Abstract
Distraction and rumination are distinct response styles that determine how an individual deals with negative thoughts and feelings. Rumination is accompanied by an elevated self-focus, which is associated with increased resting state functional connectivity and decreased reactivity within the default mode network. Interestingly, the NMDA receptor antagonist ketamine reduces functional connectivity in this network, while its effects on blood oxygenation level-dependent (BOLD) responses during stimulus perception are not known. Ketamine might lead to a more variable processing of the external world with an attenuated self-focus by reducing the resting state connectivity. Here, we used an emotional picture-viewing task in combination with functional magnetic resonance imaging to test the hypothesis that a single ketamine administration to healthy subjects increases BOLD reactivity to negative stimuli. We found a region specific increase in BOLD reactivity in the pregenual anterior cingulate cortex and not in a posterior control region after ketamine compared with placebo administration. Moreover, a linear regression revealed that the increase in BOLD reactivity was more pronounced for subjects with a low ability to apply distraction during negative experiences. Our results implicate that ketamine attenuates a potentially pathological increased self-focus during negative experiences.
Keywords: ketamine, response styles, fMRI, resting state connectivity, DMN
Introduction
Inability to employ distraction from and a tendency to ruminate about unpleasant experiences or sad feelings plays a role in the emergence and maintenance of depressed mood. A number of studies reported unfavorable effects of rumination as well as beneficial effects of distraction on depression-related emotional and cognitive processes (e.g. Nolen-Hoeksema and Morrow, 1991; Nolen-Hoeksema and Parker, 1994; Lyubomirsky et al., 1999; Bagby and Parker, 2001; Lam et al., 2003; Chang, 2004; Donaldson and Lam, 2004; Lavender and Watkins, 2004; Rimes and Watkins, 2005; Sutherland and Bryant, 2007; Huffziger and Kuehner, 2009; Kuehner et al., 2009). Distraction is thought to be adaptive during unpleasant situations or feelings and involves behaviors that help guide one’s attention away from negative emotions and turn it to pleasant or benign thoughts and absorbing activities (Nolen-Hoeksema and Morrow, 1991; Nolen-Hoeksema et al., 2008).
Distraction is often contrasted in the literature with rumination, the repetitive focusing of attention on negative feelings and thoughts in response to negative mood (Nolen-Hoeksma and Morrow, 1991). A predominant tendency to ruminate appears to contribute to pessimism about the future and a negative reflection of the self, which many theorists argue are key to depression (Abramson and Alloy, 1989; Lavender and Watkins, 2004; Verplanken et al., 2007; Takano and Tanno, 2009). Prospective longitudinal studies revealed that engaging in rumination when distressed increases the risk to develop depressive disorders and may prolong periods of depression (Just and Alloy, 1997; Nolan et al., 1998; Kuehner and Weber, 1999; Nolen-Hoeksema and Davis, 1999; Nolen-Hoeksema et al., 1999). Furthermore, it has been suggested that an increased self-focus during ruminative states results in more exacerbated depressed mood (for review see Nolen-Hoeksema et al., 2008). However, distraction from dysphoria seems to brighten one’s sad mood. The experimental manipulation of distraction or external focus significantly relieves depressed mood (Lyubomirsky and Nolen-Hoeksema, 1993, 1995). The tendency for distraction or rumination seems to be relatively stable even in individuals who experience changes in depression severity, supporting the assumption that rumination and distraction do not merely represent state-dependent epiphenomena of depressed mood but rather trait-like characteristics (Kuehner and Weber, 1999).
Rumination and an increased negative self-focus have been associated with changes in functional connectivity and activity within regions of the default mode network (DMN; Raichle et al., 2001). The DMN comprises anterior and posterior medial cortical regions such as pregenual anterior cingulate cortex (pACC) and posterior cingulate cortex (PCC) and is characterized by high functional resting-state connectivity and activity when individuals direct attention internally, e.g. during mind wandering and random thoughts (Gusnard et al., 2001) . Conversely, deactivation in DMN regions, i.e. negative blood oxygenation level-dependent (BOLD) responses (NBRs), has been shown during various emotional–cognitive tasks that require subjects to attend to external stimuli (Northoff et al., 2004; Raichle and Gusnard, 2005; Fox and Raichle, 2007; Raichle and Snyder, 2007; Buckner et al., 2008). Patients with major depressive disorder (MDD) not only show increased resting state activity in anterior and posterior DMN regions, but also a failure to deactivate these regions during emotional processing (Grimm et al., 2009; Sheline et al., 2009). Decreased NBRs of MDD patients correlate with depression severity and feelings of hopelessness (Grimm et al., 2009), which indicates that they might represent a lack of modulation of these patients’ abnormally negative emotions. Schneider et al. (2008) suggest that increased resting state activity in anterior parts of the DMN is specifically associated with self-relatedness. Accordingly, decreased NBRs in pACC have been observed in MDD patients during self-related judgments and, unlike in healthy subjects, NBRs in pACC are not parametrically modulated by the degree of self-relatedness, which may reflect that patients are no longer able to down-regulate the heightened resting state activity and consecutively their abnormally high degree of self-relatedness (Grimm et al., 2011). Furthermore, increasing levels of DMN dominance have been associated with higher levels of maladaptive rumination (Hamilton et al., 2011). Underscoring the role of increased resting state activity within the DMN for emergence and maintenance of negative mood states are findings showing an attenuation of DMN activity in MDD patients with remission after treatment with the selective serotonin reuptake inhibitor (SSRI) sertraline (Anand et al., 2005).
Contrarily to the immediate effect of ketamine, SSRIs have a delayed onset of action. Investigating the treatment effect requires therefore an appropriate time lag between baseline and follow-up measurement, which however may result in various cofounding variables. In the last decade, the NMDA receptor antagonist ketamine has been established as a research tool for the investigation of the neurobiology of depressive disorders. Evidence from a vast amount of studies has accumulated, demonstrating an antidepressant response within 24 h after intravenous administration (for a review and meta-analysis see Fond et al., 2014). The rapid onset of action allows the examination of neurophysiological effects that can be allocated more precisely to the drug administration.
