Abstract
The adult heart is able to activate cardioprotective programmes and modifies its architecture in response to physiological or pathological changes. While mammalian cardiac remodelling often involves hypertrophic expansion, the adult zebrafish heart exploits hyperplastic growth. This capacity depends on the responsiveness of zebrafish cardiomyocytes to mitogenic signals throughout their entire life. Here, we have examined the role of inflammation on the stimulation of cell cycle activity in the context of heart preconditioning and regeneration. We used thoracotomy as a cardiac preconditioning model and cryoinjury as a model of cardiac infarction in the adult zebrafish. First, we performed a spatio-temporal characterization of leucocytes and cycling cardiac cells after thoracotomy. This analysis revealed a concomitance between the infiltration of inflammatory cells and the stimulation of the mitotic activity. However, decreasing the immune response using clodronate liposome injection, PLX3397 treatment or anti-inflammatory drugs surprisingly had no effect on the re-entry of cardiac cells into the cell cycle. In contrast, reducing inflammation using the same strategies after cryoinjury strongly impaired cardiac cell mitotic activity and the regenerative process. Taken together, our results show that, while the immune response is not necessary to induce cell-cycle activity in intact preconditioned hearts, inflammation is required for the regeneration of injured hearts in zebrafish.
Keywords: thoracotomy, cryoinjury, cardiac muscle, cardiomyocyte, non-mammalian animal model, leucocytes
1. Introduction
Preconditioning refers to the induction of cellular survival and pro-regenerative programmes by brief exposure to non-lethal noxious stimuli, which subsequently increase the resistance of tissues to further harmful injuries. Our laboratory has recently developed two independent models of cardiac preconditioning in the adult zebrafish. In the first model, we used an incision through the chest (thoracotomy) as a preconditioning stimulus. Our second model used a more remote stimulus. In this case, cardioprotection is elicited by the intraperitoneal injection of immunogenic particles [1]. In contrast to mammalian models of cardiac preconditioning, thoracotomy or injection of immunogenic particles not only invoked the expression of cardioprotective genes but also increased the mitotic activity in the adult ventricle, leading to a long-term architectural modification of the ventricle, illustrated by a thickening of the compact myocardium wall [1]. Similar to mammals, preconditioning of the adult zebrafish heart enhances regeneration. When fish are subjected to preconditioning within the week preceding the heart injury, the re-entry of cardiomyocytes (CMs) into the cell cycle is more efficient; CM dedifferentiation and cell survival are promoted [1].
Although preconditioning has proved to be remarkably reproducible in human ischaemic disorders, the underlying triggering mechanisms are still unclear [2,3]. The current hypothesis suggests that both neural and humoral aspects are at stake to elicit this phenomenon. The humoral hypothesis is supported by studies performed in rabbits or rats, where the protected phenotype could be transferred from preconditioned to naive individuals by blood transfusion [4,5]. Several effector molecules, such as bradykinin, hypoxia-inducible factor 1α (HIF-1α) or stromal-derived factor 1α (SDF-1α), have been identified by proteomic approaches; however, their use as a preconditioning factor did not give convincing results [6]. An explanation for these disappointing outcomes might come from the multifactorial nature of preconditioning [2,6,7]. For that reason, studying an upstream stimulus acting at a systemic level, such as inflammation, could be of great interest to reach a better understanding of the mechanisms underlying preconditioning.
In contrast to mammals, zebrafish are able to efficiently repair their damaged myocardium. Fibrosis is only transient, and the injured cardiac muscle is fully reconstituted by new CMs within 30–60 days [8,9]. This spectacular regenerative capacity depends on the remarkable responsiveness of zebrafish CMs to mitogenic signals throughout their adult life [8,10]. Even though many local paracrine factors promoting CM proliferation after heart injury have already been described [11–16], the role of systemic factors such as innervation or inflammation on zebrafish CM proliferation has only recently been addressed [17–19].
The innate immune system, which comprises neutrophils and macrophages, is the first defence against harmful factors and rapidly responds to signals released from damaged tissues. According to current evidence, preconditioning stimuli can activate resident phagocytic cells and cause the release of pro-inflammatory cytokines to induce the pro-survival programmes [20,21]. Nevertheless, the role of the immune response on heart preconditioning is still under debate. Contradictory results have been obtained so far, showing both a pro- and an anti-inflammatory effect of remote cardiac conditioning [22]. The exact role of inflammation on the induction of a protected phenotype in the heart needs therefore to be further investigated. Interestingly, the function of the immune response is also under debate in the context of cardiac injury. Decreasing inflammation has led to both beneficial and damaging effects on cardiac repair programmes [23,24].
In this study, we proposed to examine the role of inflammation in both heart preconditioning and regeneration. In the first part of the study, we used a thoracotomy model to investigate the mechanisms of heart preconditioning after a systemic challenge without any physical damage to the myocardium. In contrast to mammals, the systemic reaction set in motion after preconditioning in the zebrafish promoted the re-entry of cardiac cells into the cell cycle and led to long-term morphological modifications of the myocardium [1]. To address the involvement of inflammation in this phenomenon, we first characterized the spatio-temporal dynamics of leucocytes and mitotic cardiac cells after thoracotomy. We observed a correlation between the CM cell-cycle activity and the presence of leucocytes in preconditioned hearts. Unexpectedly, decreasing the immune response after thoracotomy by means of several distinct approaches had no effect on the re-entry of cardiac cells into the cell cycle, indicating that leucocytes do not act directly to promote the mitotic activity observed in preconditioned intact hearts. In the second part, we used cryoinjury as a model of cardiac infarction to assess the role of inflammation in cardiac regeneration. In regenerating hearts, we observed a similar correlation between the presence of leucocytes and cell-cycle activity to that after cardiac preconditioning. However, the analysis of cryoinjured hearts revealed an important detrimental impact of a decreased immune response on both cell mitotic activity and repair of the cryoinjured area, suggesting that immune cells are in this context a prerequisite for an efficient regeneration.
