Summary
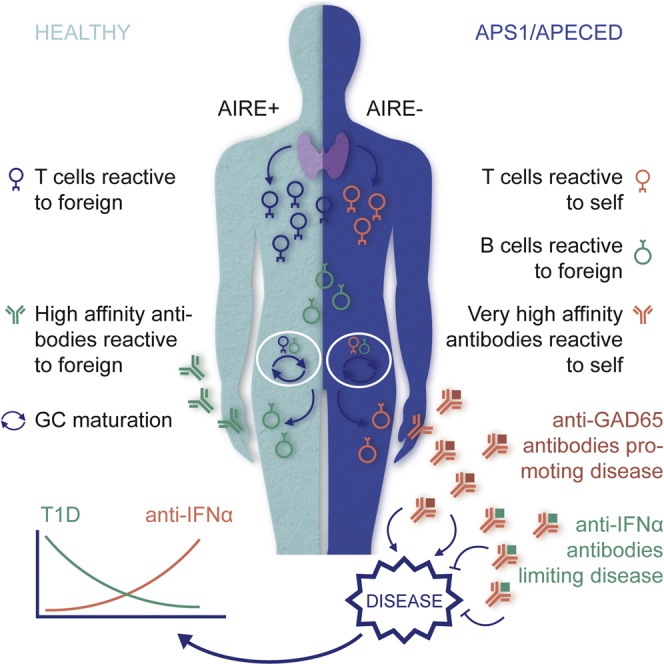
APS1/APECED patients are defined by defects in the autoimmune regulator (AIRE) that mediates central T cell tolerance to many self-antigens. AIRE deficiency also affects B cell tolerance, but this is incompletely understood. Here we show that most APS1/APECED patients displayed B cell autoreactivity toward unique sets of approximately 100 self-proteins. Thereby, autoantibodies from 81 patients collectively detected many thousands of human proteins. The loss of B cell tolerance seemingly occurred during antibody affinity maturation, an obligatorily T cell-dependent step. Consistent with this, many APS1/APECED patients harbored extremely high-affinity, neutralizing autoantibodies, particularly against specific cytokines. Such antibodies were biologically active in vitro and in vivo, and those neutralizing type I interferons (IFNs) showed a striking inverse correlation with type I diabetes, not shown by other anti-cytokine antibodies. Thus, naturally occurring human autoantibodies may actively limit disease and be of therapeutic utility.
Graphical Abstract
Highlights
-
•
Each AIRE-deficient patient has a private repertoire of autoantibody reactivities
-
•
Loss of B cell tolerance occurs during T cell-dependent somatic hypermutation
-
•
Patient autoantibodies have unprecedented affinities for conformational epitopes
-
•
Patient autoantibodies can display disease-ameliorating properties in vivo
Self-reactive antibodies specific for type I interferons are associated with protection against type I diabetes in patients with an autoimmune syndrome caused by mutations in AIRE.
Introduction
T lymphocyte tolerance is essential for limiting autoimmune disease. Tolerance occurs “centrally” when developing thymocytes with strongly self-reactive T cell receptors (TCRs) are deleted following engagement of self-antigen-derived peptides presented by major histocompatibility complex (MHC) antigens. The expression of thousands of tissue-specific self-antigens (TSAs) by medullary thymic epithelial cells (mTEC) is directly promoted by AIRE, a poorly understood transcriptional regulator (Mathis and Benoist, 2009, Klein et al., 2014). Reflecting its importance, AIRE deficiency is defined by the APS1/APECED syndrome for which autoimmune polyendocrinopathy and chronic mucocutaneous candidiasis are pathognomonic (Nagamine et al., 1997).
There are also several mechanisms of peripheral T cell tolerance, including requirements for co-stimulatory signals for the activation of naive T cells; the expression of molecular “brakes” (e.g., CTLA-4, PD-1) by activated T cells; and the suppression of effector T cells in trans by FOXP3-expressing T-regulatory (T-reg) cells. Reflecting its importance, FOXP3 deficiency is defined by early-onset, life-threatening autoimmunity (Bennett et al., 2001, Wildin et al., 2001).
Central and peripheral tolerance mechanisms have likewise been hypothesized to shape the B cell compartment. Thus, self-reactive B cells developing in the bone marrow may be censored by clonal deletion, clonal anergy, or B cell receptor (BCR) editing in which secondary gene rearrangements replace the initial BCR with a new specificity (Goodnow et al., 2010, Pillai et al., 2011, Übelhart and Jumaa, 2015). Peripheral B cell tolerance is less well characterized, although some checkpoints have been inferred. For example, immature transitional B cells recently emigrated from the bone marrow contain many autoreactive and polyreactive cells, whereas there are relatively few among mature naive B cells, strongly suggesting that tolerance is imposed as transitional B cells differentiate into naive B cells (Wardemann et al., 2003).
Interestingly, this B cell checkpoint is T cell dependent, as reflected by its impairment in patients with T-reg deficiencies (Kinnunen et al., 2013). Likewise, CD40L and MHC class II deficiencies that each impair T-B interactions also display more autoreactive B cells (Meffre and Wardemann, 2008). These considerations raise the possibility that B cell tolerance is largely governed by the state of T cell tolerance.
Certainly, any autoreactive B cell that might progress through to the naive B cell compartment of a healthy individual should lack cognate autoreactive T cells to help it mature. Likewise, T cell help is required in the germinal center (GC) reaction in which B cells undergo somatic hyper-mutation (SHM) of the immunoglobulin (Ig) variable (V) region genes, thereby driving T cell-dependent selective expansion of clones with increased antigen affinity (Brink, 2014). The question that then arises is whether major defects in central T cell tolerance provoke wide-ranging losses of B cell tolerance at either or both of these stages.
An approach to assessing this is to examine B cell reactivities in AIRE-deficient APS1/APECED patients whose under-expression of TSAs in the thymus is predicted to lead to increased numbers of peripheral autoreactive T cells. Thus, there are reports of APS1/APECED patients carrying autoantibodies against twenty-five TSAs, with prevalence ranging from 6% to 69% (Kisand and Peterson, 2015). Their specificities include steroidogenic enzymes, consistent with the patients’ polyendocrinopathies (Krohn et al., 1992, Uibo et al., 1994, Winqvist et al., 1993). In addition, most patients display autoreactivities toward type I IFNs and T helper (Th)-17-related cytokines, antibodies to which limit resistance to Candida infection (Kisand et al., 2010, Meager et al., 2006, Puel et al., 2010).
These findings notwithstanding, there has been no large-scale analysis of the scope and nature of autoantibodies in APS1/APECED patients, thereby resolving how T cell tolerance impacts upon B cell tolerance in humans. By analyzing 81 APS1/APECED patients, we found that each was much more likely than a healthy relative or an unrelated control to harbor strong serum reactivities toward ∼100 human proteins. About 10 of those, including type I IFNs and interleukin-22 (IL22), were recognized by almost all patients, whereas others were mostly “private specificities.” Hence, 81 patients collectively harbored antibodies toward >3,700 human proteins.
Focusing on antibodies to type I IFNs, IL22, and IL17, we found unexpectedly that most were reactive to conformational determinants and included highly mutated antibodies of sub-picomolar affinity. Because their gemline counterparts were not self-reactive, B cell autoreactivity was most probably driven by self-reactive T cells in the GC reaction. The autoantibodies commonly neutralized their targets in vivo, and APS1/APECED patients with signature type 1 diabetes (T1D)-associated antibodies (e.g., anti-GAD65) commonly failed to develop T1D so long as they harbored powerfully neutralizing IFNα-specific antibodies. Thus, autoantibodies naturally arising in subjects with defective central T cell tolerance may be disease ameliorating.
Results
High-Titer Autoreactivities in APS1/APECED
Sera from 81 APS1/APECED patients from discrete Finnish, Norwegian, Slovenian, and Sardinian cohorts were directed against a ProtoArray displaying ∼9000 immobilized recombinant human proteins or protein fragments. Because some patients were sampled longitudinally, 97 sera were assayed in total. Control sera were from healthy first-degree relatives (n = 9) and healthy unrelated volunteers (n = 12) across the same age range. Data readouts for the binding of individual sera were normalized by applying robust linear modeling (Sboner et al., 2009), whereafter each signal was assigned a Z score denoting the number of standard deviations (SD) above or below the mean of the combined healthy relatives and controls.
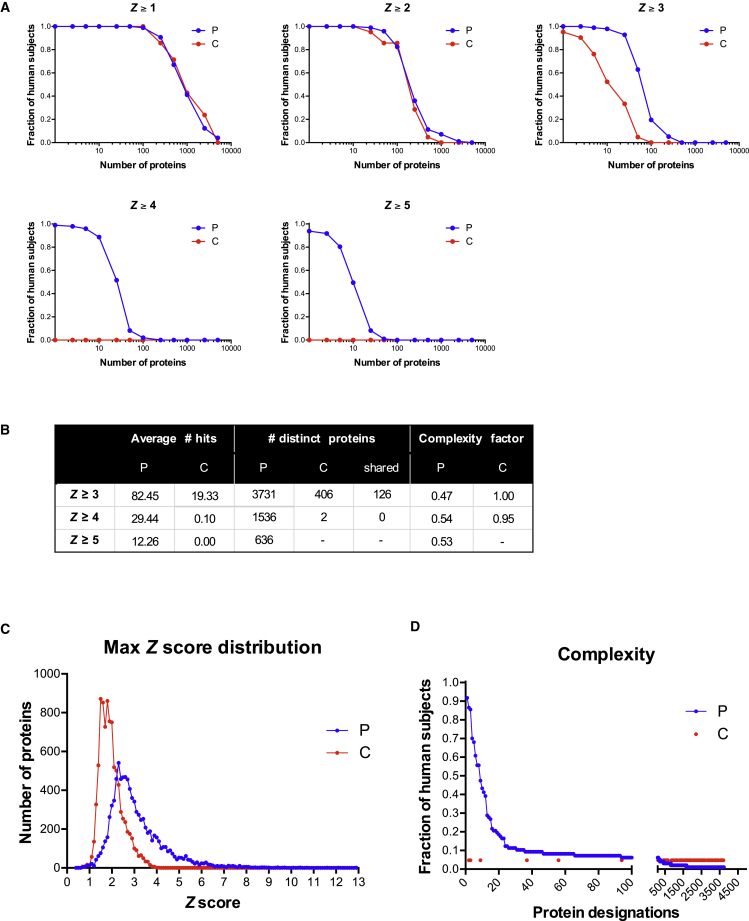
Most patients and the combined controls displayed Z scores of 1–2 for ∼200 proteins (Figure 1A). However, when the convention was employed of defining Z ≥ 3 as bona fide positives, the patients segregated from the two control cohorts, considered either jointly or separately. Thus, each control serum displayed reactivities of Z ≥ 3 toward an average of ≤20 proteins, with most recognizing < 10 (Figures 1A, 1B, and S1A). Given that there was inter-individual variation, the 21 control sera collectively displayed Z ≥ 3 reactivities toward 406 distinct proteins, i.e., ∼5% of those displayed on the array (Figure 1B). For only 2 proteins was Z ≥ 4, and for none was Z ≥ 5 (Figures 1A and 1B). Hence, as expected, the control cohorts largely lacked high-titer serum autoreactivities.
Figure 1.
Immune Response Profiling of APS1/APECED
(A) Distributions of hits between patients and controls at different Z scores.