In line with the aforementioned findings, the administration of ketamine has been shown to reduce the functional connectivity between anterior (pACC) and posterior parts of the DMN (PCC) in healthy subjects, suggesting that the reduction of increased levels of DMN dominance is involved in a putative antidepressant effect of ketamine (Scheidegger et al., 2012).
This study aimed to investigate the effect of a pharmacological ketamine challenge on resting state connectivity and NBRs during emotional processing in anterior and posterior DMN regions and its modulation by response style (rumination and distraction). We used an emotional picture viewing task to test the hypothesis that NBRs in pACC are increased 24 h after the administration of a single dose of intravenous ketamine and that ketamine effects on NBRs are modulated by the subjects’ response style. A ruminative response style is closely related to increased self- focus, which in turn has been associated with increased resting state connectivity in the DMN and reduced NBRs in the pACC (Raichle et al., 2001). The pACC is activated during the establishment of mood states and particularly involved in the processing of negative stimuli (Murphy et al., 2003). In order to test for valence-specific effects on NBRs in the pACC, we therefore included both negative and positive stimuli. On the other hand, posterior DMN regions, i.e. dorsal PCC (dPCC), should not be specific with regard to self-relatedness and affective processing, resulting in less or no effects of ketamine and response style on NBRs. As our previous study demonstrated that ketamine decreases resting state functional network connectivity (Scheidegger et al., 2012), we furthermore aimed at elucidating the association between NBRs in pACC/dPCC and resting state functional connectivity between these regions. Finally, previous work suggests that the reduction of depressive symptoms following the ketamine infusion is associated with acute dissociative side effects (Luckenbaugh et al., 2014) which in turn might reflect the amount of network disruption within the DMN (Walter et al., 2014). Therefore, we hypothesized that the psychotomimetic experience during the ketamine infusion might be related to changes in functional connectivity measured 24 h after its administration.
Materials and methods
Subjects
Healthy subjects [n = 19, mean age, 40.5 + 7.5 (s.d.)] without any psychiatric, neurological or medical illness were self-referred from online study advertisements. All subjects underwent a medical examination and psychiatric interview based on the Brief Psychiatric Rating Scale (Rhoades and Overall, 1988) and the Hamilton Rating Scale for Depression. Only medication-free subjects that were healthy according to the physical examination, electrocardiogram and blood and urine analyses were included in the study. Exclusion criteria were a history of psychiatric/neurological diseases, drug abuse, concurrent medication, cardiovascular disease, anemia and thyroid disease, any somatic disease affecting drug metabolism and excretion (e.g. renal or liver disease), MR exclusion criteria, pregnancy and left handedness.
Study design
Seventeen out of 19 subjects (one study dropout per ketamine or placebo run) completed a total of four functional magnetic resonance imaging (fMRI) sessions. The order of placebo and ketamine administration was randomly assigned by an external third party. Throughout the whole experiment, the investigators confined strictly to a double-blind assessment The baseline fMRI scan was followed by an intravenous (i.v.) infusion (45 min) of either S-ketamine (0.25 mg/kg, Ketanest (Registered Trade Mark) S, Pfizer, Zurich, Switzerland) or saline (0.90% w/v of NaCl) outside the scanner. Previous clinical trials mostly used an i.v. dose of 0.5 mg/kg of racemic ketamine (R/S enantiomer ratio of 1:1). The S(+)-isomer of ketamine is characterized by a 3–4 times higher affinity or potency at specific receptors, so that a dose reduction of 50% is recommended (Sinner and Graf, 2008). Since the effect of ketamine on depressive symptoms and emotional processing is most pronounced after 1 day (Zarate et al., 2006), the follow-up fMRI scans were scheduled 24 h after ketamine or placebo infusion in order to assess the related effects on neuronal network dynamics that might contribute to the understanding of its antidepressant efficacy (Figure 1). To avoid possible carry-over effects, the time lag between the two baseline measurements was set to at least 10 days. The time of day for all the imaging sessions was kept constant for every participant.
Fig. 1.
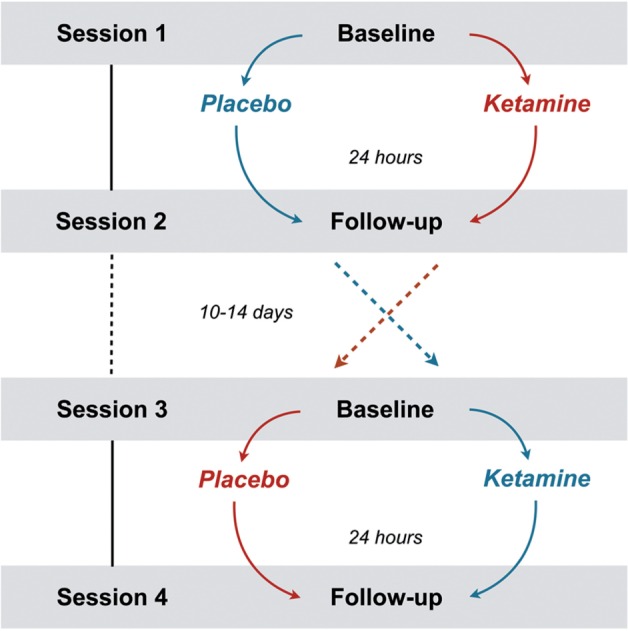
The randomized, double-blind, placebo controlled crossover design. Session 1 and 2 were completed by n = 19, session 3 and 4 by n = 17. The red and blue path indicates the randomly assigned order of administration.