2. Material and methods
2.1. Animal procedures
This work was performed with fully grown adult fish at the age of 12–24 months. Wild-type fish were AB (Oregon), transgenic fish lines were cmlc2:DsRed2-nuc, CD41:EGFP and mpeg1:EGFP [25–27]. Before every procedure, fish were anaesthetized in 0.1% tricaine (Sigma-Aldrich). Chest wound injuries were performed as described previously [9,28]. Briefly, fish were placed ventral side up on a damp sponge, and a small incision was made through the thorax skin with iridectomy scissors. Cryoinjuries were performed as described previously [9,28]. The phagocyte population was depleted by intraperitoneal injection of 5 µl suspension of clodronate- (5 mg ml−1) or PBS-containing liposomes (Encapsula NanoSciences LLC) 24 h before the thoracotomy or cryoinjury procedure. The injection was repeated at 4 days post-thoracotomy (dpt) or at 4 days post-cryoinjury (dpci) to ensure a constant low level of phagocytes in clodrosome-injected fish. The inflammatory response was blocked by immersing fish in 0.05% DMSO, 500 nM PLX3397 (Plexxikon), 5 µM flumethasone (Santa Cruz Biotechnologies) or 10 µM pranlukast (Sigma-Aldrich). For all drug treatments, fish were immersed 3 days before the thoracotomy or the cryoinjury and maintained under those conditions for the following 7 days. During all treatments, fish were fed, and solutions were changed every third day. For L-plastin staining, 5 µl of 2% paraformaldehyde was injected into the thorax of euthanized animals 15 min before heart collection. This prefixation was performed to avoid corruption of the results by immune cells that are carried in the blood and that adhere to the surface of the ventricle just at the moment of heart collection. The cantonal veterinary office of Fribourg approved this experimental research on animals.
2.2. Immunohistochemistry and histology
At the end of each experiment, the hearts were collected and fixed overnight at 4°C in 2% paraformaldehyde. They were then rinsed in PBS and equilibrated in 30% sucrose before embedding in tissue-Tek OCT compound (Sakura Finetek Europe B.V.) and cryosectioned at a thickness of 16 µm. The immunohistochemistry procedures were performed as previously described [9]. The following primary antibodies were used: chicken anti-L-plastin at 1 : 500 (kindly provided by P. Martin, Bristol) [29], rabbit anti-MCM5 at 1 : 5000 (kindly provided by Soojin Ryu, Heidelberg), rabbit anti-DsRed (Clonetech, 632496) at 1 : 200 and mouse anti-tropomyosin (developed by Jim Jung-Ching Lin and obtained from the Developmental Studies Hybridoma Bank, University of Iowa). The Alexa-Fluor-conjugated secondary antibodies (Jackson Immunoresearch) were used at 1 : 500, and DAPI was used at 1 : 2000. Aniline blue, acid Fuchsin and Orange G (AFOG) staining were performed as previously described [16].
2.3. Image analysis and quantification
After antibody staining, cardiac tissue imaging was performed at 20× magnification with confocal microscopes (Leica TCS-SP5 and Leica TCS-SPE-II). At least three different pictures were taken for each heart. n represents the number of fish used in the experiment. ImageJ software was used to perform the subsequent image analysis. The number of proliferating non-CM nuclei was obtained by subtracting the number of cmlc2:DsRed2-nuc-positive nuclei from the total number of nuclei. To quantify the number of proliferating nuclei, the images of cell cycle marker (MCM5) were superimposed with the images of nuclei markers (DAPI; cmlc2:DsRed2-nuc). L-plastin-positive area was normalized to the total area of the ventricle. All results are expressed as the mean ± standard error of the mean. Unless specified, p-values were obtained by performing t-tests. Regeneration scores were assigned to 30 dpci regenerating hearts based on AFOG histological staining, which differentiates between the myocardium, fibrin-rich tissue and collagen fibres [17]. The scores follow the following criteria: complete regeneration = absence or residual collagen fibres; partial regeneration = advanced regeneration with persisting collagen fibres; blocked regeneration = large injured area containing provisional fibrotic matrix. Statistical significance was obtained by Fisher's exact test.
2.4. Protein homology analysis
To test if the binding pockets of c-Kit and c-FMS proteins are conserved through vertebrates, using T-Coffee [30] we aligned the sequences of Homo sapiens c-FMS (NP_005202.2) and c-Kit (NP_000213.1) with their Danio rerio orthologues c-FMS (NP_571747.1), c-Kita (NP_571128.1) and c-Kitb (NP_001137390.1).
3. Results
3.1. Cardiomyocyte proliferation correlates with the presence of leucocytes after thoracotomy
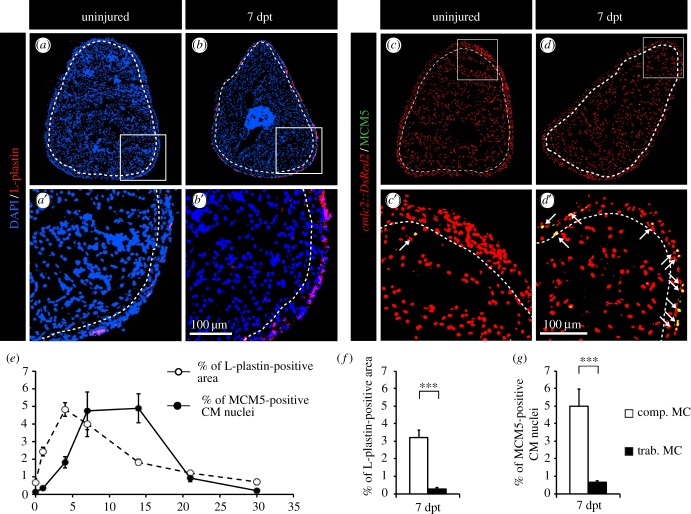
We recently established thoracotomy as a model of heart preconditioning in the adult zebrafish [1]. While leaving the heart untouched, the thoracotomy procedure exposes the cardiac structures to many aggressions, such as mechanical and osmotic stress or bacterial contamination, triggering an inflammatory response. To test whether inflammation might cause the increased CM mitotic activity seen after thoracic incision, we decided to characterize the distribution of leucocytes after thoracotomy. For this, we used an antibody against L-plastin, a leucocyte-specific actin-bundling protein [31]. To validate L-plastin as a suitable leucocyte marker, transgenic reporter lines for specific haematopoietic lineages were used: mpeg1:EGFP fish were used to visualize macrophages, whereas CD41:EGPF fish were selected to identify thrombocytes [26,27]. Remarkably, all mpeg1:EGFP-positive cells were L-plastin-positive (electronic supplementary material, figure S1a). At 7 dpt, mpeg1:EGFP-positive cells accounted for approximately 10% of L-plastin-positive cells (data not shown). In contrast, no overlap was found between L-plastin staining and the thrombocytes marker CD41:EGFP (electronic supplementary material, figure S1b). Moreover, no L-plastin was detected in the blood clots occasionally trapped in the lumen of the heart (electronic supplementary material, figure S1c). In summary, these co-stainings showed that L-plastin does not label thrombocytes or erythrocytes, but colocalizes with the specific leucocyte transgenic reporter, mpeg1. We concluded that L-plastin is a valid leucocyte marker.
