and found highly improbable values for D M in all subjects. For this reason, the present study used α = 0.0058 and β = 0.73 as recommended by Ceridon et al. (2010), which probably provides the best estimation of V C and D M during exercise. Regardless of the assumptions for θco determination, the same value was used for both trained and untrained groups, and therefore the calculated differences in V C and D M are probably not a result of the value given for θco.
The American Thoracic Society (1995) guidelines for DLCO measurements recommend a 10 s single breath‐hold technique. The present study employed a 6 s single breath hold because our subjects had difficulty with relatively long breath holds during high intensity exercise, and thus it is possible that our measurement of DLCO may be underestimated. However, a 6 s breath hold has not been shown to affect single breath DLCO measurement in healthy individuals (Graham et al. 1985). Importantly, all participants in the present study followed the same technique, and thus the shortened breath‐hold time would probably not have affected between‐group differences.
Every 1% increase in CO backpressure (evaluated by % COHb) would diminish DLCO by 1% (Cotes et al. 1972; Graham et al. 2002). However, we did not adjust for CO backpressure, consistent with other reports in the literature (Sansores et al. 1995; Smith et al. 2015). We conducted additional pilot work and determined that COHb increases less than 3% over the course of a typical study day, whereby subjects perform six DLCO manoeuvres during exercise. Based on these results, as well as previous studies (Graham et al. 2002; Ceridon et al. 2010), the resultant effect on V C and D M calculation is less than 3% and probably inconsequential.
Although we corrected DLCO for baseline (resting) [Hb] (Marrades et al. 2011), there are reports suggesting that exercise causes an increase in [Hb], with the increase similar in both sedentary and endurance trained subjects (Martin et al. 1985). In pilot work, we found a 1.6% increase in [Hb] with exercise, which, if unaccounted for, would underestimate D M and V C by 3% and 5%, respectively. Importantly, there is no evidence that the [Hb] response to exercise is different in trained individuals (Martin et al. 1985).
The present study was designed to minimize the potential effect of increasing COHb and exercise [Hb] on DLCO, V C, and D M measurements: (1) the order of FIO2‐DLCO manoeuvres was randomized; (2) the order of exercise intensity was also randomized; (3) at least 2 min separated DLCO manoeuvres; (4) subjects did not perform more than six breath holds in a single testing session; (5) data collection was spread over 3 days (with at least 1 week between tests); and (6) importantly, there is no evidence that the rise in COHb following multiple DLCO manoeuvres is different in highly fit athletes compared to sedentary subjects, and thus the between‐group differences observed in the present study would not be obscured by an increasing COHb. Therefore, although the CO back‐pressure and [Hb] changes with exercise may have had a minor effect on the data, these potential changes would not have been sufficient to explain the differences observed between our LO and HI‐Fit groups.
The baseline measurements of DLCO, V C and D M were taken during the preliminary day, before the incremental exercise test. However, Q and ventilation during baseline in both groups were greater than would be expected for true resting values. Although we made considerations in providing a quiet, resting environment for all of our study participants, it is not certain whether the pre‐exercise levels of arousal are similar between fitness groups and, thus, the differences in DLCO and V C at baseline may not reflect true resting values. However, the pre‐exercise absolute values for DLCO and V C are consistent with previous data showing that aerobic fitness is positively correlated with a greater resting V C (Lalande et al. 2012).
Finally, the cross‐sectional design of the present study only allows for speculation of the effect of endurance training on diffusion capacity and the pulmonary VC response during exercise. To our knowledge, only one study has examined longitudinal changes in lung function and diffusion at rest and during exercise, finding that prepubescent swimmers had greater exercise DLCO, secondary to greater total lung volume, after 3 years of training compared to non‐trained peers (Andrew et al. 1972). Future studies should investigate changes in the pulmonary vascular with chronic exercise training of previously un‐trained adult humans, as well as the associated changes in pulmonary V C and D M.
