Abstract
Criminal behaviour poses a big challenge for society. A thorough understanding of the neurobiological mechanisms underlying criminality could optimize its prevention and management. Specifically,elucidating the neural mechanisms underpinning reward expectation might be pivotal to understanding criminal behaviour. So far no study has assessed reward expectation and its mechanisms in a criminal sample. To fill this gap, we assessed reward expectation in incarcerated, psychopathic criminals. We compared this group to two groups of non-criminal individuals: one with high levels and another with low levels of impulsive/antisocial traits. Functional magnetic resonance imaging was used to quantify neural responses to reward expectancy. Psychophysiological interaction analyses were performed to examine differences in functional connectivity patterns of reward-related regions. The data suggest that overt criminality is characterized, not by abnormal reward expectation per se, but rather by enhanced communication between reward-related striatal regions and frontal brain regions. We establish that incarcerated psychopathic criminals can be dissociated from non-criminal individuals with comparable impulsive/antisocial personality tendencies based on the degree to which reward-related brain regions interact with brain regions that control behaviour. The present results help us understand why some people act according to their impulsive/antisocial personality while others are able to behave adaptively despite reward-related urges.
Keywords: psychopathy, dorsomedial prefrontal cortex, connectivity, reward, ventral striatum, criminality
Introduction
Criminal behaviour causes great individual suffering as well as large social and economic costs (Wickramasekera et al., 2015). There is a pressing need to understand this behaviour to improve risk assessment, prevention and treatment strategies (van der Gronde et al., 2014). Here, we add to this understanding by advancing recent insights in the neurobiology of reward processing derived from studying impulsive/antisocial traits in healthy community samples compared with a criminal sample.
In the perspective of risk assessment regarding recurrent criminal behaviour, the construct of psychopathy is of particular interest. It is highly associated with violent criminal behaviour (Porter and Woodworth, 2006; Blais et al., 2014): For example, people fulfilling the criteria for psychopathy are overrepresented in the US prison population: ∼25% of inmates are diagnosed with psychopathy compared with 1% of the general population (Hare, 2003; Porter and Woodworth, 2006). It might therefore not be surprising, that tools developed to assess psychopathy have found to be useful in predicting future criminal behaviour (e.g. Camp et al., 2013; Whittington et al., 2013). Especially, the impulsive/antisocial factor of psychopathy has repeatedly been shown to be predictive of violence (e.g. Edens et al., 2008; Kennealy et al., 2010; Camp et al., 2013; Blais et al., 2014).
Interestingly, recent advances in neurobiological research elucidate the neural underpinnings of this impulsive/antisocial factor (Buckholtz et al., 2010a): Functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) evidence suggest that reward expectancy and its underlying mesolimbic dopamine system, might be key to understanding impulsive/antisocial traits (Buckholtz et al., 2010a; Bjork et al., 2012). These seminal findings were however collected from healthy control, community samples and therefore precluded direct conclusions about its relevance for understanding overt criminality. Here, we will fill this gap by assessing the neurobiological underpinnings of reward expectancy in a low and high impulsive/antisocial non-criminal group and a (psychopathic) criminal group also scoring high on impulsive/antisocial traits. This will allow us to further our understanding of the relation between impulsive/antisociality, reward expectancy and, critically, overt criminality on a neurobiological level.
More specifically, recent work on reward expectation has shown that non-criminal volunteers with impulsive/antisocial personality traits [assessed with the Psychopathy Personality Inventory (PPI) (Lilienfeld and Andrews, 1996)] exhibit enhanced reward expectancy-related blood oxygen level dependent (BOLD) signal in the ventral striatum (Buckholtz et al., 2010a; Bjork et al., 2012) as well as enhanced ventral striatal dopamine release (Buckholtz et al., 2010a). Buckholtz et al. (2010a) used a monetary incentive delay (MID) task to assess the association between reward anticipation and impulsive/antisocial traits in a mixed gender community sample. During reward anticipation, the right nucleus accumbens signal correlated positively with the impulsive antisocial factor of psychopathy. The authors proposed that this neural hyper-reactivity to reward expectation is either a direct consequence of aberrant firing of midbrain dopamine neurons (ventral tegmental area) or a result of decreased regulatory control of ventral striatal activity through a broad inhibitory failure of prefrontal areas. These results have been extended by Bjork et al. (2012), who showed that impulsive/antisocial traits correlate positively not only with ventral striatal activity during instrumentally obtained rewards, but also with anticipation of passively obtained rewards in the anterior mesofrontal cortex. To advance these findings to a forensic level, involving overt and severe criminality, it is pivotal to test criminal, impulsive/antisocial individuals. This enables direct assessment of whether enhanced neural processing of reward expectation in non-criminal impulsive/antisocial adults extends to criminal impulsive/antisocial individuals. Therefore, the aim of this study was to investigate the neural mechanism underlying reward expectation in a group of criminals scoring high on antisocial/impulsivity factor of the psychopathic personality inventory. Specifically, we assessed whether these criminals show similar (or even greater) increases in ventral striatal reward expectancy-related BOLD signal as do (than) non-criminal healthy controls with high impulsive and antisocial traits (following Buckholtz et al. 2010a). If the ventral striatal reactivity is related to the level of impulsive/antisociality as measured by the PPI, but not directly related to criminality, we expect no group differences in ventral striatal reactivity between the criminal and non-criminal high impulsive/antisocial groups. In addition, overt criminality might only emerge in high-impulsive antisocial persons if the relatively high level of ventral striatal reactivity to reward expectation is not accompanied by appropriate regulation of other brain areas. We tested this latter hypothesis by assessing differences in neural connectivity between the healthy control group scoring high on impulsive/antisocial traits and the criminal group.