To detect any correlation between mitotic activity and inflammation, we performed a spatio-temporal description of the distribution of leucocytes and cycling CMs after thoracotomy (figure 1). The presence of leucocytes was increased rapidly after thoracotomy to peak at 4 dpt. The inflammatory response then slowly decreased to reach its normal level by 30 dpt (figure 1e). CM cell-cycle activity was assessed by counting the number of MCM5-positive CMs. Remarkably, after thoracic incision, CM mitotic activity slowly increased to reach a plateau between 7 and 14 dpt with approximately 5% of cycling CMs, and finally decreased to reach its normal level at 30 dpt (figure 1e).
Figure 1.
CM proliferation is correlated with the distribution of phagocytic immune cells. (a,b) Representative sections of the hearts reveal a few L-plastin-positive cells in uninjured fish in comparison to fish at 7 days post-thoracotomy (dpt) with the abundant L-plastin staining in the compact myocardium. A white dashed line separates compact (comp. MC) and trabecular (trab. MC) myocardium. (c,d) Representative sections of the heart of cmlc:DsRed2-nuc transgenic fish (red, marker of CM nuclei) labelled with the G1/S-phase marker MCM5 (green) reveal an increased mitotic activity at 7 dpt. Arrows indicate double-positive cells. (e) Quantification of the L-plastin-positive area (white circles) and of the number of proliferative CMs (black circles) in sections of uninjured hearts, and at 1, 4, 7, 14, 21 and 30 dpt. (f,g) Quantification of L-plastin-labelled leucocytes and MCM5-positive CMs in the two distinct myocardium compartments reveals a spatial correlation of both distributions at 7 dpt. (n ≥ 4 hearts; ≥2 sections per heart; ***p < 0.001).
The zebrafish ventricle is composed of two distinct compartments: a dense cortical layer (compact myocardium) that surrounds a spongy inner structure (trabecular myocardium) [32]. We have previously described that most cycling CMs are located in the compact myocardium after preconditioning. Based on this observation, we asked whether leucocytes were evenly scattered through both myocardial compartments. Strikingly, we observed that leucocytes accumulated in the compact myocardium (figure 1a,b,f), where cycling CMs were observed (figure 1c,d,g). The concomitance of the inflammatory response and of the entry of CMs into the cell cycle suggests a correlation between these two processes.
3.2. Leucocyte accumulation is not required to stimulate cardiomyocyte proliferation after thoracotomy
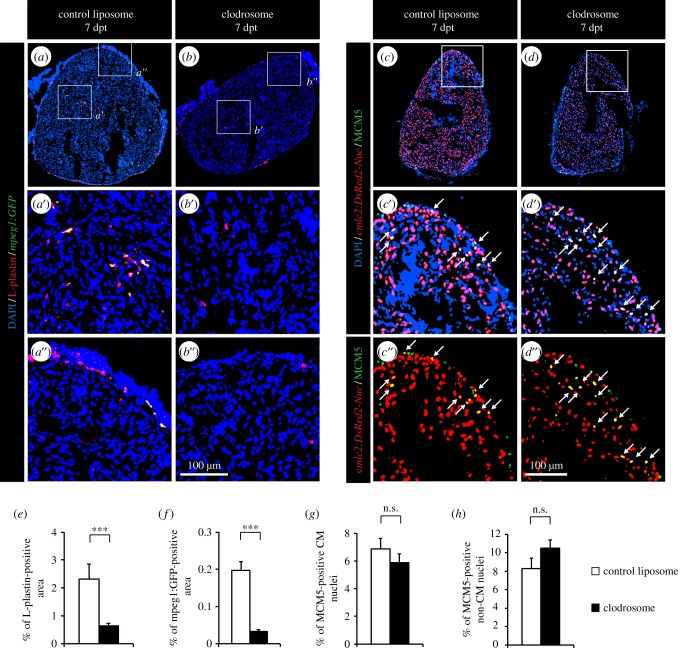
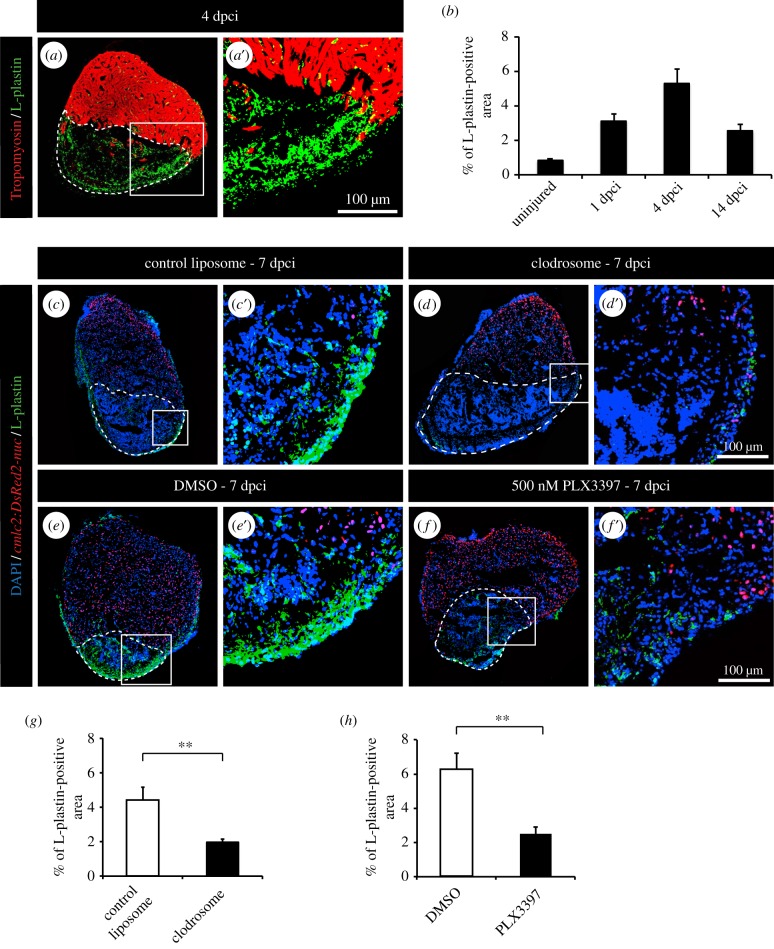
To test the role of the leucocytes in the initiation of the mitotic response induced in our cardiac preconditioning model, we applied diverse experimental approaches to modulate the immune response. First, the overall phagocyte population was depleted by an intraperitoneal injection of clodrosomes, which are cytotoxic clodronate encapsulated liposomes, one day before the thoracotomy and at 4 dpt. In the mouse heart, this method specifically reduces the cardiac mononuclear phagocyte population without altering neutrophils [23]. We found that clodrosome-injected fish displayed a fourfold reduction of L-plastin-positive area in the heart at 7 dpt, compared with fish injected with control liposomes (figure 2a,b,e). Similarly, very few mpeg1:EGFP-positive cells could be detected after clodrosome injections (figure 2a,b,f). These results support clodrosome injection as a valid method to deplete phagocytes in the zebrafish. Surprisingly, this decreased immune response had no effect on the cell-cycle activity of CM or non-CM nuclei (figure 2c,d,g,h).
