Abstract
Despite functional brain imaging research pointing to the role of prefrontal cortex in cognitive reappraisal, the structural correlates of habitual engagement of reappraisal are unclear. Functional imaging studies of reappraisal have shown broad engagement of bilateral middle frontal cortex (MFC) and left superior frontal cortex (SFC), and specific engagement of the right SFC. However, volumetric studies have not identified clear associations between reappraisal and these regions. This discrepancy between functional and structural studies suggests that broad functional engagement associated with reappraisal might not be detectable at a structural level using highly localized volumetric measures. This study addressed the discrepant structural findings by assessing the relation between reappraisal and grey matter volume, using methods that allow both region-level broad/diffuse assessments (surface-based morphometry), and voxel-level specific/localized (voxel-based morphometry) measures. Results were consistent with diffuse positive volumetric associations with reappraisal in the right MFC and left SFC, and a localized positive volumetric association in the right SFC, thus resolving the discrepancy between functional and structural studies. This study provides novel evidence supporting the idea that functional engagement related to transient manipulations of reappraisal can be linked to structural associations related to habitual engagement of similar operations, within the same brain regions.
Keywords: emotion, individual differences, neuroimaging, neuroanatomy, personality
Introduction
The way a person controls his or her emotions, or ‘emotion regulation’, is often studied in two complementary dimensions, cognitive reappraisal and expressive suppression (Gross and John, 2003). Reappraisal refers to the ability to change one’s view of a particular situation in order to see it in a different light, whereas suppression refers to the tendency to inhibit one’s emotional responses to keep them inside (Gross and John, 2003). Although these dimensions of emotion regulation have been extensively studied in functional brain imaging studies (e.g. Ochsner et al., 2002; Ochsner et al., 2004; Goldin et al., 2008; Buhle et al., 2013), relatively little is known about the relation between the habitual engagement of emotion regulation and structural neural markers, such as cortical grey matter volume. Given evidence that reappraisal is a particularly effective emotion regulation strategy (Augustine and Hemenover, 2009; Webb et al., 2012), associated with positive indicators of mental health (Gross and John, 2003; John and Gross, 2004; Aldao et al., 2010; Llewellyn et al., 2013; Hu et al., 2014), understanding its neural associations is a critical step in the development of clinical interventions and educational practices to support emotional well-being. The goal of the present investigation was to clarify the relation between the habitual engagement of emotion regulation through reappraisal and structural associations in the brain, using a volumetric approach.
Reappraisal has been shown to have both functional (Ochsner and Gross, 2008; Ochsner et al., 2012; Buhle et al., 2013) and structural correlates in the brain (Welborn et al., 2009; Giuliani et al., 2011b; Hermann et al., 2014). However, functional imaging and volumetric studies of the brain have yielded seemingly inconsistent results regarding the association between reappraisal and key regions involved in emotion regulation and emotion-cognition integration in the prefrontal cortex (PFC; Kühn et al., 2011; Hermann et al., 2014). Hence, the link between brain function and structure with respect to reappraisal remains unclear. Functional brain imaging literature has systematically associated reappraisal with PFC engagement (Ochsner and Gross, 2008; Ochsner et al., 2012; Buhle et al., 2013), and available evidence suggests a possible distinction between regions showing a relatively broad engagement and regions showing relatively localized effects. Overall, these studies emphasize the role of both the middle frontal cortex (MFC) and superior frontal cortex (SFC) in emotion regulation through reappraisal, but point to more broad engagement identified in bilateral MFC and left SFC, with peak voxel activations identified in Brodmann areas (BAs) 6, 8, 9, 10 and 46 (Buhle et al., 2013; Goldin et al., 2008; Kanske et al., 2011; Mcrae et al., 2008; Ochsner et al., 2002; Phan et al., 2005), and more localized engagement identified in the right SFC, with peak voxel activations primarily restricted to BAs 6 and 8 (Kanske et al., 2011; Silvers et al., 2015).
Functional imaging evidence identifying the neural correlates associated with individual differences in the tendency to use reappraisal (Drabant et al., 2009) suggests that the habitual ways in which individuals regulate their emotions impacts neural processing and might, over time, influence the structure of the underlying brain regions. Although there is no clear indication of the direction of the relation between the grey matter volume and individual differences in personality traits, a growing body of evidence suggests that there is a relation between the volume of a brain region and its level of use (Draganski et al., 2004; Boyke et al., 2008). This account, called ‘use-dependent plasticity’ (Nudo et al., 1996; Bütefisch et al., 2000; for reviews of relevant studies in humans see Draganski and May, 2008; May, 2011), may describe a result of Hebbian learning, which posits that repeated patterns of neuronal firing lead to increased synaptic connectivity (Hebb, 1949), and suggests that these structural changes might lead to increases in grey matter volume (Draganski et al., 2006). This would suggest that individual differences in the habitual engagement of reappraisal would be associated with differences in grey matter volume in the underlying brain structures.
In contrast to functional studies, previous anatomical studies (Kühn et al., 2011; Hermann et al., 2014) have not identified clear associations between reappraisal and MFC and SFC volumes, despite identifying structural associations with other brain regions (Welborn et al., 2009; Giuliani et al., 2011b; Hermann et al., 2014). One possible explanation for the discrepancy between functional and structural studies is that the latter used highly localized, voxel-based methods (Kühn et al., 2011; Hermann et al., 2014; but see Giuliani et al., 2011a,b), which might have prevented the identification of the broader prefrontal areas shown to support reappraisal in functional studies. Thus, it remains unclear how the MFC and SFC regions are related to the habitual engagement of reappraisal at a structural level.