Note that the impulsive/antisocial traits assessed here are an integral part of the psychopathy construct (Neumann et al., 2005; Hare and Neumann, 2008), but do not specifically distinguish psychopathic criminals from other criminals (Patrick et al., 2009). Here, we nevertheless focus our analyses on these traits, rather than on the interpersonal and affective traits of psychopathy, firstly because we aim to further the findings of Buckholtz et al. (2010a) who were able to convincingly couple these traits to reward-expectation and its underlying neurobiology. Second, the aim to advance insight in overt criminality seems to be best served by assessing the impulsive/antisocial traits as measured by the PPI: These traits reflect past violence and predict future violence more consistently and with larger effect sizes than the interpersonal/affective factor (e.g. Edens et al., 2008; Kennealy et al., 2010; Camp et al., 2013; Blais et al., 2014).
Materials and methods
Participants
We assessed BOLD signal with fMRI in 34 subjects using an MID task, known to induce reward-related BOLD signal in the ventral striatum (Knutson et al., 2001; Hoogman et al., 2013). These 34 subjects consisted of 20 healthy subjects without criminal record and 14 psychopathic criminals (Table 1). The latter group was part of a group of 18 patients recruited on a voluntary basis from the inpatient population of a high security forensic psychiatric hospital in the Netherlands based on available information about clinical status and prior history. Two criminals had to be excluded due to technical problems and two withdrew from participation during the study. The remaining 14 psychopathic criminals were between 18 and 55 years of age (mean age = 40.14, s.d. = 8.82, 3 left handed) and diagnosed with a psychopathy score of ≥ 26 according to the Hare Psychopathy Check List-Revised [PCL-R (Hare, 2003); mean total score = 30.6, s.d. = 3.9]. We assessed IQ levels using the Dutch version of the National Adult Reading Test (Schmand et al., 1991). Twenty healthy men (three left-handed) matched for age and IQ (mean age = 40.8, s.d. = 9.86) without criminal records and/or a history of current psychiatric disorders were recruited by advertisement among employees of the high security forensic psychiatric hospital.
Table 1.
Group characteristics (mean, standard deviation) of the group of psychopathic criminals (PP) and healthy matched control subjects scoring high on the PPI_IA factor (HChigh) and healthy matched controls scoring low on the PPI_IA factor (HClow)
| PP (n = 14) | HClow (n = 10) (<146 on PPI_IA) | HChigh (n = 10) (>146 on PPI_IA) | Statistics (P-value) | |
|---|---|---|---|---|
| Age | 40.1 (8.8) | 42.5 (10.22) | 39.1 (9.7) | 0.715 |
| IQ (NLV) | 100.1 (11.0) | 101.30 (10.60) | 103.1 (5.4) | 0.733 |
| PCL-R total | 30.6 (3.8) | — | — | — |
| PCL-R Factor 1 | 11.9 (2.8) | — | — | — |
| PCL-R Factor 2 | 13.9 (2.0) | — | — | — |
| PPI total | 362.2 (42.5) | 323.7 (29.8) | 368.3 (20.8) | 0.01 |
| PPI_FD (factor 1) | 142.6 (19.9) | 133.5 (23.7) | 145.6 (14.6) | 0.366 |
| PPI_IA (factor 2) | 165.1 (28.4) | 132.8 (9.7) | 169.2 (14.7) | 0.001 |
NLV, Dutch reading test; PCL-R. Psychopathy checklist revised; PP, psychopathy group; PPI_FD, factor 1 of the PPI ‘fearless dominance’; PPI_IA, factor 2 of the PPI ‘impulsive antisociality.
Participants in both groups were checked for drug use and for medical history. Furthermore, all subjects (n = 34) were assessed with the PPI (Lilienfeld and Andrews, 1996). Following Buckholtz et al. (2010a), we focused on the second factor (impulsive/antisocial traits) and divided the healthy control group by median split (median = 146) in a high and a low scoring group.
The above procedure resulted in three groups: (i) a non-criminal control group with low impulsive/antisocial traits, (ii) a non-criminal control group with high impulsive/antisocial traits and (iii) a psychopathic criminal group (Table 1). The three groups did not differ in IQ and age (both P > 0.715; Table 1). Of note is that impulsive/antisocial trait scores did not differ between the non-criminal healthy high impulsive/antisocial and the criminal psychopathy group (Table 1).
Psychopathy assessment
In both groups psychopathic traits were assessed using the PPI, and additionally in the criminal group using the PCL-R.
Psychopathy checklist—revised
The PCL-R is a 20-item instrument for assessing criminal psychopathy in research, clinical and forensic settings. In contrast to the PPI, this is not a self-report questionnaire, but items are scored by at least two independent, trained raters based on file information, collateral reports and extensive interviewing.
Psychopathic personality inventory
The PPI is a 187 item self-report questionnaire designed to measure psychopathy in community samples (Lilienfeld and Andrews, 1996). Items are answered on a 4-point Likert scale (1 = false, 4 = true). Eight subscales are scored which can be further reduced into two factors [respectively, fearless-dominance; and impulsive-antisocial behaviour (IA)], which in turn are summed into a total score representing a global index of psychopathy. Note that the IA factor was used to split the healthy control group into high and low scoring subjects.