Conclusions
We examined the effect of aerobic fitness on diffusion capacity, pulmonary V C and D M responses to exercise. We found that athletes with a high have an increased diffusion capacity during high intensity exercise, secondary to a greater D M, and do not have a greater pulmonary V C compared to non‐athletes. These findings suggest that endurance‐trained athletes appear to have differences within the alveolar‐capillary membrane that facilitate the increased O2 demand required during high‐intensity exercise.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
VT, MMB and MKS contributed to the conception and design of the experiments; collection, analysis and interpretation of data; and drafting of the article or its critical revision for important intellectual content. The study was performed in the Clinical Physiology Laboratory at the University of Alberta. All authors approved the final version of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The present study was funding by the Natural Sciences and Engineering Research Council (NSERC – M. K. Stickland). M. K. Stickland is supported by a Heart and Stroke Foundation of Canada New Investigator Salary Award. M. M. Bouwsema was supported by Alberta Innovates Health Solutions (AIHS) Summer Studentship Award and NSERC Undergraduate Student Research Award.
Acknowledgements
The authors gratefully acknowledge the contributions of Desi Fuhr for his technical expertise; graduate students Bradley Byers and Sophie Collins; undergraduate students Christine Christianson and Gillian Altheim; and the hard work of all participants in the present study.
References
- American Thoracic Society, American College of Chest Physicians (1995). American Thoracic Society. Single‐breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique‐1995 update. Am J Respir Crit Care Med 152, 2185–2198. [DOI] [PubMed] [Google Scholar]
- Andrew GM, Becklake MR, Guleria JS & Bates DV (1972). Heart and lung functions in swimmers and nonathletes during growth. J Appl Physiol 32, 245–251. [DOI] [PubMed] [Google Scholar]
- Armour J, Donnelly PM & Bye PT (1993). The large lungs of elite swimmers: an increased alveolar number? Eur Respir J 6, 237–247. [PubMed] [Google Scholar]
- Bartesaghi M, Beretta E, Pollastri L, Scotti V, Mandolesi G, Lanfranconi F & Miserocchi G (2014). Inter‐individual differences in control of alveolar capillary blood volume in exercise and hypoxia. Respir Physiol Neurobiol 190, 96–104. [DOI] [PubMed] [Google Scholar]
- Blakemore WS, Forster RE, Morton JW & Ogilvie CM (1957). A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J Clin Invest 36, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Wise RA, Kirk G, Drummond MB & Mitzner W (2015). Lung density changes with growth and inflation. Chest 148, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceridon ML, Beck KC, Olson TP, Bilezikian JA & Johnson BD (2010). Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple‐ and single‐inspired oxygen tension methods. J Appl Physiol 109, 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch HJ & Ng CK (1992). Estimating alveolar surface area during life. Respir Physiol 88, 163–170. [DOI] [PubMed] [Google Scholar]
- Cotes JE, Dabbs JM, Elwood PC, Hall AM, McDonald A & Saunders MJ (1972). Iron‐deficiency anaemia: its effect on transfer factor for the lung (diffusiong capacity) and ventilation and cardiac frequency during sub‐maximal exercise. Clin Sci 42, 325–335. [DOI] [PubMed] [Google Scholar]
- Degens H, Rittweger J, Parviainen T, Timonen KL, Suominen H, Heinonen A & Korhonen MT (2013). Diffusion capacity of the lung in young and old endurance athletes. Int J Sports Med 34, 1051–1057. [DOI] [PubMed] [Google Scholar]
- Fick A (1855). Ueber diffusion. Annalen der Physik. [Google Scholar]
- Forster RE (1987). Diffusion of gases across the alveolar membrane. Compr Physiol 4, 71–88. [Google Scholar]
- Gehr P, Bachofen M & Weibel ER (1978). The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol 32, 121–140. [DOI] [PubMed] [Google Scholar]
- Graham BL, Mink JT & Cotton DJ (1985). Effect of breath‐hold time on DLCO(SB) in patients with airway obstruction. J Appl Physiol 58, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Graham BL, Mink JT & Cotton DJ (2002). Effects of increasing carboxyhemoglobin on the single breath carbon monoxide diffusing capacity. Am J Respir Crit Care Med 165, 1504–1510. [DOI] [PubMed] [Google Scholar]