On the one hand, it is possible that volumetric correlates reflecting more broad/diffuse associations in certain regions might have a larger extent but be relatively weaker in strength, and thus they may not be captured by voxel-level approaches, such as voxel-based morphometry (VBM), using strict thresholds. Instead, such diffuse associations may be better captured by volumetric methods involving more region-level assessments, such as surface-based morphometry (SBM), using region of interest (ROI) approaches. On the other hand, volumetric correlates reflecting more specific/localized and relatively stronger associations may be better captured by VBM. It is, therefore, important to consider combining complementary volumetric techniques that allow for both the detection of differences in the extent and the strength of such associations. Previous research combining ROI and VBM approaches has shown effects for another emotion regulation strategy that were differentially captured by the two methods (Giuliani et al., 2011a), consistent with the idea that ROI approaches might be sensitive to more diffuse associations and that these effects may not be apparent in VBM at strict thresholds. This suggests that volumetric correlates reflecting more diffuse, region level associations could in some cases be capturing additive effects of relatively weaker associations across a larger extent, while volumetric correlates reflecting more localized, voxel-level associations, might be washed down at the level of individual ROIs.
This study addressed this issue using a comprehensive methodological investigation based on two complementary volumetric methods: (i) A ROI approach (SBM), which allows identification of more diffuse, region-level, associations, and (ii) A voxel-based approach (VBM), which allows identification of more localized, voxel-level, associations that might be washed down and missed by ROI-level approaches. Specifically, diffuse associations were expected to be reflected in a relatively larger extent, more easily identified by whole-region analyses (SBM) and likely only at lower significance thresholds by voxel-level analyses (VBM). On the other hand, localized associations were expected to be reflected in a relatively smaller extent, less likely to be identified by SBM, but surviving higher significance thresholds in VBM analyses. Based on the functional literature and the theory of association between brain function and structural plasticity, we tested the hypothesis that habitual engagement of reappraisal is positively associated with brain volume in the MFC and SFC, but that this association is (i) more diffuse in the bilateral MFC and left SFC, regions that are consistently reported as having broad increased activation for reappraisal and (ii) more localized in the right SFC, the region reported in more specific/localized engagement of reappraisal.
Methods
Subjects
Data were collected from a sample of 85 healthy young participants (18–34 years old; M = 23.25 years old, SD = 3.95, 48 females), who had undergone MRI scanning. No participants had previously been diagnosed with any neurological, psychiatric or personality disorders. Two participants were excluded from final analyses, one because of incomplete neuropsychological measures and the other because of outlier reappraisal score; participants with outlier anatomical measures were also removed analysis-wise (see below and Results section). Outlier values were determined using a criterion of 3 SDs (Osborne and Overbay, 2004) for both trait scores and for SBM/VBM measures. The experimental protocol was approved for ethical treatment of human participants by the institutional Health Research Ethics Board, and participants provided written consent and were compensated with either course credit or money.
Emotion regulation and control measures
Habitual engagement of cognitive reappraisal was assessed using the Emotion Regulation Questionnaire (Gross and John, 2003). This questionnaire assesses the habitual engagement of two emotion regulation strategies, reappraisal and suppression, using a seven-point Likert scale that ranges from ‘strongly disagree’ to ‘strongly agree’. Examples of statements from the reappraisal dimension include ‘I control my emotions by changing the way I think about the situation I’m in’, and statements from the suppression dimension include ‘I keep my emotions to myself’ (Gross and John, 2003). Reappraisal score was measured by six items (Cronbach’s alpha = 0.735, n = 83) and suppression score was measured by four items (Cronbach’s alpha = 0.787, n = 83). The distribution of reappraisal scores used for final analyses (M = 30.82, SD = 5.48, n = 83) was assessed for normality by performing a Shapiro-Wilk test. This confirmed that the frequency distribution of reappraisal scores did not significantly differ from a normal distribution (P > 0.3). For consistency with previous studies (Giuliani et al., 2011a,b), we also included a measure of trait negative affect as a control variable, which was assessed using the Positive and Negative Affect Scale (Watson et al., 1988). This questionnaire assesses the extent to which a person feels a certain way right now or during a longer period of time, using a five-point Likert scale that ranges from ‘very slightly or not at all’ to ‘extremely’. Trait affect is concerned with the measures for a longer period of time. The trait negative affect was measured by 10 negative affective aspects (e.g. ‘irritable’, ‘upset’; Cronbach’s alpha = 0.791, n = 81).
To ensure that the variables targeted for the current study were appropriate for multiple regression analysis and not substantially collinear, correlations were computed between reappraisal and the control variables. Also, for consistency with previous studies (Giuliani et al., 2011a,b), follow-up analyses included additional control variables of suppression and trait negative affect.
Brain imaging and processing procedures
Anatomical images (3D MPRAGE, TR = 1600 ms; TE = 3.82 ms; FOV = 256 × 256 mm; volume size = 112 slices; voxel size = 1 × 1 × 1 mm³) were obtained using a 1.5 Tesla Siemens Sonata scanner. To test predicted diffuse vs localized volumetric associations, brain imaging data were processed using two procedures, a surface-based segmentation procedure (SBM) and a VBM procedure (for more details about data processing see Supplementary Materials). SBM output included whole-region ROI definitions in order to test diffuseness of associations at a more global level. Surface-based cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite (Freesurfer Version 5.3; Fischl, 2012), which is freely available for download online (http://surfer.nmr.mgh.harvard.edu/). To address sensitivity at a more localized level than whole-region SBM, VBM was performed in addition to the SBM processing, providing complementary analysis at a voxel level. VBM was performed in SPM8 (Ashburner et al., 2008) with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/; Kurth et al., 2010) using MATLAB 7.4 (Mathworks, Inc., Released 2007). VBM8 default settings are documented elsewhere (Kurth et al., 2010) and were used in processing unless otherwise noted. A Gaussian smoothing kernel of 10 mm full width at half maximum was used on the grey matter maps to correct for registration inaccuracies inherent to the normalization process.
