Abstract
The reinforcement-sensitivity theory proposes that behavioural activation and inhibition systems (BAS and BIS, respectively) guide approach and avoidance behaviour in potentially rewarding and punishing situations. Their baseline activity presumably explains individual differences in behavioural dispositions when a person encounters signals of reward and harm. Yet, neurochemical bases of BAS and BIS have remained poorly understood. Here we used in vivo positron emission tomography with a µ-opioid receptor (MOR) specific ligand [11C]carfentanil to test whether individual differences in MOR availability would be associated with BAS or BIS. We scanned 49 healthy subjects and measured their BAS and BIS sensitivities using the BIS/BAS scales. BAS but not BIS sensitivity was positively associated with MOR availability in frontal cortex, amygdala, ventral striatum, brainstem, cingulate cortex and insula. Strongest associations were observed for the BAS subscale ‘Fun Seeking’. Our results suggest that endogenous opioid system underlies BAS, and that differences in MOR availability could explain inter-individual differences in reward seeking behaviour.
Keywords: BAS, BIS, carfentanil, opioid, PET
Introduction
The reinforcement-sensitivity theory (RST) proposes that two neurophysiologically separable systems guide human behaviour during potentially beneficial and harmful encounters (Gray, 1970; Gray, 1987). The behavioural activation/approach system (BAS) is an appetitive–motivational system that is activated by reward consumption and conditioned signals of reward or non-punishment, triggering approach behaviour. The behavioural inhibition system (BIS), on the other hand, is an aversive motivational system activated by signals of punishment, loss of reward, novelty, or uncertainty, and it inhibits behaviour that might lead to negative outcomes. (Pickering and Gray, 1999) Relative activities of these systems presumably explain whether individuals approach potentially rewarding targets or whether they inhibit or withdraw their behaviour because of the associated risks (Gray, 1970). Previous studies have shown that the sensitivity (i.e. baseline activity) of these systems broadly influences individuals’ behavioural tendencies: People with high BAS sensitivity eagerly seek reward, are socially outgoing and open to new experiences, while low BAS sensitivity is associated with introversion and depression (Carver and White, 1994; Heubeck et al., 1998; Kasch et al., 2002; Caseras et al., 2003). On the other hand, high BIS sensitivity is associated with anxiousness and avoidance behaviour, and individuals with high BIS sensitivity frequently avoid anxiety-provoking situations (Carver and White, 1994).
RST postulates separable yet interacting neuroanatomical systems underlying BAS and BIS (Gray, 1987; Pickering and Gray, 1999). The brain’s dopaminergic reward circuits, including the ventral tegmental area, ventral striatum and their projections to the prefrontal cortex, have been suggested to subserve BAS (Gray, 1987; Pickering and Gray, 1999; Depue and Collins, 1999). A bulk of fMRI studies support this proposition by showing that BAS sensitivity—as measured with self-reports—is positively associated with reward-cue-triggered haemodynamic responses in reward circuits (Beaver et al., 2006; Hahn et al., 2009; Simon et al., 2010; Costumero et al., 2013). The most important anatomical substrate of BIS is the septo-hippocampal system, consisting of the hippocampus proper, the dentate gyrus, the entorhinal cortex, the subicular area and the posterior cingulate cortex (Gray and McNaughton, 2000). Also amygdala and prefrontal and cingulate cortices have been implicated in BIS function (Gray, 1987; Gray and McNaughton, 2000).
Despite these advances in understanding the functional brain mechanisms associated with BAS and BIS, the neurochemical mechanisms serving them are still unclear. While dopaminergic neurotransmission is often linked to BAS (Gray, 1987; Pickering and Gray, 1999), findings from both animal research and human molecular neuroimaging studies suggest that the endogenous opioid system, and particularly µ-opioid receptors (MORs), might be another important neurochemical pathway supporting BAS functions. In animals, the opioidergic system modulates approach behaviour and reward functions (Van Ree et al., 2000; Papaleo et al., 2007), both of which are essential features of BAS. Human positron emission tomography (PET) studies also suggest that individual differences in BAS-driven behaviour are linked with the MOR system, as MOR availability is associated with the trait impulsivity (Love et al., 2009). Further, high BAS sensitivity may predispose individuals for developing addictions (Johnson et al., 2003) and, in turn, both alcohol (Heinz et al., 2005; Weerts et al., 2011) and cocaine (Zubieta et al., 1996; Gorelick et al., 2005) dependencies are associated with increased MOR availability in reward circuits. Finally, MOR availability is also associated with the closeness of interpersonal relationships (Nummenmaa et al., 2015), which fits with the previously established association between BAS and prosociality (Carver and White, 1994; Heubeck et al., 1998; Caseras et al., 2003).