(B) Z scores for all samples against all protein features and mean hits for each group calculated for Z ≥ 3, Z ≥ 4, and Z ≥ 5. The number of distinct proteins targeted in each group (P, n = 97; C, n = 21) at Z scores denoted. The complexity factor was calculated by dividing the number of distinct proteins by average number of hits per patient.
(C) The max Z score distribution of all proteins in patient and control groups.
(D) Fraction of patients recognizing each of 3,731 proteins at Z ≥ 3. Red dots depict 126 proteins shared between patients and controls.
Figure S1.
Related to Figure 1
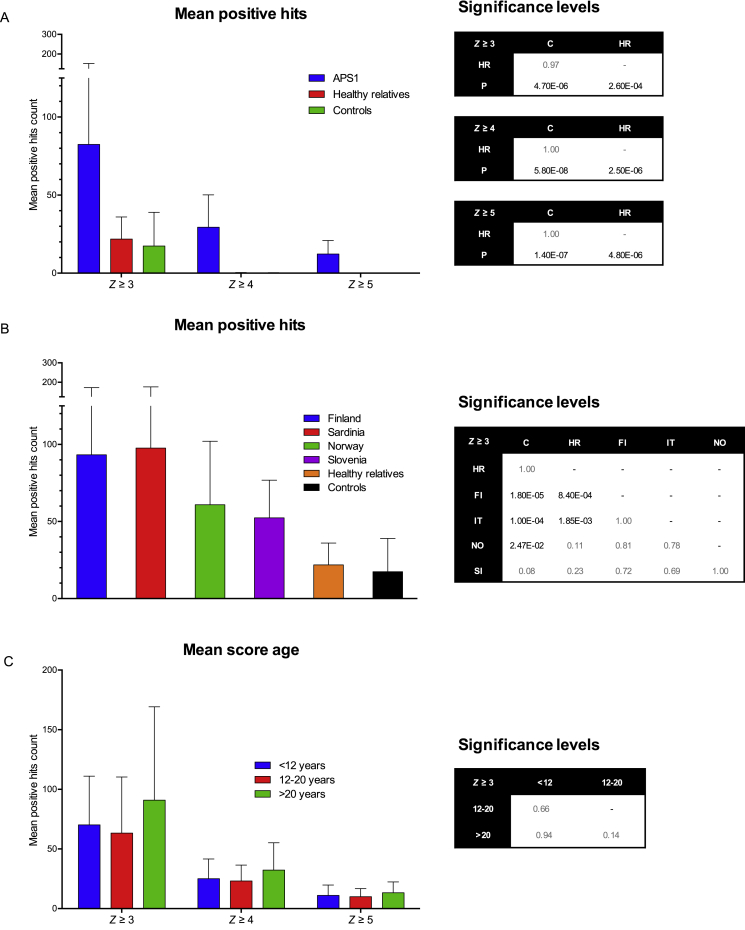
(A) Z scores for all samples against all protein features were calculated as described in experimental procedures and the mean hits for each group were calculated for either Z score ≥ 3, Z score ≥ 4, and Z score ≥ 5. There is a significant difference between patients and healthy relative and controls but no significant difference between healthy relatives and controls.
(B) Mean hits score for Z ≥ 3 were calculated for each geographical location. There is no significant difference between the patient cohorts but all cohorts are significantly different from the controls with the exception of the Slovenian and significantly different from the healthy relatives with exception of the Norwegian and Slovenian.
(C) Mean hits score for Z ≥ 3 were calculated for age-grouped patients with no significant difference between the groups.
Data are represented as mean ± SEM and statistical significance levels were calculated by Kruskal-Wallis testing.
Conversely, most patients at any one time displayed Z ≥ 3 autoreactivities toward ≥ 80 proteins (Figures 1A, 1B, and S1A). These data were re-analyzed with stringent procedures to minimize false-positives, including exclusion of any signals that might have arisen from cross-sample print contamination. With this achieved, the patients’ “private” autoantibody repertoires collectively detected 3,731 distinct targets (Figure 1B). Furthermore, almost all patients displayed Z ≥ 4 scores for at least 10 proteins (mean of ∼30), collectively recognizing > 1,500 proteins, and > 50% of patients displayed Z ≥ 5 scores for ≥ 10 proteins (mean of > 12), collectively recognizing 636 proteins (Figures 1A and 1B). Hence, high-level reactivity toward multiple self-proteins was a disease-defining property. This was further illustrated by the qualitative difference in Z score distribution curves for patients versus controls, which cannot simply be explained by there being 5-fold more patient sera (Figure 1C). Thus, whereas sampling greater numbers would likely have increased the protein species detected by control cohorts at Z ≥ 4, it would not bridge the 1,000-fold gap between two proteins detected by 21 control sera versus > 1,500 proteins detected by 97 patient sera (Figure 1B).
In sum, 81 different patients collectively displayed strong reactivities to >40% of human proteins arrayed. For most proteins (blue dots 13–3731, Figure 1D), reactivities were spread across the cohort, reflecting high inter-patient variation, whereas ∼12 proteins (blue dots 1–12, Figure 1D), including several type I IFNs, were recognized by > 60% of patients, as reported (Meager et al., 2006). However, the “public specificities” were not enriched among the 126 reactivities shared between patients and controls at z > 3 (red dots, Figure 1D), emphasizing that their common autoantigenicity is unique to the patients. Patient autoreactivity frequencies were largely comparable across geographical locations, albeit somewhat less in Norway and Slovenia, and age ranges (Figures S1B and S1C). Indeed, most anti-IFN autoantibodies of APECED patients were reported to increase early in life and remain stable thereafter (Meager et al., 2006, Wolff et al., 2013).
The collective targets of patient antibodies included intracellular, trans-membrane, and secreted proteins. Because many proteins displayed on the ProtoArray may be denatured, there may be false-negatives that underestimate patient reactivities to conformational determinants. Although a detailed analysis of the types of proteins targeted will be presented, it is evident that the proteins most commonly detected by patient sera included numerous cytokines, particularly type I IFNs, for which reason this study focuses on the nature of those autoreactivities.
Strong, Selective Anti-Cytokine Reactivities
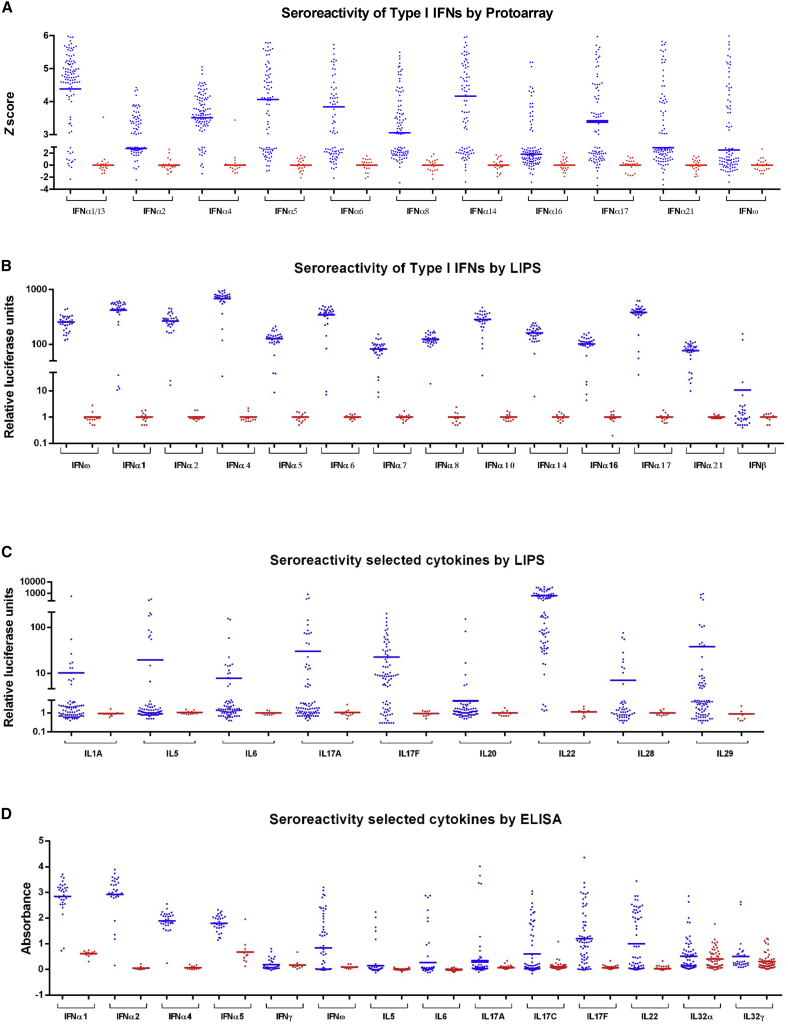
Human type I IFN genes include 13 IFNα genes, 1 IFNβ gene, and 1 IFNω gene. There is also a type II IFNγ gene and three type III IFNλ genes. IFNγ is largely limited to lymphocytes, whereas type I and type III IFNs are broadly expressed, with their functional uniqueness and/or redundancy unresolved (Ivashkiv and Donlin, 2014). As assessed by ProtoArray, patient sera showed significantly stronger reactivities than controls toward all IFNα subtypes, albeit the reactivities to some (e.g., α1/13, α5, and α14) were higher than those to others (e.g., α2, α16, and α21) (Figure 2A). The differential between patients versus controls was emphasized by luciferase-based immunoprecipitation (LIPS) in which many target proteins were recognized in their native conformations (Figure 2B). Many patients showed strong reactivities to IFNω but rarely toward IFNβ (Figure 2B) and never toward IFNκ and IFNε, two phylogenetically distant type I IFNs (data not shown). By contrast, patient sera harbored reactivities significantly above controls toward IL1α, IL5, IL6, IL17A, IL17F, IL20, IL22, IL28A (IFNλ2), IL28B (IFNλ3), and IL29 (IFNλ1) (Figure 2C). Whereas reactivities toward some targets (e.g., IL17F, IL22) were common to most patients, reactivities toward others (e.g., IL20, IL28, IL6) were not (Table S1), and with the exception of IL5, patient sera mostly did not detect either Th2 cytokines (e.g., IL4 and IL13) or IL21, a Tfh (T follicular helper) cell cytokine that drives high-affinity antibody maturation. There were also no reactivities toward G-CSF and GM-CSF (Table S1), which drive the development of myeloid cells associated with the patients’ inflammatory endocrinopathies.
Figure 2.
Serology of APS1/APECED to IFNs and Other Cytokines
Seroreactivity of APS1/APECED patients (blue) and contols (red) toward selected interferons and cytokines as measured in ProtoArray (A), LIPS (B and C), and ELISA (D).
Cytokine reactivities were largely validated by ELISA, which confirmed that IFNγ was only rarely and weakly recognized by patient sera (Figure 2D; Table S1) and that there was no reactivity toward TNFα (data not shown). By contrast, ELISA revealed autoantibodies toward IL32α and IL32γ, two poorly characterized proinflammatory cytokines (Figure 2D; Table S1). In sum, 81 APS1/APECED patient sera collectively displayed strong reactivities to a very selective subset of human cytokines.