The ROIs were selected as standard locations for the same regions, specifically designed for the method to which it was applied. For SBM, the Desikan-Killiany atlas (Desikan et al., 2006) implemented in Freesurfer was used. For VBM, automatic anatomical labeling in the Wake Forest University PickAtlas toolbox (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003) implemented in SPM8 was used, and ROI masks were resliced from atlas space to normalized grey matter space. Importantly, although different brain atlases are implemented in the two methods, the same anatomical landmarks are used to identify our targeted regions. For both methods, the MFC ROIs identify the region bordered by the superior frontal sulcus, the inferior frontal sulcus, and the precentral sulcus, and the SFC ROIs identify the region bordered by the superior frontal sulcus, the precentral sulcus, the paracentral sulcus, and the medial extent of the frontal lobe (Tzourio-Mazoyer et al., 2002; Desikan et al., 2006). Although, ideally, the results from the two atlases should be directly compared (e.g. by coregistering the ROIs directly between SBM and VBM), this is not a common procedure because it can cause inappropriate results. For example, it has been suggested that warping SBM segmentations to MNI space for volume-based group analyses (e.g. VBM) does not result in useful ROI masks (Greve, 2014). For this reason, the current study implemented the recommended procedures for each method, to ensure interpretable results and maximize generalizability.
Five participants were determined to have outlier anatomical volumes or poor registration, with one participant having outlier values using both methods, and were removed analysis-wise, as follows: as assessed by SBM, two participants had outlier total intracranial volumes (TIVs), one had an outlier right MFC volume, and one had an outlier right SFC volume; as assessed by VBM, two were identified as having possibly anomalous registration quality (determined by plotting covariance of normalized smoothed grey matter images with a criterion of three SDs; one of these was also an outlier for SBM TIV). Additionally, two subjects had missing values for trait negative affect, therefore follow-up analyses controlling for trait negative affect and suppression excluded these subjects.
Statistical analyses
Statistical analyses for demographic variables and the whole-region ROIs were performed in IBM SPSS Statistics 22 (IBM, Released 2013). For both the SBM and VBM approaches, multiple regression analyses were used to test the hypotheses regarding the relation between reappraisal and PFC volume (for more details about statistical methods see Supplementary Materials). In SBM, the selected ROI volume was used as the dependent variable, with the model including independent variables of sex, age, and TIV as nuisance variables, and reappraisal score as the variable of interest.
The multiple regression model was also tested at the voxel level using VBM data. The modulated smoothed grey matter segmentation for each subject was used as the dependent variable, with the regression model including the covariates of sex and age as nuisance variables, and reappraisal score as the covariate of interest. An absolute threshold mask of 0.1 was used, along with an implicit mask. For the targeted analyses, contrast maps were created by controlling for effects of sex and age and then examining the relation of emotion regulation through reappraisal within each of the selected ROIs (MFC and SFC). Initial analyses used a height threshold corrected for multiple comparisons for within the extent of the ROI at P family-wise error corrected (pFWE) ≤ 0.05. Follow-up analysis used an uncorrected threshold of P ≤ 0.001, unless otherwise described. For initial analyses, extent thresholds were determined empirically using the expected voxels per cluster as calculated by SPM8 (Ashburner et al., 2008; Kurth et al., 2010).
To quantify the relative diffuseness of the reappraisal associations in MFC and SFC, volumes were extracted from the modulated smoothed grey matter segmentations. Significant clusters were initially identified at a range of uncorrected height thresholds: P ≤ 0.0005, P ≤ 0.001, P ≤ 0.0025, P ≤ 0.005, P ≤ 0.01, P ≤ 0.025 and P ≤ 0.05 within each ROI mask for a positive association of reappraisal, while controlling for sex and age. No extent thresholds were used for this analysis. The resulting cluster maps were saved as binary masks and were then used for volume extraction using a MATLAB script. Additionally, total volume for each ROI was extracted and used to convert the cluster volumes at each significance level into proportions for comparison. Then, to test for differences between region volumes, proportional volumes were entered into repeated measures ANOVA with a factor of brain region and repeated measure of proportional volume at each significance level. ANOVA results are reported using Greenhouse-Geisser correction.
Results
Evidence for diffuse vs localized associations of cognitive reappraisal with right MFC vs right SFC volumes
Reappraisal was not correlated with control variables of age (r = −0.186, P = 0.092, n = 83), or TIV (r = −0.069, P = 0.542, n = 81), but was negatively correlated with suppression (r = −0.229, P = 0.037, n = 83). TIV was not correlated with age (r = −0.025, P = 0.823, n = 81). Also, independent sample t-tests did not reveal any significant differences between sex in reappraisal score (t = 0.301, P = 0.764, n = 83), age (t = −0.132, P = 0.896, n = 83) or TIV (t = −1.537, P = 0.129, n = 81). The lack of significant correlation between reappraisal and the control variables indicated that these would be appropriate for inclusion in the multiple regression analyses. Suppression was not correlated with age (r = −0.011, P = 0.919, n = 83), or TIV (r = 0.118, P = 0.292, n = 81), but was positively correlated with trait negative affect (r = 0.281, P = 0.011, n = 81). Trait negative affect was not correlated with age (r = 0.057, P = 0.611, n = 81), or TIV (r = −0.066, P = 0.565, n = 79), but was negatively correlated with reappraisal (r = −0.284, P = 0.010, n = 81). However, for consistency with previous studies (Giuliani et al., 2011a,b), follow-up analyses included control variables of suppression and trait negative affect.