Limited evidence links MORs also with BIS. First, anxiolytic effects of opiates (Colasanti et al., 2011) suggest that activation of opioidergic circuits may attenuate baseline BIS activity. Second, people who typically avoid harmful situations have elevated MOR availability in frontal cortex (Tuominen et al., 2012). Such behaviour is also common in high-BIS individuals (Carver and White, 1994), suggesting that BIS could be associated with tonic MOR upregulation.
Altogether, previous studies show that BAS affects drive for reward and sociality—both of which are associated with MORs—while limited evidence also suggest a link between BIS and MORs. However, the exact link between MORs and BAS (and potentially BIS) remains elusive. To investigate whether baseline MOR availability is associated with BAS and BIS sensitivities, we used in vivo PET with highly selective MOR agonist ligand [11C]carfentanil coupled with behavioural BAS and BIS scales (Carver and White, 1994). Based on their mutual involvement in reward, we specifically hypothesized that BAS would be positively associated with cerebral MOR availability in the brain’s reward circuits.
Materials and methods
Participants
The study protocol was approved by the ethics board of the hospital district of Southwest Finland, and the study was conducted in accordance with the Declaration of Helsinki. A priori power analysis based on effect sizes in a previous PET study on associations between personality trait variables and MOR availability (Tuominen et al., 2012) suggested that sample sizes exceeding N = 45 would have power higher than 0.90 for detecting statistically significant effects at r = 0.44. Consequently, we studied altogether 50 healthy adults (20 females, mean ± SD age 32 ± 6 years, range 19–58). One subject was removed from the sample because a previously non-diagnosed neurological disease was revealed from their MRI scan. Exclusion criteria were lack of compliance, smoking, alcohol consumption exceeding eight weekly doses, substance abuse determined by interview and blood tests, a history of or current psychiatric or neurological disease, current medication affecting the central nervous system, as well as standard PET and MRI exclusion criteria. Subjects signed ethics-committee-approved, informed consent forms and they were compensated for their time and travel costs. These data have originally been collected in clinical trials SleevePET2 (NCT01373892), EXEBRAIN (NCT02615756) as well as the PLEASUREPET project. Portions of the PET data unrelated to the present study have been published previously (Karlsson et al., 2015a,b; Nummenmaa et al., 2015; Tuominen et al., 2015).
Questionnaires
The participants completed the BIS/BAS scales (Carver and White, 1994) that measure individual differences in the sensitivities of BIS and BAS, reflecting affective responses to impending reward and punishment. The questionnaire consists of 20 Likert-scale questions and has been psychometrically validated to yield stable and reliable results (Carver and White, 1994) across different cultures (Leone et al., 2001). The questionnaire consists of one BIS scale and three BAS subscales: Drive, Reward Responsiveness, and Fun Seeking. All the subscales of the questionnaire have good internal consistency reliabilities (0.66…0.76) (Carver and White, 1994). As the BIS and BAS total scores were not correlated (r = –0.05), they were both used as covariates in the full-volume regression analyses (see below). Because the MOR system is associated with both mood- and anxiety-related processes (Colasanti et al., 2011; Lutz and Kieffer, 2013) participants also completed Beck Depression Inventory (BDI) II (Beck et al., 1988) and State-Trait-Anxiety Inventory (STAI) (Spielberger et al., 1983) questionnaires to rule out that anxious or depressive symptoms could explain the results.
PET imaging and analysis
Data were acquired with the Philips Ingenuity PET-MR and GE Healthcare Discovery TM 690 PET/CT scanners in Turku PET Centre. Radiotracer production has been described previously (Karlsson et al., 2015a). After a bolus of intravenous radioligand injection (targeted dose 250 MBq; mean 251 MBq, SD = 11 MBq), radioactivity in the brain was measured with the PET camera for 51 min (with increasing frame length: 1 × 3, 3 × 4, 6 × 6 min) with in-plane resolution of 3.75 mm. The subjects were lying in a supine position throughout the studies. Data were corrected for dead-time, decay and measured photon-attenuation and dynamic PET scans were reconstructed with vendor-provided standard MRAC and MRP methods (Alenius and Ruotsalainen, 1997).
To correct for head motion, dynamic PET images were first realigned frame-to-frame. High-resolution anatomical MR images (1 mm3 resolution) were acquired with the Philips Ingenuity PET-MR or Philips Gyroscan Intera scanners using T1-weighted sequences. The individual MR images were coregistered to the summation images calculated from the realigned frames. Occipital cortex was delineated manually on MR images with PMOD 3.4 software (PMOD Technologies Ltd., Zürich, Switzerland) and used as a reference region. Receptor binding was expressed in terms of BPND, which is the ratio of specific to non-displaceable binding in the brain using the occipital cortex as the reference region. BPND was calculated for each voxel using the simplified reference tissue model with reference tissue time-activity curves as input data (Gunn et al., 1997). Subject-wise parametric BPND images were normalized to the MNI space using the T1-weighted MR images, and smoothed with a Gaussian kernel of 8 mm full width at half maximum.