Figure 2.
Depleting phagocytes by injection of clodronate liposomes does not affect CM proliferation. (a,b) The impact of control and clodronate liposome injection on the presence of leucocytes was tested by labelling the hearts of mpeg1:GFP transgenic fish (green) with L-plastin (red). (c,d) CM proliferation was quantified by measuring the number of CMs co-expressing DsRed2-nuc (red) and MCM5 (green). Double-positive cells are indicated with arrows. (e,f) Quantification of L-plastin- and mpeg1:GFP-positive area. (g,h) Quantification of proliferative CM and non-CM nuclei. (n ≥ 4 hearts; ≥2 sections per heart; ***p < 0.001; n.s., non-significant).
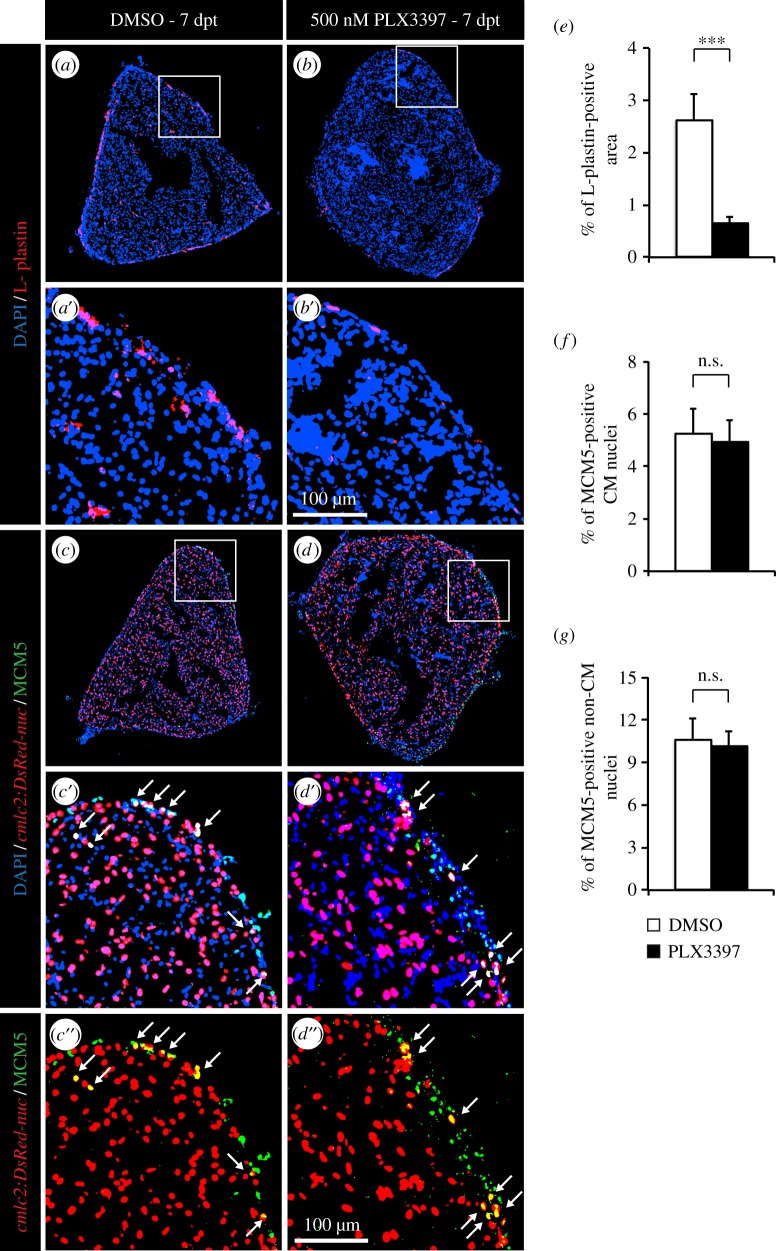
In a second approach, the immune response after thoracotomy was modulated using PLX3397, a selective inhibitor of c-Fms/CSF1R and c-Kit tyrosine kinases. These receptors regulate the proliferation of multipotent haematopoietic progenitor cells and their myeloid differentiation [33]. In mammals, PLX3397 has been shown to inhibit macrophage migration and proliferation and to decrease the production of pro-inflammatory cytokines [34]. To verify if PLX3397 might be an efficient c-Fms/CSF1R and c-Kit inhibitor in zebrafish, we aligned the H. sapiens and Danio rerio c-Fms and c-Kit orthologue proteins (electronic supplementary material, figure S2). We focused this comparison on the region that interacts with PLX3397 (H. sapiens c-Kit L521–L865; H. sapiens c-Fms L514–K870) [35]. Both proteins were well conserved between these two species, and all residues making direct contact with the inhibitor were preserved, suggesting that PLX3397 might work as a specific c-Fms/CSF1R and c-Kit inhibitor also in zebrafish. Accordingly, PLX3397-treated fish displayed a threefold decrease of the presence of leucocytes in the heart at 7 dpt (figure 3a,b,e). Despite this, CM cell-cycle activity was not affected (figure 3c,d,f,g).
Figure 3.
Depletion of leucocytes by PLX3397 treatment does not affect CM proliferation. (a,b) The impact of PLX3397 treatment on the presence of leucocytes was tested by labelling the hearts with L-plastin (red). (c,d) CM proliferation was quantified by measuring the number of CMs co-expressing cmlc:DsRed2-nuc (red) and MCM5 (green). Arrows indicate double-positive cells. (e) Quantification of L-plastin-positive area at 7 dpt. (f,g) Quantification of proliferative CM and non-CM nuclei at 7 dpt. (n ≥ 4 hearts; ≥2 sections per heart; ***p < 0.001; n.s. non-significant).