Psychometry
5D-ASC questionnaire
Psychotomimetic actions of ketamine infusion were assessed 15–30 min after the ketamine administration using the Altered States of Consciousness rating scale ‘5D-ASC’ (Dittrich, 1998). The score sums describe the following five dimensions: ‘Oceanic boundlessness’ (OB) summarizes symptoms of depersonalization and derealization; ‘Anxious ego dissolution’ (AED) focuses on anxiety and fearful delusions, arising from ego-disintegration and loss of self-control phenomena. ‘Visionary restructuralization’ (VR) consists of items measuring hallucinations, synesthesia and altered imagination. The main scale ‘auditory alterations’ (AA) measures acoustic phenomena and ‘reduction of vigilance’ (RV) subsumes states of drowsiness and impaired attention.
Response style questionnaire
The 23-item German version of the Response Style Questionnaire (RSQ-D) is designed to measure different coping styles by asking respondents what they generally do when they feel sad. The questionnaire consists of two scales: the rumination response scale (RRS), which consists of 15 items and can be subdivided into self-focused (seven items) and symptom-focused rumination (eight items), and the Distraction response scale (DRS), with eight items (Bürger and Kühner, 2007). All items are rated on a four-point Likert scale, ranging from 1, ‘almost never’, to 4, ‘almost always.’ The RRS and DRS are reliable, independent of one another, have been shown to measure trait-like coping styles and are not associated with, or confounded by, the state effects of depressed mood (Nolen-Hoeksema and Morrow, 1991; Just and Alloy, 1997).
The RSQ-D distinguishes between self- and symptom-focused rumination and includes a factorial reduction of the distraction scale, which has been shown to provide stronger predictive validity for depressive symptoms (Bürger and Kühner, 2007; Huffziger and Kuehner, 2009). The questionnaire data, however, depict an individuals’ response style as a continuous measure and is therefore not designed for determining whether an individual is a ‘distractor’ or a ‘ruminator’.
Subjects were asked to complete the RSQ-D prior to the first measurement.
Stimulus material and fMRI
The stimulus material comprised 40 different positive and negative photographs, respectively, from the International Affective Picture System (IAPS Center, 1991). Five pictures composed a block of 20 s duration. After each block of emotional stimulation, participants had to answer a question regarding the content of one of the five pictures during 8 s. This was implemented in order to control for a putative lack of concentration and to heighten the participant’s level of attention. After the rating, a resting period followed, where a fixation cross was presented for 20 s. This allowed participants to recover from emotional stimulation and served as a baseline condition. Subjects were instructed to fixate on the crosshair when it was present. The fMRI-paradigm was composed of eight blocks with negative and eight blocks with positive stimuli, resulting in 16 trials with an overall duration of 12.8 min. In order to avoid a repetition effect, for each session, the fMRI paradigm was composed of different pictures and the versions were randomly assigned to the sessions.
The experiment was presented via MRI compatible video goggles (VisuaStim, Resonance Technology, www.mrivideo.com) using Presentation software (Version 0.70, www.neurobs.com).
Picture ratings
Outside the scanner, participants rated the previously presented pictures regarding the intensity and valence on a visual analogue scale. Pictures and rating scales were presented with E-Prime 2.0 software (Psychology Software Tools, Inc., www.pstnet.com/eprime) and the rating scale was based on IAPS norms, ranging from one to nine.
fMRI data acquisition
Imaging was performed using a 3 Tesla Philips MR system (Philips Medical Systems, Eindhoven, The Netherlands) equipped with a standard eight channel SENSE head coil. Plane functional images were acquired using a T2*-weighted, single-shot, fast field echo planar imaging sequence with a repetition time (TR) of 2000 ms (Θ = 82°), an echo time (TE) of 35 ms. In total, 384 volumes were collected, consisting of 32 contiguous slices with a thickness of 4 mm, measured with a whole brain coverage. A 20 × 20 acquisition matrix, interpolated to 128 × 128, with a field of view (FOV) of 220 mm was used yielding an effective voxel size of 2.75 × 2.75 × 4. Prior to the fMRI task paradigm, brain activity at rest was measured in a 10 min run (2000 volumes) using a sensitivity-encoded single-shot echo-planar sequence (TE = 35 ms; FOV = 220 mm; acquisition matrix = 80 × 80, interpolated to 128 × 128, voxel size = 2.75 × 2.75 × 4 mm and sensitivity-encoded acceleration factor R = 2.0) sensitive to BOLD contrast (T2* weighting). Using a midsagittal scout image, 32 contiguous axial slices were placed along the anterior–posterior commissure plane covering the entire brain and acquired with a TR of 3000 ms (Θ = 82°) in ascending slice order. For the resting state measurement, participants were instructed to look at a fixation cross while staying awake, keeping their eyes open and not thinking about anything particular. Anatomical reference images of the whole brain were obtained at the beginning of the imaging session for both, task and rest fMRI, using a 3D, T1-weighted, field echo sequence (TR = 9.3 ms, TE = 4.6 ms, flip angle = 8°, in plane resolution = 1 × 1 × 1 mm, slice thickness = 2 mm, 160 slices).
Statistical analysis
Behavioral data
The four versions of the fMRI-paradigm were statistically tested for differences regarding the intensity and valence ratings of the presented pictures using a 2 × 4 repeated measures analysis of variance (ANOVA) with the factors ‘valence category’ (positive and negative) and ‘version’ (four different versions). This was done in order to avoid that within-subject differences between sessions would be ascribed to different picture content. The analysis of the effect of substance on the behavioral ratings was also conducted using repeated measures ANOVAs with the factors ‘time’ (baseline vs follow-up) and ‘substance’ (ketamine vs placebo). Where appropriate, post hoc tests were conducted using paired t-tests on delta values, reflecting changes from pre to post substance administration. Thereby, the control for baseline levels was taken into account.