Procedure
Participants received written and oral information about the experiment and signed an informed consent. All participants were invited for a screening session and a scan session with no more than 2 weeks in between the appointments. During the first appointment, they were screened for psychiatric exclusion criteria1 by trained psychologists using the Structure Clinical Interview for DSM disorders to exclude axis 2 disorder [SCID-II; Dutch version (Weertman et al., 2000)], Mini International Neuropsychiatric Interview to exclude axis 1 disorder [MINI; Dutch version (van Vliet et al., n.d.)] and the Dutch version of the National Adult Reading Test for IQ assessment (NLV; Schmand et al., 1991). Further, participants completed the PPI (Lilienfeld and Andrews, 1996) [Dutch version (Jelicic et al., 2004)]. They were instructed not to drink more than 3 units/day during in the week preceding the experimental measure, not to use of alcohol within 24 h of the measurement, not to use cannabis or other illicit drugs within the week before measurement, not to use psychotropic medication other than oxazepam during the 5 days before measurement, not to use oxazepam within 12 h before measurement and not to smoke within 1 h before measurement and no more than five cigarettes on the scan day. Furthermore, they were asked to refrain from any caffeinated drinks and chocolate on the scan day and to refrain from extensive physical exercise and heavy meals before the scan session. In the scanner, participants wore earplugs and headphones. Foam pads were placed inside the head coil to restrict movement and a heartbeat device was connected to the second toe. Before performing the MID task, participants performed an approach avoidance task reported elsewhere. Instructions and task images were projected onto a translucent screen at the end of the scan tube, which was visible via a mirror attached to the head coil. Participants received a practice block that was stopped when participants had five on-time responses (hits). After summarizing again the purpose of the task, the experimental block was started, which lasted 12 min. After a short break outside the scanner, the anatomical scan (duration: 5 min) and an unrelated task were acquired in the 3T MR scanner, which was located in an adjacent scanner room.
Experimental task
Monetary incentive delay task
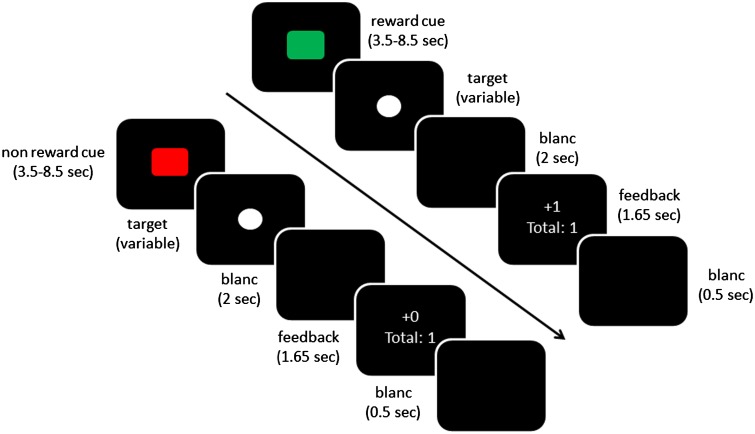
The MID task (Figure 1) consisted of 75 trials (25 potentially rewarding, 25 potentially non-rewarding and 25 baseline fixation trials). Each trial started with a cue (green square indicating reward trials and red square indicating no-reward trials), which was presented for 3500–8500 ms. Next, a white circle was presented (target) to which the participants had to respond as quickly as possible by pressing a button. The target was followed by a black screen for 2000 ms, after which the outcome was displayed (1650 ms) informing the participant about the outcome of the current trial (±1) and the total amount of points. Participants could gain one point in the reward condition and no points in the no-reward condition if they responded between 270 and 500 ms after target onset. The response window was adjusted on an individual level and separately for reward and no-reward trials (after a hit, 20 ms was subtracted from the last response window, after a miss, 10 ms was added to the last response window). For every participant, the initial response window was set to 270 ms. This procedure resulted in comparable hit rates in the reward (34%) and no-reward (30%) condition. Before the next cue was shown, a black screen was shown for 500 ms. Participants were told that the total amount of points was converted to monetary rewards (1 point resulted in 20 eurocents) and would be given as a bonus to the regular payment for participation.
Fig. 1.
Task schematics: the upper row showing a reward and the lower row showing a non-reward hit trial (i.e. responses below a variable response limit).
Behavioural analysis
The number of hits, reaction times on hits and target duration were submitted to a 2 × 3 repeated measures analysis of variance with Reward Expectation (reward, no-reward) as within-subject factor and Group (psychopathic criminals, non-criminal high-, non-criminal low-impulsive/antisocial) as between-subject factor.
MR Image acquisition and analysis
Image acquisition
Whole-brain imaging was performed on a 1.5 Tesla MR scanner (Magnetrom Sonata, Siemens Medical Solutions, Erlangen, Germany) using an 8-channel coil. Functional data were obtained using a multi-echo gradient T2*-weighted echo-planar scanning sequence (Poser et al., 2006) with BOLD contrast (34 axial-oblique slices, repetition time, 2.64 s; echo-times: 6.9, 24.2, 33, 43 and 52 ms; in plane resolution, 3.3 × 3.3 mm; slice thickness, 3.0 mm; distance factor 0.17; flip angle 80°). In addition, a high-resolution T1-weighted magnetization-prepared rapid-acquisition gradient echo anatomical scan was obtained from each subject from a 3 Tesla MR scanner Magnetrom Trio Tim, Siemens Medical Systems, Erlangen, Germany) using a 32-channel head coil (192 sagittal slices; repetition time, 2.3s; echo time, 3.03ms; voxel size 1.0 × 1.0 × 1.0 mm; field of view 256 mm).
