Abstract
Individuals with social anxiety are characterized by a high degree of social sensitivity, which can coincide with impairments in social cognitive functioning (e.g. theory of mind). Oxytocin (OT) and vasopressin (AVP) have been shown to improve social cognition, and OT has been theorized as a potential therapeutic agent for individuals with social anxiety disorder. However, no study has investigated whether these neuropeptides improve social cognitive ability among socially anxious individuals. In a randomized, double-blind, placebo controlled, between-subjects design we investigated whether social anxiety moderated the effects of OT or AVP (vs placebo) on social working memory (i.e. working memory that involves manipulating social information) and non-social working memory. OT vs placebo impaired social working memory accuracy in participants with higher levels of social anxiety. No differences were found for non-social working memory or for AVP vs placebo. Results suggest that OT administration in individuals with higher levels of social anxiety may impair social cognitive functioning. Randomized-controlled trial registration: NCT01680718.
Keywords: oxytocin, vasopressin, social anxiety, social cognition, social working memory
Individuals with social anxiety are characterized by attentional biases focused on fear of evaluation and avoidance of social situations (Clark and McManus, 2002). The social-evaluative fear at the core of social anxiety contributes to a high degree of interpersonal or social sensitivity (Marin and Miller, 2013). This high level of social sensitivity can coincide with impairments in social cognitive functioning, such as compromised theory of mind (Hezel and McNally, 2014), decreased ability to understand complex emotions (O’Toole et al., 2013) and attributional biases (Plana et al., 2014). Socially anxious individuals appear to overestimate the thoughts and feelings of others, which can lead to inaccurate inferences (Hezel and McNally, 2014). This may be why one study found evidence for higher levels of empathy in socially anxious individuals, but also decreased cognitive empathic accuracy (Tibi-Elhanany and Shamay-Tsoory, 2011).
Increasingly, efforts to elucidate the biological processes that contribute to social anxiety and social cognition have focused on the neuropeptide oxytocin (OT) (Meyer-Lindenberg et al., 2011). To date, many studies have shown that OT enhances social cognition (Meyer-Lindenberg et al., 2011), which can be impaired among individuals with social anxiety (Hezel and McNally, 2014). Although the initial reports suggested that OT enhanced social cognition in general (e.g. Domes et al., 2007), recent evidence has shown that OT benefits only some individuals (Bartz et al., 2011). For example, Bartz et al. (2010) found that OT increased empathic accuracy compared with placebo; however, this effect was only found in individuals with lower levels of social cognitive ability at baseline. Similarly, Feeser et al. (2015) found that OT improved accuracy in perspective taking, but only among individuals with lower levels of dispositional empathy at baseline. Results from these studies suggest that OT may enhance social cognitive performance in those with lower pre-existing levels of social cognitive ability. Therefore, OT may also increase social cognitive ability in socially anxious individuals, who also have impairments in social cognitive functioning (e.g. Hezel and McNally, 2014).
Another possibility, though, is that OT may decrease social cognitive ability in socially anxious individuals by exacerbating the already high level of social sensitivity that is characteristic of this population (Marin and Miller, 2013). Studies showing that OT can increase social cognition have shown this effect among individuals with autism spectrum-like traits (Bartz et al., 2010), who not only have low levels of social cognitive ability, but in some cases (when there is no comorbid social anxiety), can be conceptualized as having low levels of social sensitivity (i.e. impaired attention to or engagement in social stimuli; Chevallier et al., 2012). OT has been shown to enhance social salience or sensitivity to socially relevant stimuli (Shamay-Tsoory and Abu-Akel, 2016). Thus, it is possible that OT may impair social cognition in individuals with higher pre-existing levels of social sensitivity by effectively making them ‘hypersensitive’. This could exacerbate socially anxious individuals’ cognitive and attentional biases related to potential social scrutiny and lead to decreased social cognitive performance. However, to date, no study has examined the role of OT in more demanding, effortful social cognitive tasks in individuals with higher levels of social sensitivity, such as those with higher levels of social anxiety. Since OT is being tested as a treatment for social anxiety (Guastella et al., 2009), this is a critical area of inquiry.
OT’s potential influence on social cognitive ability in individuals with social anxiety raises the question of whether arginine vasopressin (AVP), which is structurally similar to OT, may also impact social cognition in individuals with social anxiety, or whether such an effect may be specific to OT. Like OT, AVP also regulates a broad range of social processes including social cognition (Meyer-Lindenberg et al., 2011). It is generally accepted that AVP has anxiogenic effects (Meyer-Lindenberg et al., 2011; Neumann and Landgraf, 2012), which is likely why there have been no studies of AVP’s effects in individuals with social anxiety. Nonetheless, the highly influential role of AVP on social processes and its structural similarity to OT suggests that it is important to examine whether AVP has effects on higher level social cognition, and whether these effects are moderated by varying levels of social anxiety.
We sought to clarify the way in which social anxiety may moderate the effect of OT or AVP (vs placebo) on effortful social cognition. To do so, we used a task to assess social working memory, or working memory that involves manipulating increasing amounts of social information such as traits and mental states (Meyer et al., 2012). This measure varies working memory load for social information (Meyer and Lieberman, 2012). Prior work using this task has shown that as social working memory load increases, neural regions associated with social cognition or thinking about others’ thoughts and feelings (as well as working memory regions) showed increased activation (Meyer et al., 2012, 2015). Moreover, increased activation in these social cognitive neural regions, but not neural regions associated with non-social working memory, positively correlated with another social cognitive skill, perspective-taking.