For statistical analyses referring to data from the RSQ, all subscales (distraction, self-focused rumination and symptom-focused rumination) were included. In the report of results all subscales are mentioned.
fMRI data
Functional images were pre-processed using MATLAB 2009b (The Mathworks, Natick, MA) and SPM8 (Statistical parametric mapping software, SPM; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk). The data were corrected for differences in slice acquisition time, realigned to the first volume, corrected for motion artifacts, mean-adjusted by proportional scaling, normalized into standard stereotactic space [template provided by the Montreal Neurological Institute (MNI)], and spatially smoothed using a 8 mm FWHM Gaussian kernel. The time series were high-pass filtered to eliminate low-frequency components.
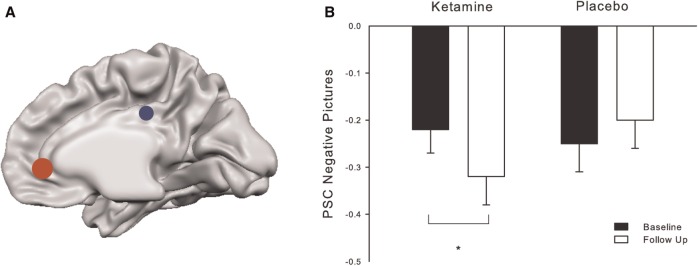
Statistical analysis was performed by modeling the three different conditions (positive picture viewing, negative picture viewing and rest period) convolved with a hemodynamic response function as explanatory variables within the context of the general linear model on a voxel-by-voxel basis. Realignment parameters were included as additional regressors in the statistical model. Region of interest [ROI: x, y, z, in MNI space] analyses were performed to investigate NBRs in pACC and dPCC (Figure 3A). On the basis of peak voxels reported in our previous papers on NBRs in DMN regions (e.g. Grimm et al., 2009; Walter et al., 2009; Grimm et al., 2011), we built spherical (radius = 6–10 mm) ROIs and carried out analyses for pACC (0, 40, 0) and dPCC (0, −30, 32). For the ROI analyses, effect sizes (% signal changes; PSCs) for the different conditions were extracted for each subject separately using MarsBaR (http://marsbar.sourceforge.net/). Signal changes during the rest period (‘fixation cross’) were subtracted from task conditions (‘negative’ or ‘positive picture viewing’).
Fig. 3.
(A) ROI placement for extraction of PSCs in the pACC (red) and dPCC (blue). (B) PSCs during negative picture viewing at the four measurements showing an increase in negative BOLD response after ketamine (paired t-test, P < 0.05).
The within-subject study design with four measurements resulted in a few drop-outs. In order to include all the collected data in the analysis, we opted against a repeated measures ANOVA and used a mixed model ANOVA instead with the two fixed factors ‘time’ (baseline vs follow-up) and ‘substance’ (ketamine vs placebo) and the random factor ‘subject’. This was conducted to compare the effects of ketamine and placebo on percent signal changes (PSCs) during emotional stimulation. Additionally, we used Pearson’s correlation analysis and linear regression to reveal possible associations between behavioral and neurophysiological data. All statistical analyses were conducted with SPSS statistical software version 21 (SPSS Inc, Chicago, IL).
Resting state fMRI data
Resting state data were analysed using a SPM8 based processing assistant for resting state fMRI (DPARSF, Yan Chao-Gan, State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, China), which includes a rsfMRI data analysis toolkit (REST; Song et al., 2011). The preprocessing steps followed the standard protocol described by Yan and Zang, 2010. Functional resting state data were corrected for differences in slice acquisition time, motion-corrected using a least squares approach and a six-parameter (rigid body) linear transformation, spatially normalized (to 3 × 3 × 3 mm isovoxels in standard space) and smoothed using a 4-mm fill-width-at-half-maximum Gaussian kernel. The data were linearly detrended and filtered by a band pass filter (0.01–0.08 Hz) to suppress cardiac and respiratory induced effects. An additional regression of nuisance covariates was applied during which the functional data were corrected for six head movement parameters and for global mean signal as well for white matter and cerebrospinal fluid signal (defined according to Yan and Zang, 2010). We limited our analysis to the a priori defined seed regions, using the same ROIs for pACC and dPCC as for the fMRI analysis. Functional connectivity maps were obtained for each subject and every session separately. Statistical tests on regional functional connectivity maps were computed after application of Fisher’s r-to-z transform, which yields variates that are approximately normally distributed. In order to test for effects of ketamine on resting state connectivity, statistical analysis relied on an ANOVA including two fixed factors ‘time’ (baseline vs follow-up) and ‘substance’ (ketamine vs placebo) and the random factor ‘subject’.
Results
Behavioral data
Ratings
Participants rated positive pictures on average over all versions with 7.2 (s.d. 1.4) on a nine-point likert-scale and negative pictures with 2.7 (s.d. 1.7). Negative pictures were experienced as significantly more intense compared with pictures with positive content (F(1,16 = 9.09, P < 0.01). However, there was no significant effect of version on the ratings of valence (F(1,14 = 0.42, P = 0.74) or intensity (F(1,14 = 0.91, P = 0.41).