Preprocessing
fMRI data analysis was performed with SPM5 software (Statistical Parametric Mapping; Wellcome Trust Centre for Cognitive Neuroimaging, London, UK). The first five volumes of each participant’s dataset were discarded to allow T1 equilibrium. First, realignment parameters were estimated for the images acquired at the first echotime and consequently applied to images resulting from the three other echoes. The echo-images were combined by applying a PAID-weight algorithm assessing the signal-to-noise ratio as described by Poser et al. (2006). Thirty volumes, acquired just after the main task (while the participant watched a black screen) were used as input for this algorithm. Thereafter, the following preprocessing steps were applied: slice-time correction, co-registration and a segmentation procedure using the tissue probability maps provided by SPM5 for grey matter, white matter and cerebrospinal fluid centered in MNI space to estimate normalization parameters based on the structural image. Structural as well as functional images were then normalized by applying these estimates. All normalized images were smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel (Worsley and Friston, 1995).
Single subject analysis
A random effects, event-related, statistical analysis was performed with SPM5. First, we specified a separate general linear model (GLM) for each participant. This GLM included eight main regressors representing different factors of the MID task: Cue for reward and no-reward trials; Instrumental Target for reward and no-reward trials; Outcome for reward-hit, reward-miss, no-reward hit and no-reward miss trials. These regressors were modeled as delta functions at their onset and were convolved with a canonical hemodynamic response function (HRF). Realignment parameters (three rigid-body translations and three rotations) were added to capture residual movement-related artefacts. High-pass filtering (128s) was applied to remove low-frequency drifts. Parameter estimates for all regressors were obtained by maximum-likelihood estimation, modelling temporal autocorrelation (AR1). The parameter estimates, derived from this fit of the model to the data, reflect the strength of covariance between the fMRI data and the canonical response function for each of the regressors.
Group level analysis
The beta estimates for the Cue reward and no-reward trials were of primary interest. These were admitted to a 2 × 3 ANOVA (full factorial) at the group-level with Reward Expectation (reward/no-reward) as within-subject factor and Group (psychopathic criminals/non-criminal high-, non-criminal low-impulsive/antisocial) as between-subject factor. Restricted Maximum Likelihood estimates of variance components were used to allow for unequal variance between subjects and possible deviations from sphericity introduced by dependencies between levels in the repeated measures factor. For the factor Group between level independence was assumed. The main effects and interactions were then calculated.
We assessed whether increased signal during reward expectation relative to no-reward expectation was different between subjects high vs low on impulsive and antisocial traits as was expected based on Buckholtz et al. (2010a). To this end, we used a planned contrast (within the full factorial, ANOVA model; contrast (1)) to compare reward- (vs no-reward-)related signal change between the healthy controls with low impulsive/antisocial traits and the compound group with high impulsive/antisocial traits (i.e. healthy controls scoring high on these traits plus the psychopathic criminals who also scored high on these traits). Next we assessed whether reward- (vs no-reward-) related signal differed between the psychopathic criminals and the high impulsive/antisocial healthy controls (at the whole brain level as well as within our small volume of interest in the ventral striatum, family wise error corrected for multiple comparisons). Finally, we assessed group differences in reward-related signal by exploring the omnibus full factorial model. Supplementary, we repeated the full factorial ANOVA, but now with the healthy control group divided in two groups based on their Fearless/Dominance score (i.e. factor 1 of the PPI, see Supplementary materials for results).
Functional connectivity analyses
Because we did not find differences between non-criminal controls with high impulsive/antisocial traits and psychopathic criminals on the contrasts described above, we anticipated that differences between criminal and non-criminal people with impulsive/antisocial traits might not lie in reward expectancy signals in the ventral striatum per se, but in how this region is connected to other brain regions. Therefore, we assessed differences between these two groups in terms of functional connectivity (Friston et al., 1997) with the ventral striatal region, in which reward-related signal was increased in (criminals and non-criminal) people with high impulsive/antisocial traits vs people with low impulsive/antisocial traits .
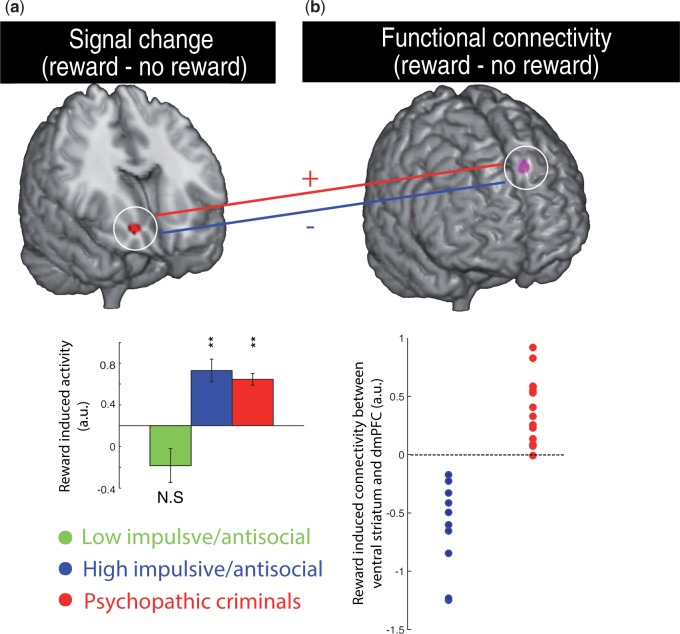
To conduct this analysis, we proceeded in several steps. First, for each individual in the psychopathic criminal and non-criminal impulsive/antisocial control group the (first principal component of the) BOLD time series was extracted from a 3 mm sphere surrounding the BOLD response peak revealed by contrast (1) (the seed, c.q. the right ventral striatum: Figure 2a). The time series was then deconvolved based on the canonical HRF to construct a time series of neural responses following the procedures outlined by Gitelman et al. (2003).
