SUMMARY
The objective of this study is to report the initial results of a prospective trial assessing instrumental deglutition function in nasopharynx and oropharynx cancers after radio or chemoradiotherapy using intensity-modulated radiotherapy (IMRT). IMRT was delivered aiming to spare the swallowing organ at risk (SWOARs) for Stage II-IV naso- and oropharynx cancer. Objective instrumental assessment included videofluoroscopy (VFS), fiberoptic endoscopic evaluation of swallowing (FEES) and oro-pharyngeal-oesophageal scintigraphy (OPES) at baseline and at 1 month after radiotherapy. Dysphagia parameter scores were calculated at each exam after liquid (L) and semi-liquid (SL) bolus intake: pre-deglutition penetration, aspiration, pharyngeal transit time (PTT) and hypopharyngeal retention index (HPRI). Overall, 20 patients (6 nasophaynx and 14 oropharynx) completed treatment and instrumental assessment after 1 month. Comparison between pre- and post-treatment HPRI score values showed a significant worsening in both FEES-L (p = 0.021) and SL (p = 0.02) and at VFS-L (p = 0.008) and SL (p = 0.005). Moreover, a relationship between HPRI worsening at FEES-L and FEES-SL (p = 0.005) as well as at VFS-L and VFS-SL (p < 0.001) was observed. PTT was not significantly affected by radiotherapy (p > 0.2). Only a few patients experienced pre-deglutition penetration (1 patient with base of tongue cancer at FEES-L and SL) and aspiration (1 patient with nasopharynx cancer at OPES-L and FEES-SL) after radiotherapy. Our early results showed that IMRT-SWOARs sparing caused a significant increase in the post-deglutition HPRI score. Longer follow-up will be necessary to evaluate if the increase of HPRI is related to a high risk of developing late aspiration.
KEY WORDS: Intensity and modulated radiotherapy, Deglutition, Fiberoptic endoscopic swallowing evaluation, Videofluoroscopy
RIASSUNTO
In questo lavoro vengono riportati i risultati a breve termine di uno studio prospettico, finalizzato alla valutazione strumentale della funzionalità deglutitoria in pazienti affetti da tumore del rinofaringe e orofaringe sottoposti a trattamento radio o radiochemioterapico con tecnica ad intensità modulata (IMRT). L' IMRT è stata finalizzata, oltre che al miglioramento della conformazione della dose radiante al volume tumorale, alla riduzione della stessa alle strutture responsabili della deglutizione (SWOARs). I criteri dello studio hanno previsto in tutti i pazienti la valutazione strumentale della deglutizione con Videofluoroscopia (VFS), Fibroscopia Endoscopica della deglutizione (FEES) e Scintigrafia Orofaringea (OPES) prima dell'inizio del trattamento e ad 1 mese dal termine dello stesso. Ogni esame è stato eseguito rispettivamente in seguito all'assunzione di un bolo liquido (L) e semiliquido (SL) e per ognuno sono stati calcolati i seguenti valori strumentali: presenza o meno di caduta pre-deglutitoria, presenza o meno di aspirazione, tempo di transito faringeo (PTT) ed indice di ritenzione ipofaringeo (HPRI). Dal Gennaio 2012 al Giugno 2013, un totale di 20 pazienti ha terminato il trattamento ed ha eseguito la valutazione strumentale a 1 mese dal termine della radioterapia. Il confronto tra i valori dell'HPRI prima e dopo il trattamento radiante ha mostrato un peggioramento significativo sia alla FEES-L (p = 0,021) e SL (p = 0,02) che alla VFS-L (p = 0,008) che SL (p = 0,005). Inoltre è stata riscontrata una significativa correlazione tra i valori dell'HPRI basale ed a 1 mese alla FEES-L e SL (p = 0,005) così come alla VFS-L e SL (p < 0,001). Diversamente, il tempo di transito faringeo (PTT) non è risultato essere influenzato dalla radioterapia (p > 0,2). Solo in pochi pazienti è stata riscontrata la comparsa di caduta pre-deglutitoria ( 1 paziente con tumore della base linguale alla FEES-L e SL) e la presenza di aspirazione (1 paziente con tumore del rinofaringe alla OPES-L e FEES-SL). Nel complesso i risultati iniziali del nostro studio mostrano che l' IMRT, finalizzata al risparmio delle SWOARs, determina soltanto un significativo incremento della ritenzione di bolo a livello del distretto ipofaringeo. Un follow-up più lungo sarà necessario per valutare se tale incremento sia associato o meno ad un maggior rischio di sviluppare fenomeni di aspirazione tardivi.
Introduction
Nowadays radiotherapy (RT) alone or most frequently combined with chemotherapy (RCT) is considered a valid alternative treatment to surgery for patients affected by head and neck cancer (HNC) in order to preserve the deglutition organ 1 2. Historically, conventional RT has been burdened by severe and potentially "life threatening" toxicity that limited the delivery of high tumour radiation dose and in most cases affected the final treatment result 3-6. In this regard radiation-induced dysphagia, as a final multifactorial side effect often requiring enteral nutrition, represents a real "Achille's heel" that occurs in more than 50% of patients and can lead to a malnutritional status and an increased risk of aspiration pneumonia 7-9. The 1-and 2-year rates of percutaneous endoscopic gastrostomy (PEG) tube dependence is reported, respectively, in 24% and 14%, whereas clinical aspiration pneumonia is reported in 3% of cases 10.
On the contrary, an organ preservation strategy should provide both the highest tumour control probability (TCP) and the minimum function impairment with the subsequent maximum therapeutic index gain.
In fact, reducing deglutition disorders related-symptoms (e.g., oropharyngeal pain, dry mouth, food stuck in the throat and choking) and deglutition disorder related-complications (pulmonary complications) can result both in a significant improvement of patient quality of life (QoL) together with a reduction in hospitalisation costs 11-13.
In the last few decades, the advancement of treatment technologies, such as intensity and modulated radiotherapy (IMRT), has shown promising results in terms of better TCP as well as a reduction of toxicity through the sparing of swallowing organs at risk (SWOARs) 14 15.
Hence, several studies have investigated the impact of RT using IMRT on the deglutition function but are mostly retrospective, addressed to an heterogeneous set of patients and lacking preatreatment swallowing evaluation 16-20.
Moreover, in most cases dysphagia was defined using a surrogate clinical endpoint, such as percutaneous tube dependence (PEG) time, aspiration pneumonia or pharingoesophageal strictures. Differently, the assessment of deglutition function typically includes both clinical and instrumental evaluation 11.