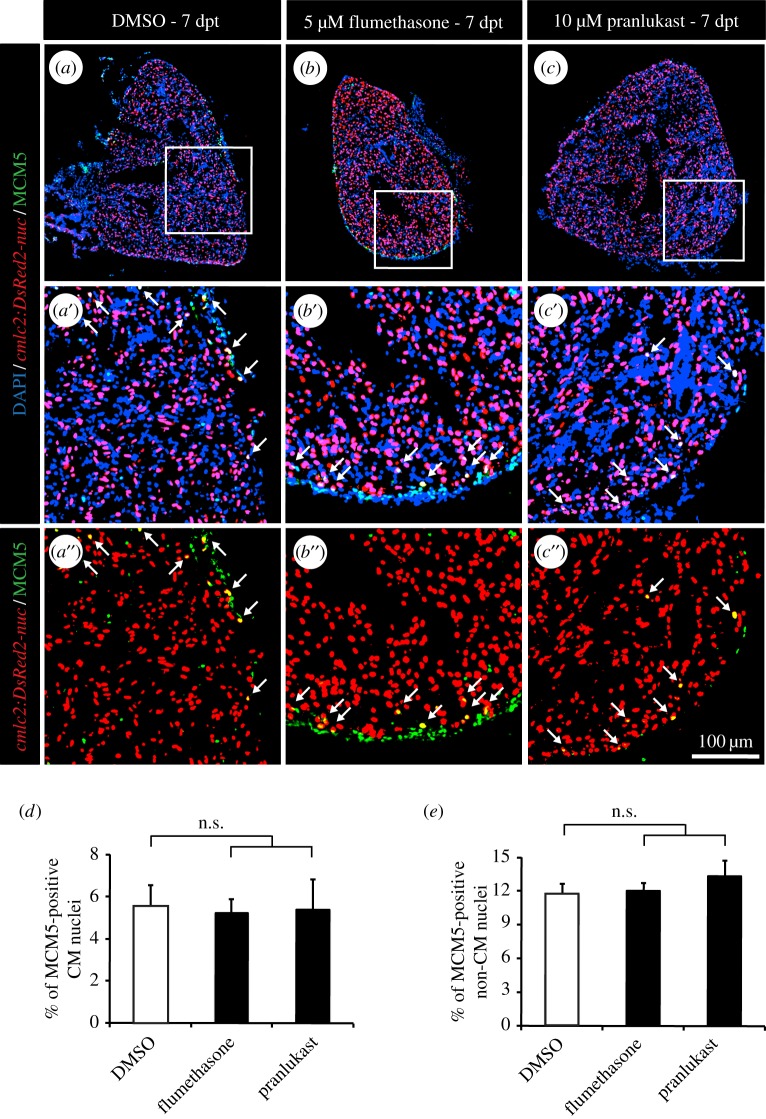
Because both methods, clodronate liposomes and PLX3397, mainly affect the macrophage lineage, widely acting anti-inflammatory drugs were used to exclude any role of other immune mechanisms in the induction of CM proliferation (figure 4). Even so, reducing the immune response using flumethasone (figure 4b) or pranlukast (figure 4c) did not affect the rate of re-entry into the cell cycle (figure 4d,e).
Figure 4.
Wide anti-inflammatory drugs do not affect CM proliferation after thoracotomy. (a–c) Representative heart images of cmlc2:DsRed-nuc fish (red) treated with 0.05% DMSO (a), 5 µM flumethasone (b) or 2.5 µM pranlukast (c) labelled with the cell cycle marker MCM5 (green) at 7 dpt. Arrows indicate double-positive cells. (d,e) Quantification of MCM5-positive CM and non-CM nuclei at 7 dpt. (n ≥ 4 hearts; ≥2 sections per heart; n.s., non-significant).
Taken together, we concluded that cardiac preconditioning is accompanied by an accumulation of leucocytes in the uninjured myocardium. However, the presence and function of these immune cells are not essential for triggering cell-cycle progression in the intact zebrafish heart.
3.3. Cycling cardiomyocytes are associated with the presence of leucocytes after cryoinjury
Although inflammation is associated but not required for heart conditioning, we decided to address the role of leucocytes during heart regeneration. To induce myocardial injury, we applied the cryoinjury model, in which approximately 20% of the ventricle is damaged by exposure to a precooled metal probe [28]. Within the first week after the procedure, the damaged part of the myocardium becomes replaced with provisional fibrotic tissue. Within four weeks, this fibrotic tissue undergoes remodelling to repair the injured ventricle and progressively withdraws to give space for the regenerating myocardium [36]. After cryoinjury, CM mitotic activity slowly increased to reach a plateau between 7 and 21 dpci with approximately 10% of proliferating CMs, and finally decreased to reach its normal level at 60 dpci [9]. The complete restoration of the cardiac tissue is achieved in four to eight weeks [9,37]. To determine the involvement of immune response in heart regeneration, we analysed the distribution of L-plastin-expressing cells at different regenerative phases. Shortly after cryoinjury, at 1 and 4 dpci, leucocytes infiltrated the myocardium and abundantly accumulated in the post-injury zone, consistent with the stimulation of immune response in the damaged tissue (figure 5a,b). At 14 dpci, the number of L-plastin-positive cells declined as regeneration progressed (figure 5b).
Figure 5.
Clodronate liposome injections and PLX3397 treatment efficiently deplete phagocyte populations after cryoinjury. (a) Representative image of cryoinjured heart at 4 days post-cryoinjury (dpci) labelled with antibody against tropomyosin (red) to demarcate the remaining myocardium and L-plastin (green) to reveal leucocytes. The cryoinjured area is identified by the absence of tropomyosin (encircled by dashed line). (b) Quantification of the L-plastin-positive area in sections of uninjured hearts, and at 1, 4 and 14 dpci. (c–f) Representative images of hearts of transgenic fish cmlc2:DsRed2-nuc at 7 dpci with L-plastin staining (green). The cryoinjured area is identify by the absence of cmlc2:DsRed2-nuc expression (encircled by dashed line). (c,d) Hearts after control and clodronate liposome (clodrosome) injections. (e,f) Hearts after 0.05% DMSO or 500 nM PLX3397 treatment. (g,h) Quantification of L-plastin-positive area after clodronate liposome injection (g) and PLX3397 treatment (h) at 7 dpci. (n ≥ 4 hearts; ≥2 sections per heart; **p < 0.01).