Fig. 2.
(a) Enhanced reward-related BOLD signal in the ventral striatum of psychopathic criminals and healthy noncriminal controls with high antisocial/impulsive traits compared with healthy controls with low antisocial/impulsive traits (peak: MNI xyz [18 22 −8]). Average signal change (reward–no-reward) extracted from the peak cluster is shown for illustrative purposes. (b) BOLD signal in the ventral striatum (3 mm sphere around MNI xyz [18 22 −8]) contributes differentially to the dorsomedial prefrontal cortex (dmPFC) during reward vs no reward expectancy in psychopathic criminals compared with healthy controls with high antisocial/impulsive traits. The scatter plot depicts individual parameter estimates of functional connectivity differences between reward and no reward expectation, extracted from the peak cluster (peak: MNI xyz [−14 34 44]). Images are displayed at a threshold of P < 0.001 uncorrected for illustration purposes.
Second, a GLM was estimated for every subject, which included the following three main regressors (as well as the six motion parameters): (1) The seed BOLD response time series; (2) a regressor representing the task-induced effect reflecting reward vs no-reward expectation and (3) the psychophysiological interaction regressor, which is the cross product of the deconvoluted regressor (1) and regressor (2). The latter regressor represents the interaction between neural signal and the two task conditions. This regressor was then convolved with the HRF. Parameter estimates for the interaction regressor were estimated by maximum-likelihood estimation, modelling temporal autocorrelation at the subject-level. The parameter estimates, derived from this fit of the model to the data, reflect the strength of the task-induced change in connectivity with the seed region (the ventral striatum). These estimates were then used at the group level in an independent sample t-test with group (two levels: psychopathic criminal/non-criminal impulsive/antisocial control) as between-subject factor to assess group differences.
Statistical thresholding of fMRI analysis
We report only those effects that survive family wise error correction for multiple comparisons at the whole brain (P<0.05, voxel-level) and where appropriate in the right ventral striatum as defined by the Harvard–Oxford Atlas [based on previous findings by Buckholtz et al.(2010a) and Bjork et al. (2012)].
Results
Behavioural results
No behavioural differences between groups
During reward expectation subjects showed more accurate responses than during the neutral condition (main effect of Reward Expectation on hits: F(1/31) = 8.3, P = 0.007) leading to shorter target duration for the reward condition than the no-reward condition (F(1/31) = 11.0, P = 0.002). Critically, there were no main or interaction effects of Group in terms of target duration (F < 1.5). Furthermore, as intended by our task design, behavioural performance on hits (i.e. accuracy) did not differ between the groups.
Neuroimaging results
First, analysis of data from all 34 subjects replicated prior studies using this task and revealed significantly greater BOLD signal in the ventral striatum during reward than no-reward cues (xyz = [12 14 −6], T = 3.49, P =0.007; small volume correction for multiple comparisons within the anatomically defined right ventral striatum, (Knutson et al., 2003)). Next, we established that, following Buckholtz et al. (2010a), reward-related BOLD signal in the ventral striatum was higher in the two groups with high impulsive/antisocial traits than in the group with low impulsive/antisocial traits (Figure 2a, whole-brain corrected for multiple comparisons: T = 5.31, P = 0.049, small volume correction for ventral striatum: T = 3.30, P = 0.011). There were no other Group × Reward effects (established by an omnibus ANOVA with a three-level group factor, and/or planned contrasts between pairs of groups). Critically, there were also no differences between the two (criminal vs non-criminal) groups with high impulsive/antisocial traits. Thus reward-related BOLD signal in the ventral striatum was enhanced in people with impulsive/antisocial traits, but did not differentiate criminal psychopathic individuals from non-criminal individuals with impulsive/antisocial traits. Furthermore, there were no correlations between task performance (reaction times/target duration on reward vs no-reward trials) and BOLD signal change (reward–no reward) in the ventral striatum.
Next, we tested the hypothesis that the difference between the non-criminal impulsive/antisocial group and psychopathic criminal group does not lie in ventral striatal signalling per se, but rather in the degree to which this reward-related neural signal interacts with neural systems that control behaviour, such as the prefrontal cortex. This task-dependent, functional connectivity analysis revealed a group effect in the dorsomedial prefrontal cortex. No other regions were revealed by this analysis. Thus, reward-related connectivity between the ventral striatum and dorsomedial prefrontal cortex was different between the psychopathic criminals and the non-criminal impulsive/antisocial group (Figure 2b, two sample t-test: T = 7.44, P = 0.018, result was corrected for multiple comparisons at the whole brain level). This difference was remarkable in terms of consistency (Figure 2b): There was no overlap between the groups, with reward-related connectivity between the ventral striatum and dorsomedial prefrontal cortex being below zero in all healthy high impulsive/antisocial individuals, but (around or) above zero in all psychopathic criminals. There were no significant correlations between connectivity differences (reward vs no-reward) within the psychopathy group and the factors of the two or four factor model of the PCL-R (within the psychopathy group, all Spearman’s rho<0.580, P>0.007, P-threshold corrected for multiple comparisons). Moreover, there were no correlations between task performance (reaction times/target durations on reward vs no-reward trials) and connectivity (reward vs no-reward).