Although several objective patient-reported instruments, such as Radiation Therapy Oncology Group (RTOG)/ European Organization for Research and Treatment of Cancer (EORTC) criteria, the Subjective Objective Management Analytic (SOMA) scale and the Common Terminology Criteria for Adverse Events (CTCAE) are available 20-23, they are variable and have been shown to underestimate deglutition impairment compared with objective instrumental assessment 24. Indeed, a clear and uniform consensus of objective instrumental deglutition assessment has yet not been clearly defined.
Videofluoroscopy (VFS) and fiberoptic endoscopic evaluation of swallowing (FEES) are considered the gold standards for dysphagia assessment, whereas oropharyngealossophageal scintigraphy (OPES) is considered optional combined with FEES and VFS 25.
Thus, due to the reliability and validity of instrumental assessment tools, the use of all available complementary procedures is suggested by the current literature to properly evaluate swallowing function 25 26.
We therefore initiated a prospective longitudinal study to assess the impact of RT on swallowing function in a homogeneous subset of HNC patients who were candidates for radio or chemoradiotherapy as a radical curative treatment.
The primary endpoint was to evaluate dysphagia parameter changes using pre and post-treatment objective instrumental assessment after IMRT aimed to reduce the radiation dose to the SWOARs.
In this study, we report our preliminary results focusing on acute dysphagia (1 month after treatment), not previously investigated, in order to assess the risk of severe complications during or soon after RT or RCT.
Materials and methods
Patient characteristics
This is an ongoing prospective study carried out by the collaboration of the Department of Radiation Oncology, the Department of Radiology and the Otorhinolaringology and Speech Language Pathologist Unit. The study was approved by the Institutional Review Board of the University of Pisa; all patients signed a study-specific informed consent form.
The eligibility criteria included all patients affected by nasopharynx and oropharynx cancer (Stage II-IVA), with histological proven diagnosis of undifferentiated nasopharyngeal- type carcinoma or squamous cell carcinoma, Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0-2 and age < 80 years old.
Exclusion criteria were the following: a different site from nasopharynx or oropharynx, a different histology from undifferentiated nasopharyngeal type or squamous cell carcinoma, ECOG Status ≥ 3, Stage IVB and C, prior induction chemotherapy or prior HN treatment (surgery and/or RT), diagnosis of concomitant comorbidity which might compromise basic deglutition function (demyelinating or degenerative diseases and connective tissue diseases) and age > 80 years.
Radiotherapy
All patients required bilateral neck irradiation and underwent whole-neck-field IMRT; the anterior low neck field abutting the upper IMRT region was not used in any patient.
The clinical target volumes (CTVs) were directly delineated by the radiation oncologist according to the guidelines of the Italian Association of Radiation Oncology- Head and Neck Working Group 27 and the corresponding planning target volumes (PTVs) were automatically created by uniform expansions of 0.3 cm.
According to our internal image guided radiotherapy (IGRT) protocol, patients underwent weekly cone beam CT (CBCT) set-up control and online correction to reduce systematic set-up errors.
The prescribed doses were 66 Gy at 2.2 Gy per fraction to the high risk gross volume PTV and 60-54 Gy at 2.0-1.8 Gy per fraction to the intermediate (optional) and low risk subclinical PTVs, respectively, delivered concomitantly in 30 daily fractions.
According to the recent computed tomography (CT)- based delineation guidelines by Christianen et al. 28, eight different SWOARs were defined in each CT slice and included in IMRT planning objective functions: superior, middle and inferior constrictor muscle (SPCM, MPCM and IPCM), supraglottic larynx (SL), glottis larynx (GL), cricopharyngeus muscle (CPM) and cervical oesophagus (CE). Thereafter, the mean dose received by each swallowing structures as well as by parotid glands and oral cavity were recorded.
In the IMRT optimisation cost function, target coverage replaced sparing of any SWOARs, parotid glands and oral cavity, but the spinal cord.
The IMRT plans set target prescription goals and spinal cord maximum dose (Dmax) as the highest priority, whereas SWOAR constraints were set as secondary.
Medical therapy, supportive care and follow-up
Chemotherapy was given weekly using cisplatin 40 mg/ m2 i.v. over 1 h during the 6-week RT course for a maximum of 6 cycles both for patients affected by nasopharynx or oropharynx cancer.
For oropharynx patients with severe comorbidities, cetuximab was administered as an induction dose of 400 mg2 over 2 h at 1 week before the start of RT and then 250 mg2 weekly over 1-hour during RT course for a maximum of 6 cycles was administered.
Patients underwent PEG positioning during or after treatment if weight loss was > 10% (grade 2) from pretreatment status.
Acute toxicity was reported according to Common Toxicity Criteria Adverse Effects (CTCAE) version 3 23, an observer-assessed validated toxicity scale scoring dysphagia between grade 1 (symptomatic but able to eat regular diet) to grade 5 (death).
Evaluation of dysphagia
Oro-pharyngeal-oesophageal scintigraphy (OPES)
OPES investigation entails the acquisition of a rapid sequence of images referring to a single voluntary deglutition
which the patient performs on command. It is preferable to carry out this scintigraphic examination after the patient has been without food for at least three hours. Before starting the OPES, the patient should be made to swallow a small amount of non-radioactive water as a test; this helps to train the patients regarding the procedure, ensures patient compliance and assesses the capacity to swallow the amount of liquid foreseen for the examination (5 cc). After about five minutes, the examination begins with the patient in an orthostatic position with his/her face in an 80° oblique projection in front of a single rectangular headed large-field-of–view (LFOV) gamma camera equipped with a low energy-high resolution (LEHR) parallel hole collimator using a 140 KeV (± 10%) energy window. The patient is administered a single bolus of 5 cc of water marked with 37 MBq (1 mCi) of 99mTc nanocolloid (Nanocoll-Amersham®, UK). Eight images per sec (0.125 sec/frame) are acquired for one min by dynamic acquisitions (with a 64 x 64 matrix and zoom at 1), including the oral region as far as the epigastric area within the imaging field. The pharyngeal region of interest (ROI ) is that between the oral cavity and the external reference corresponding to the pharyngo- oesophageal transition 29.
Two seconds after the start, the patient is invited to take the liquid bolus in one deglutition (OPES-L). At the end of the test, a static image lasting 60 sec is acquired, with the patient still in the same position to evaluate any possible tracheo-bronchial aspiration.
After an interval of 30 min, the procedure is repeated, but this time with a semi-solid bolus marked with 37 MBq (1 mCi) of 99mTc nanocolloid (OPES-SL). The acquisitions are obtained with the same method as with the liquid bolus.
Videofluoroscopy (VFS)
Digital fluoroscopy examinations were performed with a Clinodigit Compact Xframe Italray® device. The digital images were acquired by filming at a frame rate of 30/sec, which was sufficient to record the swallowing act. The acquisition resolution was 30,001 x 3001 x 14 bit.