3.4. Leucocytes promote the re-entry of cardiomyocytes into the cell cycle after cryoinjury
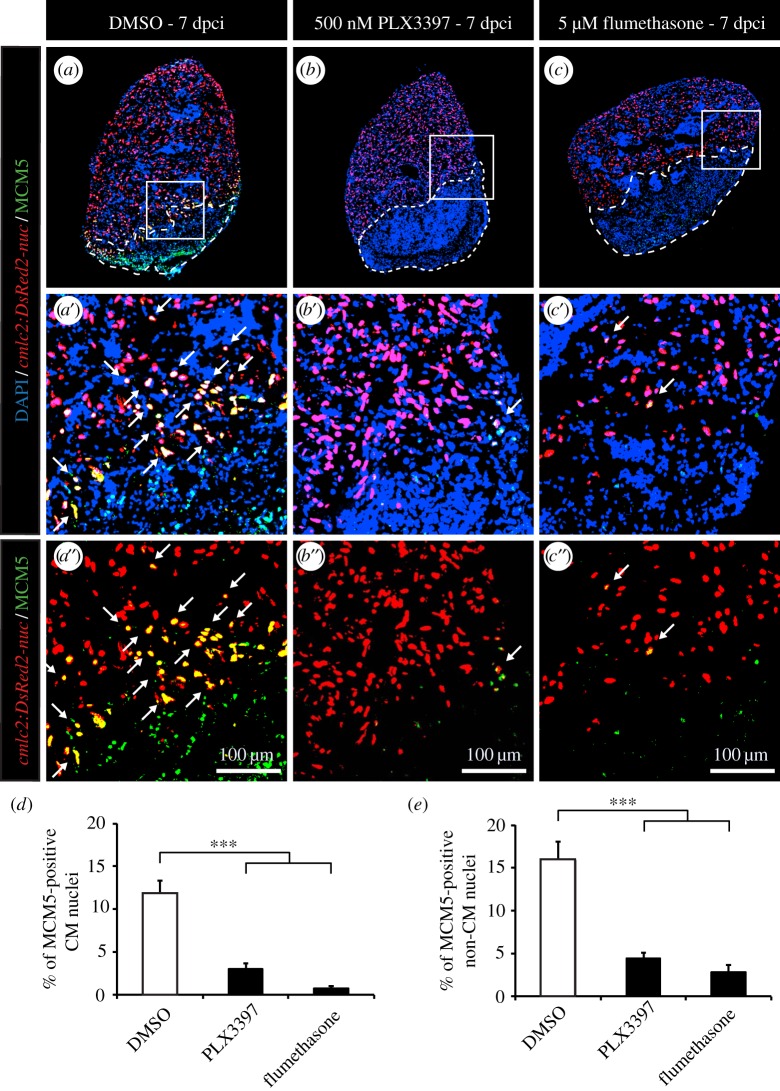
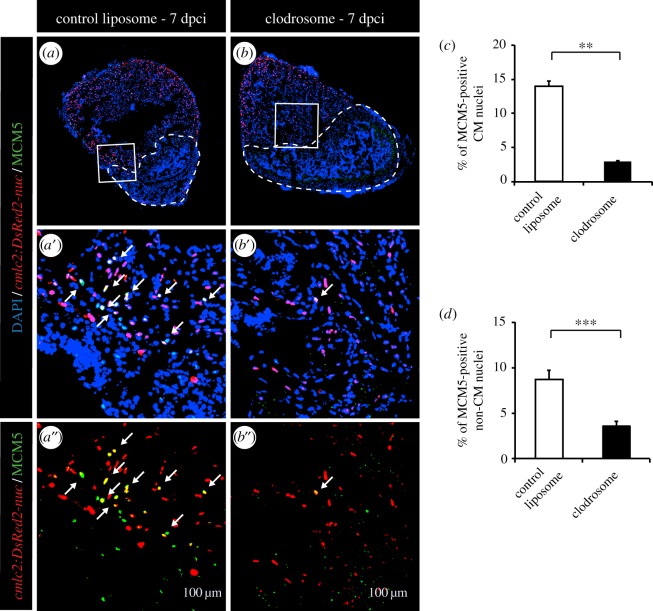
To assess the role of inflammation on CM cell-cycle activity after ventricular cryoinjury, we applied the same experimental strategies as after thoracotomy. Again, we were able to efficiently reduce the phagocyte population with PLX3397 treatment or intraperitoneal injection of clodrosomes, as illustrated by L-plastin staining (figure 5c–f). With both strategies, more than a twofold decrease of leucocytes was observed in the hearts at 7 days after cryoinjury (figure 5g,h). In this context, however, the reduced immune response strongly suppressed the stimulation of the mitotic activity (figure 6a,b,d,e and figure 7). This effect was observed for both CM and non-CM nuclei (figures 6d,e and 7c,d). Likewise, treating fish with the wide anti-inflammatory drug flumethasone severely impaired the re-entry of cardiac cells into the cell cycle at 7 dpci (figure 6c,d,e).
Figure 6.
Inhibition of inflammation strongly decreases CM proliferation after cryoinjury. (a–c) Representative images of the hearts of cmlc2:DsRed-nuc fish (red) treated with 0.05% DMSO (a), 500 nM PLX3397 (b) or 5 µM flumethasone (c) labelled with the cell cycle marker MCM5 (green). The cryoinjured area is detected by the absence of cmlc2:DsRed-nuc (encircled with a dashed line). Arrows indicate double-positive cells. (d,e) Quantification of MCM5-positive CMs and non-CMs at 7 dpci. (n ≥ 4 hearts; ≥2 sections per heart; ***p < 0.001).
Figure 7.
Phagocyte depletion strongly decreases CM proliferation after cryoinjury. (a,b) Representative images of the hearts of cmlc2:DsRed-nuc fish (red) injected with control liposomes (a) or injected with clodronate liposomes (b) labelled with the cell cycle marker MCM5 (green). The cryoinjured area is detected by the absence of cmlc2:DsRed-nuc (encircled with a dashed line). Arrows indicate double-positive cells. (c,d) Quantification of MCM5-positive CMs and non-CMs at 7 dpci. (n ≥ 4 hearts; ≥2 sections per heart; **p < 0.01; ***p < 0.001).