Discussion
The present data suggest that not reward expectation per se, but the way in which reward expectations are communicated to frontal areas might be key to understanding the overt criminality in impulsive/antisocial people. This suggests that criminality in impulsive/antisocial individuals is accompanied by abnormal contribution of reward signalling to regions regulating the cognitive control of behaviour (Ridderinkhof, 2004).
We go beyond earlier studies that assessed reward expectation in relation to impulsive/antisocial traits (Buckholtz et al., 2010a; Bjork et al., 2012) by assessing high impulsive/antisocial criminals (compared with healthy control samples) and by assessing task-related connectivity. Our results show differential task-dependent coupling between the anterior ventral striatum and dorsomedial prefrontal cortex (c.q. superior frontal gyrus) in the high impulsive/antisocial healthy control group compared with the high impulsive/antisocial criminals. Noteworthy is the difference between these groups, which was striking in terms of its nature and robustness: First, there was no overlap between the groups when assessing individual connectivity patterns. Second, all impulsive/antisocial healthy controls showed a clear negative coupling, whereas all the criminal individuals showed (near zero or) positive coupling.
These results advance findings from earlier studies showing abnormal reward processing in healthy, non-criminal volunteers with impulsive/antisocial traits (Buckholtz et al., 2010a; Bjork et al., 2012) to a criminal sample. For example, Buckholtz et al. (2010a) have reported a positive association between impulsive/antisocial trait scores and neural signal in the right ventral striatum during reward expectation. The ventral striatum well connected with (para)limbic, cortical and ventral tegmental areas as well as motor effector sides, which enables this region to functions as an interface between cognition, emotion and action (Cardinal et al., 2002; Floresco, 2015). It has an established function in reward-related processes such as expectation of reward (Knutson et al., 2001) and ventral striatal deficits may be involved in impulsivity (Basar et al., 2010), sensation seeking and heightened reward sensitivity all associated with antisocial behaviour (Glenn and Yang, 2012). Recently, greater reactivity in the ventral striatum has been directly linked with increased retaliatory aggression (Chester and DeWall, 2015). Moreover, there is one case description of deep brain-stimulation in bilateral ventral striatum resolving pathological (self-directed) aggression (Harat et al., 2015). Furthermore, the finding that enhanced reward signalling in the ventral striatum is not specific to impulsive/antisocial criminals, but extends to non-criminal, but impulsive/antisocial individuals is not surprising given previous studies showing enhanced reward signalling in the ventral striatum of healthy individuals with high impulsive (Buckholtz et al., 2010b) or impulsive/antisocial traits (Buckholtz et al., 2010a).
Our findings concur with a growing body of research that suggests that subjects comparable in terms of overt criminality and PCL-score to our sample (i.e. psychopathic criminals) are characterized by aberrant connectivity within networks that underpin the interaction between affective and cognitive processes (Yang et al., 2012; Contreras-Rodríguez et al., 2014; Motzkin et al., n.d.). In fact psychopathic criminals have been shown to exhibit abnormal functional and structural connectivity patterns in particular with the dorsomedial prefrontal cortex (Yang et al., 2012; Contreras-Rodríguez et al., 2014). This part of the prefrontal cortex has long been implicated in the cognitive control of behaviour, especially in signalling the need for performance adjustment (e.g. Ridderinkhof, 2004), self-inhibition of movements (Brass and Haggard, 2007) and impulse control (Cho et al., 2013). Interestingly, non-invasive stimulation of the dorsomedial prefrontal cortex via repetitive transcranial magnetic stimulation has been shown to enhance inhibitory control over prepotent responses (Obeso et al., 2013), to improve subjective choice for delayed rewards, and to interfere with striatal dopamine (Cho et al., 2015). These observations raise the hypothesis that psychopathic criminals might exhibit a failure to adjust performance due to aberrant impact of reward expectation. This hypothesis should be tested in future studies with behavioural tasks that are optimized for detecting aberrant behaviour in psychopathic criminals. We hypothesize that tasks involving both reward anticipation and adjustment of behaviour based on punishment and/or of negative (facial) emotional cues (cf. Blair, 2008) might be particularly sensitive.
The present results might be relevant in the context of theorizing about differences between ‘successful’ from ‘unsuccessful’ psychopathic individuals, with the former referring to individuals with psychopathic personality traits who do not have any criminal convictions (Gao and Raine, 2010). Unlike previous studies that focused on reward expectation in healthy people with psychopathic traits (Buckholtz et al., 2010a; Bjork et al., 2012), this study included also a sample of criminal, non-successful psychopathic individuals rather than a community sample of people with high PPI scores. As such our data concur with previous suggestions that there are substantial differences between non-successful and successful psychopathic individuals at neural, physiological, cognitive and behavioural levels (Gao and Raine, 2010). Specifically, non-successful psychopathic individuals have been argued to exhibit greater frontal impairments and greater high-level cognitive deficits than do successful psychopathic individuals (Gao and Raine, 2010). Our results provide the first direct evidence for this hypothesis. Note that the comparison between successful and non-successful impulsive/antisocial individuals is necessarily confounded by overt criminal history. As such, we cannot and do not claim specificity of our findings to psychopathic criminals compared with non-psychopathic criminals.