Digitalised imaging permits the creation of a PACS (Picture Archiving and Communication System), which is a computerised system where images are uploaded, together with the relative supplied by the various diagnostic tools available in the hospital, thus allowing the images to be archived and shared. Furthermore, PACS permits viewing information about any previous investigation the patient was submitted to whenever a new examination was necessary. An image was enlarged on the neck region of the patient in an orthostatic latero-lateral position, and contrast medium was administered. The contrast medium used was Prontobario HD (Bracco®): the packaging supplied contains 98.45 g powder for oral suspension, 340 g barium sulphate.
The powder was diluted in 65 ml of water for the liquid consistency (VFS-L) and in 30 ml of water for the semisolid bolus (VFS-SL); for each density, the patient was invited to take three 5 cc sips 30-32.
Fibreoptic endoscopic evaluation of swallowing (FEES)
FEES was performed with a flexible fibreoptic rhinopharyngolaryngoscope (Olympus ENF-P3®) connected to a CCD camera and colour monitor and recorded digitally on the Digital Swallowing Workstation (Kay Pentax Ltd®, Montvale, NJ, USA). The examination was carried out by a phoniatrician and a speech therapist and every patient was administered two or more semi-solid (viscous water) or liquid boluses (water marked with blue methylene for easy detection), swallowing 5 cc of each type of bolus 30-32.
Four different dysphagia parameters were calculated and reported at each exam both after liquid (L) and semi-liquid (SL) bolus intake. Pre-deglutition penetration, hypopharyngeal retention index (HPRI) and penetration/aspiration were reported at each exam, whereas pharyngeal transit time (PTT) and white-out phase (WOP) only at VFS/ OPES and at FEES, respectively. The evaluation of both pre-deglutition penetration and penetration/aspiration was scored 0 if it was absent and 1 if was present. The latter was defined at FEES and VFS once the bolus entered the upper airways above the vocal cords (penetration) or passed below the vocal cords into the subglottis (aspiration).
The OPES detected only the transit of bolus into the tracheobronchial tree (aspiration), also giving the possibility for semi quantitative measurement of the aspirate.
Thereafter, the penetration/aspiration was classified in 8 different scores according to the worldwide used Penetration- Aspiration Scale at VFS 33.
The PTT was calculated at the end of the test by evaluation of the images recorded (normal < = 1 sec; Score 0), slightly long (≥ 1 to < 1.5 sec; Score 1), long (≥ 1.5 to < 2 sec; Score 2) and very long (≥ 2 sec; Score 3).
The WOP was defined as the total amount of time that the entire view screen was completely white.
The endoscopic examination was reviewed in a frameby- frame analysis (ATMOS recording system), which allowed marking the examination film at specific points using a running frame-by-frame counter with a capture film rate of 30 frames per sec.
Swallowing initiation was defined as the time from when the bolus reached the horizontal level at the tip of the epiglottis to the start of the complete "white out." The HPRI was calculated as the amount of residue (pooling amount) in the hypopharynx against the Farneti pooling-score scale. Score 0 was considered normal, whereas scores 1, 2 and 3 pathological (mild, moderate and severe, respectively). Dysphagia parameters scores are shown in Table I.
Table I.
Videofluoroscopy, fibreoptic endoscopic evaluation of swallowing and oro-pharyngeal-oesophageal scintigraphy dysphagia parameter scores.
| Absent | Present | |||||
|---|---|---|---|---|---|---|
| Pre-swallowing penetration | 0 | 1 | ||||
| Penetration/aspiration | 0 | 1 | ||||
| PTT a /WOP b | Normal | Mild | Moderate | Severe | ||
| HPRI c | 0 | 1 | 2 | 3 | ||
| Videofluoroscopy | 0 | 1 | 2 | 3 | ||
| (Dyer et al. 34) | (< 3%) | (≥ 3 to < 25%) | (≥ 25 to < 55%) | (≥ 55%) | ||
| Scintigraphy | 0 | 1 | 2 | 3 | ||
| (Fattori et al. 35) | (< 5%) | (≥ 5 to < 20%) | (≥ 20 to < 40%) | (≥ 40%) | ||
| Fibreoptic evaluation | 0 | 1 | 2 | 3 | ||
| (Farneti et al. 36) |
PTT = Pharyngeal transit time. Values are numbers and (percentage)
WOP = White-out phase. Values are numbers and (percentage)
HPRI = Hypopharyngeal retention index. Values are numbers and (percentage)
Statistical parameter
We studied three main factors: dysphagia temporal variations, baseline dysphagia and the effect of liquid-semiliquid bolus. Before testing of inferential statistics, a graphical exploration was always performed.
Dysphagia temporal variations
To detect the significant changes in the dysphagia scores, measured at time zero and after 1 month, we used the Wilcoxon test (for continuous variables) and the McNemar test (for dichotomous variables). For continuous variables, worsening score was considered as the transition from a lower to a higher score (e.g. from 0 to 1 or from 1 to 2).
Evaluation baseline dysphagia
To evaluate the association among the primary tumour site (nasopharynx, oropharynx), T stage and N stage with the baseline dysphagia score (low, medium-high) measured by several instrumental analysis, two-tailed chi-square test and Fisher's exact test were used.
Effect of liquid-semiliquid bolus
To evaluate the correlation between deglutition worsening scores (from before to 1 month after treatment) for different bolus consistencies (L and SL) at the same exam (FEES/VFS/OPES), we used a non-parametric correlation analysis.
All statistical analyses, including those on the radiation doses received by the SWOARs (box-plots) and the variations between scores changes (bar graph), were performed using SPSS 21.
Results
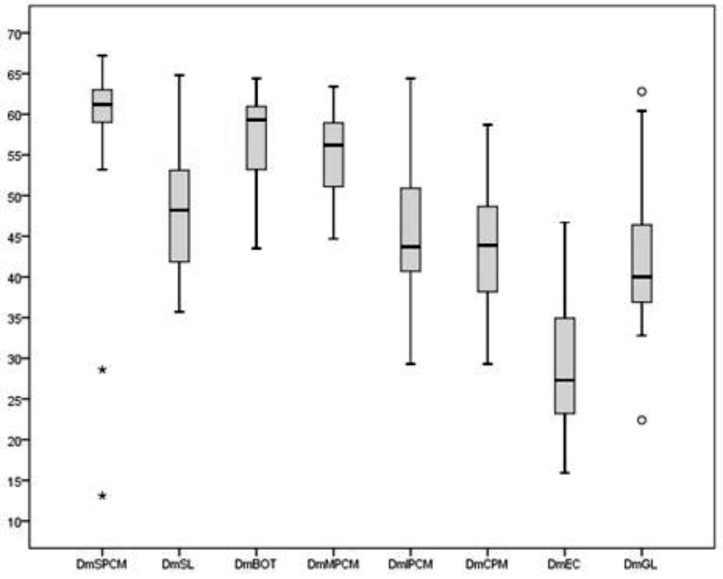
Between June 2012 and December 2013, 20 patients with nasopharynx (n = 6) or oropharynx (n = 14) cancer were enrolled. The summaries of baseline patient and tumour characteristics are detailed in Table II. Average ± standard deviation mean doses to the SPCM, MPCM, IPCM, BOT, SL, GL, CPM and CE were 56.7 ± 13.7Gy, 53.4Gy ± 8.2Gy, 44.7 ± 11Gy, 55.7 ± 8.7Gy, 47.5 ± 11.2 Gy, 41.5 ± 11.3Gy, 41.7 ± 12.9Gy and EC 27.8 ± 10.3 Gy, respectively (Fig. 1).