3.5. The deposition of a transient scar is dependent on leucocytes after cryoinjury
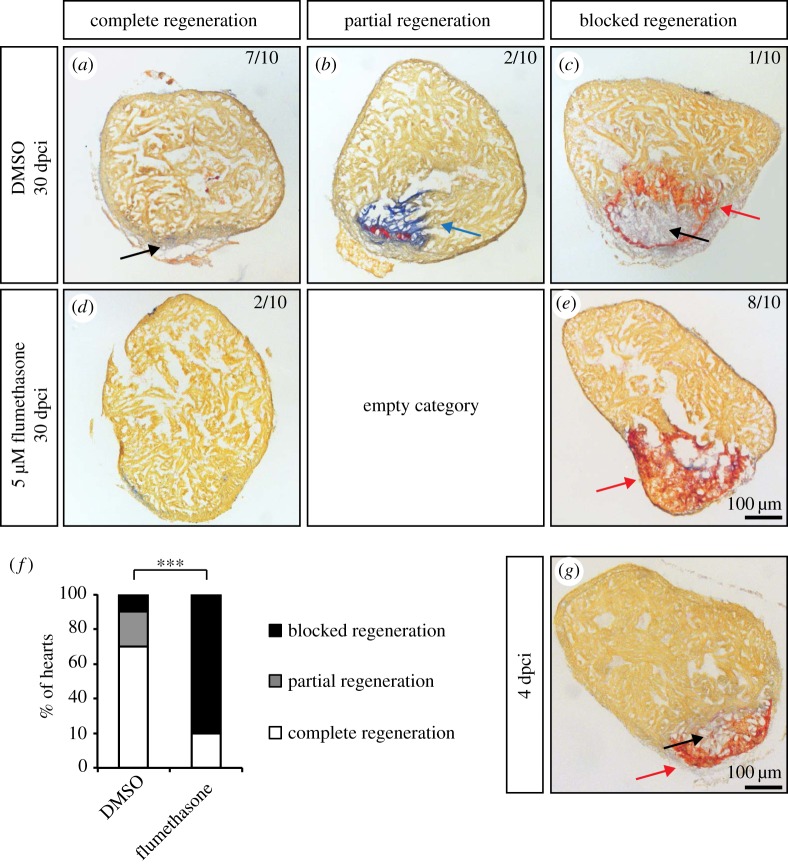
Moreover, fish treated with flumethasone for one month after cryoinjury clearly exhibited a blocked regenerative process, as revealed by histological staining (figure 8a–e). In the majority of control fish, regeneration was almost completed (figure 8d–f). At this stage, fibrin deposits were completely resolved, and only small size scars retaining collagen fibres were observed (figure 8a–c). In contrast, the flumethasone-treated fish displayed blocked regeneration (figure 8d,e,f), which was reminiscent of the early regenerative phase, such as 4 dpci (figure 8g). Fibrin-like material persisted in the cryoinjured areas, and the deposition of a collagen network necessary to support the migration of the newly formed cardiac cells was severely impaired (figure 8e). To exclude any side effect of the flumethasone treatment, we decided to validate our observations using the clodronate liposome approach. For this, fish were injected with control or clodronate liposomes 24 h before the cryoinjury procedure. Liposome injections were then repeated every 4 days for 2 weeks. Again, an impairment of regeneration was found in fish in which the immune response was blocked by clodronate liposomes (data not shown), confirming a specific role of leucocytes in the initiation of cardiac healing after injury. Taken together, our results demonstrate thus that the key regenerative processes of transient scar deposition and CM proliferation are dependent on the appropriate immune response after heart injury.
Figure 8.
Flumethasone treatment inhibits matrix remodelling after cryoinjury and heart regeneration. (a–e) Representative sections of cryoinjured hearts at 30 dpci labelled with AFOG staining, which detects collagen in blue (blue arrow), fibrin-like matrix in red (red arrows) and muscle in orange, after 30 days of treatment with 0.05% DMSO and 5 µM flumethasone. The newly formed myocardium is indicated by black arrows. (f) Quantification of heart regeneration according to the criteria described in Materials and methods. (g) Representative example of a heart at 4 dpci labelled with AFOG staining. The cryoinjured zone in the heart at 4 dpci contains a provisional fibrotic matrix (red), which displays a similarity to the matrix of flumethasone-treated hearts at 30 dpci. (n ≥ 10; ***p < 0.001, Fisher's exact test).
4. Discussion
In mammalian studies, conflicting data have been obtained so far on the role of inflammation on heart preconditioning [22]. For instance, it has been demonstrated that a brief forearm ischaemia suppresses pro-inflammatory genes expression in circulating human leucocytes [38], whereas neutrophil adhesion and phagocytosis are reduced [39]. At the same time, an increased production of tumour necrosis factor-α, interleukin-1β and -8 has been detected in patients subjected to remote ischaemic preconditioning before cardiopulmonary bypass [40]. Further studies are therefore needed to precisely define the role of inflammation during cardiac preconditioning.
To address this question in the zebrafish, we first characterized the spatio-temporal dynamics of the immune response after thoracotomy. We observed a correlation between the presence of inflammatory cells and mitotic nuclei after heart preconditioning, suggesting a link between these two processes. We used two distinct strategies that effectively depleted the macrophage population and two different immunosuppressive drugs. None of these techniques, however, had an effect on cardiac cell proliferation. Thus, leucocytes are not necessary to induce proliferation of cardiac cells in the context of heart preconditioning.
In contrast to this result, the immune response is necessary for mitotic activity, the transient scar deposition and heart regeneration after cryoinjury. These results are consistent with previously published studies. In neonatal mice, injections of liposomal clodronate robustly impair heart regeneration after myocardial infarction [23,24]. Likewise, inhibiting phagocyte recruitment with the glucocorticoid beclomethasone strongly reduces zebrafish heart regenerative capacities after ventricular resection [18]. Taken together, these observations highlight the importance of leucocytes in the context of a regenerative process, where the immune response cleans the damaged area and creates a favourable environment for tissue rebuilding. These immune cells are essential to ensure the re-entry of cardiac cells into the cell cycle and to pilot the injured heart towards an efficient regeneration.
Besides, the clear phenotype observed in the cryoinjury model confirms that the absence of effect of an altered immune system after heart preconditioning was not due to any dosage issues. We applied the same protocols and drug dosage on both models. Moreover, we achieved a better reduction of L-plastin-positive cells after preconditioning than after cryoinjury. Therefore, we can exclude the possibility that the negative results obtained with our thoracotomy model were caused by a too gentle depletion of the phagocytes.
In summary, we discovered that the function of inflammation on the mitotic activity of cardiac cells is context-dependent. In intact preconditioned heart, the entry of CM and non-CM cells into the cell cycle is unresponsive to leucocyte accumulation. These results suggest that the factors triggering the mitotic cycle of cardiac cells are not secreted by local leucocytes. In contrast, in injured hearts, phagocytic cells are key components of the regenerative process. When their function is depleted, cellular debris and fibrin are not efficiently cleared. The persisting damaged tissue is likely to impair remodelling of the post-infarcted ventricle, which directly or indirectly blocks cardiac cell proliferation and migration to achieve heart regeneration. Although organ regeneration requires the inflammatory phase, the preconditioning evokes regenerative programmes through leucocyte-independent mechanisms that remain yet uncharacterized.
Supplementary Material
Supplementary Material
Acknowledgements
We thank V. Zimmermann for excellent technical assistance and for fish care and J. Bertrand for the mpeg1:eGFP fish. We are grateful to Gideon Bollag, Plexxikon, for providing PLX3397 compound.
Authors' contributions
A.S.P.C. carried out laboratory work, performed data analysis, carried out sequence alignments, designed the study and drafted the manuscript; T.B. carried out laboratory work and performed data analysis; F.B. carried out laboratory work and initiated the study; J.M. performed laboratory work; P.S. performed laboratory work; A.J. conceived the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no financial or non-financial competing interests.
Funding
This work was supported by the Swiss National Science Foundation (grant nos. 310030_159995 and CRSII3_147675).