The effects of BAS and BIS scores on MOR availability were assessed in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) using linear regression model with PET camera as a covariate. Statistical threshold was set at P < 0.05, FDR-corrected at cluster level. We also conducted a complementary region of interest (ROI) analysis for ten a priori anatomical ROIs involved in reward and emotion processing (anterior cingulate cortex, amygdala, dorsolateral prefrontal cortex, hippocampus, insula, nucleus accumbens, orbitofrontal cortex, pallidum, putamen and thalamus). FreeSurfer 5.3 (http://surfer.nmr.mgh.harvard.edu/) was used to segment an MNI template brain, and the FreeSurfer-generated segments were then combined to generate ROI masks. Subsequently, the masks were used to extract the voxels within each ROI in the normalized BPND images. Mean ROI-wise BPND were calculated and then predicted with BAS and BIS scores using linear regression and stepwise linear regression analyses.
Results
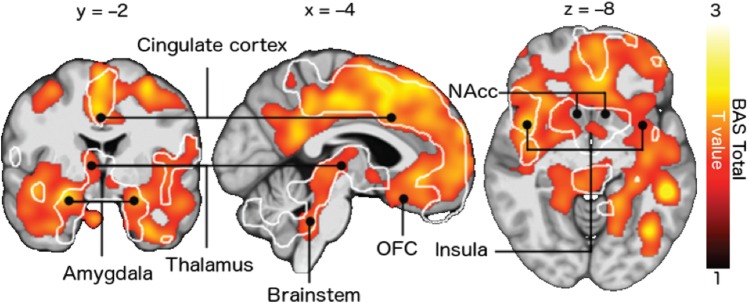
Full-volume analysis revealed a positive association between BAS sensitivity and [11C]carfentanil BPND in a large cluster extending in both hemispheres from the frontal lobe to the parieto-occipital sulcus (Figure 1). Significant associations were also observed in insula, thalamus, amygdala, brainstem and temporal cortices. Additional analysis revealed that the BAS subscale Fun Seeking was mainly driving the effect, with statistically significant associations in largely overlapping regions with those observed in the analysis of total BAS scores. The other subscales and BIS did not correlate statistically significantly with MOR availability in full-volume analysis. The results remained essentially unchanged when subject age, total intracranial volume (derived from DARTEL-segmented T1 images), STAI or BDI scores were included as nuisance covariates.
Fig. 1.
Association between BAS total scores and [11C]carfentanil BPND. The data are thresholded at P < 0.05, FDR-corrected at the cluster level. The white outlines show regions where the BAS subscale Fun Seeking was associated with BPND. OFC = orbitofrontal cortex, NAcc = nucleus accumbens.
Because morbid obesity is associated with lowered BMI (Karlsson et al., 2015a,b) we also tested whether BMI would predict MOR availabilities in the present sample of non-obese subjects (mean BMI = 22.9). However, this analysis revealed no significant clusters. Similarly, including BMI as a covariate to the main analysis did not alter the results.
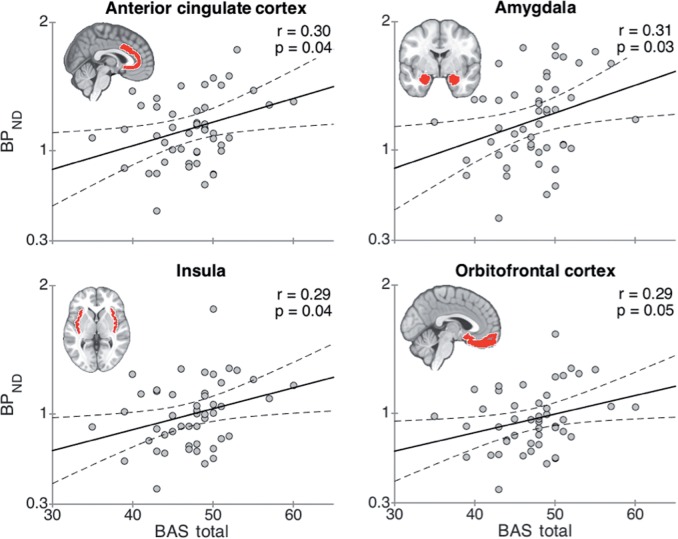
Table 1 shows the ROI-level-associations between the BIS/BAS scales and MOR availabilities. [11C]carfentanil BPND was significantly correlated with the BAS total scale in anterior cingulate cortex, amygdala, insula and orbitofrontal cortex (Figure 2), and with the BAS subscale Fun Seeking in the amygdala (Pearson correlation r = 0.29, P = 0.04) and nucleus accumbens (r = 0.29, P = 0.04). No significant negative correlations were found between BPND and BIS scores. Stepwise regression analyses revealed that BPND in the amygdala emerged as the sole predictor for BAS scores (β = 4.36; R2 = 0.09; P < 0.05).