We investigated the moderating role of social anxiety on OT and AVP’s effects on social and non-social working memory in a group of female and male participants. Based on evidence suggesting the effects of OT on social behavior are more beneficial in social compared with non-social contexts (Declerck et al., 2010), we hypothesized that OT would influence social working memory, but not non-social working memory. However, the specific moderating role of social anxiety on the effects of OT on social working memory is not yet known. One possibility is that OT may increase social working memory accuracy in those with social anxiety, in the same way that social cognition was enhanced in other populations of individuals who exhibit social cognitive impairments (Bartz et al., 2010; Feeser et al., 2015). Another possibility, however, is that OT administration may decrease social working memory accuracy in individuals with higher levels of social anxiety, perhaps by exacerbating already high levels of social sensitivity. This study tested these competing possibilities. In addition, because of its structural similarity to OT and its well-known effects on social processes, we also explored how AVP interacted with social anxiety to affect social working memory.
Methods
As reported in our previous study in which we found interaction effects of intranasal AVP and paternal warmth on empathic concern (Tabak et al., 2015), several tasks were included post-administration in randomized order including the working memory task that we report in this study. Thus, data from the same sample (although a different task) has previously been published in Tabak et al. (2015).
Participants
As described in Tabak et al. (2015), participants initially included 125 undergraduate students from the University of California, Los Angeles (90 female; 35 male, age range of all participants = 18–31 years, Mean age of all participants = 20.88, SD = 2.71). They were randomly assigned to receive intranasal AVP (n = 42; 30 female, 12 male), OT (n = 42; 30 female, 12 male) or placebo (n = 41; 30 female, 11 male). However, due to computer error, data for the social and non-social working memory task were not recorded for 27 participants. This resulted in 98 participants (69 female; 29 male, age range of all participants = 18–31 years, Mean age of all participants = 20.93, SD = 2.8) who were randomly assigned to receive OT (n = 36; 25 female, 11 male), AVP (n = 28; 20 female, 8 male) or placebo (n = 34; 24 female, 10 male). Exclusion criteria included present or history of medical illness, present psychiatric diagnosis, present use of medications (e.g. selective serotonin reuptake inhibitors (SSRIs)), pregnancy, breastfeeding, and smoking >15 cigarettes per day (for further details see Tabak et al., 2015 and Supplementary Figure S1). Participants were asked to refrain from using medication or alcohol for 24 h, caffeine for 4 h and food or drinks (except water) for 2 h preceding the experiment. Participants self-identified as Asian (58.2%), White (19.4%), Hispanic (12.2%), Black or African American (5.1%) and ‘Other’ (5.1%). Participants who completed all aspects of the study were paid $40–$50 depending on their choices in another task not relevant to this study. Informed consent was obtained from all participants and the UCLA Institutional Review Board approved this study.
Procedure
As in Tabak et al. (2015), participants completed two separate sessions. In the first session, participants completed several self-report questionnaires that included three measures of social anxiety (described below). In the second session (completed on average 19.2 days after the first session, SD = 17.59), participants arrived in groups of 2–15 at a computer lab where they each had their own computer terminal. Participants completed the second session between 2:00 and 5:30 pm. They first completed a set of questionnaires pre-administration including measures of positive/negative affect and state anxiety (described below). Participants also provided a urine sample, which was tested for drug use and possible pregnancy (if female). Research nurses then checked all participants’ temperature, heart rate and blood pressure, to ensure that they were in the accepted limits: systolic blood pressure: 90–130, diastolic blood pressure: 60–90, heart rate: 55–100 beats per minute, and temperature <100°F. If vital signs were out of range, participants rested for 10–15 min and measurements were repeated to see if readings were then within acceptable limits; one participant was excluded on basis of abnormal vital signs and did not receive OT/AVP/placebo.
In preparation for each drug-administration session, a third-party research coordinator unrelated to this study used an online random number generator (www.random.org) to randomly assign participants to the OT, AVP or placebo condition (blocked on gender) and communicated this information to the UCLA pharmacy. A UCLA pharmacist prepared the drug or placebo for each participant with no indication on the label as to what was received (to maintain the blind).
Approximately 1 h after arriving, participants received OT/AVP/placebo using a randomized, double-blind, placebo-controlled, between-subjects procedure. We used sterile 6-ml amber glass bottles with metered nasal pumps from Advantage Pharmaceuticals, Inc. Participants first received instructions on how to use the nasal sprays from the first author and a UCLA research nurse. Participants were then instructed to deliver one spray per nostril in an alternating fashion when prompted (every 30 s).
OT (Syntocinin) was provided by Novartis Pharmaceuticals, Switzerland. OT (24 IU/ml) was transferred into the bottles with attached intranasal applicators (1 puff = 0.1 ml). Participants self-administered five puffs per nostril (2.4 IU/puff) for a total dose of 24 IU. AVP was provided by American Regent Laboratories, Shirley, NY, USA. The pharmacist transferred AVP (20 IU/ml) into the bottles with attached intranasal applicators (1 puff = 0.1 ml). Participants self-administered five puffs per nostril (2 IU/puff) for a total dose of 20 IU. Placebo (used previously by Tabak et al., 2015) consisted of 2 ml glycerine and 3 ml purified water (methylparaben and propylparaben mixed according to purified water formula) for a total of 5 ml. This was filtered with a 5 ml filter and transferred to the bottles with attached intranasal applicators (1 puff = 0.1ml). Participants self-administered five puffs per nostril.