As expected, reappraisal score showed positive associations with whole-region but not voxel-level volumes in the right MFC and left SFC, and voxel-level but not whole-region volumes in the right SFC. Consistent with a diffuse volumetric association, reappraisal was significantly associated with right MFC whole-region volume (β = 0.208, P = 0.035, n = 80), and was marginally associated with left SFC volume (β = 0.199, P = 0.056, n = 81); reappraisal was not associated with left MFC volume (β = 0.153, P = 0.134, n = 81). At a voxel level, no association was shown in the right MFC, left MFC or left SFC that survived both the corrected height and empirically-determined extent threshold, suggesting that there were no localized effects detectable within these regions. However, consistent with the prediction of a more diffuse association, there were extended clusters associated with reappraisal in the right and left MFC when the height threshold was lowered. Additionally, when the threshold was lowered to uncorrected P ≤ 0.001, a cluster was shown in the left SFC (Tmax = 3.51, empirically-determined extent threshold of 152 voxels; MNI coordinates: x = −14, y = 11, z = 70; k = 403; n = 81), suggesting a more diffuse, lower threshold volumetric association. Cluster volumes were extracted and quantified for testing in subsequent analyses with right SFC, as described below.
Follow-up analyses controlling for trait negative affect and suppression confirmed that the identified associations were specific to reappraisal. These analyses still showed a significant association between the right MFC and reappraisal in SBM (β = 0.213, P = 0.045, n = 78), and the marginal association between the left SFC and reappraisal became significant (β = 0.237, P = 0.035, n = 79); the left MFC remained non-significant (P > 0.05). In VBM, the left SFC still showed a cluster associated with reappraisal (Tmax = 3.47, height threshold of P ≤ 0.001 uncorrected and empirically-determined extent threshold of 147 voxels; MNI coordinates: x = −15, y = 9, z = 70; k = 148; n = 79), and the right and left MFC did not show a significant association at these thresholds.
For the right SFC, the voxel-level results were consistent with a localized volumetric association. Specifically, the regression model showed a significant positive association between reappraisal score and right SFC (Tmax = 4.62, height threshold of pFWE ≤ 0.05 corrected for within the extent of the right SFC ROI and empirically-determined extent threshold of 82 voxels; MNI coordinates: x = 29, y = 14, z = 61; k = 95; n = 81). For a display of this result see Figure 1. At a whole-region level, reappraisal score did not show a significant association with right SFC volume (β = 0.159, P = 0.134, n = 80), suggesting that there was not a diffuse volumetric association with reappraisal in this region. Again, follow-up analyses controlling for trait negative affect and suppression confirmed that the identified association was specific to reappraisal. These analyses still showed a significant association between the right SFC volume and reappraisal in VBM (Tmax = 4.25, height threshold of P ≤ 0.001 uncorrected and empirically-determined extent threshold of 147 voxels; MNI coordinates: x = 29, y = 15, z = 61; k = 293; n = 79); the right SFC remained non-significant in SBM (P > 0.05). For additional follow-up analyses, see Supplementary Materials.
Fig. 1.
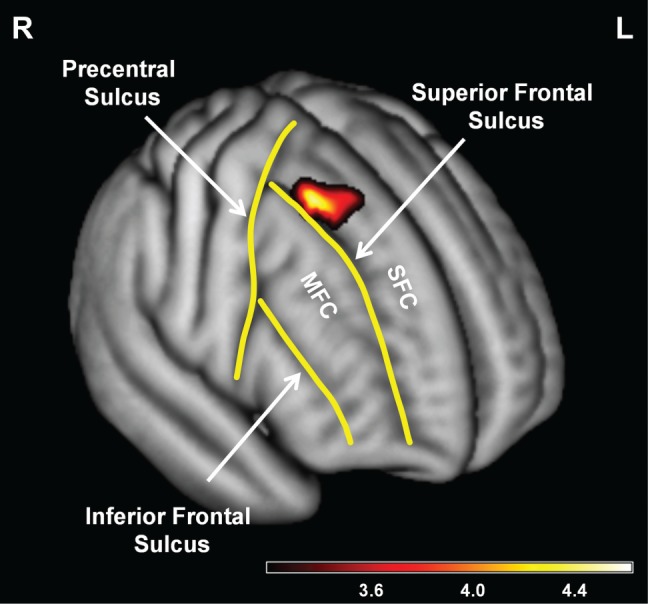
Evidence for localized volumetric association with reappraisal in the right SFC. Consistent with a localized volumetric association, voxel-level results from the multiple regression model in VBM analyses, controlling for sex and age, showed that reappraisal was significantly associated with right SFC (for display purposes, a height threshold of P ≤ 0.001 uncorrected and an empirically-determined extent threshold of 152 voxels were used). Key borders for the SFC and MFC are outlined on a rendering of the average brain from subjects used in the final voxel-level analyses (n = 81). The color bar indicates T values. R, right; L, left; MFC, middle frontal cortex; SFC, superior frontal cortex; VBM, voxel-based morphometry.