Table 1.
Pearson correlations between BIS/BAS scores and regional BPND
| Region of interest | BAS Total | BAS fun seeking | BAS drive | BAS reward responsiveness | BIS |
|---|---|---|---|---|---|
| ACC | 0.30* | 0.18 | 0.24 | 0.21 | 0.12 |
| Amygdala | 0.31* | 0.29* | 0.18 | 0.15 | 0.10 |
| DLPFC | 0.27 | 0.16 | 0.21 | 0.22 | 0.17 |
| Hippocampus | 0.26 | 0.19 | 0.18 | 0.19 | 0.13 |
| Insula | 0.29* | 0.21 | 0.22 | 0.18 | 0.09 |
| NAcc | 0.23 | 0.29* | 0.10 | 0.07 | 0.06 |
| OFC | 0.29* | 0.22 | 0.20 | 0.16 | 0.14 |
| Pallidum | 0.19 | 0.09 | 0.14 | 0.17 | 0.10 |
| Putamen | 0.23 | 0.14 | 0.18 | 0.16 | 0.15 |
| Thalamus | 0.25 | 0.25 | 0.11 | 0.15 | 0.14 |
Statistically significant (P < 0.05) correlations are marked with boldface and an asterisk.
*ACC = anterior cingulate cortex, DLPFC = dorsolateral prefrontal cortex, NAcc = nucleus accumbens, OFC = orbitofrontal cortex.
Fig. 2.
Least-squares (LS) regression lines for [11C]carfentanil BPND as a function of BAS total score in anatomically determined regions of interest in anterior cingulate cortex, amygdala, insula and orbitofrontal cortex. The dashed lines represent 95-% confidence intervals for the LS lines.
Discussion
We demonstrated that individual differences in BAS sensitivity are positively associated with MOR availability in multiple brain regions involved in reward and pain, including ventral striatum, orbitofrontal cortex, brainstem, cingulate cortex, insular cortex and thalamus. However, no associations were observed between MOR availability and BIS, trait anxiety or BMI, suggesting that MOR availability is independent of more general mood processes and body weight within the non-obese range. Together with results of previous human and animal studies on the role of the endogenous opioid system in both incentive motivation and hedonic functions (Berridge et al., 2010), these data suggest that individual variation in MOR availability is associated with sensitivity to reward cues. Furthermore, the present results suggest that BIS does not depend on the MOR system.
Opioidergic basis of BAS
BAS sensitivity was positively associated with MOR availability widely within the brain. Such extensive association is not surprising, because MOR expression, as quantified with [11C]carfentanil BPND, is consistent across different cortical and subcortical sites (Tuominen et al., 2014). The present results thus imply that BAS sensitivity is associated globally with MOR tone in the brain, instead of regionally specific alterations. Even though the Fun Seeking subscale had most consistent associations with MOR availability, it must be noted that all the subscales showed a similar, yet slightly less consistent associations with MOR availability (See Table 1). These effects thus likely reflect the general association between overall BAS sensitivity and the MOR system.
Regional associations between BAS and MOR availability were observed in prefrontal, cingulate and insular cortices, as well as subcortical regions, such as ventral striatum, amygdala and brainstem. These regions process both hedonic and nociceptive signals (Leknes and Tracey, 2008). Our data accord with a previous human PET study showing that baseline MOR availability in these regions is associated with impulsiveness (Love et al., 2009), a personality trait closely related to BAS (Caseras et al., 2003). Animal studies have also established that the MOR system drives motivated behaviour and reward functions (Van Ree et al., 2000; Berridge and Robinson, 2003). Specifically, the opioid system mediates liking responses to rewarding stimuli (Berridge and Kringelbach, 2008). Microinjections of morphine into MOR-dense nucleus accumbens increases liking reactions in rats (Peciña and Berridge, 2000), suggesting that MORs mediate hedonic sensations resulting from reward consumption. Accordingly, our results show that an individual’s MOR availability may alter their capacity for experiencing hedonic sensations, and, consequently influence their sensitivity to reward signals.