Very High-Affinity Human Antibodies
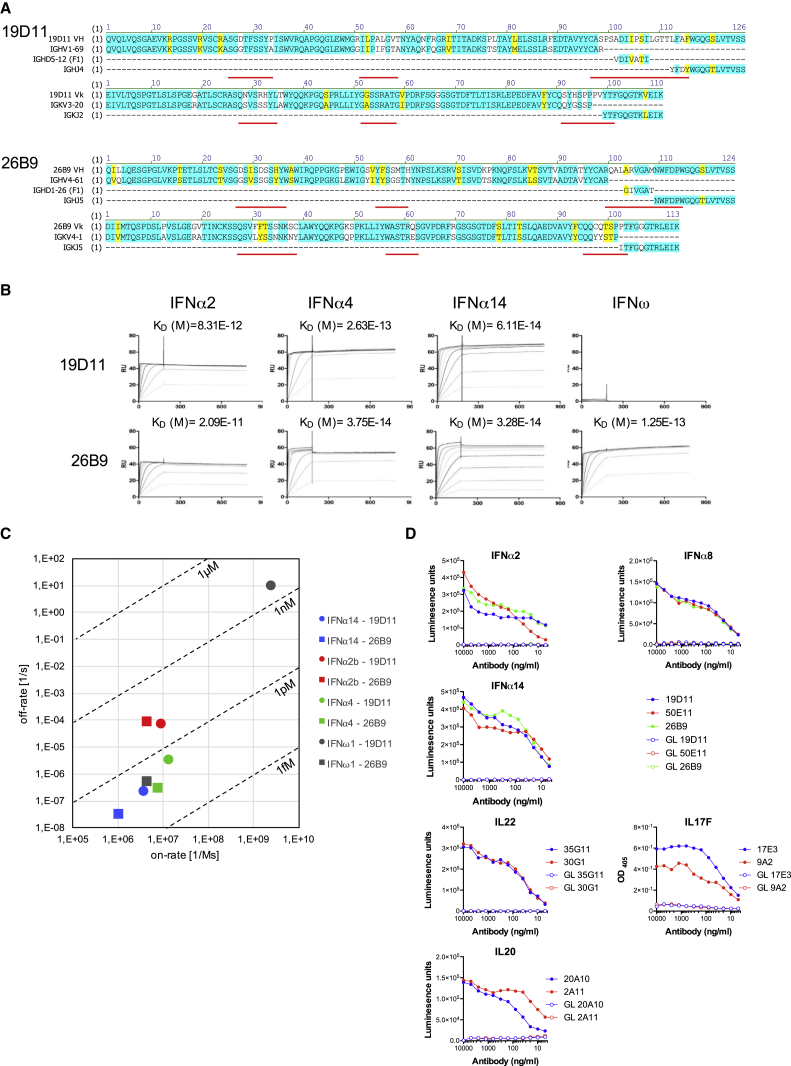
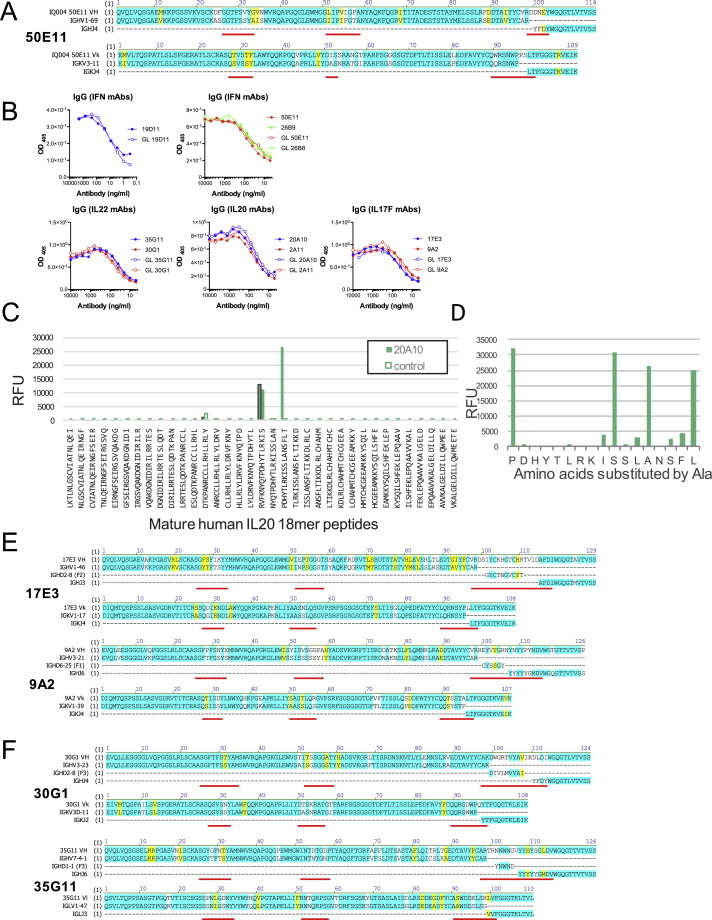
To understand the nature of patient serum reactivities, nine IFNα-specific monoclonal antibodies (mAbs) were derived by limit-dilution cloning from memory B cells of four patients. Two were characterized in detail (26B9 and 19D11), whereas a more limited analysis of the others strongly argued that the properties of 26B9 and 19D11 were generally representative of patients’ cytokine-specific antibodies. First, their VH and Vκ sequences were highly mutated relative to their germline counterparts, with non-conservative replacements enriched in complementarity-determining regions (CDRs), as expected (white; Figure 3A). The antibodies bore no obvious resemblance to each other in V-gene segment or CDR3 usage. Conversely, a third anti-IFNα antibody, 50E11, shared with 19D11 the same VH (IGHV1-69) and junctional (IGHJ4) segments and a very similar light chain (IGKV3-11 versus V3-20) (Figure S2A). However, there were very different template-independent nucleotide insertions in the VH CDR3s of 19D11 and 50E11, and the somatic mutation patterns were different: whereas 19D11 and 26B9 showed high mutation frequencies in VH CDR2 and Vκ CDR1, 50E11 did not (Figures 3A and S2A).
Figure 3.
Affinity of Patient-Derived mAbs
(A) Amino acid sequences of 26B9 and 19D11 anti-IFN antibodies aligned with closest corresponding germline IgVH, DH, JH, VL, and JL sequences. Identities highlighted in blue; conservative mutations in yellow; non-conservative in white; CDRs underlined in red.
(B) Plasmon resonance data: antibodies 19D11 and 26B9 were immobilized on Biacore chips; different concentrations of recombinant human IFNα2b, IFNα4, IFNα14, and IFNω were passed over; response units were recorded; and dissociation constants (KD) calculated.
(C) Scatter chart of KD values derived from (B).
(D) Binding determined by LIPS of APS1/APECED-derived mAbs and of germline counterparts to IFNα2, IFNα8, IFNα14 (19D11, 50E11, and 26B9), IL22 (35G11 and 30G1), and IL20 (20A10 and 2A11). Binding to immobilized IL17F (17E3 and 9A2) was determined by ELISA.
Figure S2.
Related to Figure 3
(A) Corresponding closest germlines and heavy chain diversity regions of APS1 patient-derived mAb 50E11 targeting IFNαs.
(B) IgG concentration of APECED/APS1 derived mAbs and its closest germline antibodies as measured by ELISA.
(C) Mapping of the epitope of the patient-derived anti-IL20 antibody 20A10 by primary peptide array. The antibody specifically binds to the peptide comprising amino acids P101 to T118. Signals at the 18-mer peptides starting with R93 and D73 are caused by binding of the detection antibody as shown in the control.
(D) Alanine-scan of the epitope comprising residues P101 to L117. Alanine substitutions at positions D102, H103, Y104, T105, L106, R107, K108, S111, N114, S115, and F116 lead to a breakdown of mAb binding.
(E and F) Corresponding closest germlines and heavy chain diversity regions of APS1 patient-derived mAbs. (E) anti-IL17F mAbs. (F) anti-IL22 mAbs. Identical amino acids are highlighted in blue, mutated but similar amino acids in yellow and CDRs are underlined in red.
The recombinant antibodies 26B9 and 19D11 harvested from transfected CHO cells were immobilized on surface plasmon resonance (SPR) chips over which were run recombinant human IFNα2b, IFNα4, IFNα14, and IFNω, the latter being recognized by 26B9 but not by 19D11 (Figure 3B). These experiments revealed very slow off-rates reflecting extremely high affinities of the antibodies for their targets, ranging from KD = 3.28e−14M for 26B9 toward IFNα14 to KD = 2.09e−11M for 26B9 toward IFNα2b (Figures 3B and 3C). Sub-picomolar/near-femtomolar dissociation constants were likewise shown by 19D11 (Figures 3B and 3C). Thus, APS1/APECED patients harbor some of the strongest affinity antibodies described.
18-mer peptides spanning IFNα2b and IFNω were used to map linear epitopes recognized by 26B9 and 19D11. However, no specific reactivities were detected (data not shown), consistent with the antibodies binding conformational determinants shared by several type I IFNs. Also, the antibodies reacted poorly or not at all to mouse IFNs (Table S2).
To investigate the origins of the high-affinity, conformation-specific antibodies, germline counterparts for 19D11, 26B9, and 50E11, albeit with the same CDR3-VDJ sequences, were expressed and tested by LIPS against recombinant human IFNα2b, IFNα8, and IFNα14. There was no measurable interaction with any target (Figure 3D), although the antibodies’ quality was evident from their comparable detection by anti-human IgG (Figure S2B). These data argue that the strong autoreactivity toward IFNs developed de novo during affinity maturation, rather than being an intrinsic property of the germline repertoire that is enhanced by affinity maturation.
The high affinities of 26B9 and 19D11 were not unique. Thus, a patient-derived IgGκ mAb (20A10) specific for IL20 (which is not a target detected by most patients; Figure 2C; Table S1) displayed a KD of 9.1e−14M, (Table S3). Likewise one IgGκ mAb (17E3) and one IgGλ mAb (24D3), each specific for IL17F, displayed dissociation constants of <10 pM, and one IgGκ antibody (30G1) and one IgGλ antibody (35G11) specific for IL22 displayed dissociation constants of 37 pM and 39 pM, respectively. As a comparison, a CHO cell-expressed form of fezakinumab, a humanized anti-IL22 mAb tested in the clinic, displayed a KD of 54 pM (Table S3). The only exception to this pattern was 2C2, an IgGλ mAb specific for IL32γ (for which no human antibody has been reported), which displayed nanomolar dissociation (Table S3).
Similar to IFNα antibodies, most cytokine-specific antibodies did not detect linear peptides from relevant target proteins, strongly suggestive of complex conformational determinants (data not shown). The one exception was 20A10, which bound to an IL20 peptide and within which key amino acids were identified by mutagenesis (Figures S2C and S2D).
The antibody sequences of IL17F-reactive 17E3 and 9A2 and of IL22-reactive 30G1 and 35G11 displayed myriad non-conservative mutations enriched in the CDRs. Again their germline counterparts did not detect the respective targets (Figures 3D, S2E, and S2F). Moreover, neither patient-derived antibodies nor their germline counterparts showed any general autoreactivity (judged by immunofluorescent staining of tissue sections or HEp-2 cells) or reactivity to Candida albicans, thus arguing against candida infection being the trigger for autoantibody generation (data not shown).