As in previous research (Rilling et al., 2012; Tabak et al., 2015), following completion of administration, participants waited ∼40 min before beginning the tasks. During this time, participants were asked to sit quietly and read from a stack of 10 magazines (e.g. Newsweek). They were also instructed to turn off their phones and refrain from speaking to one another. Participants then completed measures of positive/negative affect and state anxiety. Next, they completed a series of tasks including the social and non-social working memory tasks as well as several other tasks that were unrelated to social or non-social working memory, which were presented in randomized order to minimize potential order effects. This study is focused on the social and non-social working memory tasks, which occurred back to back in a counterbalanced order. Study personnel and research nurses were blind to the drug condition (there were participants in each condition in each group session). The first author supervised the procedure and was present throughout every session.
To assess accuracy on the social working memory task, participants first completed a questionnaire ∼1 month prior to the experimental session in which they ranked 10 of their closest friends on a 1–100 scale (1 = the least, 100 = the most) on several different positive adjectives (e.g. funny; for further details see, Meyer et al., 2012). The answers to these questions were then used to create the social and non-social working memory trials (described below). Participants were aware that the information provided would be used for their second session, but they did not know precisely how this information would be used.
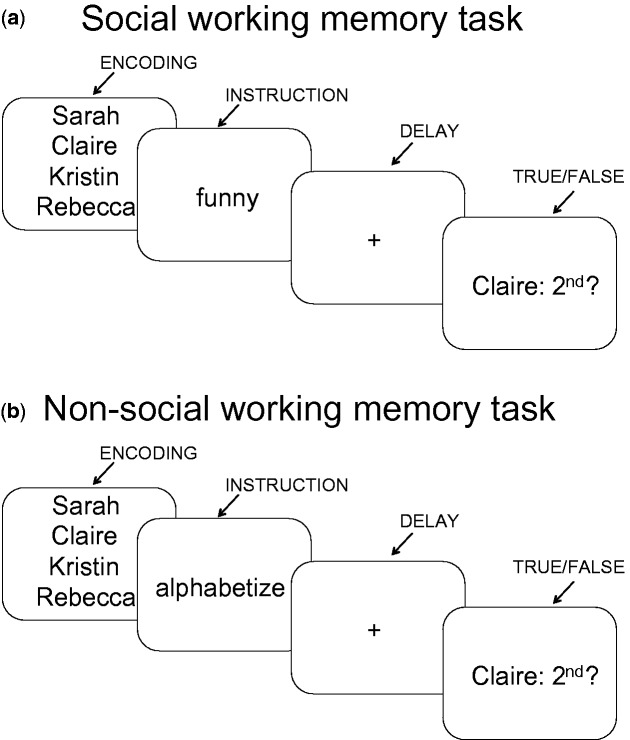
As in Meyer et al. (2012), on the day of the experimental session, the social working memory task was personalized to include names of each participant’s friends (see Figure 1). During social working memory trials, participants first saw some of their own friends’ names (‘encoding’, 4 s). For a given trial, participants saw two, three or four of their friends’ names, which was the working memory load manipulation. Next, participants saw a trait word (1.5 s), followed by a delay period (6 s). During the delay period, participants were instructed to rank the previously encoded friends along the trait dimension, from most-to-least. After the delay period, participants were asked a true/false question about their ranking. For example, the true/false question shown in Figure 1a (Claire: 2nd?) asks whether Claire is the second funniest friend of the set of previously shown friends.
Fig.1.
(a, b) Example trials for the social working memory task and non-social working memory task.
To ensure that the only factor influencing social working memory trial difficulty was the number of friends considered, and not how close participants’ friends fell along a trait dimension (e.g. ranking two friends that are similarly funny may be more challenging than ranking two friends that vary greatly in how funny they are), an algorithm was used to select which friends’ names to show for a given trial. The algorithm used a rule of selecting friends ranked no more than 25 points apart, or within 5 points on the original friend trait ranking questionnaire (which used a 1–100 scale). The ranked position in each true or false question was randomized across trials to avoid mental set effects. In addition, participants completed practice social working memory trials with celebrities’ names to ensure that they understood the task; all subjects received the same practice trials.
In addition to social working memory trials, participants completed non-social working memory trials in which they alphabetized two, three or four friends’ names in working memory during the delay period. Here, the true/false question asked about the alphabetical position of the friend’s name, relative to the other friends’ names encoded on that trial (Figure 1b). Participants completed one block of the social working memory trials (18 trials; 6 per load level) and one block of the non-social working memory trials (18 trials; 6 per load level). The order of social and non-social working memory blocks was randomized across participants.
Answers to the social working memory trials were considered accurate if a participant’s answer to the true/false question was consistent with their trait rankings from the online questionnaire. Thus, social working memory accuracy was represented by the average of correct responses across trials with a load of two, three or four. Our focus was on the most demanding or effortful portion of the task (i.e. social working memory accuracy when manipulating three or four friends’ names). Therefore, difference scores were computed by subtracting a participant’s average accuracy on the lowest load trials (i.e. when two friends’ traits were considered) from their average accuracy on the highest load trials (i.e. when four friends’ traits were considered) as well as the medium load trials (i.e. when three friends’ traits were considered). This variable allowed us to isolate the variability across subjects specifically associated with the social working memory manipulation, beyond the variance associated with performing a social cognition task more generally. We also created an analogous non-social working memory performance variable, in which we subtracted each participant’s average accuracy on the lowest load trials (i.e. when two names were alphabetized) from their average accuracy on the highest load trials (i.e. when four names were alphabetized) as well as the medium load trials (i.e. when three names were alphabetized). Examining non-social working memory allowed us to investigate whether the effects of either neuropeptide influenced working memory involving social information as well as working memory processes more broadly.