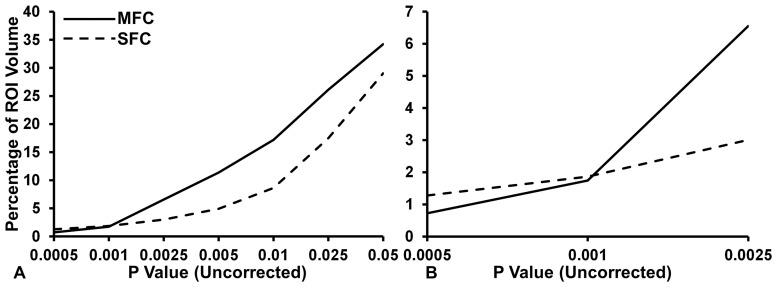
Based on the previous results identifying a diffuse association in right MFC and a localized association in right SFC, an additional analysis was performed to assess the gradient of diffuseness of association between reappraisal and proportional region volume using volumes extracted from VBM (n = 81). A repeated measures ANOVA showed a significant main effect of brain region, F(1, 80) = 4180.055, P < 0.001, of significance threshold, F(1.100, 87.961) = 51237.126, P < 0.001, and a significant interaction between brain region and significance threshold F(1.282, 102.561) = 4173.756, P < 0.001, indicating that there was a difference in the average percentage of ROI volume associated with reappraisal across significance levels. Results for the ANOVA are shown in Figure 2.
Fig. 2.
More diffuse volumetric association with reappraisal in the right MFC than right SFC. (A) The estimated marginal means plot for significance threshold and percentage of ROI volumes. The right MFC shows a smaller percentage of ROI volume associated with reappraisal at higher significance levels compared with right SFC, but larger increase in volume associated with reappraisal compared with right SFC. (B) The cross-over interaction between the right MFC and right SFC occurs around the significance threshold of P ≤ 0.001. ROI, region of interest; MFC, middle frontal cortex; SFC, superior frontal cortex.
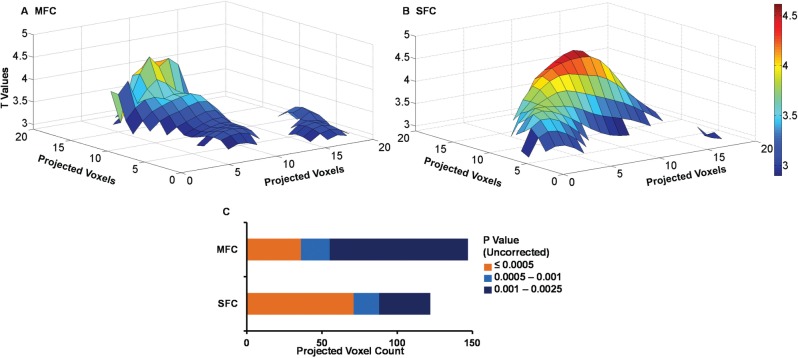
Post-hoc comparisons showed that, consistent with the expectation of a more diffuse association in right MFC, mean proportional volume of right MFC (13.990% ± 0.080%) was significantly larger than for right SFC (9.459% ± 0.056%, P < 0.001, Bonferroni corrected). The interaction between region and significance threshold provided additional clarification about this effect. Consistent with the expectation of diffuse (relatively large extent, lower significance) association of reappraisal in right MFC compared with localized (relatively smaller extent, high significance) association of reappraisal in right SFC, right MFC showed a numerically lower proportional volume associated with reappraisal at a significance level of P ≤ 0.0005 compared with right SFC, and showed a numerically higher proportional volume compared with right SFC at a significance level of P ≤ 0.0025. This suggests that, for these data, the cross-over point between diffuse compared with localized associations was around the typical exploratory significance threshold of P ≤ 0.001. To visualize the relative diffuseness of the effects in right MFC and SFC, T-maps from the initial VBM analysis were converted to maximum intensity plots and then projected as surfaces in MATLAB. As can be seen in Figure 3, the MFC association showed more diffuse, lower significance cluster(s), while the SFC showed a more localized, higher significance cluster. Consistent with the ANOVA analysis, this visualization indicated that the significance level around P ≤ 0.001 is the point where significance and volume effects interact.
Fig. 3.
Differential intensity distribution of volumetric association with reappraisal in the right MFC (A) compared with the right SFC (B). T-maps for the right MFC and SFC were converted to maximum intensity plots and mapped as surfaces in MATLAB. The matrix values have been thresholded at the critical T value associated with a contrast at P ≤ 0.0025. The x and y axes show projected voxel dimensions, the z axis and the color bar show T values. (C) Projected voxel counts for MFC and SFC. MFC, middle frontal cortex; SFC, superior frontal cortex.
Discussion
In this report, diffuse and localized volumetric associations of reappraisal were examined in the MFC and SFC using two complementary methods: one allowing whole-region (SBM) and the other allowing voxel-level (VBM) assessments. Results showed novel positive volumetric associations between habitual engagement of reappraisal and these regions, identifying a diffuse volumetric association in the right MFC and left SFC, and a localized association in the right SFC. These findings provide structural evidence consistent with the idea of use-dependent plasticity as a possible mechanism explaining the link between brain function and structure described by Hebbian learning (Hebb, 1949): neurons that ‘fire together, wire together’. Consistent with this idea, suggesting that systematic differences in function may also be associated with systematic differences in structure, if the bilateral MFC and left SFC are broadly engaged by general reappraisal processing, then the structural association with reappraisal should also be evident across diffuse neuronal populations. Furthermore, if the right SFC is focally engaged by specific reappraisal processing, then the structural association with reappraisal should be evident in a localized neuronal population. The present results support this idea in the right MFC, showing volumetric associations of reappraisal at a whole-region level, and in the right SFC, showing volumetric association of reappraisal at a voxel level. As discussed below, these results reconcile the seeming discrepancy between functional and structural brain imaging studies of reappraisal effects in the PFC.