- Green DJ, Naylor LH, George K, Dempsey JA, Stickland M & Katayama K (2008). Cardiovascular and pulmonary adaptations to endurance training. Physiological Bases of Human Performance During Work and Exercise. Publisher Elsevier Health, Atlanta, GA. [Google Scholar]
- Hagberg JM, Yerg JE & Seals DR (1988). Pulmonary function in young and older athletes and untrained men. J Appl Physiol 65, 101–105. [DOI] [PubMed] [Google Scholar]
- Hlastala MP, McKenna HP, Franada RL & Detter JC (1976). Influence of carbon monoxide on hemoglobin‐oxygen binding. J Appl Physiol 41, 893–899. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Herazo LF & Johnson RL (1992). Cardiopulmonary adaptations to pneumonectomy in dogs. I. Maximal exercise performance. J Appl Physiol 73, 362–367. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Herazo LF, Ramanathan M & Johnson RL (1994). Cardiopulmonary adaptations to pneumonectomy in dogs. IV. Membrane diffusing capacity and capillary blood volume. J Appl Physiol 77, 998–1005. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Herazo LF, Ramanathan M, Johnson RL & Wagner PD (1993). Cardiopulmonary adaptations to pneumonectomy in dogs. II. VA/Q relationships and microvascular recruitment. J Appl Physiol 74, 1299–1309. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Johnson RL & Shah D (1999). Red cell distribution and the recruitment of pulmonary diffusing capacity. J Appl Physiol 86, 1460–1467. [DOI] [PubMed] [Google Scholar]
- Hsia CC, McBrayer DG & Ramanathan M (1995). Reference values of pulmonary diffusing capacity during exercise by a rebreathing technique. Am J Respir Crit Care Med 152, 658–665. [DOI] [PubMed] [Google Scholar]
- Hsia CCW (2002). Recruitment of lung diffusing capacity: update of concept and application. Chest 122, 1774–1783. [DOI] [PubMed] [Google Scholar]
- Johnson RL & Hsia CC (1994). Functional recruitment of pulmonary capillaries. J Appl Physiol 76, 1405–1407. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Heigenhauser GJF, Hsia CW, Jones NL & Wagner PD (2010). Determinants of gas exchange and acid‐base balance during exercise. In: Comprehensive Physiology. Compr Physio: Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems: 515–584. [Google Scholar]
- Johnson RL, Spicer WS, Bishop JM & Forster RE (1960). Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol 15, 893–902. [DOI] [PubMed] [Google Scholar]
- La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt J‐U, Heidbüchel H & Prior DL (2010). Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol 109, 1307–1317. [DOI] [PubMed] [Google Scholar]
- Lalande S, Yerly P, Faoro V & Naeije R (2012). Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol 590, 4279–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce‐Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D & Duncker DJ (2013). Peripheral Circulation ‐ Handbook of Physiology In: Comprehensive Physiology, ed. Terjung R. Wiley, Hoboken, NJ, USA. [DOI] [PubMed] [Google Scholar]
- Levine BD, Lane LD, Buckey JC, Friedman DB & Blomqvist CG (1991). Left ventricular pressure‐volume and Frank‐Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84, 1016–1023. [DOI] [PubMed] [Google Scholar]
- Lewis BM, Lin TH, Noe FE & Komisaruk R (1958). The measurement of pulmonary capillary blood volume and pulmonary membrane diffusing capacity in normal subjects; the effects of exercise and position. J Clin Invest 37, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley S, Puderbach M, Risse F, Ley‐Zaporozhan J, Eichinger M, Takenaka D, Kauczor H‐U & Bock M (2007). Impact of oxygen inhalation on the pulmonary circulation: assessment by magnetic resonance (MR)‐perfusion and MR‐flow measurements. Invest Radiol 42, 283–290. [DOI] [PubMed] [Google Scholar]
- MacIntyre N (2005). Standardisation of the single‐breath determination of carbon monoxide uptake in the lung. Eur Respir J 26, 720–735. [DOI] [PubMed] [Google Scholar]
- Marrades RM, Diaz O, Roca J, Campistol JM, Torregrosa JV, Barberà JA, Cobos A, Félez MA & Rodriguez‐Roisin R (2011). Adjustment of DLCO for hemoglobin concentration. Am J Respir Crit Care Med 155, 236–241. [DOI] [PubMed] [Google Scholar]
- Martin DG, Ferguson EW, Wigutoff S, Gawne T & Schoomaker EB (1985). Blood viscosity responses to maximal exercise in endurance‐trained and sedentary female subjects. J Appl Physiol 59, 348–353. [DOI] [PubMed] [Google Scholar]