Measures
Social anxiety
On a separate day prior to the drug administration, participants completed the Social Phobia Scale (Mattick and Clark, 1998), the Social Interaction Anxiety Scale (Mattick and Clark, 1998) and the Liebowitz Social Anxiety Scale (Liebowitz, 1987). As in Niles et al. (2014), the three scales were Z-scored, mean centered, and a mean composite was created to represent social anxiety (α = 0.87). No differences were found between the OT, AVP or placebo groups on social anxiety (P > 0.75).
State Positive and Negative Affect
We measured self-reported positive/negative affect pre-administration and 40 min post-administration in the OT, AVP and placebo groups using the 10-item PANAS (Thompson, 2007). Items on the PANAS were rated using a 5-point Likert-type scale (1 = not at all; 5 = extremely). Mean composites were created to represent positive affect (pre-administration α = 0.81; post-administration α = 0.80) and negative affect (pre-administration α = 0.61; post-administration α = 0.69). Change scores (post- and pre-administration) were then computed to examine differences in positive and negative affect before and after drug-administration.
State Anxiety
We measured state anxiety pre-administration and 40 min post-administration in the OT, AVP, and placebo groups using the state version of the State Trait Anxiety Inventory (STAI; Spielberger et al., 1983). Items on the STAI were rated using a 4-point Likert-type scale (1 = not at all; 4 = very much). A mean composite was created for pre-administration (α = 0.91) and post-administration (α = 0.91).
Statistical analysis
First, to examine whether there were differences in accuracy at higher vs lower loads, we conducted paired sample t-tests for social working memory and non-social working memory accuracy scores in the placebo condition. We then used hierarchical linear regression analyses to examine non-specific drug effects. In doing so we examined the main effect of drug condition (OT vs placebo and AVP vs placebo) and social anxiety, as well as their interaction, on pre–post changes in state anxiety as well as positive/negative affect. Additional hierarchical linear regression analyses examined the main effect of drug condition (OT vs placebo and AVP vs placebo) and social anxiety on social and non-social working memory accuracy. Then we examined the interaction between drug condition (OT vs placebo or AVP vs placebo) and social anxiety on social and non-social working memory accuracy. Post-hoc tests of specific interaction effects were conducted using PROCESS (Hayes, 2013). P-values < 0.05 (two-tailed) were considered statistically significant.
Due to computer error, five participants did not rate their post-administration state anxiety. In addition, one participant unintentionally began completing post-administration questionnaires and tasks ∼25-min post-administration instead of ∼40 min post-administration. Results remained unchanged when removing this participant from analyses. Analyses were conducted following removal of outliers on major study variables based on scores lower than the 25th percentile—1.5 × the interquartile range and scores higher than the 75th percentile + 1.5 × the interquartile range. Social anxiety, social and non-social working memory accuracy were continuous variables. All analyses were conducted using SPSS version 20 and Figure 2 was created using Stata version 13. See Tabak et al. (2015) for sample size determination.
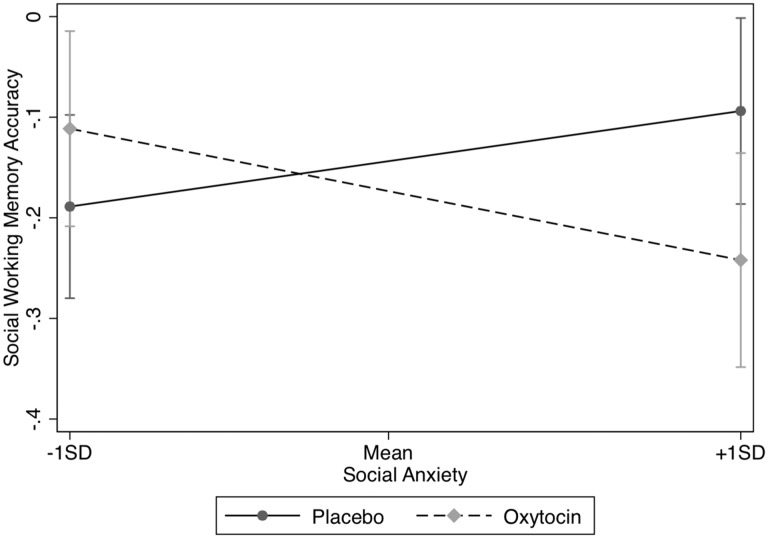
Fig. 2.
There was a significant interaction of drug condition (OT vs placebo) × social anxiety on social working memory accuracy. At +1 SD above the mean of social anxiety, individuals given OT were significantly less accurate compared with those on placebo. Error bars represent 95% CIs.