In addition to reward circuits, BAS sensitivity was also associated with MOR availability in brainstem, insula and anterior cingulate cortex. All these regions are involved in processing nociceptive signals, and MOR has an important role in their transmission (Fields, 2004). MOR availability in striatum is also positively associated with pain threshold (Hagelberg et al., 2012), indicating that MOR density may influence an individual's pain sensitivity.
This dual role of MORs in both reward and pain-relief could explain why individuals with high baseline MOR availability are motivated to approach high-incentive targets possibly leading to physical or social pain. First, high baseline MOR availability may intensify pleasure responses upon reward reception. In line with this, mice with lowered MOR density consume more opiates than their controls (Zhang et al., 2015), suggesting that they need a larger opiate dose for achieving the desired pleasure response. Similarly, human studies have proposed that low MOR availability might promote overeating and obesity, as it may yield individuals unresponsive to the pleasure triggered by palatable food consumption (Karlsson et al., 2015a,b). Second, high MOR density also provides enhanced capacity for relieving the possible physical (Zubieta et al., 2001) or social (Hsu et al., 2013) harm experienced while trying to obtain the rewards (Peciña et al., 2015). This combination of MOR-dependent elevated euphoric capacity and high pain tolerance may make high-BAS individuals well-equipped to impulsively approach rewards (Love et al., 2009).
It is however clear that the MOR system alone does not determine human reward-seeking tendencies. In addition to the MOR system, dopamine and endocannabinoid systems contribute to motivational and reward functions (Mahler et al., 2007; Fields and Margolis, 2015). Indeed, already the early formulations of the RST suggested that dopaminergic circuits would contribute to BAS function (Gray, 1987; Pickering and Gray, 1999). The present data thus suggest that human reward-sensitivity may be determined by interactions between multiple neurotransmitter systems; this idea is also supported by prior work highlighting how dopamine-opioid interactions are critical for reward and motivation system related conditions such as addiction (Mick et al., 2015) and obesity (Tuominen et al., 2015).
Previous studies have shown that high BAS sensitivity may predispose individuals to addiction disorders (Johnson et al., 2003; Franken et al., 2006), and functional neuroimaging studies suggest that this association might be mediated by increased neural sensitivity to reward signals in high-BAS individuals (Beaver et al., 2006; Hahn et al., 2009; Simon et al., 2010; Costumero et al., 2013). Our data suggest that high MOR availability may increase an individual’s sensitivity to reward, thus promoting behaviours that more frequently activate the MOR system and possibly making the individual more vulnerable to addiction. Supporting this explanation, people with genetically determined lowered MOR availability (Peciña et al., 2015) have reduced likelihood to develop a drug addiction (Schwantes-An et al., 2015). Furthermore, MOR availability is elevated during early stages of abstinence in cocaine and alcohol abusers (Zubieta et al., 1996; Gorelick et al., 2005; Heinz et al., 2005; Weerts et al., 2011), suggesting that upregulation of MORs explains craving for these substances. In line with these clinical observations, MOR availability in healthy individuals is also associated with personality factor trait impulsivity (Love et al., 2009), indicating that the clinical examples may be simply extreme forms of the same phenomenon. Together, these data suggest that MOR availability may partially explain human reward-sensitivity in both healthy individuals and in patient populations.
MOR availability has been previously linked with obesity, which is also associated with altered BAS sensitivity (Dietrich et al., 2014). Specifically, obese individuals have significantly lower global MOR availability than normally weighted controls (Karlsson et al., 2015a,b). However we found no association between MOR availability and BMI in the present sample of normal-weight individuals. This result suggests that obesity may alter MOR function and BAS sensitivity only above certain weight threshold, whereas these processes remain uncoupled in the normal-weight population.
Our experimental design does not allow distinguishing the direction of causality. It is indeed possible that the present results reflect the genetically determined role of endogenous MOR system in regulating appetitive behaviour, or alternatively the neuroplastic changes resulting from such behaviour. It is also possible that the effect is bidirectional. Indeed, other lines of evidence support this explanation. First, the OPRM1 gene is known to affect MOR expression (Zhang et al., 2005; Mague et al., 2009) and [11C]carfentanil binding (Peciña et al., 2015). Second, both BAS sensitivity (Salavert et al., 2007) and MOR availability (Karlsson et al., 2015b) of an individual may fluctuate depending on their internal state and environment. Consequently, changes in behaviour may override the genetic tendencies and modify the baseline tone of both MORs and BAS.