References
- 1.de Preux Charles A-S, Bise T, Baier F, Sallin P, Jaźwińska A. 2016. Preconditioning boosts regenerative programmes in the adult zebrafish heart. Open Biol. 6, 160101 (doi:10.1098/rsob.160101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heusch G. 2015. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 116, 674–699. (doi:10.1161/CIRCRESAHA.116.305348) [DOI] [PubMed] [Google Scholar]
- 3.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. 2012. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317. (doi:10.1016/B978-0-12-394309-5.00006-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. 1999. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J. Thromb. Thrombolysis 8, 123–129. (doi:10.1023/A:1008911101951) [DOI] [PubMed] [Google Scholar]
- 5.Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. 2011. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res. Cardiol. 106, 135–145. (doi:10.1007/s00395-010-0133-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aimo A, Borrelli C, Giannoni A, Pastormerlo LE, Barison A, Mirizzi G, Emdin M, Passino C et al. 2015. Cardioprotection by remote ischemic conditioning: mechanisms and clinical evidences. World J. Cardiol. 7, 621–632. (doi:10.4330/wjc.v7.i10.621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Yellon DM. 2009. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis 204, 334–341. (doi:10.1016/j.atherosclerosis.2008.10.029) [DOI] [PubMed] [Google Scholar]
- 8.Poss KD. 2007. Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36–45. (doi:10.1016/j.semcdb.2006.11.009) [DOI] [PubMed] [Google Scholar]
- 9.Chablais F, Veit J, Rainer G, Jaźwińska A. 2011. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev. Biol. 11, 21 (doi:10.1186/1471-213X-11-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallin P, de Preux Charles AS, Duruz V, Pfefferli C, Jaźwińska A. 2014. A dual epimorphic and compensatory mode of heart regeneration in zebrafish. Dev. Biol. 399, 27–40. (doi:10.1016/j.ydbio.2014.12.002) [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. 2011. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 20, 397–404. (doi:10.1016/j.devcel.2011.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. 2006. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607–619. (doi:10.1016/j.cell.2006.08.052) [DOI] [PubMed] [Google Scholar]
- 13.Chablais F, Jaźwińska A. 2012. The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling. Development 139, 1921–1930. (doi:10.1242/dev.078543) [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Borikova AL, Ben-Yair R, Guner-Ataman B, MacRae CA, Lee RT, Burns CG, Burns CE. 2014. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl Acad. Sci. USA 111, 1403–1408. (doi:10.1073/pnas.1311705111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y, Gupta V, Karra R, Holdway JE, Kikuchi K, Poss KD. 2013. Translational profiling of cardiomyocytes identifies an early Jak1/Stat3 injury response required for zebrafish heart regeneration. Proc. Natl Acad. Sci. USA 110, 13 416–13 421. (doi:10.1073/pnas.1309810110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi WY, Gemberling M, Wang J, Holdway JE, Shen MC, Karlstrom RO, Poss KD. 2013. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development 140, 660–666. (doi:10.1242/dev.088526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoud AI, et al. 2015. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev. Cell 34, 387–399. (doi:10.1016/j.devcel.2015.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang WC, Yang CC, Chen IH, Liu YM, Chang SJ, Chuang YJ. 2013. Treatment of glucocorticoids inhibited early immune responses and impaired cardiac repair in adult zebrafish. PLoS ONE 8, e66613 (doi:10.1371/journal.pone.0066613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallin P, Jaźwińska A. 2016. Acute stress is detrimental to heart regeneration in zebrafish. Open Biol. 6, 160012 (doi:10.1098/rsob.160012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita N, Hoshida S, Otsu K, Taniguchi N, Kuzuya T, Hori M. 2000. The involvement of cytokines in the second window of ischaemic preconditioning. Br. J. Pharmacol. 131, 415–422. (doi:10.1038/sj.bjp.0703594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong JW, Vallejo JG, Tzeng HP, Thomas JA, Mann DL. 2010. Innate immunity mediates myocardial preconditioning through Toll-like receptor 2 and TIRAP-dependent signaling pathways. Am. J. Physiol. Heart Circul. Physiol. 298, H1079–H1087. (doi:10.1152/ajpheart.00306.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivaraman V, Pickard JM, Hausenloy DJ. 2015. Remote ischaemic conditioning: cardiac protection from afar. Anaesthesia 70, 732–748. (doi:10.1111/anae.12973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. 2014. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392. (doi:10.1172/JCI72181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. 2014. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA 111, 16 029–16 034. (doi:10.1073/pnas.1406508111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rottbauer W, et al. 2002. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell 111, 661–672. (doi:10.1016/S0092-8674(02)01112-1) [DOI] [PubMed] [Google Scholar]
- 26.Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. 2011. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49–e56. (doi:10.1182/blood-2010-10-314120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin HF, Traver D, Zhu H, Dooley K, Paw BH, Zon LI, Handin RI. 2005. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood 106, 3803–3810. (doi:10.1182/blood-2005-01-0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chablais F, Jaźwińska A. 2012. Induction of myocardial infarction in adult zebrafish using cryoinjury. J. Visu. Exp. 18, 3666 (doi:10.3791/3666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. 2010. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 8, e1000562 (doi:10.1371/journal.pbio.1000562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, Notredame C. 2011. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 39(web server issue), W13–W17. (doi:10.1093/nar/gkr245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morley SC. 2012. The actin-bundling protein L-plastin: a critical regulator of immune cell function. Int. J. Cell Biol. 2012, 935173 (doi:10.1155/2012/935173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta V, Poss KD. 2012. Clonally dominant cardiomyocytes direct heart morphogenesis. Nature 484, 479–484. (doi:10.1038/nature11045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander WS, Nicola NA. 1998. Hemopoietic growth factor receptor abnormalities in leukemia. Leukemia Res. 22, 1097–1111. (doi:10.1016/S0145-2126(98)00110-6) [DOI] [PubMed] [Google Scholar]
- 34.Prada CE, et al. 2013. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 125, 159–168. (doi:10.1007/s00401-012-1056-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, et al. 2013. Design and pharmacology of a highly specific dual FMS and KIT kinase inhibitor. Proc. Natl Acad. Sci USA 110, 5689–5694. (doi:10.1073/pnas.1219457110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaźwińska A, Sallin P. 2016. Regeneration versus scarring in vertebrate appendages and heart. J. Pathol. 238, 233–246. (doi:10.1002/path.4644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. 2011. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138, 1663–1674. (doi:10.1242/dev.060897) [DOI] [PubMed] [Google Scholar]
- 38.Konstantinov IE, et al. 2004. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol. Genomics 19, 143–150. (doi:10.1152/physiolgenomics.00046.2004) [DOI] [PubMed] [Google Scholar]
- 39.Shimizu M, et al. 2010. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J. Surg. Res. 158, 155–161. (doi:10.1016/j.jss.2008.08.010) [DOI] [PubMed] [Google Scholar]
- 40.Albrecht M, et al. 2013. Remote ischemic preconditioning regulates HIF-1α levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res. Cardiol. 108, 314 (doi:10.1007/s00395-012-0314-0) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.