Results
Effects of drug on changes in state anxiety and affect
We first examined the effect of OT (vs placebo) and trait social anxiety on self-reported changes in state anxiety and state positive/negative affect. There were no main effects of drug or social anxiety and no drug by anxiety interactions on self-reported changes in state anxiety (P’s > 0.453), positive affect (P’s > 0.098), or negative affect (P’s > 0.163). Similarly, there were no main effects of AVP (vs placebo), no main effects of social anxiety and no drug by anxiety interactions on self-reported changes in state anxiety (P’s > 0.205) positive affect (P’s > 0.186) or negative affect (P’s > 0.095). Hence, any effects of OT or AVP and social anxiety on social cognition are likely not being driven by simple changes in affective states.
Effects of working memory load
Next, we examined differences in social and non-social working memory accuracy for a load of two, three and four in the placebo condition. Accuracy for both tasks at all levels in the placebo condition was significantly greater than chance (P’s < 0.05). In the social working memory task participants were more accurate on trials with a load of two (M = 0.76, SD = 0.14) compared with trials with a load of three (M = 0.57, SD = 0.17; t(33) = 5.42, P < 0.001, Cohen’s d = 0.954) or four (M = 0.62, SD = 0.16; t(33) = 4.5, P < 0.001, Cohen’s d = 0.776) As in prior research (Meyer et al., 2012), the difference between accuracy for social working memory trials with a load of three and four was not statistically significant [t(33) = −1.12, P = 0.27, Cohen’s d = −0.2).
In the non-social working memory task, participants were more accurate on trials with a load of two (M = 0.91, SD = 0.12) compared with trials with a load of three (M = 0.84, SD = 0.13; t(33) = 2.34, P = 0.026, Cohen’s d = 0.435); however, the difference between accuracy with a load of two compared with four (M = 0.88, SD = 0.18) as well as three compared to four was not statistically significant (P’s > 0.198). The relatively high level of accuracy in the non-social working memory task for trials with a load of four was unanticipated since our previous studies have typically found lower rates of accuracy in the non-social task with a load of four (e.g. M = 0.78, SD = 0.15; Meyer et al., 2015).
Importantly, there was a significant incremental load-dependent increase in reaction time in both the social and non-social working memory task (i.e. load 2 < load 3 < load 4, P’s < 0.001). This suggests that effort did in fact increase as a function of load even if accuracy scores did not always reflect this stepwise pattern.
Because our interest was in the more effortful aspect of social cognitive ability, we then computed difference scores for a load of four vs two, as well as a load of three vs two on social and non-social working memory accuracy. Due to the significant differences in reaction time between load three and four on both the social and non-social tasks, we examined these outcomes in separate analyses.
Effect of OT and social anxiety on social working memory and non-social working memory
Effects on social working memory accuracy
We first investigated the effect of OT vs placebo (dummy coded: OT = 1, placebo = 0) and social anxiety on social working memory for the highest load of four (vs two). There was no main effect of OT, b = −0.03, SE = 0.05, P = 0.562, R2 = 0.01 or social anxiety, b = 0.01, SE = 0.03, P = 0.857, R2 < 0.01, R2 change < 0.01, on social working memory accuracy. However, there was a significant interaction between drug condition (OT vs placebo) and social anxiety on social working memory accuracy, b = −0.14, SE = 0.06, P = 0.022, R2 = 0.08, Interaction R2 ‘change = 0.08. To further explore the significant interaction, we examined mean differences in social working memory accuracy between the OT and placebo groups at one SD above and below the mean of social anxiety. There was a significant difference in social working memory accuracy between the OT and placebo group at higher levels of social anxiety (+1 SD), b = −0.15, SE = 0.07, P = 0.04, but no significant difference at lower levels (−1 SD), b = 0.09, SE = 0.07, P = 0.209 (see Figure 2). Thus, among individuals with higher levels of social anxiety, OT decreased social working memory accuracy. However, OT did not have a significant effect on social working memory accuracy for individuals with lower levels of social anxiety. Analysis of simple slopes showed no association between social anxiety and social working memory accuracy in the OT group, b = −0.08, SE = 0.05, P = 0.128, R2 = 0.07, but a marginal positive association in the placebo group, b = 0.06, SE = 0.03, P = 0.077, R2 = 0.10.
Next, we examined the effect of OT vs placebo and social anxiety on social working memory accuracy for the middle load of three (vs two). There was no main effect of OT vs placebo, b = −0.04, SE = 0.06, P = 0.533, R2 = 0.01 and no main effect of social anxiety, b = 0.04, SE = 0.04, P = 0.274, R2 = 0.02, R2 change = 0.02. There was also no interaction between drug condition (OT vs placebo) and social anxiety on social working memory accuracy, b = −0.02 SE = 0.08, P = 0.85, R2 = 0.02, Interaction R2 change < 0.01. Thus, there was an interaction between OT and social anxiety at the highest social working memory load, but not at the medium load.
Effects on non-social working memory accuracy
We also examined the main and interaction effects of OT (vs placebo) and social anxiety on non-social working memory accuracy. Starting with the load of four (vs two), there was no main effect of OT vs placebo, b = −0.02, SE = 0.03, P = 0.648, R2 < 0.01, no main effect of social anxiety, b = 0.02, SE = 0.02, P = 0.222, R2 = 0.03, R2 ‘change’ = 0.02, and no interaction between drug condition (OT vs placebo) and social anxiety on non-social working memory accuracy, b = −.004, SE = .04, p = .929, R2 = 0.03, Interaction R2 change < 0.01.