Previous studies have shown enhanced reward expectancy signalling in the ventral striatum of healthy individuals with high impulsive (Plichta and Scheres, 2014) or impulsive/antisocial traits (Buckholtz et al., 2010a). Nevertheless, it is far from trivial that psychopathic criminals show similar hypersensitivity, because several other patient groups characterized by impulsivity, such as ADHD, show reduced ventral striatal neural signals during reward expectancy (Plichta and Scheres, 2014). This result contributes to our understanding of why some people act according to their impulsive/antisocial personality while others are able to behave adaptively despite reward-related urges. The enhanced reward expectation processing in psychopathic criminals concurs very well with the clinical observation that psychopathic criminals exhibit ruthless reward-driven behaviours and that they, unlike healthy people, are not inhibited by immoral or otherwise aversive signals. Moreover, this account would accord with the finding that psychopathic criminals fail to adapt behaviour based on aversive information when reward is at stake (Newman et al., 1990).
It is important to emphasize that our findings cannot be interpreted as being ‘specific’ to either criminality, psychopathy or their interaction (i.e. psychopathic criminality): a non-criminal control group with high psychopathy severity (in terms of PCL-R scores) nor a criminal control group with low psychopathy severity was included. Crucially, our findings enhance the understanding of the neural mechanism underlying overt criminality within impulsive/antisocial populations, given the comparison of two (one criminal, one non-criminal) equally impulsive/antisocial groups.
Furthermore, we highlight that the sample size in this study was relatively modest, which is a necessary consequence of the limited availability of clean, drug- and tattoo-free psychopathic criminals. Indeed our sample size is comparable with that in other fMRI studies with psychopathic criminals. Nevertheless, we argue that our results are reliable, given that it includes a general replication of prior findings (Buckholtz et al., 2010a), and given that our novel result on connectivity reaches statistical significance even after correction for multiple comparisons at the whole-brain level. As such, the present mechanistic study raises a promising target for future clinical work, which could advance our findings to a diagnostic level, by replicating in a larger population our finding that reward expectancy-based connectivity fully dissociated criminals from high impulsive/antisocial non-criminals.
Supplementary Material
Acknowledgements
D.G. acknowledges the Netherlands Organisation for Health Research and Development (ZON-MW AGIKO grant 92003576). R.C. acknowledges the Netherlands Organisation for Scientific Research (VIDI grant NWO- 452-08-009). R.-J.V. acknowledges the Netherlands Organisation for Scientific Research (grant NWO-056-24-011). The authors declare no competing financial interests.
Footnotes
Psychiatric exclusion criteria: recent major depressive disorder, bipolar disorder, schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional and other psychotic disorders, schizoid or schizotypical personality disorder, current alcohol and substance intoxication, first degree relatives with DSM IV axis I schizophrenia or schizophreniform disorder.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interests. None declared.
References
- Basar K., Sesia T., Groenewegen H., Steinbusch H.W.M., Visser-Vandewalle V., Temel Y. (2010). Nucleus accumbens and impulsivity. Progress in Neurobiology, 92, 533–57. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Chen G., Hommer D.W. (2012). Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biological Psychology, 89, 408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R. (2008). The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363, 2557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais J., Solodukhin E., Forth A.E. (2014) A meta-analysis exploring the relationship between psychopathy and instrumental versus reactive violence. Criminal Justice and Behavior, 41, 797–821. [Google Scholar]
- Brass M., Haggard P. (2007). To do or not to do: the neural signature of self-control. Journal of Neuroscience, 27, 9141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., et al. (2010a). Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience, 13, 419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., et al. (2010b). Dopaminergic network differences in human impulsivity. Science, 329, 532.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp J.P., Skeem J.L., Barchard K., Lilienfeld S.O., Poythress N.G. (2013). Psychopathic predators? Getting specific about the relation between psychopathy and violence. Journal of Consulting and Clinical Psychology, 81, 467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews, 26, 321–52. [DOI] [PubMed] [Google Scholar]
- Chester D.S., DeWall C.N. (2015) The pleasure of revenge: retaliatory aggression arises from a neural imbalance toward reward. Social Cognitive and Affective Neuroscience, nsv082 Doi: 10.1093/scan/nsv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.S., Koshimori Y., Aminian K., et al. (2015). Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology, 40, 546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.S., Pellecchia G., Aminian K., et al. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26, 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodríguez O., Pujol J., Batalla I., et al. (2014). Functional connectivity bias in the prefrontal cortex of psychopaths. Biological Psychiatry, 78(9), 647–55. [DOI] [PubMed] [Google Scholar]