Our results suggest that individual differences in MOR system do not explain BIS-dependent behaviour. This accords with our previous study that found no association between attachment anxiety and MOR availability (Nummenmaa et al., 2015). Indeed, both human and animal studies suggest that instead of MOR, the serotonergic system is a plausible neuromolecular candidate subserving BIS-dependent avoidance functions. Activation of the serotonergic system is critical for avoidance behaviour in rodents (Deakin and Graeff, 1991), and genetic variations in expression of serotonin transporter influence fear circuit’s responsiveness to acute threat signals in humans (Hariri et al., 2002). Finally, acute lowering of serotonin levels by tryptophan depletion abolishes punishment-dependent inhibition (Crockett et al., 2009), suggesting that individual differences in the serotonin system could underlie BIS. This however needs to be tested in future studies.
Limitations
The most obvious limitation of our study is that instead of direct measures, we used self-reports to assess sensitivity to reward and punishment signals. However, prior studies have established that these self-reports accurately reflect the tendencies to engage in approach/avoidance behaviour (Johnson et al., 2003; Coplan et al., 2006; Kimbrel et al., 2010) and they are also associated with functioning of the corresponding neural circuits, as revealed by neuroimaging experiments (Beaver et al., 2006; Barrós-Loscertales et al., 2006a,b; Hahn et al., 2009; Simon et al., 2010; Costumero et al., 2013). It must also be stressed that our outcome measure (BPND) does not distinguish between receptor density, affinity and the amount of endogenous neurotransmitter occupancy. However, MOR density itself is largely determined by long-term endogenous neurotransmitter tone: low opioid tone leads to high receptor density and visa versa (McConalogue et al., 1999; Lesscher et al., 2003; Rajashekara et al., 2003). Thus, it is can be assumed that baseline MOR availability is associated with long-term endogenous MOR tone.
Conclusions
We show that opioidergic system mediates BAS but not BIS function in human healthy adults. High BAS sensitivity was manifested as elevated cerebral MOR availability in brain regions involved in processing reward and pain information. BAS and BIS thus appear to have independent neuromolecular basis. Altogether these data suggest that individual differences in baseline cerebral MOR availability may explain why some people seek reward more actively than others, and thus provide a possible neurobiological explanation for our behavioural dispositions when encountering signals of reward.
Funding
This research was supported by the Academy of Finland (MIND program grant 265915 to L.N., 138145 to I.P.J. and 218072 to R.H.), ERC Starting Grant 313000 to L.N. and ERC Advanced Grant 232946 to R.H. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest. None declared.
References
- Alenius S., Ruotsalainen U. (1997). Bayesian image reconstruction for emission tomography based on median root prior. European Journal of Nuclear Medicine, 24, 258–65. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A., Meseguer V., Sanjuán A, et al. (2006a). Striatum gray matter reduction in males with an overactive behavioral activation system. European Journal of Neuroscience 24, 2071–4. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A., Meseguer V., Sanjuán A, et al. (2006b). Behavioral inhibition system activity is associated with increased amygdala and hippocampal gray matter volume: a voxel-based morphometry study. NeuroImage, 33, 1011–5. [DOI] [PubMed] [Google Scholar]
- Beaver J.D., Lawrence A.D., van Ditzhuijzen J, et al. (2006). Individual differences in reward drive predict neural responses to images of food. The Journal of Neuroscience, 26, 5160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Garbin M.G. (1988). Psychometric properties of the Beck Depression Inventory - twenty-five years of evaluation. Clinical Psychology Review, 8, 77–100. [Google Scholar]
- Berridge K.C., Robinson T.E. (2003). Parsing reward. Trends in Neurosciences, 26, 507–13. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Kringelbach M.L. (2008). Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology, 199, 457–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Ho C.Y., Richard J.M., DiFeliceantonio A.G. (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research, 1350, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral inhibition, behavioal activation, and affective responses to impending reward and punishment. Journal of Personality and Social Psychology, 67, 319–33. [Google Scholar]
- Caseras X., Ávila C., Torrubia R. (2003). The measurement of individual differences in behavioural inhibition and behavioural activation systems: a comparison of personality scales. Personality and Individual Differences, 34, 999–1013. [Google Scholar]
- Colasanti A., Rabiner E., Lingford-Hughes A., Nutt D. (2011). Opioids and anxiety. Journal of Psychopharmacology, 25, 1415–33. [DOI] [PubMed] [Google Scholar]