For a load of three (vs two), there was a marginally significant main effect of OT vs placebo, b = −0.06, SE = 0.03, P = 0.056, R2 = 0.06, but no main effect of social anxiety, b = −0.01, SE = 0.02, P = 0.714, R2 = 0.06, R2 change < 0.01. In addition, there was no interaction between drug condition and social anxiety on non-social working memory accuracy, b = 0.02, SE = 0.04, P = 0.572, R2 = 0.06, Interaction R2 change = 0.01. Further decomposing the marginally significant main effect revealed that subjects in the OT condition showed a larger drop in accuracy from a load of two (M = 0.93, SD = 0.12) to a load of three (M = 0.77, SD = 0.19), t(35) = 6.86, P < 0.001, Cohen’s d = 1.33, than did subjects in the placebo condition (load of two: M = 0.91, SD = 0.12; load of three: (M = 0.84, SD = 0.13), t(33) = 2.34, P = 0.026, Cohen’s d = 0.44.
Effect of AVP and social anxiety on social and non-social working memory
Effects on social working memory accuracy
We also investigated the effect of AVP vs placebo (dummy coded: AVP = 1, placebo = 0) and social anxiety on social working memory for a load of four (vs two), but found no significant effects here. Thus, we found no main effect of drug condition, b = −0.001, SE = 0.06, P = 0.99, R2 < 0.01, no main effect of social anxiety b = 0.03, SE = 0.03, P = 0.35, R2 = 0.02, R2 change = 0.02, and no interaction between drug condition (AVP vs placebo) and social anxiety on social working memory accuracy, b = −0.07, SE = 0.06, P = 0.257, R2 = 0.04, Interaction R2 change = 0.02.
Similar results appeared for a load of three (vs two) with no main effect of AVP vs placebo, b = 0.01, SE = 0.06, P = 0.915, R2 < 0.01, no main effect of social anxiety, b = 0.01, SE = 0.03, P = 0.691, R2 < 0.01, R2 change < 0.01, and no interaction between drug condition (AVP vs placebo) and social anxiety on social working memory accuracy, b = −.07, SE = .06, p = .254, R2 = .03, Interaction R2 change = 0.02.
Effects on non-social working memory
We also examined the effect of AVP vs placebo and social anxiety on non-social working memory for a load of four (vs two). There was no main effect of AVP vs placebo, b = −0.04, SE = 0.04, P = 0.352, R2 = 0.02, no main effect of social anxiety, b = 0.02, SE = 0.02, P = 0.379, R2 = 0.03, R2 change = 0.02, and no interaction between drug condition and social anxiety on non-social working memory accuracy, b = −0.02, SE = 0.05, P = 0.714, R2 = 0.03, Interaction R2 change < 0.01.
Similarly, when examining AVP vs placebo for a load of three (vs two), there was no main effect of AVP vs placebo, b = −0.004, SE = 0.04, P = 0.906, R2 < 0.01, no main effect of social anxiety, b = −0.004, SE = 0.02, P = 0.832, R2 < 0.01, R2 change < 0.01, and no interaction between drug condition (AVP vs placebo) and social anxiety on non-social working memory accuracy, b = 0.02 SE = 0.04, P = 0.508, R2 = 0.01, Interaction R2 change = 0.01.Thus, we found no effect for AVP with either outcome.1
Task order effects
Social and non-social working memory tasks were presented in random order, but to ensure that the significant interaction between drug condition and social anxiety was not affected by task order, we examined the three-way interaction between OT (vs placebo), social anxiety, and task order on working memory accuracy for a load of three and four. There were no interactions with gender for either social or non-social working memory accuracy (P’s > 0.603). We then examined the three-way interaction between AVP (vs placebo), social anxiety and task order on working memory accuracy and found no interactions with order for social or non-social working memory accuracy for a load of three or four (P’s > 0.099). Thus, task order did not affect the present results.
Gender effects on working memory
Based on previous studies identifying gender-specific effects of OT and AVP (Rilling et al., 2014) we also examined the three-way interaction between OT (vs placebo), social anxiety and gender on working memory accuracy for a load of three and four. There were no interactions with gender for either social or non-social working memory accuracy (P’s > 0.218). We then examined the three-way interaction between AVP (vs placebo), social anxiety and gender on working memory accuracy and found no interactions with gender for social or non-social working memory accuracy for a load of three and four (P’s ≥ 0.06). Thus, gender did not affect the present results.
Nonetheless, because the majority of our sample was female, we reanalyzed our primary finding (i.e. the significant interaction between OT vs placebo and social anxiety on social working memory accuracy for a load of four) in only female participants. Main effects of OT vs placebo, b = −0.001, SE = 0.06, P = 0.993, and social anxiety, b = 0.03, SE = 0.04, P = 0.48 were not significant, but the interaction remained significant, b = −0.18, SE = 0.07, P = 0.019, R2 = 0.13, Interaction R2 change = 0.07.
Discussion
The present results are the first to demonstrate that social anxiety moderates the effect of OT on effortful social cognition in the form of social working memory accuracy. Specifically, OT decreased social working memory accuracy in a sample of female and male participants with higher levels of social anxiety. However, we did not find an effect of OT and social anxiety on non-social working memory accuracy. In addition, no main or interaction effects were found with AVP for either outcome.