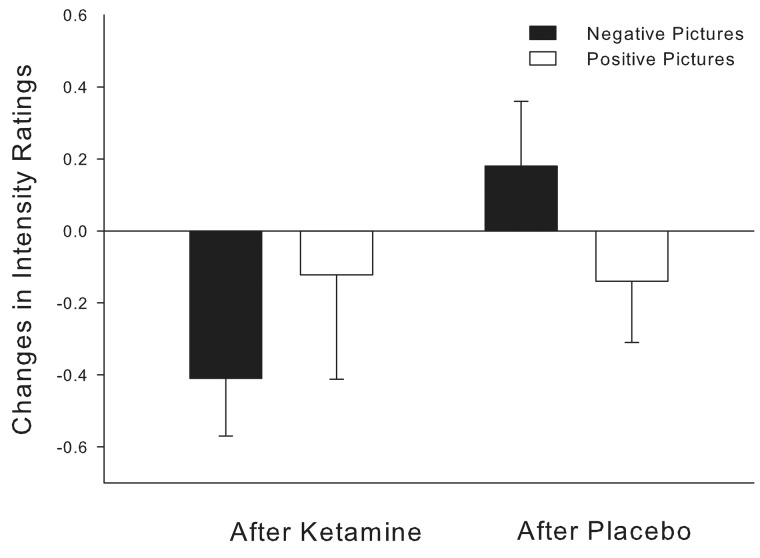
The analysis of changes in intensity ratings showed a trend towards a significant effect of ‘substance’ (F(1,63) = 3.63, P = 0.062) resulting in reduced ratings after the administration of ketamine. Post hoc tests revealed that the two baseline intensity ratings did not differ significantly (t = 1.41, P > 0.05). However, while the ratings were not altered after the administration of placebo (t = −0.77, P > 0.4), the changes in mean ratings for negative pictures after ketamine (M = −0.41, SEM = 0.16) were significantly reduced compared with the changes after placebo (M = 0.18, SEM = 0.18; t = −2.23, P < 0.05; Figure 2). Intensity ratings for positive and valence ratings for positive and negative pictures were unaffected by the administration of ketamine or placebo.
Fig. 2.
Changes in intensity ratings for negative and positive pictures after placebo and ketamine administration.
No correlations of valence and intensity ratings were found with the subscales of the RSQ or the 5D-ASC questionnaire.
5D-ASC questionnaire
Compared with placebo, subjects reported a significant increase in psychotomimetic experiences following ketamine administration as assessed by the altered state of consciousness rating scale ‘5D-ASC’. Following the infusion of ketamine subjects reported significant changes regarding ‘RV’ (n = 17, paired t-test: P < 0.001), ‘OB’ (P = 0.005), ‘AED’ (P = 0.009) and ‘VR’ (P = 0.022).
Additionally, we observed that the more subjective experiences during the ketamine infusion were altered in the sub scales ‘OB’ and ‘AA’, the stronger were the intensity ratings for negative pictures reduced 24 h post ketamine (OB: r = −0.656, P < 0.005 and AA: r = −0.485, P < 0.05). There was no correlation between the subscales of the RSQ and the 5D-ASC.
Negative BOLD responses
According to our hypothesis, 24 h after the stimulation with ketamine but not after placebo administration, significantly increased NBRs in the pACC could be observed during emotional stimulation with negative pictures (F(1,15) = 6.33, P = 0.025; Figure 3B). However, ketamine did not alter the NBRs during viewing of positive pictures (F(1,15 =1.425, P = 0.252; Figure 3D).
In order to ensure that this effect was not based on differences in PSCs between baseline measurements, we compared the two baselines statistically. They did not differ significantly (positive pictures: t(16) = 0.18, P > 0.8; negative pictures: t(16) = 0.75, P > 0.5).
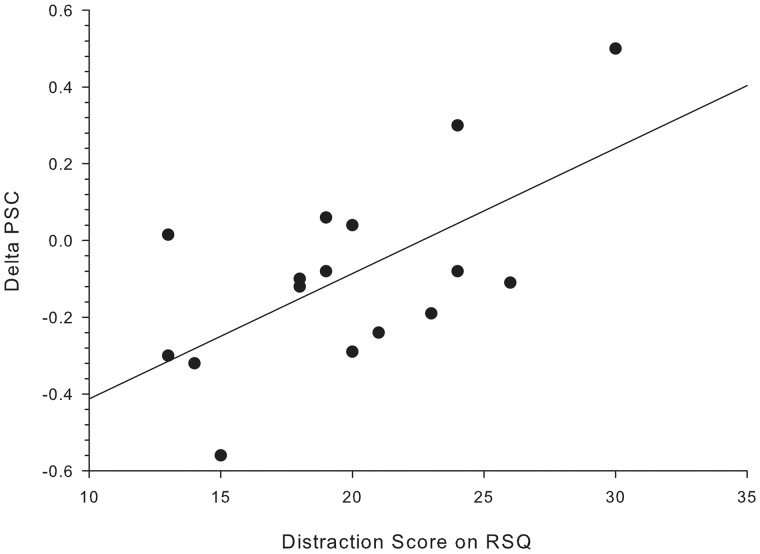
In general, viewing of negative pictures resulted in a stronger deactivation of the pACC, as compared with positive pictures. The difference in PSCs was significant during both baseline sessions (BslKet: t(16) = −2.36, P < 0.05; BslPlc: t(16) = −0.256, P < 0.05). A stepwise linear regression was used to test if one or several RSQ subscales significantly predict the alteration in NBRs. Therefore, a delta score for the NBRs in the pACC during negative picture viewing (delta_pACCneg) was calculated indicating the change from baseline to follow-up ketamine measurement. The results indicated that only one predictor explains 33.1% of the variance (R2 = 0.331, F(1,13) = 6.42, P < 0.05). It was found that distraction significantly predicts delta_pACCneg (b = 0.575, P < 0.05; Figure 4). The positive correlation between the two variables (r = 0.58, P < 0.05) implies that subjects with low distraction scores show a stronger increase in NBRs after ketamine. However, neither differences in symptom- nor self-focused rumination scores provide additional explanatory power (P > 0.5).
Fig. 4.
Correlation between distraction score in RSQ and changes in PSC from baseline to post ketamine administration (r = 0.52, P < 0.05).
In order to control for the specificity of the pACC in emotional processing, we also investigated NBRs in the dPCC. No changes in NBRs after ketamine could be observed during negative (F(1,15) = 0.304, P > 0.5; Figure 3C) or positive picture viewing (F(1,15) = 0.308, P > 0.5; Figure 3F).