The right MFC findings support the broad functional role of this region in reappraisal (Ochsner et al., 2012), and extend this association to the structural level. These findings provide novel empirical support for interpretations of previous comparisons between different anatomical methods (Giuliani et al., 2011a), suggesting that some associations are more diffuse, and therefore may be captured by more holistic ROI methods such as SBM or manual tracing. The SFC results support the idea that the right SFC may be specifically and more focally engaged when particularly intense emotions are being regulated, whereas the left SFC may broadly handle regulation for lower and higher intensity emotions (Silvers et al., 2015). This has implications for future volumetric studies, as it indicates that the context in which a function engages a brain region, and the extent of the region that is engaged by a function, should be taken into account when generating predictions, selecting methodological approaches, or defining ROIs. Together, the SBM and VBM results suggest a possible explanation as to why some volumetric literature has shown seeming discrepancies with functional literature and has failed to detect effects in some regions while successfully detecting them in others. A combined SBM–VBM approach might therefore be useful for the study of other individual differences, where associations might be more distributed, and might be a complementary analysis to methods targeting individual differences across many regions, or at the level of networks. For example, the extant literature which has focused on individual differences within the framework of personality neuroscience has depended largely on voxel-level analyses (DeYoung et al., 2010). Additional clarifications may emerge if a multi-method approach is taken to investigations in these areas.
The results of the confirmatory analysis on the relative diffuseness of associations between reappraisal and volume provide further support for the diffuse effect in right MFC and localized effect in right SFC. Consistent with the Hebbian learning model (Hebb, 1949), one possible mechanism that could underlie volumetric effects is changes in synaptic connectivity and dendritic arborization. However, it has also been noted that volumetric effects could represent differences in cell size (Draganski et al., 2006). In either case, the relative diffuseness of association is informative about what regions are more broadly involved in emotion regulation, and may suggest possible regions where structural changes may occur related to affective disorders. Indeed, the current results are consistent with previous research that has shown decreased MFC and SFC volume in patients with depression (Chang et al., 2011). Although volumetric studies provide partial insight into the underlying anatomy, these findings are also consistent with postmortem investigations which have shown decreased density and size of neurons and glial cells within the PFC of subjects with depression (Rajkowska et al., 1999), which points to possible changes that might underlie observed volumetric effects. Additionally, several recent lesion studies have examined the PFC and reappraisal performance (as compared to habitual emotion regulation), and have shown that reappraisal performance was impaired by PFC lesions (Falquez et al., 2014; Salas et al., 2014). Together, the present results and the extant literature suggest that interventions that involve training for emotion regulation through reappraisal might result in beneficial structural changes.
Reappraisal has also been shown to be a protective emotion regulation factor against other biological risk factors. For example, the influence of genetic polymorphisms associated with risk of depression have been shown to be moderated by reappraisal (Ford et al., 2014), suggesting that the benefits of emotion regulation fit within a larger biological framework of individual differences. This is consistent with extant models positing that individual differences at the level of genotype, experience, and personality influence the neural correlates of emotion processing (Hamann and Canli, 2004). Thus, targeting emotion regulation through reappraisal to improve the health of brain regions at a structural and functional level could improve the contribution of emotion regulation to relevant outcomes, such as resilience against developing clinical conditions (Aldao et al., 2010; Llewellyn et al., 2013) and academic resilience (Graziano et al., 2007; Schelble et al., 2010).
Interestingly, this study showed the strongest results in the right hemisphere, which is consistent with previous evidence regarding hemispheric lateralization of emotion regulation effects. For example, the left PFC has been associated with a more generic role, consistent with its involvement in reappraising both positive and negative stimuli, with the goal of both decreasing and increasing the emotional response, whereas the right PFC has primarily been associated with a more specific role in reappraisal with the goal of decreasing the emotional response, and in particular to negative emotions (Ochsner et al., 2012). This suggests that the associations observed in our data might be more related to the tendency to decrease response to negative emotions. Since the role of the left hemisphere is more heterogeneous, it is possible that some of the effects are being washed down, or that the relation between reappraisal and this hemisphere is more complex, and therefore less apparent in these data.
The current volumetric findings might also be considered in regard to the lateralization of appetitive and aversive processing. Specifically, our results showing the strongest associations in the right hemisphere appear consistent with the idea that the right PFC is involved in a system facilitating avoidance of aversive stimuli (Davidson, 1983; reviewed in Spielberg et al., 2008). However, a recent review (Miller et al., 2013) suggests that the associations between hemispheric laterality and emotional valence and motivation might be more complex than the traditional lateralization theories first proposed. Furthermore, other research has shown that reappraisal is linked to approach coping (Ferguson and Cox, 1997) and promotion goal orientation (Llewellyn et al., 2013), suggesting that the relation between reappraisal and appetitive and aversive processing might be more complex. Thus, future work is necessary to clarify the laterality of associations with habitual engagement of reappraisal and the relation to appetitive vs aversive processing and approach vs avoidance motivational/temperamental tendencies.
It should also be noted that the relation between instructed and habitual cognitive reappraisal is potentially complex. It is possible that the way in which people regulate their emotions in an instructed lab task is slightly different from the way they habitually regulate emotion. Thus, the potential link between functional and structural MRI studies could be weakened by these possible differences. It is, therefore, notable that the current and previous studies have successfully identified significant associations using hypotheses informed by fMRI. Overall, convergence between functional and structural findings is consistent with the framework of use-dependent plasticity (Nudo et al., 1996; Bütefisch et al., 2000), and is also consistent with previous studies showing that instructed and uninstructed emotion regulation lead to similar consequences (Egloff et al., 2006).