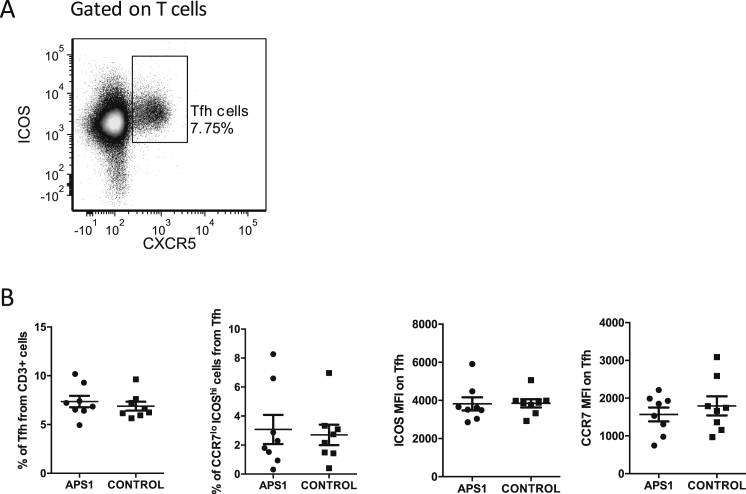
The highly mutated CDRs of all studied antibodies suggested that they derived from GC reactions that partially rely on Tfh cells. Aberrant generation and/or activation of Tfh cells has been described in several autoimmune diseases (Ueno et al., 2015), but when four pediatric and four adult APS1/APECED were compared to controls, we found no differences in the percentages of circulating CXCR5+ Tfh cells, or their activation state, as judged by ICOS (inducible costimulator) and CCR7 levels (Figure S3).
Figure S3.
Related to Figure 3
(A) Tfh cells were gated as CD3+, CD8−, TCR Vδ1−, TCR Vδ2−, CXCR5+, ICOS+ cells.
(B) PBMCs from 8 APS1/APECED patients (4 children and 4 adults) and 8 age-matched healthy individuals were tested. No statistically significant differences were revealed in circulating Tfh cell percentages, neither of their CCR7lo ICOShi subset nor in the mean fluorescence index (MFI) of ICOS or CCR7 among Tfh cells.
Biologically Active Human Antibodies
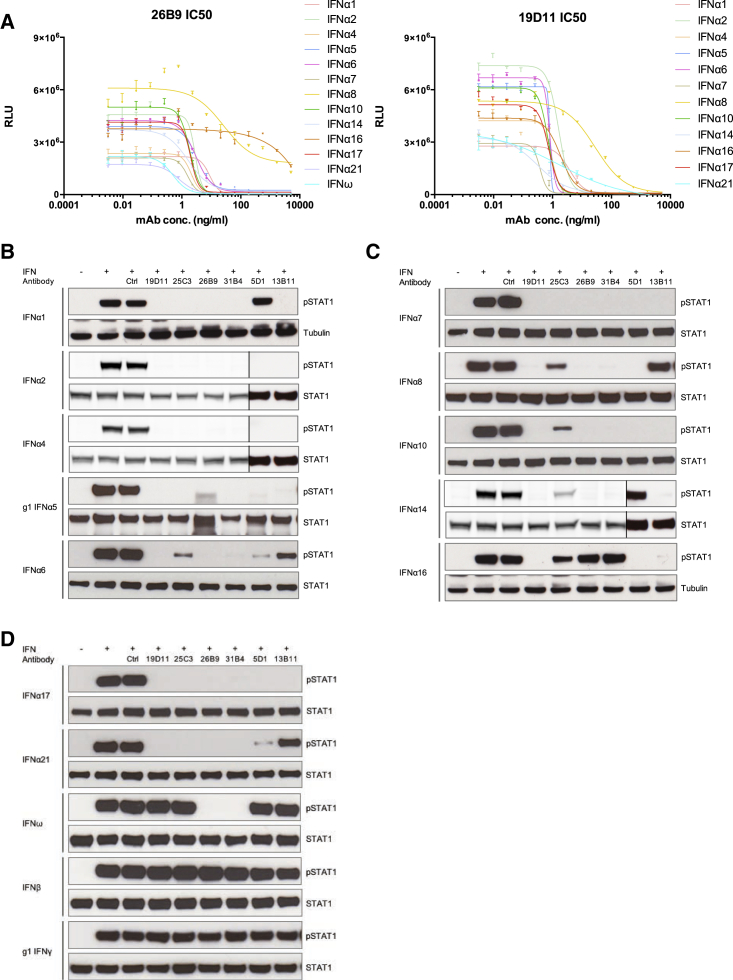
To test the biological activities of 19D11 and 26B9, HEK293 cells transfected with type I IFN-stimulated response elements (ISRE) fused to firefly luciferase were treated with recombinant forms of each of 12 IFNα subtypes and IFNω in the presence or absence of increasing concentrations of 19D11 or 26B9. Following treatment, firefly luciferase values were normalized to those of co-transfected Renilla luciferase, so as to control for variations in transfection efficiency. Both antibodies strongly inhibited the IFN-dependent response, with median IC50 values of 2.83 ng/ml for 26B9 and 0.9 ng/ml for 19D11 (Figure 4A; Table S4). By comparison, median IC50 values of 76.24 ng/ml and 10.86 ng/ml, respectively, were displayed by in-house-generated recombinant sifalimumab and rontalizumab, two anti-IFN mAbs used in clinical trials for systemic lupus erythematosus patients (Table S4).
Figure 4.
In Vitro Neutralization
(A) IC50 analysis of APS1/APECED-derived anti-IFN mAbs 19D11 and 26B9 in HEK293T MSR cells transfected with ISRE dual-luciferase reporter constructs and treated with IFNα subtypes shown. Error bars correspond to SEM of multiple measurements.
(B–D) IFN-induced STAT1 tyrosine phosphorylation detected by western blot and normalized to total STAT1 or to tubulin levels as loading controls. Vertical lines in (B) and (C) denote cropped lanes.
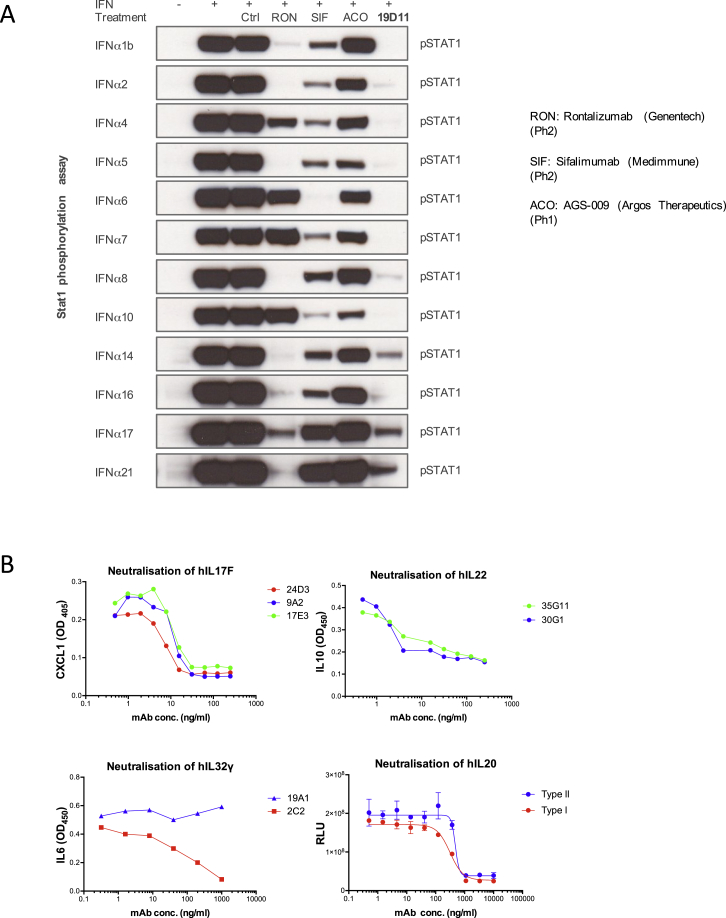
Predictably, the antibodies varied in their inhibition of IFN-stimulated responses. Thus, 26B9 neutralized IFNω, but not IFNα16, and only poorly inhibited IFNα8 (Figure 4A; Table S4). Likewise, in the same assay, rontalizumab failed to efficiently neutralize IFNα6, IFNα7 and IFNα10, whereas sifalimumab neutralized several IFNα subtypes only weakly. By contrast, 19D11 neutralized all 12 IFNα subtypes tested (Table S4).
Patient-derived IFN-specific mAbs were also assessed for their capacity to inhibit STAT1 phosphorylation in cells treated with each of 12 IFNα subtypes, IFNω, IFNβ, or IFNγ (Figures 4B, 4C, and 4D). As predicted from the luciferase assay, 19D11 inhibited STAT1 phosphorylation levels (normalized to total STAT1 or tubulin) driven by all IFNα subtypes but did not affect responses to IFNω, IFNβ, or IFNγ. By contrast, 25C3, an additional patient-derived mAb (Table S2), was highly selective for discrete IFNα subtypes, whereas other antibodies tested, including 26B9, showed neutralization profiles between those of 19D11 and 25C3 (Figures 4B–4D). Only 26B9 and 31B4 neutralized IFNω, and none neutralized IFNβ or IFNγ. By comparison, sifalimumab, rontalizumab, and AGS-009 (another IFNα-targeting mAb in clinical development) showed variable and less uniform inhibition of STAT1 phosphorylation induced by different IFNα subtypes (Figure S4A).
Figure S4.
Related to Figure 4
(A) Neutralization of IFNα induced STAT1 signaling by APS/APECED patient-derived and other mAbs.
(B) Neutralization of IL17F, IL22, IL32α, and IL20 by the antibodies indicated as described in Experimental Procedures. For IL20 neutralization, data are shown for cells expressing type I and type II IL20 receptors, respectively.
Error bars denote standard error of the mean of multiple parallel measurements.
The striking biological activities of patient mAbs were not limited to those specific for IFNs in that potent functional target neutralization was shown by mAbs targeting IL17F, IL22, IL32γ, and IL20, respectively (Figure S4B).
Biologically Active Human Antibodies In Vivo
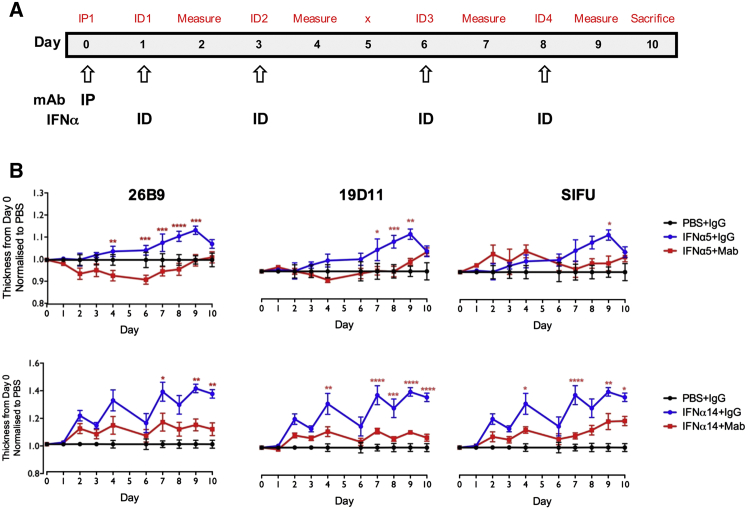
We next asked whether patient autoantibodies could functionally neutralize targets in vivo. To test this, mice were treated intraperitoneally (i.p.) with a single aliquot of antibodies 26B9, 19D11, or sifalimumab, and their ears inoculated intradermally (i.d.) on days 1, 3, 6, and 8 with recombinant human IFNα5 or IFNα14 (Figure 5A) and IFNω (data not shown). Relative to repeated inoculation with vehicle/PBS, the cytokines induced ear swelling, reflecting an inflammatory response that includes rapid TNFα and IFNγ induction (Figures S5A and S5B). This ear swelling was significantly inhibited by single injections of antibodies (Figure 5B). Again, neutralization varied toward the effector IFNα subtype: 26B9 and 19D11, but not sifalimumab, largely ablated the IFNα5 response, whereas all three partially yet significantly limited swelling induced by IFNα14 (Figure 5B).