Resting state functional connectivity
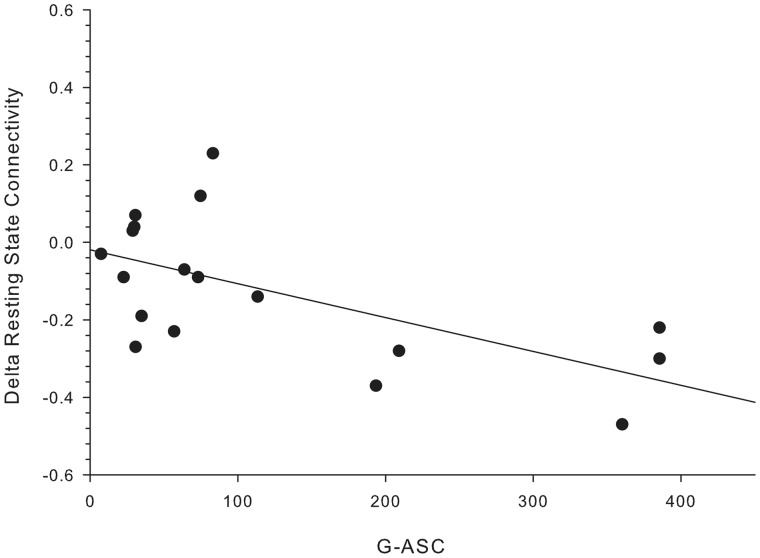
Additional resting-state analysis (rsfMRI) revealed that 24 h after the administration of ketamine, the functional connectivity between pACC and the dPCC was significantly reduced (F(1,15 = 7.304, P = 0.016). For further correlation analyses, the ketamine induced changes in functional connectivity were calculated for each subject resulting in the variable Delta_pACC_dPCC. All four subscales of the 5D-ASC questionnaire revealed a highly predictive power for the individual alteration in connectivity. The stronger the psychotomimetic effects of ketamine were experienced during the infusion, the more reduced the resting state connectivity between pACC and dPCC reduced was (OB: r = −0.678, P < 0.01; VR: r = −0.672, P < 0.01; AA: r = −0. 616, P < 0.01; AED: r = −0.492, P < 0.05; RV: r = −0.482, P < 0.05). The global 5D-ASC subsuming the subscales OB, AED and VR indicates the mean intensity of the psychotomimetic drug effects and correlated as well highly significantly with the reduced connectivity between pACC and dPCC after ketamine (G-ASC: r = −0.624, P < 0.01; Figure 5). However, the changes in connectivity were neither correlated with altered PSCs after ketamine administration nor the RSQ subscales.
Fig. 5.
Correlation between general 5D-ASC score and changes in resting state connectivity (r = 0.63, P < 0.01).
Discussion
We here investigated the effect of a pharmacological ketamine challenge on resting state connectivity and NBRs during emotional processing in anterior and posterior DMN regions and its modulation by response style (rumination and distraction).
In previous studies, high resting state neural activity in anterior DMN regions has been suggested as a marker for high degrees of self-relatedness (Schneider et al., 2008). In accordance with that finding, deactivation (i.e. NBRs) in the pACC has been found to be decreased in MDD patients during emotional stimulation, which was positively correlated with elevated self-focus (Grimm et al., 2009, 2011). Here, we report as our key finding a significant increase in NBRs to negative and aversive stimuli in the same brain region in healthy subjects 24 h after ketamine administration compared with placebo. Considering that the antidepressant effect of ketamine is most pronounced within that time range as well (Zarate et al., 2006), our finding suggests that ketamine enhances NBRs during emotional processing. The finding that increased NBRs were only observable during the presentation of negative and not positive stimuli emphasizes the involvement of the pACC in the processing of negative stimuli. Moreover, by applying the same analysis to the dPCC, a region belonging to the DMN but to a lesser extent involved in affective processing, we controlled for the specificity of the pACC. However, the NBRs in the dPCC were unchanged over the four measurements, suggesting a local region specific effect of ketamine and a general effect on cerebral blood flow seems less likely.
Additionally, we were able to reveal that the increased NBRs in the pACC from pre to 24 h post ketamine administration were particularly pronounced for subjects with low levels on the distraction scale. Distraction is considered an adaptive and effective coping mechanism during the experience of unpleasant situations (Nolen-Hoeksema et al., 2008) and is often insufficiently applied by depressive patients, while its integration in therapeutic learning reduces depressive symptoms (Fennell and Teasdale, 1984; Fennell et al., 2009). On a continuous scale, subjects with an inability to apply distraction appropriately might be more prone to develop depressed mood. Reacting with distraction during an unpleasant situation reduces chances to internalize negative feelings related to the experience, while an inability to use distraction results in an increased affective load and self-focus. The attenuation of NBRs after ketamine, particularly in subjects with an impaired ability to use distraction, might therefore reflect a reduction of involvement and self-focus during negative emotional experiences. Following this reasoning, it is conceivable that participants who apply distraction to a lesser extent benefit more from ketamine’s effect, whereby the pACC’s ability to respond variably during disturbing experiences is restored.