Table II.
Patient and tumour characteristics.
| Characteristic | Patients | |
|---|---|---|
| N | % | |
| Age | 43-77 | |
| Mean | 62 | |
| Range | ||
| Sex | ||
| Male | 16 | 80 |
| Female | 4 | 20 |
| ECOG Status | ||
| 0 | 16 | 80 |
| 1 | 4 | 20 |
| Smoking Status | ||
| No | 8 | 40 |
| < 1 packet | 7 | 35 |
| >1 packet | 5 | 25 |
| Alcohol Intake | ||
| No | 9 | 45 |
| < 1 litre/day | 7 | 35 |
| > 1 litre/day | 4 | 20 |
| HPV Status * | ||
| Negative | 7 | 50 |
| Positive | 2 | 14 |
| Unknown | 5 | 36 |
| Primary Site | ||
| Tonsil | 7 | 35 |
| Base of tongue | 5 | 25 |
| Soft palate | 2 | 10 |
| Nasopharynx | 6 | 30 |
| T Stage | ||
| 1 | 4 | 20 |
| 2 | 8 | 40 |
| 3 | 3 | 15 |
| 4 | 5 | 25 |
| N Stage | ||
| 0 | 7 | 35 |
| 1 | 3 | 15 |
| 2 | 10 | 50 |
| AJCC Stage ** | ||
| II | 6 | 30 |
| III | 4 | 20 |
| IV | 10 | 50 |
| Medical therapy | ||
| None | 4 | 20 |
| Cisplatin | 13 | 65 |
| Cetuximab | 3 | 15 |
HPV status was assessed for patients with oropharynx cancer
AJCC Stage = American Joint Committee on Cancer
Fig. 1.
Dose to the SWOARs.
Abbreviations: SPCM: superior constrictor muscle; MPCM: middle constrictor muscle; IPCM inferior constrictor muscle; SL supraglottic larynx; GL glottic larynx; CMP cricopharyngeal muscle; EC cervical esophagus; Dm = mean dose
All patients but one, who stopped chemotherapy after two administrations due to a high grade long-term nausea and vomit, received at least five of the planned six cycles of concurrent medical therapy. No patient experienced a significant weight loss (≥ grade 2) requiring PEG positioning during or soon after treatment.
Mucositis G1 was reported in 7 patients (35%) and was G2 in 11 patients (55%) and G3 in 2 patients (10%). Eight patients reported G1 dysphagia (40%), 10 patients G2 (50%) and 2 patients G3 (10%), whereas 10 patients reported G1 xerostomia (50%) and 10 patients G2 xerostomia (50%).
Moreover, 4 patients (20%) referred no pain during the course of treatment, whereas 7 patients referred G1 (35%), 8 patients G2 (40%) and 1 patient G3 (5%).
Variations of swallowing parameters between baseline and 1 month after RT
The examination of the differences between the pre- and post-treatment HPRI score was found to be statistically significant both at FEES-L (p = 0.021) and SL (p = 0.02) and at VFS-L (p = 0.008) and SL (p = 0.005); OPES did not confirm these results (Table III).
Table III.
Comparison between pre- and post-treatment HPRI for the three different exams used.
| Parameter | Exam | Median (range) | p-value | |
|---|---|---|---|---|
| Pretherapy | 1 month | |||
| HPRIa 0-1b L c | FEES e | 0 (0-1) | 1 (0-2) | 0.021 |
| VFS f | 1 (0-3) | 2 (0-3) | 0.008 | |
| OPES g | 1(0-2) | 1 (0-1) | 0.480 | |
| HPRI 0-1 SL d | FEES | 0 (0-2) | 1 (0-2) | 0.020 |
| VFS | 1 (0-3) | 3 (1-3) | 0.005 | |
| OPES | 1(0-2) | 1 (1-2) | 0.058 | |
HPRI= Hypopharyngeal Retention Index;
0-1= parameter worsening score between baseline and 1 month after treatment;
L=liquid;
SL=semiliquid;
FEES= Fibreoptic endoscopic evaluation of swallowing;
VFS=Videofluoroscopy;
OPES=Oropharyngeal oesophageal scintigraphy
HPRI worsening scores from baseline to 1 month after treatment are shown in Tables IV and V.
Table IV.
HPRI scores after L bolus by the three different exams used.
| Time | Parameter | Exam | Total | Score | Score Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||||||
| N | % | N | % | N | % | N | % | Mean | SD | ||||
| Pretherapy | HPRI | FEES | 20 | 17 | 85 | 3 | 15 | 0 | 0 | 0 | 0 | 0.16 | 0.37 |
| 1 month | HPRI | 20 | 9 | 45 | 9 | 45 | 2 | 10 | 0 | 0 | 0.65 | 0.67 | |
| Pretherapy | HPRI | VFS | 20 | 6 | 30 | 5 | 25 | 4 | 20 | 5 | 25 | 1.47 | 1.17 |
| 1 month | HPRI | 20 | 1 | 5 | 4 | 20 | 6 | 30 | 9 | 45 | 2.21 | 0.92 | |
| Pretherapy | HPRI | OPES | 20 | 8 | 40 | 11 | 55 | 1 | 5 | 0 | 0 | 0.68 | 0.58 |
| 1 month | HPRI | 20 | 8 | 40 | 12 | 60 | 0 | 0 | 0 | 0 | 0.60 | 0.50 | |
Abbreviations FEES=Fiberoptic endoscopic swallowing evaluation; VFS=Videofluoroscopy; OPES=Oro-pharyngeal-oesophageal scintigraphy; HPRI=Hypopharyngeal retention Index; SD=Standard deviation
Table V.