Figure 5.
Biological Activity of IFN mAbs
(A) Experimental timeline: mAb administered i.p. at day 0; human IFNα administered i.d. on days 1, 3, 6, and 8. Ear thickness measured on all days (prior to cytokine injection) except for day 5.
(B) I.p.-administered IFN mAbs reduced IFNα-induced ear inflammation.
Significance calculated by two-way ANOVA, with ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ∗∗∗∗p ≤ 0.0001. Error bars denote SEM.
Figure S5.
Related to Figure 5
(A) Intradermal injection of human IFNα creates a inflammatory response causing ear swelling.
(B) The inflammatory response caused by i.d. injection of human IFNα caused a significant increase in mRNA levels of TNFα and IFNγ.
Error bars denote standard error of the mean of multiple parallel measurements.
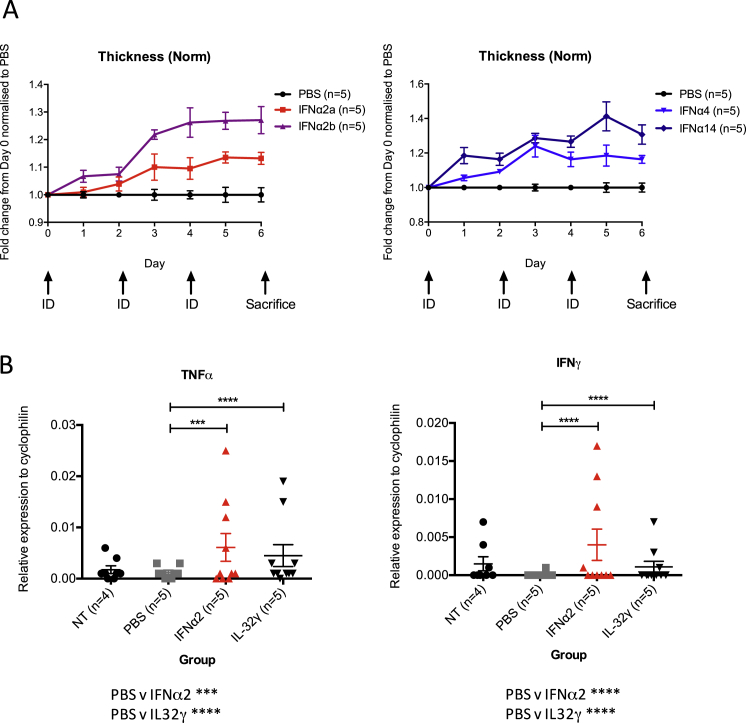
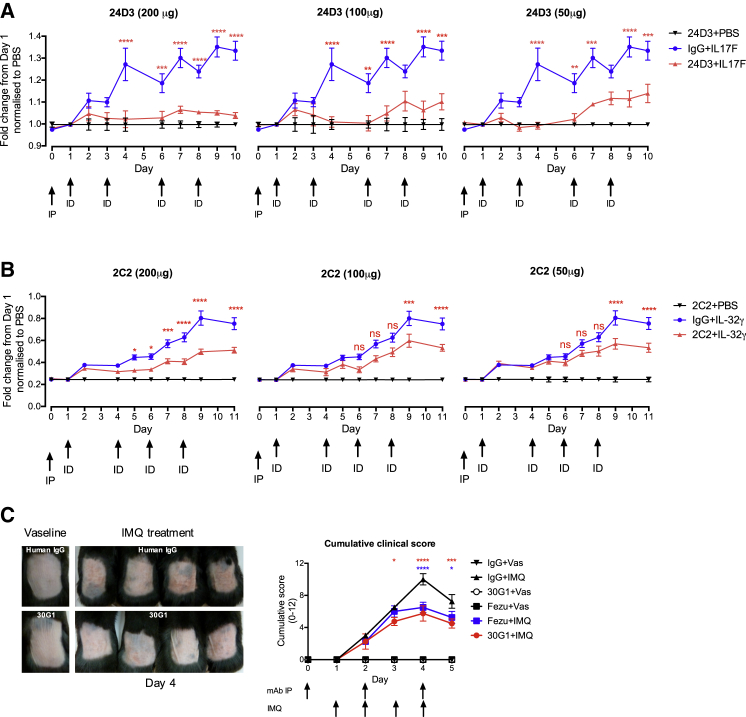
Specific, antibody-mediated neutralization in vivo was likewise seen when the same assay was applied to human IL17F or IL32γ (Figures 6A and 6B). For IL17 neutralization, the data are clearly consistent with the known capacity of APS1/APECED patients’ antibodies to neutralize Th17-family cytokines (Kisand et al., 2010, Puel et al., 2010), thereby predisposing to Candida infection.
Figure 6.
In Vivo Activity of Cytokine-Reactive mAbs
(A) mAb administered i.p. at day 0, and human IL17F administered i.d. on days 1, 3, 6, and 8. Ear thickness measured on all days (prior to cytokine injection) except day 5.
(B) As in (A), but with human IL32γ administered i.d.
(C) anti-IL22-specific mAb injected i.p. into 9-week mice prior to and during IMQ treatment. Efficacy measured by Psoriasis Area and Severity Index (PASI).
Significance calculated by two-way ANOVA, with ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ∗∗∗∗p ≤ 0.0001. Error bars denote SEM.
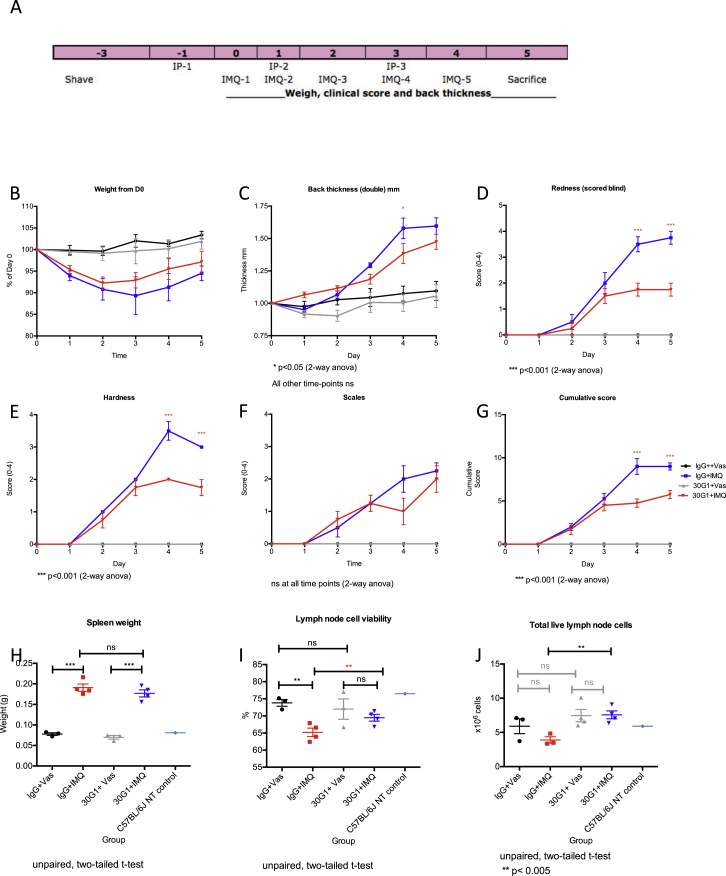
Additionally, the detection of mouse IL22 by antibody 30G1 offered an opportunity to measure its bio-activity toward endogenous IL22, a primary effector of imiquimod (IMQ)-induced dermatitis used to model psoriasis (van der Fits et al., 2009). IMQ-induced pathology measured by modified PASI scoring was significantly ameliorated by 30G1 relative to IgG control, particularly following an initial inflammatory response (Figures 6C and S6). Again, 30G1 was at least as effective as an in-house-expressed anti-IL22 antibody, fezakinumab (see above) (Figure 6C). Collectively these data establish the capacity of patient anti-cytokine antibodies to limit pathologies induced by their targets in vivo.
Figure S6.
Related to Figure 6
(A) Experimental setup of Imiquimod-induced psoriasiform lesion in vivo model. Skin of 9-week-old C57BL/6Jax mice were shaved prior to antibody administration i.p. at day −1, day 1, and day 3. The mice were treated locally with IMQ from day 0 to day 4.
(B) Treatment with IMQ and/or 30G1 does not cause any changes in body weight.
(C–G) efficacy of 30G1 by Psoriasis Area and Severity Index (PASI).
(H) There is no significant difference in spleen weight in mice treated with 30G1 versus IgG.
(I) Lymph node cell viability and (J) total live lymph node cells are significantly different in mice treated with 30G1 versus IgG.
Error bars denote standard error of the mean of multiple parallel measurements.
Clinical Correlates of Neutralizing Antibodies
Given the results from animal models, it was appropriate to consider the potential impact of APS1/APECED antibodies in the patients themselves. Because circulating IFNα levels are extremely low in human peripheral blood, even following vaccination (Sobolev et al., 2016), circulating IFN levels do not offer robust bio-markers of anti-IFNα antibodies. Neither does measurement of interferon-stimulated genes (ISGs) because many, e.g., CXCL10, can be upregulated by type II IFNs (Welcher et al., 2015). By contrast, antibody activities may be reliably reflected in discrete pathologies, as in the correlation of anti-IL22 with candidiasis.