Another consideration for interpreting the seeming discrepancies between the functional and structural literatures is the possibly different imaging parameters and measurement techniques that might exist across studies. For example, it is informative to note whether the MR scanner is 1.5, 3, or greater Tesla strength. Previous research comparing volumetric data based on MRI from 1.5 and 3 T machines has suggested that VBM measures are consistent between these field strengths, although to a lesser extent compared with when volumes are measured manually (Briellmann et al., 2001; Scorzin et al., 2008). Thus, such aspects should be carefully considered when interpreting results across the extant literature.
Caveats
One limitation of this study is the focus on only two key brain regions. Although their selection was informed by the extant functional literature, another PFC area that has been indicated in emotion regulation is the inferior frontal/ventrolateral PFC (Wager et al., 2008; Ochsner et al., 2012; Buhle et al., 2013; Silvers et al., 2015). However, this region has been indicated as an area that overlaps across a number of functional networks (Corbetta and Shulman, 2002; Dolcos et al., 2011; Power and Petersen, 2013), and hence it may be functionally more heterogeneous; therefore, it was not targeted in this study. Another caveat of this study is the cross-sectional design, which does not allow us to assess the directionality of the associations between habitual reappraisal and the identified brain structures. This also limits the extent to which the use-dependent plasticity framework could be tested directly. Future research using longitudinal or intervention designs are needed to clarify this issue. However, this study provides basic findings that could be used to inform future studies.
Conclusion
In summary, by using complementary volumetric methods to assess the relation between habitual engagement of reappraisal and grey matter volume in the MFC and SFC, this study provides novel empirical evidence reconciling the seeming discrepancy between functional and structural brain imaging studies of reappraisal. As predicted, results are consistent with diffuse volumetric associations with reappraisal in the right MFC and left SFC, and a localized volumetric association in the right SFC. These results provide novel evidence supporting the idea that functional engagement related to transient manipulations of emotion regulation is paralleled by structural associations of habitual engagement of similar operations, within the same brain regions.
Supplementary data
Supplementary data are available at SCAN online.
Acknowledgements
The authors wish to thank members of the Dolcos Lab for assisting with data collection, and Monica Fabiani and Karen Rudolph for feedback on an earlier version of the report.
Funding
This work was supported by funds from a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, a Research Award from the Canadian Psychiatric Research Foundation, the University Hospital Foundation, and the University of Illinois (to F.D.). M.M. was supported by a National Science Foundation Fellowship, through the University of Illinois Integrative Graduate Education and Research Traineeship Program (National Science Foundation Grant No. 0903622). A.D.I. was supported by a Dissertation Completion Fellowship from the University of Illinois.
Conflict of interest. None declared
References
- Aldao A., Nolen-Hoeksema S., Schweizer S. (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–37. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Barnes G., Chen C., et al. (2008). SPM8 Manual. London: Functional Imaging Laboratory, Institute of Neurology, University College London. [Google Scholar]
- Augustine A.A., Hemenover S.H. (2009). On the relative effectiveness of affect regulation strategies: A meta-analysis. Cognition and Emotion, 23(6), 1181–220. [Google Scholar]
- Boyke J., Driemeyer J., Gaser C., Büchel C., May A. (2008). Training-induced brain structure changes in the elderly. The Journal of Neuroscience, 28(28), 7031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briellmann R.S., Syngeniotis A., Jackson G.D. (2001). Comparison of hippocampal volumetry at 1.5 tesla and at 3 tesla. Epilepsia, 42(8), 1021–4. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2013). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch C.M., Davis B.C., Wise S.P., et al. (2000). Mechanisms of use-dependent plasticity in the human motor cortex. Proceedings of the National Academy of Sciences of the United States of America, 97(7), 3661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Yu S.C., McQuoid D.R., et al. (2011). Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Research, 193(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–15. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. (1983). Hemispheric specialization for cognition and affect In: Gale A., Edwards J., editors. Physiological Correlates of Human Behavior. London: Academic Press. [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–80. [DOI] [PubMed] [Google Scholar]
- DeYoung C.G., Hirsh J.B., Shane M.S., Papademetris X., Rajeevan N., Gray J.R. (2010). Testing predictions from personality neuroscience: Brain structure and the big five. Psychological Science, 21(6), 820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Iordan A.D., Dolcos S. (2011). Neural correlates of emotion-cognition interactions: A review of evidence from brain imaging investigations. Journal of Cognitive Psychology, 23(6), 669–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant E.M., McRae K., Manuck S.B., Hariri A.R., Gross J.J. (2009). Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry, 65(5), 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. (2004). Neuroplasticity: Changes in grey matter induced by training. Nature, 427(6972), 311–2. [DOI] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Kempermann G., et al. (2006). Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of Neuroscience, 26(23), 6314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., May A. (2008). Training-induced structural changes in the adult human brain. Behavioural Brain Research, 192(1), 137–42. [DOI] [PubMed] [Google Scholar]
- Egloff B., Schmukle S.C., Burns L.R., Schwerdtfeger A. (2006). Spontaneous emotion regulation during evaluated speaking tasks: Associations with negative affect, anxiety expression, memory, and physiological responding. Emotion, 6(3), 356–66. [DOI] [PubMed] [Google Scholar]
- Falquez R., Couto B., Ibanez A., et al. (2014). Detaching from the negative by reappraisal: The role of right superior frontal gyrus (BA9/32). Frontiers in Behavioral Neuroscience, 8, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E., Cox T. (1997). The functional dimensions of coping scale: Theory, reliability and validity. British Journal of Health Psychology, 2(2), 109–29. [Google Scholar]
- Fischl B. (2012). FreeSurfer. Neuroimage, 62(2), 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B.Q., Mauss I.B., Troy A.S., Smolen A., Hankin B. (2014). Emotion regulation moderates the risk associated with the 5-HTT gene and stress in children. Emotion, 14(5), 930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Drabant E.M., Bhatnagar R., Gross J.J. (2011a). Emotion regulation and brain plasticity: Expressive suppression use predicts anterior insula volume. Neuroimage, 58(1), 10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Drabant E.M., Gross J.J. (2011b). Anterior cingulate cortex volume and emotion regulation: Is bigger better? Biological Psychology, 86(3), 379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P.A., Reavis R.D., Keane S.P., Calkins S.D. (2007). The role of emotion regulation and children’s early academic success. Journal of School Psychology, 45(1), 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D. (2014). Warping to MNI space. Message posted to https://mail.nmr.mgh.harvard.edu/pipermail//freesurfer/2014-February/035970.html.