HPRI scores after SL bolus at the three different exams used.
| Time | Parameter | Exam | Total | Score | Score Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||||||
| N | % | N | % | N | % | N | % | Mean | SD | ||||
| Pretherapy | HPRI | FEES | 20 | 16 | 80 | 3 | 15 | 1 | 5 | 0 | 0 | 0.28 | 0.57 |
| 1 month | HPRI | 20 | 6 | 30 | 14 | 70 | 0 | 0 | 0 | 0 | 0.70 | 0.47 | |
| Pretherapy | HPRI | VFS | 20 | 6 | 30 | 5 | 25 | 4 | 20 | 5 | 5 | 1.47 | 1.17 |
| 1 month | HPRI | 20 | 0 | 0 | 3 | 16 | 6 | 32 | 9 | 10 | 2.37 | 0.76 | |
| Pretherapy | HPRI | OPES | 20 | 5 | 25 | 10 | 50 | 5 | 25 | 0 | 0 | 1.05 | 0.70 |
| 1 month | HPRI | 20 | 0 | 0 | 12 | 60 | 8 | 40 | 0 | 0 | 1.40 | 0.50 | |
Abbreviations FEES = Fiberoptic endoscopic swallowing evaluation; VFS = Videofluoroscopy; HPRI = Hypopharyngeal retention index; SD = Standard deviation
A total of 11 (55%), 19 (95%) and 12 (60%) patients experienced poorer scores at FEES-L, VFS-L and OPES-L.
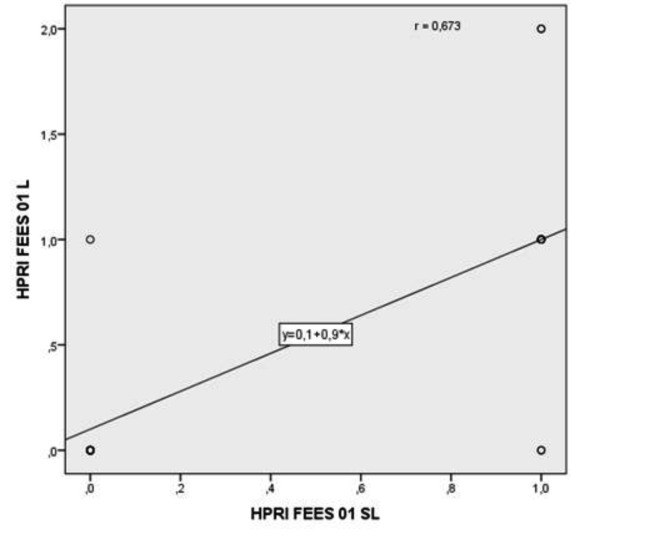
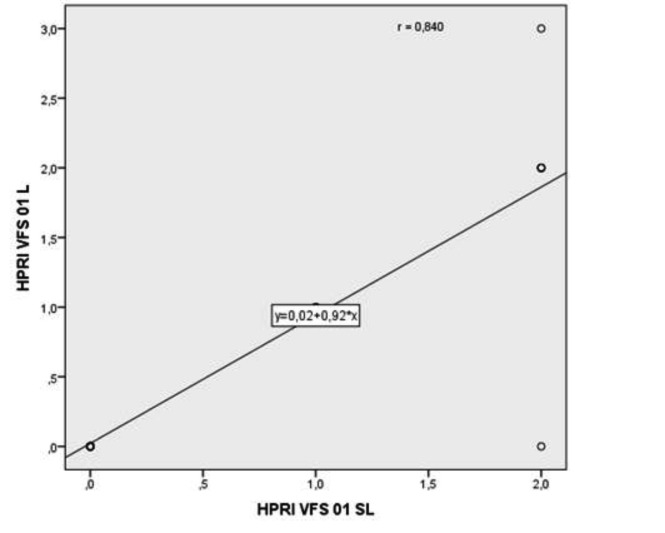
All patients showed a poorer HPRI score at VFS-SL and OPES-SL, as well as 14 (70%) at FEES-SL. In this regard, 9 (45%) and 10 (50%) patients showed a severe HPRI score (grade 3) after treatment at VF-L and SL, respectively. Furthermore, the relationship between HPRI worsening at FEES-L and FEES-SL (p = 0.005) as well as at VFS-L and VFS-SL (p < 0.001), was statistically significant (Figs. 2, 3). On the contrary, PTT and WPO was not significantly affected by RT either after L or SL bolus intake, as shown in Table VI.
Fig. 2.
Relationship between HPRI worsening score at FEES-L and SL.
Abbreviations: 0-1 = parameter worsening score between baseline and 1 month post-treatment. L = Liquid; SL = Semiliquid; HPRI = hypopharyngeal retention index; FEES = Fibreoptic endoscopic evaluation of swallowing.
Fig. 3.
Relationship between VFS worsening score at VFS-L and SL.
Abbreviations: 0-1 = parameter worsening score between baseline and 1 month post-treatment. L = Liquid; SL = Semiliquid; HPRI = Hypopharyngeal retention index; VFS=Videofluoroscopy.
Table VI.
Comparison between pre and post-treatment PTT for the three different exams.
| Parameter | Exam | Median (range) | p-value | |
|---|---|---|---|---|
| Pretherapy | 1 month | |||
| WOPa 0-1 L | FEES f | 0 (0-1) | 0 (0-1) | 0.219 |
| PTTb 0-1c L d | VFS g | 0 (0-3) | 0 (0-0) | 1.0 |
| PTT 0-1 L | OPES h | 0 (0-0) | 0 (0-1) | 1.0 |
| WOP 0-1 SL | FEES | 0 (0-1) | 0 (0-2) | 0.454 |
| PTT 0-1 SL e | VFS | 0 (0-1) | 0 (0-0) | 0.756 |
| PTT 0-1 SL | OPES | 0 (0-0) | 0 (0-0) | 1.0 |
WOP = White-out phase;
PTT = Pharyngeal transit time;
0-1 = parameter worsening score between baseline and 1 month after treatment;
L = Liquid;
SL = Semiliquid;
FEES = Fibreoptic endoscopic evaluation of swallowing;
VFS = Videofluoroscopy;
OPES = Oro-pharyngeal-oesophageal scintigraphy.
Six patients (2 nasopharynx, 1 base of tongue, 1 tonsil and 1 soft palate) showed a worsening of the PTT score resulting in a mild prolongation time (grade 1) at FEES-L and only 1 patient (tonsil) at OPES-L. A mild (grade 1) PTT prolongation was observed in 4 patients (1 tonsil, 1 base of tongue, 1 nasopharynx and 1 soft palate) and 3 patients (1 base of tongue, 1 nasopharynx and 1 soft palate) at FEES-SL and VFS-SL, respectively. Moreover, one patient (tonsil) experienced a moderate (grade 2) PTT prolongation at FEES-SL. No case of PTT prolongation time at 1 month after RT was seen at VFS-L and at OPES-SL.
Finally, analysis of the development of pre-deglutition penetration and aspiration was limited owing to the restricted number of patients with pre-swallowing penetration (only 1 patient at FEES-L and 1 patient at FEES-SL) and aspiration (only 1 patient at OPES-L and 1 patient at FEES-SL) after RT, which did not reach statistical significance.