However, to address this issue more comprehensively, future studies should also assess the individual self-relatedness of the stimuli. Moreover, the above reasoning can be linked to our resting state connectivity findings. We were able to show that the resting-state functional connectivity between pACC and dPCC was reduced 24 h after ketamine administration. It is conceivable that a disentanglement of pACC from the DMN allows for a more varied response to external stimulation e.g. negative vs positive pictures. Additionally, a reduction of an increased DMN activity during external stimulation might permit processing without an elevated self-focus, which could be of particular importance during confrontation with negative experiences. The unchanged valence ratings suggest that negative stimuli are still perceived as aversive but the self-referential aspect might be attenuated. This is a crucial difference and can decisively alter everyday experiences by creating a sufficient distance to unpleasant situations. We did not observe a baseline difference in resting state connectivity between pACC and dPCC modulated by different response styles. This might be due to the drawback that only the comparison between particularly pronounced distracting or ruminative response styles reveals significant differences in connectivity measures and the between-subject variance was simply too low. While small differences in behavior are not detectable under normal circumstances with neurophysiological measures, the ketamine administration challenges the neurochemical system and accentuates them. This clearly underscores the necessity to investigate the effects of ketamine in a population of MDD patients displaying a greater behavioral variety when handling unpleasant experiences. Our findings suggest that particularly in patients with an elevated self-focus, ketamine might reduce resting state functional connectivity in affective compartments of the DMN and NBRs in the pACC during emotional stimulation, allowing for emotional processing while shifting the attention away from the self. If the antidepressant effect of ketamine is indeed closely related to an attenuated self-focus during negative experiences, its administration might open a plasticity window for psychotherapeutic learning, in which the attributions of memories and perceptions are disentangled from the self and reviewed under a new light.
Interestingly, our data also revealed a strong positive correlation between changes in connectivity and the psychotomimetic effects of ketamine. A recent study has shown that the reduction of depressive symptoms following the ketamine infusion was positively correlated with acute dissociative side effects (Luckenbaugh et al., 2014). It has been suggested that pathologically restricted network dynamics, as e.g. hyper-connectivity within the DMN in MDD, need to be directly disrupted and that acute psychotomimetic effects may reflect the amount of network disruption (Walter et al., 2014).
Based on recent findings from clinical trials that ketamine’s antidepressant effects peak ∼24 h after infusion before it slowly decays across the following 3–7 days, we investigated particularly the effects after 1 day. However, it is conceivable that scanning the effects in a higher temporal resolution across this 24 h time window would reveal the mechanisms leading to changes reported here. Especially in depressed patients, it would be of great interest to investigate how the effects of ketamine on the neurobiology and behavior evolve, when they reach a climax and how resting state and task related changes are intertwined over time. For research investigating the effect of psychedelic substances on neuronal activity, as with e.g. ketamine, the choice of an adequate placebo control is an unresolved issue. The administration of saline does not provoke similar psychotomimetic effects as ketamine. Therefore—even though the study was conducted in a double-blind fashion—it is possible that the symptoms were an indicator for the experimental condition. However, the subjects experienced the exact same setting and thus the placebo condition allows controlling for effects of the infusion procedure and general setting effects. Furthermore, the time lag between administration and measurement of 24 h should have minimized drug effects on task and ratings that could be attributed to reduced attention.
To conclude, the current study indicates for the first time that ketamine increases NBRs in the pACC during processing of negative stimuli. This alteration was neither observed in a control region nor during positive picture viewing, a finding that underscores the specific involvement of the pACC in negative affective processing. For subjects with a low ability to apply distraction during unpleasant experiences, the increase of NBRs was more pronounced. Moreover, the extent of reduction in resting state functional connectivity between pACC and dPCC after ketamine administration was predicted by the psychotomimetic effects during the ketamine infusion. Taken together, these results suggest that ketamine helps the pACC, a region that is over-activated and hyper-connected in depressed patients, to regain a variable responsiveness to negative stimuli and this effect seems critically depend on inter-individual differences in the ability to distract and psychotomimetic effects during drug administration.
Conflict of interest. Imaging was carried out using an MR scanner financed by the “highly specialized medicine” grant of the canton of Zurich. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Abramson M., Alloy L.B. (1989). Hopelessness depression: a theory-based subtype of depression. Psychological Review, 96(2), 358. [Google Scholar]
- Anand A., Li Y., Wang Y., Lowe M. J., Dzemidzic M. (2009). Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Research: Neuroimaging, 171(3), 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby R., Parker J.D. (2001). Relation of rumination and distraction with neuroticism and extraversion in a sample of patients with major depression. Cognitive Therapy and Research, 25(1), 91–102. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain’s default network. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Bürger C., Kühner C. (2007). Copingstile im umgang mit depressiver stimmung: faktorenstruktur und psychometrische Gütekriterien der deutschen version des Response Styles Questionnaire (RSQ). Zeitschrift Fur Klinische Psychologie Und Psychotherapie, 36(1), 36–45. [Google Scholar]
- Chang E.C. (2004). Distinguishing between ruminative and distractive responses in dysphoric college students: does indication of past depression make a difference? Personality and Individual Differences, 36, 845–55. [Google Scholar]
- Donaldson C., Lam D. (2004). Rumination, mood and social problem-solving in major depression. Psychological Medicine, 34, 1309–18. [DOI] [PubMed] [Google Scholar]
- Fennell M.J.V., Teasdale J.D. (1984). Effects of distraction on thinking and affect in depressed patients. British Journal of Clinical Psychology, 23, 65–6. [DOI] [PubMed] [Google Scholar]
- Fennell M.J.V., Teasdale J.D., Jones S., Damlé A. (2009). Distraction in neurotic and endogenous depression: an investigation of negative thinking in major depressive disorder. Psychological Medicine, 17(2), 441. [DOI] [PubMed] [Google Scholar]