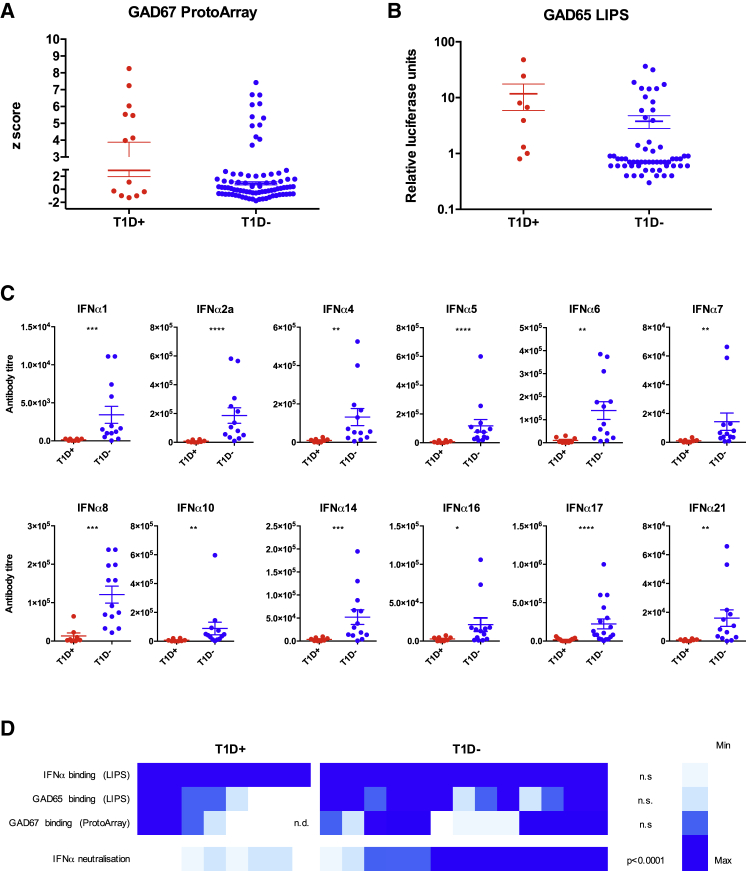
In this regard, many datasets, particularly in mouse models, suggest that type I IFN contributes to type 1 diabetes (T1D) (Carrero et al., 2013, Downes et al., 2010, Foulis et al., 1987, Huang et al., 1995, Li et al., 2008). Although APECED/APS1 patients by definition suffer from polyendocrinopathy, T1D affects only ∼10%–20% of patients and manifests primarily in adulthood (Husebye et al., 2009, Kisand and Peterson, 2015). This is despite the fact that radioimmunoassays have revealed that many APS1/APECED patients carry GAD65-reactive autoantibodies, a clinically applied biomarker for likely onset of T1D (Ziegler et al., 2013). Consistent with this, ProtoArray and LIPS data showed that many patients carried GAD65- and/or GAD67-reactive antibodies, but among them relatively few presented with T1D (red dots, Figures 7A and 7B). Collectively, these many observations suggest that patients at risk of T1D, as judged by anti-GAD65/GAD67, might fail to develop T1D if they harbored powerfully neutralizing anti-IFNα antibodies. Indeed, we reported a seemingly exceptional APS1/APECED patient, completely lacking IFNα-neutralizing antibodies and presenting with T1D (Kisand et al., 2008).
Figure 7.
Clinical Correlation of T1D and IFN Neutralization
(A and B) Seroreactivity to GAD67 and GAD65 measured by ProtoArray and LIPS in APS1/APECED patients with (red) or without (blue) T1D.
(C) IFNα-neutralizing titers in patients with T1D (n = 8) and anti-GAD65 seropositive patients without T1D (n = 13). y axis shows inhibitory concentration IC50 reflecting serum dilutions at which IFN activity was reduced 50%.
(D) Heatmap of seroreactivity toward GAD67, GAD65, and IFNα analyzed by ProtoArray and LIPS combined with neutralization capacity in patients with and without T1D.
Significance calculated by Mann Whitney using GraphPad Prism v.6, with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Error bars denote SEM. Significance values in (D) compare T1D+ and T1D− groups for each parameter.
To investigate this, the 8 patients presenting with T1D (red dots, Figure 7B; mean age ± SD, 48 ± 11 years) were compared with an available cohort of 13 patients without T1D but with strong GAD65 reactivity (relative luciferase units > 5) (blue dots, Figure 7B; mean age ± SD, 31 ± 12 years). Consistent with T1D developing in adult APS1/APECED patients, GAD65 reactivities mostly arose post-adolescence, and hence the patient cohorts comprised 20 adults and one 8 year old.
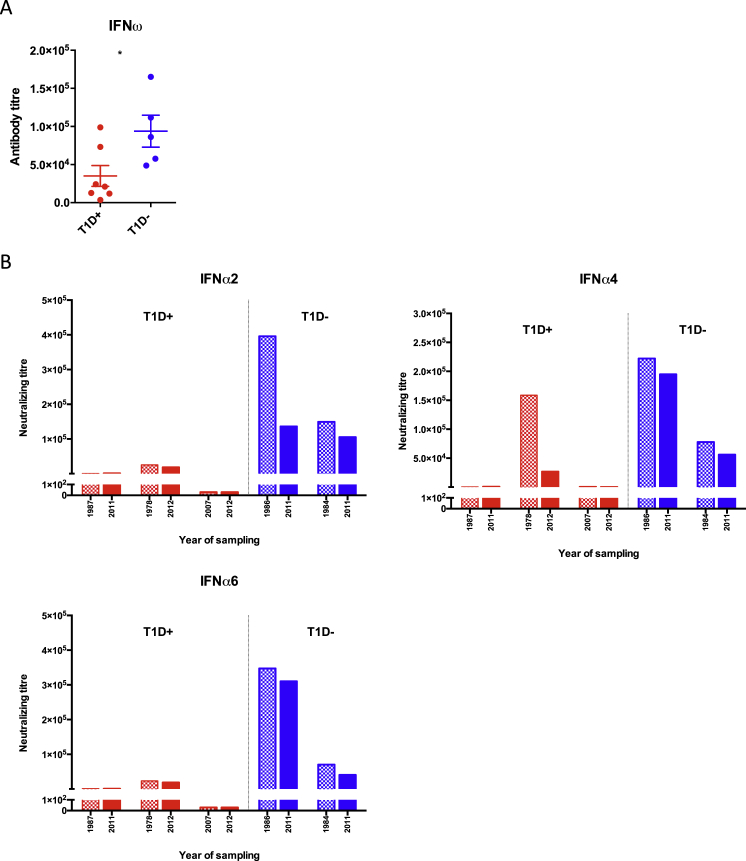
As expected, all 21 patients harbored antibodies to IFNα and IFNω (see Figure 2B), but when tested for IFNα and IFNω neutralization, the antibodies showed a striking segregation with clinical status (Figures 7C, 7D, and S7A): patients without T1D collectively neutralized all IFNα subtypes, whereas those with T1D showed only low or negligible neutralization. Particularly strong differences were seen vis-a-vis IFNα1, IFNα2, IFNα5, IFNα8, IFNα14, and IFNα17 neutralization (Figure 7C). The two subgroups of the 21 patients also showed statistically significant differences in neutralizing IFNω, but the difference was weaker than for IFNα (Figure S7A). Interestingly, the two GAD65-reactive non-diabetics who displayed relatively low IFNα neutralization were young adults who may be en route to developing T1D.
Figure S7.
Related to Figure 7
(A) IFNω-neutralizing titers in APS1/APECED patients with T1D (n = 7) and anti-GAD65 seropositive APS1/APECED patients without T1D (n = 5). y axis represents the neutralizing capacity in inhibitory concentration IC50 showing the serum dilution in which the activity of the IFNω was reduced to its half. ∗p < 0.05. Error bars denote standard error of denoted patient groups.
(B) IFNα-neutralizing titers in longitudinal samples of APS1/APECED patients. Three patients from the T1D+ group (red) and two patients from the T1D− group (blue) were tested in two different time points as indicated on x scale. The latest time point is identical to the one in Figure 7C.
In a small subcohort of GAD65/67-reactive patients for whom longitudinal samples were available, the three T1D patients (red bars) again showed lower IFNα neutralization relative to the two patients without T1D. Moreover, one patient was able to neutralize IFNα4 in 1978 but by 2012 could no longer do so and presented with T1D (Figure S7B).
Such striking correlations with T1D (Figure 7D) were not evident for any other naturally arising anti-cytokine antibodies, supporting the view that IFNα may contribute critically to the natural progression of T1D. Moreover, although the data do not prove that active anti-IFN antibodies underpin selective protection from T1D, they provide a firm foundation for exploring the potentials of APS1/APECED-derived autoantibodies to ameliorate other major diseases that are rarely if ever present in APS1/APECED patients.
Discussion
This analysis of the impact of AIRE deficiency on human B cells has revealed a signature pattern of humoral autoreactivity with general implications for our understanding of autoimmunity. First, the autoantibodies studied were mostly extremely high affinity and specific for native conformational epitopes. These properties were shared by antibodies specific for cytokines targeted by most patients (e.g., IFNα, IL17, IL22) and by antibodies specific for IL20 to which few patients displayed reactivity. Because such properties are very rare among antibodies raised by immunization, when B cells are primed de novo to antigen for short periods of time, it seems inappropriate to continue to model one type of mAb on the other.
Second, essentially all 81 APS1/APECED patients studied showed strong reactivities toward a common set of 10–15 proteins, coupled with patient-specific reactivity profiles toward 80–90 additional proteins. This limited frequency (< 1% of proteins displayed on the array) is consistent with a recent report that B cell tolerance was not globally disrupted in 51 APS1/APECED patients sampled (Landegren et al., 2016). Nonetheless, the patient-to-patient variation in reactivity profiles meant that the 97 sera analyzed in our study collectively harbored antibodies toward over 3,500 proteins.
The patient-to-patient variation argues that B cell autoimmunity resulting from AIRE deficiency is not simply an amplification of sporadic, low-level autoreactivities seen in healthy controls but has distinct origins. By this perspective, defects in central T cell tolerance may underpin other autoimmune and autoinflammatory pathologies attributed to high-affinity autoantibodies. Whereas this contrasts with the widely held view that autoimmune diseases mostly reflect peripheral tolerance defects, it aligns with data that central tolerance defects contribute to the NOD mouse model of T1D (Geng et al., 1998, Zucchelli et al., 2005). Moreover, wherever autoantibodies reflect central T cell tolerance defects, donor-to-donor variation is to be expected, as individuals will generate distinct TCR repertoires via quasi-random gene rearrangements, will be exposed to different physiologic and environmental triggers that promote the selective outgrowth of autoreactive T cell clones, and will differ in immune response modifier genes (e.g., HLA) that regulate the magnitude of antigen-specific responses.
Autoantibodies to some non-tissue-restricted antigens, including multiple type I IFNα subtypes, are displayed by almost all patients, sometimes early post-partum (Wolff et al., 2013). Most likely, the immunogenicity of these proteins arises by mechanisms distinct from those shaping patient-specific autoantibody repertoires. Possibly the public autoantibodies arise from a direct impact of AIRE deficiency on B cell tolerance, for example, via the dysregulation of AIRE-expressing thymic B cells that resemble GC B cells by several criteria (Yamano et al., 2015). Arguing against this, however, autoantibodies to type I IFNs, Th17 cytokines, and additional self-proteins are found in thymoma patients with AIRE-sufficient B cells (Kisand et al., 2011, Meager et al., 1997, Wolff et al., 2014). This likewise argues against autoantibodies to type I IFNs and Th17 cytokines originating from defects in lymph node AIRE+ cells termed eTACs (Gardner et al., 2008). Although studies in mice have suggested tolerizing roles of eTACs, the functions of their rare human counterparts are unknown (Poliani et al., 2010).
AIRE deficiency may, however, act indirectly on thymic B cells, for example by hyperactivity of functionally competent thymic γδ cells (Ribot et al., 2009) that may likewise be dysregulated in thymoma. Such cells may create an intra-thymic milieu favoring priming rather than tolerance of thymic B cells toward proteins highly expressed in the thymus (Dudakov et al., 2012, Meager et al., 2006).