- Coplan R.J., Wilson J., Frohlick S.L., Zelenski J. (2006). A person-oriented analysis of behavioral inhibition and behavioral activation in children. Personality and Individual Differences, 41, 917–27. [Google Scholar]
- Costumero V., Barrós-Loscertales A., Bustamante J.C, et al. (2013). Reward sensitivity is associated with brain activity during erotic stimulus processing. PLoS ONE, 8, e66940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett M.J., Clark L., Robbins T.W. (2009). Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. The Journal of Neuroscience, 29, 11993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin J.F., Graeff F.G. (1991). 5-HT and mechanisms of defence. Journal of Psychopharmacology, 5, 305–15. [DOI] [PubMed] [Google Scholar]
- Depue R.A., Collins P.F. (1999). Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences, 22, 491–517. [DOI] [PubMed] [Google Scholar]
- Fields H.L. (2004). State-dependent opioid control of pain. Nature Reviews Neuroscience, 5, 565–75. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Federbusch M., Grellmann C, et al. (2014). Body weight status, eating behavior, sensitivity to reward/punishment, and gender: relationships and interdependencies. Frontiers in Psychology, 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H.L., Margolis E.B. (2015). Understanding opioid reward. Trends in Neurosciences, 38, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken I., Muris P., Georgieva I. (2006). Gray's model of personality and addiction. Addictive Behaviors, 31,399–403. [DOI] [PubMed] [Google Scholar]
- Gorelick D.A., Kim Y.K., Bencherif B, et al. (2005). Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biological Psychiatry, 57, 1573–82. [DOI] [PubMed] [Google Scholar]
- Gray J.A. (1970). The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy, 8, 249–66. [DOI] [PubMed] [Google Scholar]
- Gray J.A. (1987). Perspectives on anxiety and impulsivity: a commentary. Journal of Research in Personality, 21, 493–509. [Google Scholar]
- Gray J.A., McNaughton N. (2000) The Neuropsychology of Anxiety: an Enquiry into the Functions of the Septo-hippocampal System. 2nd edition New York: Oxford University Press; p. 1–36. [Google Scholar]
- Gunn R.N., Lammertsma A.A., Hume S.P., Cunningham V.J. (1997). Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage, 6, 279–87. [DOI] [PubMed] [Google Scholar]
- Hagelberg N., Aalto S., Tuominen L, et al. (2012). Striatal µ-opioid receptor availability predicts cold pressor pain threshold in healthy human subjects. Neuroscience Letters, 521, 11–4. [DOI] [PubMed] [Google Scholar]
- Hahn T., Dresler T., Ehlis A.C, et al. (2009). Neural response to reward anticipation is modulated by Gray's impulsivity. NeuroImage, 46, 1148–53. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A, et al. (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science, 297, 400–3. [DOI] [PubMed] [Google Scholar]
- Heinz A., Reimold M., Wrase J, et al. (2005). Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. JAMA Psychiatry, 62, 57–64. [DOI] [PubMed] [Google Scholar]
- Heubeck B.G., Wilkinson R.B., Cologon J. (1998). A second look at Carver and White's (1994) BIS/BAS scales. Personality and Individual Differences, 25, 785–800. [Google Scholar]
- Hsu D.T., Sanford B.J., Meyers K.K, et al. (2013). Response of the µ-opioid system to social rejection and acceptance. Molecular Psychiatry, 18, 1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.L., Turner R.J., Iwata N. (2003). BIS/BAS levels and psychiatric disorder: an epidemiological study. Journal of Psychopathology and Behavioral Assessment, 25, 25–36. [Google Scholar]
- Karlsson H.K., Tuominen L., Tuulari J.J, et al. (2015a). Obesity is associated with decreased µ-opioid but unaltered dopamine D2 receptor availability in the brain. The Journal of Neuroscience, 35, 3959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H.K., Tuulari J.J., Tuominen L, et al. (2015b). Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Molecular Psychiatry, 21, 1057–62. [DOI] [PubMed] [Google Scholar]
- Kasch K.L., Rottenberg J., Arnow B.A., Gotlib I.H. (2002). Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology, 111, 589–97. [DOI] [PubMed] [Google Scholar]
- Kimbrel N.A., Mitchell J.T., Nelson-Gray R.O. (2010). An examination of the relationship between behavioral approach system (BAS) sensitivity and social interaction anxiety. Journal of Anxiety Disorders, 24, 372–8. [DOI] [PubMed] [Google Scholar]
- Leknes S., Tracey I. (2008). A common neurobiology for pain and pleasure. Nature Reviews Neuroscience, 9, 314–20. [DOI] [PubMed] [Google Scholar]
- Leone L., Perugini M., Bagozzi R.P, et al. (2001). Construct validity and generalizability of the Carver–White behavioural inhibition system/behavioural activation system scales. European Journal of Personality, 15, 373–90. [Google Scholar]
- Lesscher H.M.B., Bailey A., Burbach J.P.H, et al. (2003). Receptor-selective changes in µ-, δ - and κ-opioid receptors after chronic naltrexone treatment in mice. European Journal of Neuroscience, 17, 1006–12. [DOI] [PubMed] [Google Scholar]