Specifically, pre-deglutition penetration at 1 month was detected in only 1 patient (base of tongue) at FEES-L and SL and aspiration at 1 month was detected in only 1 patient (nasopharynx) by OPES-L and FEES-SL, respectively.
Baseline dysphagia assessment and tumour characteristics
Association between primary site (nasopharynx vs oropharynx), T stage (T1-2 vs. T3-T4), N stage (N0 vs N1-2) and baseline dysphagia parameters using the three different exams was assessed after L and SL bolus intake. No significant association was found between primary site, T and N stage with the baseline HPRI (p > 0.405) or PTT (p > 0.314).
Moreover, analysis of the association with baseline pre-deglutition penetration and aspiration showed a limited number of patients with baseline pre-deglutition penetration and/or aspiration, which did not reach statistical significance.
In more detail, baseline HPRI was altered in 14 patients (70%) at VFS-L and SL and in 13 patients (65%) and 15 patients (75%) at OPES-L and SL, respectively.
These data were not confirmed at FEES-L and SL, as shown in Table VII.
Table VII.
Baseline HPRI scores with the three different exams.
| HPRI | Grade 0 | % | Grade 1 | % | Grade 2 | % | Grade 3 | % |
|---|---|---|---|---|---|---|---|---|
| FEESa 0dLe | 17 | 85 | 3 | 15 | - | - | - | - |
| VFSb 0L | 6 | 30 | 5 | 25 | 4 | 20 | 5 | 25 |
| OPESc 0L | 7 | 35 | 12 | 60 | 1 | 5 | - | - |
| FEES 0SLf | 16 | 80 | 3 | 15 | 1 | 5 | - | - |
| VFS 0SL | 6 | 30 | 5 | 25 | 4 | 20 | 5 | 25 |
| OPES 0SL | 5 | 25 | 10 | 30 | 5 | 25 | - | - |
FEES = Fiberoptic endoscopic swallowing evaluation;
VFS = Videofluoroscopy;
OPES = Oro-pharyngeal-oesophageal scintigraphy;
0 = Baseline evaluation;
L = Liquid;
SL = Semiliquid.
In this regard, 3 patients (1 tonsil, 1 base of tongue and 1 nasopharynx) showed a mild HPRI at FEES-L and SL, and 1 patient (tonsil) showed a moderate HPRI at FEESSL. In contrast, baseline PTT after L bolus intake was normal in all patients but one (tonsil cancer), in whom mild prolongation (grade 1) was seen at FEES.
Furthermore, moderate PTT prolongation (grade 2) was observed in 3 patients at FEES-SL (2 with tonsil and 1 with base of tongue cancer) and in 2 patients at VFS-SL (tonsil cancer). The PTT was normal in all patients at OPES-SL. Baseline pre-deglutition penetration was detected in only 1 patient (nasopharynx cancer) at FEES-L, VFS-L and OPES-L, in 3 patients at FEES-SL (2 with nasopharynx cancer and 1 with tonsil cancer) and in only 1 patient (nasopharynx cancer) at VFS-SL and OPES-SL. Finally, aspiration was observed in only 2 patients (10%) affected by tonsil and base of tongue cancer, respectively, at OPES-L.
Discussion
The results of our study showed that post-deglutition HPRI was the most sensitive parameter, independently of the exam and consistency of the bolus.
Our data showed a significant number of patients who experienced an increased HPRI score from baseline to 1 month after RT, especially using VFS and FEES rather than OPES. This difference is probably due to the poor anatomical resolution of OPES together with the difficulty for the nuclear physician to correctly create a region of interest (ROI ) with the subsequent risk to overestimate the pattern of dysphagia.
Our explanation is supported by the high percentage of patients with baseline increased HPRI score at OPES-L and SL (65% and 75%, respectively), which probably justifies the lack of statistical difference between pre- and post-RT. Hence, we believe that OPES should be considered as a complementary exam in assessment of radiationinduced dysphagia. Indeed, this is supported by a recent systematic review of oropharyngeal dysphagia assessment by Speyer et al. 25.
On the other hand, the significant worsening of HPRI score seen at FEES and VFS was probably related to inflammatory oedema of pharyngeal mucosa, constrictor muscles and base of tongue causing a reduction of tongue strength and motion, pharyngeal contraction and laryngohyoid elevation with a consequent increased number of swallows needed to clear the bolus 37.
Lazaurus et al., Wu et al., and Jensen et al. 38-40 have published similiar results, reporting a significant amount of pharyngeal retention (88-93.5%) and post-deglutition aspiration (59-77.4%) after RT alone for patients affected by different HN cancer sites. Similar results were reported by most studies on patients submitted to concomitant chemo-radiation protocols 41-44.
In our preliminary experience, a linear relationship was observed between HPRI worsening score after L and SL bolus using the same exam. This finding might be explained by a similar muscular effort in the deglutition act for the two different consistencies of bolus. As reported by most literature data, the instrumental assessment of dysphagia is based on deglutition evaluation after L and SL bolus intake 45 46. Likewise, most patients undergoing radio- or radiochemotherapy for HN cancer favour a soft diet owing to acute radiation-induced mucositis and xerostomia 47.
Thus, dysphagia assessment using solid bolus might have shown a higher percentage of HPRI worsening due to the requirement of a stronger muscular propulsion in this set of patients.
On the contrary, PTT changes from baseline to 1 month after RT were not statistically significant. Among the four deglutition parameters, PTT is the most difficult to disclose regardless of the type of exam. In our experience, no case of severe PTT prolongation was observed either at baseline or 1 month after RT. In addition, only a few patients experienced mild or moderate PTT prolongation after L (30% at FEES and 5% at OPES, respectively) and SL bolus intake (25% at FEES and 15% at VFS, respectively). This result is probably related to the low specificity of the three exams in revealing such a subtle parameter, causing a significant percentage of false negative patients and lack of variations from before to after treatment.
Our clinical study was aimed to prospectively evaluate the impact of RT using IMRT on deglutition function through complementary instrumental assessment.
This issue has been addressed by only few studies 10 16 17 19, mostly reporting retrospective clinical results on patients affected by tumours arising from different HN sites, which limited the validity of the final data.
According to the recent recommendations by the Italian Association of Radiation Oncology, objective instrumental evaluation was performed using FEES, VFS and OPES before and after RT 48. Selection criteria were defined to properly assess the impact of RT in a homogeneous subset of HN cancer patients to maximally avoid selection bias.
Firstly, we excluded the patients affected by oral, larynx and hypopharynx cancer due to the high prevalence of baseline cancer-related dysphagia 49 as well as those who underwent previous surgery and/or RT in HN region or with deglutition-related comorbidities (i.e. neurological or rheumatological diseases).