- Edens J.F., Poythress N.G., Lilienfeld S.O., Patrick C.J., Test A. (2008). Further evidence of the divergent correlates of the psychopathic personality inventory factors: prediction of institutional misconduct among male prisoners. Psychological Assessment, 20, 86–91. [DOI] [PubMed] [Google Scholar]
- Floresco S.B. (2015). The nucleus accumbens: an interface between cognition, emotion, and action. Annual Review of Psychology, 66, 25–52. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6, 218–29. [DOI] [PubMed] [Google Scholar]
- Gao Y., Raine A. (2010). Successful and unsuccessful psychopaths: a neurobiological model. Behavioural Science and the Law, 28, 194–210. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. (2003). Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage, 19, 200–7. [DOI] [PubMed] [Google Scholar]
- Glenn A.L., Yang Y. (2012). The potential role of the striatum in antisocial behavior and psychopathy. Biological Psychiatry, 72, 817–22. [DOI] [PubMed] [Google Scholar]
- Harat M., Rudas M., Zielinski P., Birska J., Sokal P. (2015). Deep brain stimulation in pathological aggression. Stereotactic and Functional Neurosurgery, 93, 310–5. [DOI] [PubMed] [Google Scholar]
- Hare R.D. (2003) Manual for the Hare Psychopathy Checklist-Revised. 2nd edn. Toronto, ON, Canada: Multi Health Systems. [Google Scholar]
- Hare R.D., Neumann C.S. (2008). Psychopathy as a clinical and empirical construct. Annual Review of Clinical Psychology, 4, 217–46. [DOI] [PubMed] [Google Scholar]
- Hoogman M., Onnink M., Cools R., et al. (2013). The dopamine transporter haplotype and reward-related striatal responses in adult ADHD. European Neuropsychopharmacology: the Journal of the European College of Neuropsychopharmacology, 23, 469–78. [DOI] [PubMed] [Google Scholar]
- Jelicic M., Merckelbach H., Timmermans M., Candel I. (2004) De Nederlandstalige versie van de psychopathic personality inventory. Psychodiagnostisch Gereedschap. De Psycholoog, 12, 604–8. [Google Scholar]
- Kennealy P.J., Skeem J.L., Walters G.D., Camp J. (2010). Do core interpersonal and affective traits of PCL-R psychopathy interact with antisocial behavior and disinhibition to predict violence? Psychological Assessment, 22, 569–80. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21, RC159.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Bennett S.M., Adams C.M., Hommer D. (2003). A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage, 18, 263–72. [DOI] [PubMed] [Google Scholar]
- Lilienfeld S.O., Andrews B.P. (1996). Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personal Assessment, 66, 488–524. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Newman J.P., Kiehl K.A., Koenigs M. (n.d.) Reduced Prefrontal Connectivity in Psychopathy. Journal of Neuroscience, 31(48), 17348–57. [DOI] [PMC free article] [PubMed]
- Neumann C.S., Vitacco M.J., Hare R.D., Wupperman P. (2005). Reconstruing the “reconstruction” of psychopathy: a comment on Cooke, Michie, Hart, and Clark. Journal of Personality Disorder, 19, 624–40. [DOI] [PubMed] [Google Scholar]
- Newman J.P., Patterson C.M., Howland E.W., Nichols S.L. (1990). Passive avoidance in psychopaths: the effects of reward. Personality and Individual Differences, 11, 1101–14. [Google Scholar]
- Obeso I., Cho S.S., Antonelli F., et al. (2013). Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimulation, 6, 769–76. [DOI] [PubMed] [Google Scholar]
- Patrick C.J., Fowles D.C., Krueger R.F. (2009). Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology, 21, 913.. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Scheres A. (2014). Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neuroscience and Biobehavioral Reviews, 38, 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S., Woodworth M. (2006) Psychopathy and aggression In: Patrick C.J., editor. Handbook of Psychopathy, 1st edn New York: The Guilford Press, 481–94. [Google Scholar]
- Poser B.A., Versluis M.J., Hoogduin J.M., Norris D.G. (2006). BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel-acquired inhomogeneity-desensitized fMRI. Magnetic Resonance in Medicine, 55, 1227–35. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R. (2004). The role of the medial frontal cortex in cognitive control. Science, 306, 443–7. [DOI] [PubMed] [Google Scholar]
- Schmand B., Bakker D., Saan R., Louman J. (1991). [The Dutch reading test for adults: a measure of premorbid intelligence level]. Tijdschrift Voor Gerontologie En Geriatrie, 22, 15–9. [PubMed] [Google Scholar]
- van der Gronde T., Kempes M., van El C., Rinne T. (2014) Neurobiological correlates in forensic assessment: a systematic review. PLoS ONE, 9(10). [DOI] [PMC free article] [PubMed]
- van Vliet I.M., Leroy H., van Megen H.J.G.M. (n.d.) M.I.N.I Internationaal Neuropsychiatrisch Interview, 5th edn. Available: http://www.tijdschriftvoorpsychiatrie.nl/assets/measuringinstruments/meetinstrumenten_90pdf.pdf.
- Weertman A., Arntz A., Kerkhofs M. (2000) Handleiding Gestructureerd Klinisch Interview Voor DSM-IV AS-II Persoonlijkheidsstoornissen. Lisse, The Netherlands: Swets test publishers. [Google Scholar]
- Whittington R., Hockenhull J.C., McGuire J., et al. (2013). A systematic review of risk assessment strategies for populations at high risk of engaging in violent behaviour: update 2002–8. Health Technology Assessment, 17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasekera N., Wright J., Elsey H., Murray J., Tubeuf S. (2015). Cost of crime: a systematic review. Journal of Criminal Justice, 43, 218–28. [Google Scholar]
- Worsley K.J., Friston K.J. (1995). Analysis of fMRI time-series revisited–again. NeuroImage, 2, 173–81. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raine A., Joshi A.A., et al. (2012). Frontal information flow and connectivity in psychopathy. The British Journal of Psychiatry, 201, 408–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.