- Love T.M., Stohler C.S., Zubieta J.K. (2009). Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. JAMA Psychiatry, 66, 1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P.E., Kieffer B.L. (2013). Opioid receptors: distinct roles in mood disorders. Trends in Neurosciences, 36, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague S.D., Isiegas C., Huang P, et al. (2009). Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proceedings of the National Academy of Sciences of the United States of America, 106, 10847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Smith K.S., Berridge K.C. (2007). Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in Nucleus Accumbens Shell Enhances ‘Liking' of a Sweet Reward. Neuropsychopharmacology, 32, 2267–78. [DOI] [PubMed] [Google Scholar]
- McConalogue K., Grady E.F., Minnis J, et al. (1999). Activation and internalization of the µ-opioid receptor by the newly discovered endogenous agonists, endomorphin-1 and endomorphin-2. Neuroscience, 90, 1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick I., Myers J., Ramos A.C, et al. (2015). Blunted endogenous opioid release following an oral amphetamine challenge in pathological gamblers. Neuropsychopharmacology, 41, 1742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L., Manninen S., Tuominen L, et al. (2015). Adult attachment style is associated with cerebral µ-opioid receptor availability in humans. Human Brain Mapping, 36, 3621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F., Kieffer B.L., Tabarin A., Contarino A. (2007). Decreased motivation to eat in µ-opioid receptor-deficient mice. European Journal of Neuroscience, 25, 3398–405. [DOI] [PubMed] [Google Scholar]
- Peciña S., Berridge K.C. (2000). Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Research, 863, 71–86. [DOI] [PubMed] [Google Scholar]
- Peciña M., Love T., Stohler C.S., Goldman D., Zubieta J.K. (2015). Effects of the mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology, 40, 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A.D., Gray J.A. (1999) The neuroscience of personality. In: Pervin, L.A., John, O.P., editors. Handbook of Personality: Theory and Research. 2nd edition New York: The Guilford Press: p. 277-99. [Google Scholar]
- Rajashekara V., Patel C.N., Patel K, et al. (2003). Chronic opioid antagonist treatment dose-dependently regulates µ-opioid receptors and trafficking proteins in vivo. Pharmacology Biochemistry and Behavior, 75, 909–13. [DOI] [PubMed] [Google Scholar]
- Salavert J., Caseras X., Torrubia R, et al. (2007). The functioning of the behavioral activation and inhibition systems in bipolar I euthymic patients and its influence in subsequent episodes over an eighteen-month period. Personality and Individual Differences, 42, 1323–31. [Google Scholar]
- Schwantes-An T.H., Zhang J., Chen L.S, et al. (2015). Association of the OPRM1 variant rs1799971 (A118G) with non-specific liability to substance dependence in a collaborative de novo meta-analysis of european-ancestry cohorts. Behavior Genetics, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J.J., Walther S., Fiebach C.J, et al. (2010). Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage, 49, 1868–74. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R, et al. (1983) Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Tuominen L., Salo J., Hirvonen J, et al. (2012). Temperament trait Harm Avoidance associates with µ-opioid receptor availability in frontal cortex: A PET study using [11C]carfentanil. NeuroImage, 61, 670–6. [DOI] [PubMed] [Google Scholar]
- Tuominen L., Nummenmaa L., Keltikangas-Järvinen L, et al. (2014). Mapping neurotransmitter networks with PET: an example on serotonin and opioid systems. Human Brain Mapping, 35, 1875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen L., Tuulari J.J., Karlsson H, et al. (2015). Aberrant mesolimbic dopamine-opioid interaction in obesity. NeuroImage, 122, 80–6. [DOI] [PubMed] [Google Scholar]
- Van Ree J.M., Niesink R.J.M., van Wolfswinkel L, et al. (2000). Endogenous opioids and reward. European Journal of Pharmacology, 405, 89–101. [DOI] [PubMed] [Google Scholar]
- Weerts E.M., Wand G.S., Kuwabara H, et al. (2011). Positron emission tomography imaging of mu- and delta-opioid receptor binding in alcohol-dependent and healthy control subjects. Alcoholism: Clinical and Experimental Research, 35, 2162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang D.X., Johnson A.D, et al. (2005). Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. Journal of Biological Chemistry, 280, 32618–24. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Picetti R., Butelman E.R, et al. (2015). Mouse model of the OPRM1 (A118G) polymorphism: differential heroin self-administration behavior compared with wild-type mice. Neuropsychopharmacology, 40, 1091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J.K., Gorelick D.A., Stauffer R, et al. (1996). Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nature Medicine, 2, 1225–9. [DOI] [PubMed] [Google Scholar]
- Zubieta J.K., Smith Y.R., Bueller J.A, et al. (2001). Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science, 293, 311–5. [DOI] [PubMed] [Google Scholar]