Moreover, patients with Stage IVB (T4b or N3) or C (M1) disease were not enrolled mainly due to the poor prognosis that significantly reduces the importance of deglutition evaluation.
Aiming to strictly assess the radiation-induced dysphagia, patients who previously underwent neoadjuvant chemotherapy were excluded due to the significant percentage of chemotherapy-related dysphagia (20-40%) that could worsen deglutition function before the beginning of standard radio- or radiochemotherapy 50.
In fact, the lack of significant correlation between pretreatment dysphagia parameters and tumour characteristics (primary site, T and N Stage) demonstrates the validity of our selection criteria.
Furthermore, our study investigated both acute (1 month) and late (6 and 12 months) radiation-induced dysphagia.
In this paper, we report the results of acute dysphagia (at 1 month after RT). Our preliminary findings showed that IMRT aimed to SWOARs-sparing caused an increase of postswallowing HPRI, but did not significantly influence the occurrence of pre-deglutition penetration and aspiration.
In this regard, we believe that the post-treatment increase of HPRI may also be related to mucositis and xerostomia, which occurs during the course of RT, with a consequent increased difficulty to the transit of bolus through the pharyngeal region 51-53.
Therefore, the radiation oncologist must pay attention to maximally reduce the dose delivered to the major salivary glands and pharyngeal uninvolved mucosa rather than to the SWOARs.
In contrast, the use of IMRT aimed to spare SWOARs irradiation, probably contributed to the low incidence (only 1 patient) of post-deglutition penetration and aspiration.
In this regard, Feng et al. 54 initially and Eisbruch et al. (55) afterwards, reported the data of the only prospective study by the University of Michigan on 73 oropharyngeal cancer patients undergoing IMRT and evaluated at 3, 12 and 24 months using both clinical (CTCAE scale) and instrumental (videofluoroscopy) assessment criteria. The authors reported a slight worsening of VFS scores (mild to moderate dysphagia) from pre-therapy to soon after therapy (3 months) that did not improve at subsequent follow-up (12 and 24 months).
Conclusions
At present, our early preliminary data seem to confirm the experience reporting no cases of severe side effects (PEG positioning or clinical aspiration) as well as a low percentage of major instrumental dysfunction (pre-deglutition penetration or aspiration). In our opinion, IMRT significantly limits severe acute deglutition sequelae in HN cancer patients compared with historical literature data (56,57). Indeed, longer follow-up and larger sample size are needed to further evaluate if the observed increase of hypopharyngeal retention of food is subsequently related to a high risk of developing late aspiration (6 and 12 months).
References
- 1.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamouscell carcinoma: three meta-analyses of updated individual data . Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Delaney GP, Fisher RJ, Smee RI, et al. Split-course accelerated therapy in head and neck cancer: an analysis of toxicity . Int J Radiat Oncol Biol Phys. 1995;32:763–768. doi: 10.1016/0360-3016(95)00093-E. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TD, Panis X, Froissart D, et al. Analysis of late complications after rapid hyperfractionated radiotherapy in advanced head and neck cancers . Int J Radiat Oncol Biol Phys. 1988;14:23–25. doi: 10.1016/0360-3016(88)90045-4. [DOI] [PubMed] [Google Scholar]
- 5.Olmi P, Cellai E, Chiavacci A, et al. Accelerated fractionation in advanced head and neck cancer: results and analysis of late sequelae . Radiother Oncol. 1990;17:199–207. doi: 10.1016/0167-8140(90)90204-a. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JS, Fu K, Marks J, et al. Late effects of radiation therapy in the head and neck region . Int J Radiat Oncol Biol Phys. 1995;31:1141–1164. doi: 10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NP, Sallah S, Karlsson U, et al. Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues . Cancer. 2002;94:1131–1141. doi: 10.1002/cncr.10257. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NP, Moltz CC, Frank C, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer . Ann Oncol. 2004;15:383–388. doi: 10.1093/annonc/mdh101. [DOI] [PubMed] [Google Scholar]
- 9.Batth SS, Caudell JJ, Chen AM. Practical considerations in reducing swallowing dysfunction following concurrent chemoradiotherapy with intensity-modulated radiotherapy for head and neck cancer . Head and Neck. 2014;36:291–298. doi: 10.1002/hed.23246. [DOI] [PubMed] [Google Scholar]
- 10.Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head and neck cancer. Int J Radiat Oncology Biol Phys. 2009;73:410–415. doi: 10.1016/j.ijrobp.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Raber-Durlacher JA, Brennan MT, Verdonck-de Leeuw IM, et al. Swallowing dysfunction in cancer patients. Support Cancer Care. 2012;20:433–443. doi: 10.1007/s00520-011-1342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gluck I, Feng FY, Lyden T, et al. Evaluating and reporting dysphagia in trials of chemoirradiation for head-and neckcancer . Int J Radiat Oncol Biol Phys. 2010;77:727–733. doi: 10.1016/j.ijrobp.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy BA. Clinical and economic consequences of mucositis induced by chemotherapy and/or radiotherapy . J Support Oncol. 2007;5(Suppl4):13–21. [PubMed] [Google Scholar]
- 14.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and after chemoradiotherapy for head and neck cancer: which anatomic structures are affected and can they be spared by IMRT? . Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 15.O'Sullivan B, Rumble RB, Warde P, et al. Intensity-modulated radiotherapy in the treatment of head and neck cancer. Clin Oncol (R Coll Radiol) 2012;24:474–487. doi: 10.1016/j.clon.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 17.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck . Int J Radiat Oncol Biol Phys. 2010;76:403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term and speech related quality of life. Int J Radiat Oncol Biol Phys. 2007;68:750–757. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 19.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Li D, Lau DH, et al. Clinical-dosimetric analysis of measures of dysphagia including gastrostomy-tube dependence among head and neck cancer patients treated definitively by intensity-modulated radiotherapy with concurrent chemotherapy. Radiat Oncol. 2009;4:52–52. doi: 10.1186/1748-717X-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox JD, Stetz J, Pajak TF, et al. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 22.LENT SOMA scales for all anatomic sites. Int J Radiat Oncol Biol Phys. 1995;31:1049–1091. doi: 10.1016/0360-3016(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 23.Denis F, Garaud P, Bardet E, et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: comparison of LENT/SOMA, RTOG/ EORTC, and NCI-CTC scoring system. Int J Radiat Oncol Biol Phys. 2003;55:93–98. doi: 10.1016/s0360-3016(02)03819-1. [DOI] [PubMed] [Google Scholar]