- Mostyn EM, Helle S, Gee JB, Bentivoglio LG & Bates DV (1963). Pulmonary diffusing capacity of athletes. J Appl Physiol 18, 687–695. [DOI] [PubMed] [Google Scholar]
- Presson RG, Hanger CC, Godbey PS, Graham JA, Lloyd TC & Wagner WW (1994). Effect of increasing flow on distribution of pulmonary capillary transit times. J Appl Physiol 76, 1701–1711. [DOI] [PubMed] [Google Scholar]
- Reeves JT & Taylor AE (2011). Pulmonary hemodynamics and fluid exchange in the lungs during exercise. Compr Physiol 585–613. [Google Scholar]
- Reeves JT, Linehan JH & Stenmark KR (2005). Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 288, L419–L425. [DOI] [PubMed] [Google Scholar]
- Rose GL, Cassidy SS & Johnson RL (1979). Diffusing capacity at different lung volumes during breath holding and rebreathing. J Appl Physiol 47, 32–36. [DOI] [PubMed] [Google Scholar]
- Roughton FJ & Forster RE (1957). Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 11, 290–302. [DOI] [PubMed] [Google Scholar]
- Sansores RH, Abboud RT, Kennell C & Haynes N (1995). The effect of menstruation on the pulmonary carbon monoxide diffusing capacity. Am J Respir Crit Care Med 152, 381–384. [DOI] [PubMed] [Google Scholar]
- Short AC, Montoya ML, Gebb SA, Presson RG, Wagner WW & Capen RL (1996). Pulmonary capillary diameters and recruitment characteristics in subpleural and interior networks. J Appl Physiol 80, 1568–1573. [DOI] [PubMed] [Google Scholar]
- Smith JR, Brown KR, Murphy JD & Harms CA (2015). Does menstrual cycle phase affect lung diffusion capacity during exercise? Respir Physiol Neurobiol 205, 99–104. [DOI] [PubMed] [Google Scholar]
- Staub NC, Bishop JM & Forster RE (1962). Importance of diffusion and chemical reaction rates in O2 uptake in the lung. J Appl Physiol 17, 21–27. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Lindinger MI, Olfert IM, Heigenhauser GJF & Hopkins SR (2013). Pulmonary gas exchange and acid‐base balance during exercise. Compr Physiol 3, 693–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Welsh RC, Petersen SR, Tyberg JV, Anderson WD, Jones RL, Taylor DA, Bouffard M & Haykowsky MJ (2006). Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol 100, 1895–1901. [DOI] [PubMed] [Google Scholar]
- Tamhane RM, Johnson RL & Hsia CC (2001). Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest 120, 1850–1856. [DOI] [PubMed] [Google Scholar]
- Taylor BJ, Carlson AR, Miller AD & Johnson BD (2014). Exercise‐induced interstitial pulmonary edema at sea‐level in young and old healthy humans. Respir Physiol Neurobiol 191, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turino GM, Bergofsky EH, Goldring RM & FIshman AP (1963). Effect of exercise on pulmonary diffusing capacity. J Appl Physiol 18, 447–456. [DOI] [PubMed] [Google Scholar]
- Wagner PD (1996. a). A theoretical analysis of factors determining VO2 MAX at sea level and altitude. Respir Physiol 106, 329–343. [DOI] [PubMed] [Google Scholar]
- Wagner PD (1996. b). Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58, 21–50. [DOI] [PubMed] [Google Scholar]
- Wagner WW & Latham LP (1975). Pulmonary capillary recruitment during airway hypoxia in the dog. J Appl Physiol 39, 900–905. [DOI] [PubMed] [Google Scholar]
- Warren GL, Cureton KJ, Middendorf WF, Ray CA & Warren JA (1991). Red blood cell pulmonary capillary transit time during exercise in athletes. Med Sci Sports Exerc 23, 1353–1361. [PubMed] [Google Scholar]
- Warren J & Jennings S (1984). Normal human airway response to exercise. J Appl Physiol 56, 1686. [DOI] [PubMed] [Google Scholar]
- West JB (2012). Respiratory Physiology: The Essentials . Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Womack CJ, Harris DL, Katzel LI, Hagberg JM, Bleecker ER & Goldberg AP (2000). Weight loss, not aerobic exercise, improves pulmonary function in older obese men. J Gerontol A Biol Sci Med Sci 55, M453–M457. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS (2013). The rise in carboxyhemoglobin from repeated pulmonary diffusing capacity tests. Respir Physiol Neurobiol 186, 103–108. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS, Smoliga JM, Longo LD, Uhranowsky KA, Cadman CR, Duffin J & Fisher JA (2012). Increased carbon monoxide clearance during exercise in humans. Med Sci Sports Exerc 44, 2118–2124. [DOI] [PubMed] [Google Scholar]