In this study, there was a marginally significant positive correlation between social anxiety and social working memory accuracy among participants in the placebo condition. Although many studies have shown social cognitive impairments in socially anxious individuals, some studies have shown the opposite (i.e. ‘enhanced’ social cognitive ability in individuals with social anxiety; Tibi-Elhanany and Shamay-Tsoory, 2011). Results from these studies suggest that impairments in social cognition associated with social anxiety (O’Toole et al., 2013; Hezel and McNally, 2014; Plana et al., 2014) are not the result of processing deficits (Sutterby et al., 2012), or lack of attention or motivation as in autism spectrum disorders (Chevallier et al., 2012). Rather, by increasing attention to social stimuli, OT may enhance the pre-existing high degree of social sensitivity present in individuals with social anxiety (Marin and Miller, 2013). For individuals with higher levels of social anxiety, the process of ranking one’s friends may contribute to ‘overthinking’, which can harm social cognitive functioning.
Our primary finding showed an interaction between OT and social anxiety at the highest load, but not the medium load in our social working memory task. It is possible that the addition of only one more friend (i.e. from two names to three names) is not enough additional social information to be processed for OT to influence performance among individuals with higher levels of social anxiety. This is consistent with our proposal that OT has a deleterious effect on social cognitive ability among individuals with higher levels of social anxiety only when the task becomes more effortful or demanding in the social cognitive domain.
The anxiolytic effects of OT in animals (Neumann and Landgraf, 2012), as well as the potentially beneficial effects of OT on social cognition (Meyer-Lindenberg et al., 2011) suggest that increased levels of OT should help individuals with social anxiety. However, several recent studies further bolster the proposition that increased or higher levels of OT may predict or negatively impact individuals with social anxiety. Radke et al. (2013) found that OT increased approach to negative social stimuli in individuals with lower-levels of social anxiety, but decreased approach behavior in those with higher-levels of social anxiety. In addition, recent evidence suggests that variation in OT system genes may heighten social sensitivity and increase risk for the development of social anxiety (Tabak et al., 2016).
Just as OT is commonly associated with anxiolytic effects, AVP is commonly associated with anxiogenic effects (Neumann and Landgraf, 2012). For this reason, it is interesting that we found no main effect of AVP and no AVP × social anxiety interaction effect on state anxiety or negative affect. Much like OT, AVP studies in humans also seem to have nuances that are inconsistent with what we might expect from the animal literature. Although one study found an increase in state anxiety following AVP administration (Thompson et al., 2006), another study did not (Shalev et al., 2011). One potential reason for these inconsistencies may relate to the social context, as Shalev et al. (2011) only found increased physiological stress reactions following AVP administration in the presence of social-evaluative threat.
Our findings add to a growing body of research on OT in humans that show moderation of OT’s effects that are specific to context and individual differences (Bartz et al., 2011; Macdonald, 2012; Tabak, 2013). Although no gender effects were found in the present investigation, our study included many more female participants than male. For this reason, future studies including a more equal ratio of female and male participants would improve the ability to detect potential gender-specific effects of OT. In addition, our tasks for social and non-social working memory accuracy involved personally relevant names of participants’ friends. Future research examining the effects of OT on working memory would benefit from including stimuli without personal meaning to determine the generalizability of the current findings (Hariri-Dahan and Bernstein, 2014). The social working memory task used in this study was also more difficult than the non-social working memory task. In the future, studies may wish to examine whether the present results are altered with a more challenging non-social working memory task.
Our study also included non-clinical undergraduate participants who were not diagnosed with social anxiety disorder. Future studies including community clinical samples will be necessary to replicate and extend the present findings. Last, there is still no definitive evidence demonstrating that intranasal administration of OT or AVP using standard methods enters the brain (Leng and Ludwig, 2016). Although results such as those in this study demonstrate cognitive and behavioral effects of intranasal OT administration, future research is needed to clarify if these effects may result from peripheral rather than central mechanisms (Leng and Ludwig, 2016).
To summarize, the present results demonstrated that among individuals with higher levels of social anxiety, OT decreased performance on an effortful social cognitive process—social working memory. These same effects were not observed for non-social working memory. Moreover, there was no interaction between AVP and social anxiety on either social or non-social working memory. Although studies have demonstrated anxiolytic effects of OT, our results add to a growing cautionary literature regarding the interaction between OT and anxiety (Macdonald and Feifel, 2014).
Supplementary Material
Acknowledgements
We would like to thank Spencer Uemura for his assistance in data collection and statistical analysis.
Footnotes
Although we had no hypotheses regarding the differential effects of OT vs AVP, it is important to note that there were no main effects of OT vs AVP and no significant interactions with social anxiety on either social working or non-social working memory accuracy for a load of three or four (P’s > 0.08).