- Fond G., Loundou A., Rabu C., et al. (2014). Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology, 231(18), 3663–76. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–11. [DOI] [PubMed] [Google Scholar]
- Grimm S., Boesiger P., Beck J., et al. (2009). Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology, 34(4), 932–843. [DOI] [PubMed] [Google Scholar]
- Grimm S., Ernst J., Boesiger P., Schuepbach D., Boeker H., Northoff G. (2011). Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. The World Journal of Biological Psychiatry, 12(8), 627–37. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(7), 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological Psychiatry, 78(4), 224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. (2011). Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry, 70(4), 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffziger S., Kuehner C. (2009). Rumination, distraction, and mindful self-focus in depressed patients. Behaviour Research and Therapy, 47(3), 224–30. [DOI] [PubMed] [Google Scholar]
- Just N., Alloy L.B. (1997). The response styles theory of depression: tests and an extension of the theory. Journal of Abnormal Psychology, 106(2), 221–9. [DOI] [PubMed] [Google Scholar]
- Kuehner C., Huffziger S., Liebsch K. (2009). Rumination, distraction and mindful self-focus: effects on mood, dysfunctional attitudes and cortisol stress response. Psychological Medicine, 39(2), 219–28. [DOI] [PubMed] [Google Scholar]
- Kuehner C., Weber I. (1999). Responses to depression in unipolar depressed patients: an investigation of Nolen-Hoeksema’s response styles theory. Psychological Medicine, 29(6), 1323–33. [DOI] [PubMed] [Google Scholar]
- Lam D., Smith N., Checkley S., Rijsdijk F., Sham P. (2003). Effect of neuroticism, response style and information processing on depression severity in a clinically depressed sample. Psychological Medicine, 33, 469–79. [DOI] [PubMed] [Google Scholar]
- Lavender A., Watkins E. (2004). Rumination and future thinking in depression. The British Journal of Clinical Psychology, 43, 129–42. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh D.A., Niciu M.J., Ionescu D.F., et al. (2014). Do the dissociative side effects of ketamine mediate its antidepressant effects? Journal of Affective Disorders, 159, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S., Nolen-Hoeksema S. (1993). Self-perpetuating properties of dysphoric rumination. Journal of Personality and Social Psychology, 65(2), 339–49. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S., Nolen-Hoeksema S. (1995). Effects of self-focused rumination on negative thinking and interpersonal problem solving. Journal of Personality and Social Psychology, 69(1), 176–90. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S., Tucker K.L., Caldwell N.D., Berg K. (1999). Why ruminators are poor problem solvers: clues from the phenome- nology of dysphoric rumination. Journal of Personality and Social Psychology, 77(5), 1041–60. [DOI] [PubMed] [Google Scholar]
- Murphy F.C., Nimmo-Smith I., Lawrence A.D. (2003). Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, and Behavioral Neuroscience, 3(3), 207–33. [DOI] [PubMed] [Google Scholar]
- Nolan S.A., Roberts J.E., Gotlib I.H. (1998). Neuroticism and ruminative response style as predictors of change in depressive symptomatology. Cognitive Therapy and Research, 22(5), 445. [Google Scholar]
- Nolen-Hoeksema S., Davis C.G. (1999). “Thanks for sharing that”: ruminators and their social support networks. Journal of Personality and Social Psychology, 77(4), 801–14. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Larson J., Grayson C. (1999). Explaining the gender difference in depressive symptoms. Journal of Personality and Social Psychology, 77(5), 1061–72. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Morrow J. (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology, 61(1), 115–21. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Parker L. (1994). Ruminative coping with depressed mood following loss. Journal of Personality and Social Psychology, 67(1), 92–104. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3(5), 400–24. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., Bermpohl F., et al. (2004). Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Human Brain Mapping, 21(3), 202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Am M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Gusnard D.A. (2005). Intrinsic brain activity sets the stage for expression of motivated behavior. The Journal of Comparative Neurology, 493(1), 167–76. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Snyder A.Z. (2007). A default mode of brain function: a brief history of an evolving idea. NeuroImage, 37(4), 1083–90. [DOI] [PubMed] [Google Scholar]
- Rhoades H., Overall J. (1988). The semistructured BPRS interview and rating guide. Psychopharmacology Bulletin, 24(1), 101–4. [PubMed] [Google Scholar]
- Rimes K.A., Watkins E. (2005). The effects of self-focused rumination on global negative self-judgements in depression. Behaviour Research and Therapy, 43(12), 1673–81. [DOI] [PubMed] [Google Scholar]
- Scheidegger M., Walter M., Lehmann M., et al. (2012). Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One, 7(9), e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F., Bermpohl F., Heinzel A., et al. (2008). The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience, 157(1), 120–31. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., et al. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner B, Graf B.M. (2008). Ketamine In Schüttler J., Schwilden H., editors. Modern Anesthetics SE—15, Vol. 182, pp. 313–33, Berlin Heidelberg: Springer. [Google Scholar]
- Song X.W., Dong Z.Y., Long X.F., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 6(9), e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland K., Bryant R. (2007). Rumination and overgeneral autobiographical memory. Behaviour Research and Therapy, 45, 2407–16. [DOI] [PubMed] [Google Scholar]
- Takano K., Tanno Y. (2009). Self-rumination, self-reflection, and depression: self-rumination counteracts the adaptive effect of self-reflection. Behaviour Research and Therapy, 47(3), 260–4. [DOI] [PubMed] [Google Scholar]
- Verplanken B., Friborg O., Wang C.E., Trafimow D., Woolf K. (2007). Mental habits: metacognitive reflection on negative self-thinking. Journal of Personality and Social Psychology, 92, 526–41. [DOI] [PubMed] [Google Scholar]
- Walter M. Henning A. Grimm S. Schulte R.F. Beck J. Dydak U. et al. (2009) The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Archives of General Psychatry, 66(5), 478–86. [DOI] [PubMed] [Google Scholar]
- Walter M., Li S., Demenescu L.R. (2014). Multistage drug effects of ketamine in the treatment of major depression. European Archives of Psychiatry and Clinical Neuroscience, 264 (Suppl S55–S65), doi:10.1007/s00406-014-0535-3 [DOI] [PubMed] [Google Scholar]
- Yan C.G., Zang Y.F. (2010). DPARSF: a MATLAB Toolbox for “‘Pipeline’” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4(13), doi:10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C.A., Singh J.B., Carlson P.J., et al. (2006). A randomized trial of an {N-methyl-D-aspartate} antagonist in treatment-resistant major depression. Archives of General Psychiatry, 63(8), 856–64. [DOI] [PubMed] [Google Scholar]