Notwithstanding this possibility, our findings suggest that high-affinity autoantibodies in APS1/APECED patients probably reflect dysregulated GC reactions, wherein autoreactive T cells, e.g., Tfh cells, that were not tolerized in the thymus promote the competitive outgrowth and affinity maturation of B cells that were initially primed to exogenous antigen(s) but whose mutated IgGs bind to self-proteins. Consistent with this, autoantibodies targeting thyroid-stimulating hormone receptor in Graves’ disease cross-react to Yersinia enterocolitica antigens (Brink, 2014, Hargreaves et al., 2013), and activated peripheral blood Tfh cells correlate positively with serum autoantibodies and disease activity/severity in multiple autoimmune diseases (Ueno et al., 2015). Although our analysis of four adult and four pediatric APS1/APECED patients revealed no alterations in Tfh cell numbers relative to age-matched healthy controls, this did not exclude Tfh cells being enriched in autoreactive specificities. Moreover, no patients displayed neutralizing autoantibodies to IL21, a major mediator of Tfh cells in the GC.
This etiology of APS1/APECED B cell autoimmunity is strikingly similar to proposed origins of highly mutated anti-desmoglein-3 antibodies in autoimmune pemphigus (Di Zenzo et al., 2012) and of anti-GM-CSF antibodies pathognomonic in pulmonary alveolar proteinosis (Piccoli et al., 2015). In those studies, as in this, the closest germline counterparts (“unmutated common ancestors” [UCAs]) showed no reactivity toward the targets of the affinity-matured autoantibodies. By contrast, germline versions of antiviral antibodies showed only slightly reduced binding to target viral antigens (Corti et al., 2011, Corti et al., 2013). Moreover, it is not the case that UCAs intrinsically lack autoreactivity, as germline counterparts of some autoantibodies with few replacement mutations showed autoantigen reactivity in pemphigus patients (Cho et al., 2014). The underlying defect(s) in T cell tolerance that dysregulate affinity maturation in pemphigus, pulmonary alveolar proteinosis, and other organ-specific autoimmune diseases may be limited to few antigens, by contrast to broad-spectrum defects in APS1/APECED.
That almost all APS1/APECED-derived mAbs were biologically active in vivo against a range of cytokine targets has profound implications for patients. Clearly, immune-effector responses may be reduced, as in the association of anti-IL22 with susceptibility to Candidiasis (Kisand et al., 2010). Likewise, gut barrier integrity may be compromised, leading to increased levels of anti-commensal antibodies (Hetemäki et al., 2016). Conversely, despite the common neutralization of IFNα and IFNω, APS1/APECED patients do not show severe viral infections, as were recently reported for a child genetically impaired in type I IFN (Ciancanelli et al., 2015). Possibly preserved IFNβ function mediates anti-viral protection in APS1/APECED patients.
On the other hand, some autoantibodies may target key mediators of immunopathologies, thereby ameliorating disease. Thus, a unique correlation was observed between antibody-mediated neutralization of IFNα and failure to develop T1D, providing a novel strand of support for animal studies arguing that targeting type I IFNs could be effective in T1D. The concept that naturally arising autoantibodies may be beneficial is not widely considered, despite its underpinning the widespread use of therapeutic mAbs. In this regard, it is striking that despite their severe flaws in central T cell tolerance, APS1/APECED patients do not present with systemic sclerosis, Sjögren’s syndrome, MS, or SLE. These pathologies are considered to involve interplays of IL17/Th17 and type I IFNs—two main targets of APS1/APECED autoantibodies (Ambrosi et al., 2012). Likewise, Th17-driven psoriasis was diagnosed in only two of our patients, each of whom lacked autoantibodies to IL17A, IL17F, and IL22 (our unpublished data). Furthermore, atopy/allergy is seemingly rare among APS1/APECED patients, although whether anti-IL5 antibodies underpin this requires more study. For now, the data presented by this study strongly suggest that antibodies recovered from APS1/APECED patients include ones with profound therapeutic and diagnostic potential.
Experimental Procedures
More details are available in the Supplemental Experimental Procedures.
Human Samples
Eighty-one APS1/APECED patients were diagnosed by mutational analysis of AIRE and by autoantibodies to type I IFNs. All provided informed consent, and many were analyzed previously (Kisand et al., 2011, Kluger et al., 2015, Meloni et al., 2012, Wolff et al., 2007). Approvals by local ethics committees are described in the Supplemental Experimental Procedures. Ages at serum sampling were 4–73 years; mean = 31.9. For protoarray there were 12 age-matched controls and 9 healthy first-degree relatives, and there were additional healthy controls for LIPS and ELISA.
Immune Response Profiling by ProtoArray
Sera of patients, healthy relatives, and controls were tested against > 9,000 human proteins displayed on the Human Protein Microarray v5.1 (ThermoFisher Scientific). Preprocessing methods were applied to account for technical variability. First, corresponding local background intensity was subtracted, whereafter values were log-transformed and subjected to robust linear normalization (Sboner et al., 2009). Z scores were calculated as the number of standard deviations of the signal from the mean of the corresponding controls and healthy relatives; Z ≥ 3 was considered positive. After scoring, stringent quality assessment was undertaken, including high correlation coefficients of duplicate spots of printed proteins (average r = 0.92), reactivity toward known autoantibody targets, and perfect correlation of signals for proteins spotted in different locations. Printing contaminants were identified as proteins showing high correlation coefficients with known APECED antibody targets and were further verified by cross-reference to another protoarray (5.0) used for 23 patients and 7 controls. Thus, 31 suspect false-positives were identified and excluded from further consideration.
Antibody Isolation and Cloning
Cloning, production, and purification of human mAbs were performed as described (patent application WO2013/098419). In brief, memory B cells (CD22+, IgD−, IgM−, CD3−, CD8−, and CD54−) were flow-sorted (MoFlo) from patient PBMC, incubated transiently with EBV-containing B95-8 supernatant (SN) for 3.5 hr at 37°C, and then incubated in Transferrin- and CpG- supplemented IMDM at 37°C, 5% CO2, at 10 cells/well in 96-well plates coated with irradiated PBMC feeders. Short-term, oligoclonal B cell culture SN were analyzed for IgG and antigen-specific antibodies detected by ELISA and/or LIPS. Positive wells were harvested, cells single-cell-sorted into reverse transcriptase (RT) buffer (Life Technologies), and RT-PCR performed using Superscript III (Life Technologies) and random hexamers. IgG VH, Vλ, and Vκ regions were amplified from cDNA by two-step nested PCR reaction using Advantage 2 cDNA polymerase (Clontech) and primer mixes specific for germline families (VBASE database). Nested primers attached restriction sites for V-region cloning into expression vectors providing IgG1, Ig-κ, or Ig-λ constant regions. Recombinant antibodies were produced in HEK293T cells and antigen specificity analyzed by ELISA. Corresponding closest germline region sequences were identified using the VBASE2 database (Retter et al., 2005). CDRs were identified by IMGT definitions (Lefranc, 2003).
Complete Ig-VH and VL regions described in US7741449 (Sifalimumab), US7087726 B2 (Rontalizumab), US8361463 (ACO-1), and US20070258982 A1 (Fezakinumab) were ordered as CHO-codon-optimized synthetic constructs (GenScript) and expressed as above.
mAb Characterization In Vitro
EC50 binding of mAbs was determined by ELISA. Neutralizing capacities of type I IFN-specific mAbs were studied using phospho-STAT1 quantification in immunoblot and ISRE-luciferase reporter assay. IL17F, IL22, IL20, and IL32 neutralization assays were performed on respective responsive cell lines. mAB affinities were measured with a Biacore T200 (GE Healthcare). Epitope mapping used overlapping 18-mer peptides.
mAb Characterization In Vivo
C57BL/6J (WT; from Charles River) mice were administered i.p. with mAbs (day 0) and inoculated i.d. on days 1, 3, 6, and 8 with cognate human cytokines, IFNα2a, IFNα2b, IFNα4, IFNα14, IL17F, and IL32γ, and their ear thicknesses measured with a micrometer. For IL22 mAbs cross-reactive to mouse, bioactivity was assessed in imiquimod-treated mice.
Consortia
The members of the APECED patient collaborative are Antonella Meloni, Nicolas Kluger, Eystein S. Husebye, Katarina Trebusak Podkrajsek, Tadej Battelino, Nina Bratanic, and Aleksandr Peet.
Author Contributions
S.M., A.M., and S.C.O. cloned monoclonal antibodies from patient samples, and K.J. and K.H. assisted. S.M., P.V., and A.M. characterized antibodies in vitro; M.W. and Y.H. did so in vivo. C.H. analyzed ProtoArray data and wrote and edited the paper. J.K. assayed neutralization by sera and Tfh subsets and performed LIPS. D.F. and H.P. analyzed ProtoArray data. K.M. and R.U. screened sera for T1D autoantibodies and tested germline antibody specificities. K. Krohn and A.R. developed the clinical database, sampled Finnish patients, and employed ELISA. A.S.B.W. sampled Norwegian patients, contributed to the clinical database, and assayed antibodies. APECED patient collaborative contributed to the clinical database and sampled respective patients. P.P., K. Kisand, and A.H. supervised research, reviewed data, and wrote and edited the paper.
Acknowledgments
We are indebted to patients; to the Finnish APECED and Addison patients’ association; and to attending physicians and carers. We thank M. Rothe, P. Adler, A. Remm, M. Pihlap, M. Karlsberg, A. Tallqvist, M. Tuukkanen, L. Prassmayer, M. Wördehoff, A. Peters, R. Repke, B. Mathis, and particularly E. Stuart and K. Henco for critical insight and support. We thank staff of the Biological Services Unit at King's College London. Funding was by the following: ImmunoQure AG, the Wellcome Trust, and CRUK (to A.H.) and Estonian Research Council grant IUT2-2 and European Union Project 2014-2020.4.01.15-0012 (to J.K., P.P., and K. Kisand). P.P., K. Krohn, K. Kisand, A.R., and A.H. are cofounders and shareholders of ImmunoQure AG, and A.M., C.H., P.V., S.C.O., and S.M. were/are employees of ImmunoQure AG.
Published: July 14 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2016.06.024.
Contributor Information
Kai Kisand, Email: kai.kisand@ut.ee.
Adrian Hayday, Email: adrian.hayday@kcl.ac.uk.