- Gross J.J., John O.P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–62. [DOI] [PubMed] [Google Scholar]
- Hamann S., Canli T. (2004). Individual differences in emotion processing. Current Opinion in Neurobiology, 14(2), 233–8. [DOI] [PubMed] [Google Scholar]
- Hebb D.O. (1949). The Organization of Behavior: A Neuropsychological Theory. New York: Wiley. [Google Scholar]
- Hermann A., Bieber A., Keck T., Vaitl D., Stark R. (2014). Brain structural basis of cognitive reappraisal and expressive suppression. Social Cognitive and Affective Neuroscience, 9(9), 1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T.Q., Zhang D.J., Wang J.L., Mistry R., Ran G.M., Wang X.Q. (2014). Relation between emotion regulation and mental health: A meta-analysis review. Psychological Reports, 114(2), 341–62. [DOI] [PubMed] [Google Scholar]
- IBM (Released 2013). IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
- John O.P., Gross J.J. (2004). Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality, 72(6), 1301–33. [DOI] [PubMed] [Google Scholar]
- Kanske P., Heissler J., Schönfelder S., Bongers A., Wessa M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21(6), 1379–88. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gallinat J., Brass M. (2011). “Keep calm and carry on”: Structural correlates of expressive suppression of emotions. Plos One 6(1), e16569.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F., Luders E, Gaser C. (2010). VBM8 Toolbox Manual. Jena: University of Jena. [Google Scholar]
- Llewellyn N., Dolcos S., Iordan A.D., Rudolph K.D., Dolcos F. (2013). Reappraisal and suppression mediate the contribution of regulatory focus to anxiety in healthy adults. Emotion 13(4), 610–5. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Mathworks, Inc. (Released 2007). MATLAB Version 7.4, R2007a. Natick, MA: Mathworks, Inc. [Google Scholar]
- May A. (2011). Experience-dependent structural plasticity in the adult human brain. Trends in Cognitive Sciences, 15(10), 475–82. [DOI] [PubMed] [Google Scholar]
- Mcrae K., Ochsner K.N., Mauss I.B., Gabrieli J.J.D., Gross J.J. (2008). Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes and Intergroup Relations, 11(2), 143–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.A., Crocker L.D., Spielberg J.M., Infantolino Z.P., Heller W. (2013). Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Frontiers in Integrative Neuroscience, 7, 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo R.J., Milliken G.W., Jenkins W.M., Merzenich M.M. (1996). Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. The Journal of Neuroscience, 16(2), 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience 14(8), 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2008). Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science, 17(2), 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23(2), 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne J.W., Overbay A. (2004). The power of outliers (and why researchers should always check for them). Practical Assessment, Research and Evaluation 9(6), 1–12. [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. (2005). Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–9. [DOI] [PubMed] [Google Scholar]
- Power J.D., Petersen S.E. (2013). Control-related systems in the human brain. Current Opinion in Neurobiology, 23(2), 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J.J., Wei J., et al. (1999). Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry, 45(9), 1085–98. [DOI] [PubMed] [Google Scholar]
- Salas C.E., Gross J.J., Turnbull O.H. (2014). Reappraisal generation after acquired brain damage: The role of laterality and cognitive control. Frontiers in Psychology, 5, 242.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelble J.L., Franks B.A., Miller M.D. (2010). Emotion dysregulation and academic resilience in maltreated children. Child and Youth Care Forum, 39(4), 289–303. [Google Scholar]
- Scorzin J.E., Kaaden S., Quesada C.M., et al. (2008). Volume determination of amygdala and hippocampus at 1.5 and 3.0T MRI in temporal lobe epilepsy. Epilepsy Research, 82(1), 29–37. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., Weber J., Wager T.D., Ochsner K.N. (2015). Bad and worse: Neural systems underlying reappraisal of high- and low-intensity negative emotions. Social Cognitive and Affective Neuroscience, 10(2), 172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Stewart J.L., Levin R.L., Miller G.A., Heller W. (2008). Prefrontal cortex, emotion, and approach/withdrawal motivation. Social and Personality Psychology Compass, 2(1), 135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063.. [DOI] [PubMed] [Google Scholar]
- Webb T.L., Miles E., Sheeran P. (2012). Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138(4), 775–808. [DOI] [PubMed] [Google Scholar]
- Welborn B.L., Papademetris X., Reis D.L., Rajeevan N., Bloise S.M., Gray J.R. (2009). Variation in orbitofrontal cortex volume: Relation to sex, emotion regulation and affect. Social Cognitive and Affective Neuroscience, 4(4), 328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.