- 24.Jensen K, Lambertsen K, Torkov P, et al. Patient assessed symptoms are poor predictors of objective findings. Results from a cross sectional study in patients treated with radiotherapy for pharyngeal cancer . Acta Oncol. 2007;46:1159–1168. doi: 10.1080/02841860701491041. [DOI] [PubMed] [Google Scholar]
- 25.Speyer R. Oropharyngeal dysphagia screening and assessment. Otolaryngol Clin North Am. 2013;46:989–1008. doi: 10.1016/j.otc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Rofes L, Arreola V, Almirall J, et al. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly . Gastroenterol Res Pract. 2011;2011 doi: 10.1155/2011/818979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merlotti A, Alterio D, Vigna Taglianti R, et al. Technical guidelines for head and neck cancer on behalf of the Italian association of radiation oncology-head and neck working group . Radiation Oncology. 2014;9:264–264. doi: 10.1186/s13014-014-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christianen ME, Langendijk JA, Westerlaan HE, et al. Delineation of organs at risk involved in swallowing for radiotherapy treatment planning . Radiother Oncol. 2011;101:394–402. doi: 10.1016/j.radonc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Silva AC, Fabio SR, Dantas RO, et al. A scintigraphic study of oral, pharyngeal, and esophageal transit in patients with stroke . Dysphagia. 2008;23:165–171. doi: 10.1007/s00455-007-9117-0. [DOI] [PubMed] [Google Scholar]
- 30.Badenduck LA, Matthews TW, Mc Donough A, et al. Fiberoptic endoscopic evaluation of swallowing to assess swallowing outcomes as a function of head position in a normal population. J Otolaryngol Head Neck Surg . 2014;43:9–9. doi: 10.1186/1916-0216-43-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neubauer PD, Rademaker AW, Leder SB. The Yale Pharyngeal Residue Severity Rating Scale: an anatomically defined and image-based tool. Dysphagia. 2015;30:521–528. doi: 10.1007/s00455-015-9631-4. [DOI] [PubMed] [Google Scholar]
- 32.Park WY, Lee TH, Ham NS, et al. Adding endoscopist-directed flexible endoscopic evaluation of swallowing to the videofluoroscopic swallowing study increased the detection rates of penetration, aspiration and pharyngeal residue . Gut Liver. 2015;9:623–628. doi: 10.5009/gnl14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenbek JC, Robbins J, Roecker EV, et al. A penetrationaspiration scale . Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 34.Dyer JC, Leslie P, Drinnan MJ. Objective computer-based assessment of valleculae residue. Is it usufeul? . Dysphagia. 2008;23:7–15. doi: 10.1007/s00455-007-9088-1. [DOI] [PubMed] [Google Scholar]
- 35.Fattori B, Grosso M, Bongioanni P, et al. Assessment of swallowing by oropharyngoesophageal scintigraphy in patients with amyotrophic lateral sclerosis . Dysphagia. 2006;21:280–286. doi: 10.1007/s00455-006-9052-5. [DOI] [PubMed] [Google Scholar]
- 36.Farneti D. Pooling score: an endoscopic model for evaluating severity of dysphagia . Acta Otorhinolaryngol Ital. 2008;28:135–140. [PMC free article] [PubMed] [Google Scholar]
- 37.Dejaeger E, Goethals P. Deglutition disorder as a late sequel of radiotherapy for pharyngeal tumor. Am J Gastroenterol. 1995;90:493–495. [PubMed] [Google Scholar]
- 38.Lazaurus CL. Effects of radiation therapy and voluntary maneuvers on swallow function in head and neck cancer patients . Clin Comm Disorders. 1993;3:11–20. [PubMed] [Google Scholar]
- 39.Wu CH, Hsiao TY, Ko JY, et al. Dysphagia after radiotherapy: endoscopic examination of swallowing in patients with nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol. 2000;109:320–325. doi: 10.1177/000348940010900315. [DOI] [PubMed] [Google Scholar]
- 40.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity, and correlation with dose and volume parameters . Radiother Oncol. 2007;85:74–82. doi: 10.1016/j.radonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Mittal B, Kepka A, Mahadevan A, et al. Tissue-dose compensation to reduce toxicity from combined radiation and chemotherapy for advanced head and neck cancers. Radiat Oncol Invest. 2001;96:61–70. doi: 10.1002/ijc.10360. [DOI] [PubMed] [Google Scholar]
- 42.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 43.Kotz T, Abraham S, Beitler JJ, et al. Pharyngeal transport dysfunction consequent to an organ-sparing protocol. Arch Otolaryngol Head and Neck Surg. 1999;125:410–413. doi: 10.1001/archotol.125.4.410. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen NP, Frank C, Moltz CC, et al. Aspiration rate following chemoradiation for head and neck cancer: an underreported occurrence . Radioth Oncol. 2006;80:302–306. doi: 10.1016/j.radonc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Kendall KA, McKenzie S, Leonard RJ, et al. Timing of events in normal swallowing: a videofluoroscopic study . Dysphagia. 2000;15:74–83. doi: 10.1007/s004550010004. [DOI] [PubMed] [Google Scholar]
- 46.Wu CH, Hsiao TY, Chen JC, et al. Evaluation of swallowing safety with fiberoptic endoscope: comparison with videofluoroscopic technique . Laryngoscope. 1997;107:396–401. doi: 10.1097/00005537-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 47.Logemann JA, Pauloski BR, Rademaker AW, et al. Xerostomia: 12 month changes in saliva production and its relationship to perception and performance of swallow function, oral intake, and diet after chemoradiation . Head Neck. 2003;25:432–437. doi: 10.1002/hed.10255. [DOI] [PubMed] [Google Scholar]
- 48.Russi EG, Corvò R, Merlotti A, et al. Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: review and recommendations of the supportive task group of the Italian Association of Radiation Oncology. Cancer Treat Rev. 2012;38:1033–1049. doi: 10.1016/j.ctrv.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head and neck cancer . Int J Radiation Oncology Biol Phys. 2009;73:410–415. doi: 10.1016/j.ijrobp.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 50.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer . N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 51.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer . J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen AM, Bardow A, Jensen SB, et al. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion . Oral Dis. 2002;8:117–129. doi: 10.1034/j.1601-0825.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 53.Rhodus NL, Moller K, Colby S, et al. Dysphagia in patients with three different etiologies of salivary dysfunction . Ear Nose Throat J. 1995;74:45–48. [PubMed] [Google Scholar]
- 54.Feng FY, Kim HM, Lyden TH, et al. Intensity modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisbruch A, Kim HM, Feng FY, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81:e93–e99. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.aurell G, Kraepelien T, Mavroidis P, et al. Stricture of the proximal esophagus in head and neck carcinoma after radiotherapy. Cancer. 2003;97:1693–1700. doi: 10.1002/cncr.11236. [DOI] [PubMed] [Google Scholar]
- 57.Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypopharyngeal/ upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808–812. doi: 10.1002/hed.20427. [DOI] [PubMed] [Google Scholar]