Funding
Funding was provided by the UCLA Jeffrey/Wenzel Term Chair in Behavioral Neuroscience (to N.I.E.). A post doctoral fellowship for B.A.T. was supported by the T32MH15750 training fellowship in Biobehavioral Issues in Physical and Mental Health at the University of California, Los Angeles.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Bartz J.A., Zaki J., Bolger N., et al. (2010). Oxytocin selectively improves empathic accuracy. Psychological Science, 21, 1426–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences, 15, 301–9. [DOI] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E.S., Schultz R.T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16, 231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.M., McManus F. (2002). Information processing in social phobia. Biological Psychiatry, 51, 92–100. [DOI] [PubMed] [Google Scholar]
- Declerck C.H., Boone C., Kiyonari T. (2010). Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Hormones and Behavior, 57, 368–74. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. (2007). Oxytocin improves “mind-reading” in humans. Biological Psychiatry, 61, 731–3. [DOI] [PubMed] [Google Scholar]
- Feeser M., Fan Y., Weigand A., et al. (2015). Oxytocin improves mentalizing - pronounced effects for individuals with attenuated ability to empathize. Psychoneuroendocrinology, 53, 223–32. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Howard A.L., Dadds M.R., Mitchell P., Carson D.S. (2009). A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology, 34, 917–23. [DOI] [PubMed] [Google Scholar]
- Hariri-Dahan O., Bernstein A. (2014). A general approach-avoidance hypothesis of oxytocin: accounting for social and non-social effects of oxytocin. Neuroscience and Biobehavioral Reviews, 47, 506–19. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. (2013). An Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press. [Google Scholar]
- Hezel D.M., McNally R.J. (2014). Theory of mind impairments in social anxiety disorder. Behavior Therapy, 45(4),530–40. [DOI] [PubMed] [Google Scholar]
- Leng G., Ludwig M. (2016). Intranasal oxytocin: Myths and delusions. Biological Psychiatry, 79, 243–50. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R. (1987). Social phobia. Modern Problems in Pharmacopsychiatry, 22, 141–73. [DOI] [PubMed] [Google Scholar]
- Macdonald K.S. (2012). Sex, receptors, and attachment: a review of individual factors influencing response to oxytocin. Frontiers in Neuroscience, 6, 194.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K.S., Feifel D. (2014). Oxytocin’s role in anxiety: a critical appraisal. Brain Research, 1580, 22–56. [DOI] [PubMed] [Google Scholar]
- Marin T.J., Miller G.E. (2013). The interpersonally sensitive disposition and health: an integrative review. Psychological Bulletin, 139, 941–84. [DOI] [PubMed] [Google Scholar]
- Mattick R.P., Clark J.C. (1998). Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy, 36, 455–70. [DOI] [PubMed] [Google Scholar]
- Meyer M.L., Lieberman M.D. (2012). Social working memory: neurocognitive networks and directions for future research. Frontiers in Psychology, 3, 571.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Spunt R.P., Berkman E.T., Taylor S.E., Lieberman M.D. (2012). Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy of Sciences of the United States of America, 109, 1883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Taylor S.E., Lieberman M.D. (2015). Social working memory and its distinctive link to social cognitive ability: an fMRI study. Social Cognitive and Affective Neuroscience, 10, 1338–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12, 524–38. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Landgraf R. (2012). Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35, 649–59. [DOI] [PubMed] [Google Scholar]
- Niles A.N., Burklund L.J., Arch J.J., Lieberman M.D., Saxbe D., Craske M.G. (2014). Cognitive mediators of treatment for social anxiety disorder: comparing acceptance and commitment therapy and cognitive-behavioral therapy. Behavior Therapy, 45, 664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole M.S., Hougaard E., Mennin D.S. (2013). Social anxiety and emotion knowledge: a meta-analysis. Journal of Anxiety Disorders, 27, 98–108. [DOI] [PubMed] [Google Scholar]
- Plana I., Lavoie M.A., Battaglia M., Achim A.M. (2014). A meta-analysis and scoping review of social cognition performance in social phobia, posttraumatic stress disorder and other anxiety disorders. Journal of Anxiety Disorders, 28, 169–77. [DOI] [PubMed] [Google Scholar]
- Radke S., Roelofs K., de Bruijn E.R. (2013). Acting on anger: social anxiety modulates approach-avoidance tendencies after oxytocin administration. Psychological Science, 24, 1573–8. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., DeMarco A.C., Hackett P.D., et al. (2012). Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology, 37, 447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., Demarco A.C., Hackett P.D., et al. (2014). Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology, 39, 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I., Israel S., Uzefovsky F., Gritsenko I., Kaitz M., Ebstein R.P. (2011). Vasopressin needs and audience: Neuropeptide elicited stress responses are contingent uopnperceived social evaluative threats. Hormones and Behavior, 60, 121–7. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Abu-Akel A. (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79, 194–202. [DOI] [PubMed] [Google Scholar]
- Spielberger C. D., Gosuch R. L., Lushene R., Vagg P. R., Jacobs G. A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Sutterby S.R., Bedwell J.S., Passler J.S., Deptula A.E., Mesa F. (2012). Social anxiety and social cognition: the influence of sex. Psychiatry Research, 197, 242–5. [DOI] [PubMed] [Google Scholar]
- Tabak B.A. (2013). Oxytocin and social salience: a call for gene-environment interaction research. Frontiers in Neuroscience, 7, 199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak B.A., Meyer M.L., Castle E., et al. (2015). Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth: A randomized controlled trial. Psychoneuroendocrinology, 51, 253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak B.A., Vrshek-Schallhorn S., Zinbarg R.E., et al. (2016). Interaction of CD38 variant and chronic interpersonal stress prospectively predicts social anxiety and depression symptoms over six years. Clinical Psychological Science, 4, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E.R. (2007). Development and Validation of an Internationally Reliable Short-Form of the Positive and Negative Affect Schedule (PANAS). Journal of Cross-Cultural Psychology, 38, 227–42. [Google Scholar]
- Thompson R.R., George K., Walton J.C., Orr S.P., Benson J. (2006). Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Science of the United States of America, 103, 7889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibi-Elhanany Y., Shamay-Tsoory S.G. (2011). Social cognition in social anxiety: first evidence for increased empathic abilities. Israel Journal of Psychiatry and Related Sciences, 48, 98–106. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.