APECED patient collaborative:
Antonella Meloni, Nicolas Kluger, Eystein S. Husebye, Katarina Trebusak Podkrajsek, Tadej Battelino, Nina Bratanic, and Aleksandr Peet
Supplemental Information
References
- Ambrosi A., Espinosa A., Wahren-Herlenius M. IL-17: a new actor in IFN-driven systemic autoimmune diseases. Eur. J. Immunol. 2012;42:2274–2284. doi: 10.1002/eji.201242653. [DOI] [PubMed] [Google Scholar]
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Brink R. The imperfect control of self-reactive germinal center B cells. Curr. Opin. Immunol. 2014;28:97–101. doi: 10.1016/j.coi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Carrero J.A., Calderon B., Towfic F., Artyomov M.N., Unanue E.R. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS ONE. 2013;8:e59701. doi: 10.1371/journal.pone.0059701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M.J., Lo A.S., Mao X., Nagler A.R., Ellebrecht C.T., Mukherjee E.M., Hammers C.M., Choi E.J., Sharma P.M., Uduman M. Shared VH1-46 gene usage by pemphigus vulgaris autoantibodies indicates common humoral immune responses among patients. Nat. Commun. 2014;5:4167. doi: 10.1038/ncomms5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancanelli M.J., Huang S.X., Luthra P., Garner H., Itan Y., Volpi S., Lafaille F.G., Trouillet C., Schmolke M., Albrecht R.A. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D., Voss J., Gamblin S.J., Codoni G., Macagno A., Jarrossay D., Vachieri S.G., Pinna D., Minola A., Vanzetta F. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- Corti D., Bianchi S., Vanzetta F., Minola A., Perez L., Agatic G., Guarino B., Silacci C., Marcandalli J., Marsland B.J. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501:439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- Di Zenzo G., Di Lullo G., Corti D., Calabresi V., Sinistro A., Vanzetta F., Didona B., Cianchini G., Hertl M., Eming R. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J. Clin. Invest. 2012;122:3781–3790. doi: 10.1172/JCI64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes K., Pekalski M., Angus K.L., Hardy M., Nutland S., Smyth D.J., Walker N.M., Wallace C., Todd J.A. Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS ONE. 2010;5:e12646. doi: 10.1371/journal.pone.0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov J.A., Hanash A.M., Jenq R.R., Young L.F., Ghosh A., Singer N.V., West M.L., Smith O.M., Holland A.M., Tsai J.J. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulis A.K., Farquharson M.A., Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987;2:1423–1427. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- Gardner J.M., Devoss J.J., Friedman R.S., Wong D.J., Tan Y.X., Zhou X., Johannes K.P., Su M.A., Chang H.Y., Krummel M.F., Anderson M.S. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L., Solimena M., Flavell R.A., Sherwin R.S., Hayday A.C. Widespread expression of an autoantigen-GAD65 transgene does not tolerize non-obese diabetic mice and can exacerbate disease. Proc. Natl. Acad. Sci. USA. 1998;95:10055–10060. doi: 10.1073/pnas.95.17.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C., Vinuesa C.G., Randall K.L., Mackay F., Brink R. Control systems and decision making for antibody production. Nat. Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- Hargreaves C.E., Grasso M., Hampe C.S., Stenkova A., Atkinson S., Joshua G.W., Wren B.W., Buckle A.M., Dunn-Walters D., Banga J.P. Yersinia enterocolitica provides the link between thyroid-stimulating antibodies and their germline counterparts in Graves’ disease. J. Immunol. 2013;190:5373–5381. doi: 10.4049/jimmunol.1203412. [DOI] [PubMed] [Google Scholar]
- Hetemäki I., Jarva H., Kluger N., Baldauf H.M., Laakso S., Bratland E., Husebye E.S., Kisand K., Ranki A., Peterson P., Arstila T.P. Anticommensal responses are associated with regulatory T cell defect in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. J. Immunol. 2016;196:2955–2964. doi: 10.4049/jimmunol.1500301. [DOI] [PubMed] [Google Scholar]
- Huang X., Yuang J., Goddard A., Foulis A., James R.F., Lernmark A., Pujol-Borrell R., Rabinovitch A., Somoza N., Stewart T.A. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44:658–664. doi: 10.2337/diab.44.6.658. [DOI] [PubMed] [Google Scholar]
- Husebye E.S., Perheentupa J., Rautemaa R., Kämpe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J. Intern. Med. 2009;265:514–529. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen T., Chamberlain N., Morbach H., Choi J., Kim S., Craft J., Mayer L., Cancrini C., Passerini L., Bacchetta R. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121:1595–1603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K., Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J. Clin. Immunol. 2015;35:463–478. doi: 10.1007/s10875-015-0176-y. [DOI] [PubMed] [Google Scholar]
- Kisand K., Link M., Wolff A.S., Meager A., Tserel L., Org T., Murumägi A., Uibo R., Willcox N., Trebusak Podkrajsek K. Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes. Blood. 2008;112:2657–2666. doi: 10.1182/blood-2008-03-144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K., Bøe Wolff A.S., Podkrajsek K.T., Tserel L., Link M., Kisand K.V., Ersvaer E., Perheentupa J., Erichsen M.M., Bratanic N. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K., Lilic D., Casanova J.L., Peterson P., Meager A., Willcox N. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur. J. Immunol. 2011;41:1517–1527. doi: 10.1002/eji.201041253. [DOI] [PubMed] [Google Scholar]
- Klein L., Kyewski B., Allen P.M., Hogquist K.A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat. Rev. Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger N., Jokinen M., Lintulahti A., Krohn K., Ranki A. Gastrointestinal immunity against tryptophan hydroxylase-1, aromatic L-amino-acid decarboxylase, AIE-75, villin and Paneth cells in APECED. Clin. Immunol. 2015;158:212–220. doi: 10.1016/j.clim.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Krohn K., Uibo R., Aavik E., Peterson P., Savilahti K. Identification by molecular cloning of an autoantigen associated with Addison’s disease as steroid 17 alpha-hydroxylase. Lancet. 1992;339:770–773. doi: 10.1016/0140-6736(92)91894-e. [DOI] [PubMed] [Google Scholar]
- Landegren N., Sharon D., Freyhult E., Hallgren Å., Eriksson D., Edqvist P.-H., Bensing S., Wahlberg J., Nelson L.M., Gustafsson J. Proteome-wide survey of the autoimmune target repertoire in autoimmune polyendocrine syndrome type 1. Sci. Rep. 2016;6:20104. doi: 10.1038/srep20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc M.P. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2003;31:307–310. doi: 10.1093/nar/gkg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Xu B., Michie S.A., Rubins K.H., Schreriber R.D., McDevitt H.O. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D., Benoist C. Aire. Annu. Rev. Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- Meager A., Vincent A., Newsom-Davis J., Willcox N. Spontaneous neutralising antibodies to interferon—alpha and interleukin-12 in thymoma-associated autoimmune disease. Lancet. 1997;350:1596–1597. doi: 10.1016/s0140-6736(05)64012-3. [DOI] [PubMed] [Google Scholar]
- Meager A., Visvalingam K., Peterson P., Möll K., Murumägi A., Krohn K., Eskelin P., Perheentupa J., Husebye E., Kadota Y., Willcox N. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre E., Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Meloni A., Willcox N., Meager A., Atzeni M., Wolff A.S., Husebye E.S., Furcas M., Rosatelli M.C., Cao A., Congia M. Autoimmune polyendocrine syndrome type 1: an extensive longitudinal study in Sardinian patients. J. Clin. Endocrinol. Metab. 2012;97:1114–1124. doi: 10.1210/jc.2011-2461. [DOI] [PubMed] [Google Scholar]
- Nagamine K., Peterson P., Scott H.S., Kudoh J., Minoshima S., Heino M., Krohn K.J., Lalioti M.D., Mullis P.E., Antonarakis S.E. Positional cloning of the APECED gene. Nat. Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- Piccoli L., Campo I., Fregni C.S., Rodriguez B.M., Minola A., Sallusto F., Luisetti M., Corti D., Lanzavecchia A. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat. Commun. 2015;6:7375. doi: 10.1038/ncomms8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Mattoo H., Cariappa A. B cells and autoimmunity. Curr. Opin. Immunol. 2011;23:721–731. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliani P.L., Kisand K., Marrella V., Ravanini M., Notarangelo L.D., Villa A., Peterson P., Facchetti F. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. Am. J. Pathol. 2010;176:1104–1112. doi: 10.2353/ajpath.2010.090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Döffinger R., Natividad A., Chrabieh M., Barcenas-Morales G., Picard C., Cobat A., Ouachée-Chardin M., Toulon A., Bustamante J. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retter I., Althaus H.H., Münch R., Müller W. VBASE2, an integrative V gene database. Nucleic Acids Res. 2005;33:D671–D674. doi: 10.1093/nar/gki088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot J.C., deBarros A., Pang D.J., Neves J.F., Peperzak V., Roberts S.J., Girardi M., Borst J., Hayday A.C., Pennington D.J., Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sboner A., Karpikov A., Chen G., Smith M., Mattoon D., Freeman-Cook L., Schweitzer B., Gerstein M.B. Robust-linear-model normalization to reduce technical variability in functional protein microarrays. J. Proteome Res. 2009;8:5451–5464. doi: 10.1021/pr900412k. [DOI] [PubMed] [Google Scholar]
- Sobolev O., Binda E., O’Farrell S., Lorenc A., Pradines J., Huang Y., Duffner J., Schulz R., Cason J., Zambon M. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat. Immunol. 2016;17:204–213. doi: 10.1038/ni.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Übelhart R., Jumaa H. Autoreactivity and the positive selection of B cells. Eur. J. Immunol. 2015;45:2971–2977. doi: 10.1002/eji.201444622. [DOI] [PubMed] [Google Scholar]
- Ueno H., Banchereau J., Vinuesa C.G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uibo R., Aavik E., Peterson P., Perheentupa J., Aranko S., Pelkonen R., Krohn K.J. Autoantibodies to cytochrome P450 enzymes P450scc, P450c17, and P450c21 in autoimmune polyglandular disease types I and II and in isolated Addison’s disease. J. Clin. Endocrinol. Metab. 1994;78:323–328. doi: 10.1210/jcem.78.2.8106620. [DOI] [PubMed] [Google Scholar]
- van der Fits L., Mourits S., Voerman J.S., Kant M., Boon L., Laman J.D., Cornelissen F., Mus A.M., Florencia E., Prens E.P., Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Welcher A.A., Boedigheimer M., Kivitz A.J., Amoura Z., Buyon J., Rudinskaya A., Latinis K., Chiu K., Oliner K.S., Damore M.A. Blockade of interferon-gamma normalizes interferon-regulated gene expression and serum CXCL10 levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2015;67:2713–2722. doi: 10.1002/art.39248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin R.S., Ramsdell F., Peake J., Faravelli F., Casanova J.L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Winqvist O., Gustafsson J., Rorsman F., Karlsson F.A., Kämpe O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison’s disease. J. Clin. Invest. 1993;92:2377–2385. doi: 10.1172/JCI116843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A.S., Erichsen M.M., Meager A., Magitta N.F., Myhre A.G., Bollerslev J., Fougner K.J., Lima K., Knappskog P.M., Husebye E.S. Autoimmune polyendocrine syndrome type 1 in Norway: phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene. J. Clin. Endocrinol. Metab. 2007;92:595–603. doi: 10.1210/jc.2006-1873. [DOI] [PubMed] [Google Scholar]
- Wolff A.S., Sarkadi A.K., Maródi L., Kärner J., Orlova E., Oftedal B.E., Kisand K., Oláh E., Meloni A., Myhre A.G. Anti-cytokine autoantibodies preceding onset of autoimmune polyendocrine syndrome type I features in early childhood. J. Clin. Immunol. 2013;33:1341–1348. doi: 10.1007/s10875-013-9938-6. [DOI] [PubMed] [Google Scholar]
- Wolff A.S., Kärner J., Owe J.F., Oftedal B.E., Gilhus N.E., Erichsen M.M., Kämpe O., Meager A., Peterson P., Kisand K. Clinical and serologic parallels to APS-I in patients with thymomas and autoantigen transcripts in their tumors. J. Immunol. 2014;193:3880–3890. doi: 10.4049/jimmunol.1401068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T., Nedjic J., Hinterberger M., Steinert M., Koser S., Pinto S., Gerdes N., Lutgens E., Ishimaru N., Busslinger M. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity. 2015;42:1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Ziegler A.G., Rewers M., Simell O., Simell T., Lempainen J., Steck A., Winkler C., Ilonen J., Veijola R., Knip M. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchelli S., Holler P., Yamagata T., Roy M., Benoist C., Mathis D. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.