Abstract
Objective:
Hydatidosis is one of the most important zoonotic diseases and surgery is still the main treatment for this problem. One of the side effects of hydatid cyst surgery is recurrence, thus, searching and assessment of some new agents such as medicinal plant extracts are very important. In the present study, the scolicidal effect of ethanolic extract of Ziziphora tenuior (Z. tenuior) was investigated.
Materials and Methods:
Protoscolices were aseptically collected from sheep livers containing hydatid cyst and used in the experiments. Z. tenuior extract was used at concentration of 3-100 mg/ml for 10-60 min. Viability of protoscolices was determined by 0.1% eosin staining.
Results:
Based on our results, Z. tenuior extract at concentration of 10 mg/ml killed all protoscolices after 20 min. However, this medicinal plant at concentration of 25 mg/ml destroyed all protoscolices in a shorter exposure time (10 min). Therefore, the scolicidal activity of the extract at 10 and 25 mg/ml concentrations was considerably effective in lower concentrations and shorter exposure times.
Conclusion:
The findings of this study showed that the ethanolic extract of Z. tenuior produces high scolicidal activity; it may be used as an appropriate and effective scolicidal agent in hydatidosis surgery. This is the first report on the protoscolicidal activity of Z. tenuior.
Key Words: Hydatidosis, Surgery, Medicinal plant, Scolicidal, Hydatid cyst, Ziziphora tenuior
Introduction
Echinococcosis is a zoonotic disease caused by the larval stages of parasitic cestode Echinococcus granulosus (McManus, et al., 2003 ▶; Eckert and Deplazes, 2004 ▶). Cystic hydatid disease (CHD) in humans and animals is an economic and public health problem in many parts of the world (Lahmar, et al., 2007 ▶; Da Silva, 2010 ▶). Hydatidosis affects many organs of the body such as liver, lung and less frequently spleen, kidney, bone, brain, and heart (Ammann and Eckert, 1996 ▶). The surgical operation is considered as the most efficient method for the treatment of hydatid disease. On the other hand, one of the side effects of hydatid cyst surgery is recurrence. Therefore, assessing and finding some agents such as medicinal plants are necessary to reduce the recurrence rate. Several scolicidal substances such as silver nitrate, cetrimide, and hypertonic saline, all with a number of complications, have been used for inactivation of the hydatid cyst contents, but most of these substances may produce a range of side effects (Adas, et al., 2009 ▶; Topcu, et al., 2006 ▶; Moazeni and Nazer, 2010 ▶). In addition, many efforts have been made to discover new antimicrobial compounds from various kinds of sources such as plants. Recently, the medicinal plants have increasingly been used to treat many disorders including several infections (Khan et al., 2010 ▶). Ziziphora genus (Lamiacaea) are consisted of four species namely Z. tenuior L., Z. Capitata L., Z. Clinopodioides, and Z. Persica Bunge (Zohrabi M, 2000 ▶). Ziziphora or Kakuti in Persian traditional medicine is recognized by the dried aerial parts of Z. tenuior L. which contains at least 1.2 % (volume/weight) of the essential oil (Davis and Newland, 1982 ▶). This medicinal plant is widely distributed in Iran, Pakistan, Afghanistan, Turkmenistan, Armenia, Syria, Anatolia, Transcaucasia, and Central Asia (Zohrabi M, 2000 ▶). In traditional medicine, Ziziphora is used for the treatment of vomiting, diarrhea, dysentery, fever, and uterus infections (Naeini et al., 2009 ▶; Naghibi et al., 2005 ▶; Zargari, 1987 ▶). Moreover, the antioxidant (Admassu Shimelis and Kumar Rakshit, 2005 ▶; Tian et al., 2011 ▶), antifungal (Shokri et al., 2012 ▶), antibacterial (Salehi et al., 2005 ▶; Sarac and Ugur, 2009 ▶), and antiprotozoal (Rota et al., 2004 ▶) effects of Ziziphora are well known. Phytochemical analysis of Ziziphora L. indicated that the major components of its essential oil are pulegone, isomenthone, thymol, menthone, and piperitone (Zargari, 1987 ▶; Sezik et al., 1991 ▶; Kasumov, 1987 ▶; de Sousa et al., 2007 ▶). These compounds are suggested to be responsible for the medicinal properties mentioned earlier. Hence, in the present study, the scolicidal effect of Z. tenuior extract was evaluated.
Materials and Methods
Collection and determination of protoscolices viability
Hydatid cysts from the liver of infected sheep were obtained from the city abattoir in Qazvin, located in the central region of Iran. Protoscolices were obtained aseptically and washed three times with normal saline. The viability of protoscolices was assessed by their motility characteristics, muscular movements, and with 0.1% eosin staining under light microscopy. The protoscolices with more than 90% viability were selected for our study (Moazeni and Nazer, 2010 ▶).
Preparation of Z. tenuior extract
Z. tenuior was purchased from the herbal market in Qazvin and authenticated by a botanist. Voucher specimens were preserved in the central herbarium of medicinal plants (ACECR).The aerial parts of plant were air-dried at room temperature and later was ground into powder. The aerial part of plant (50 g) was extracted using percolation method by ethanol (80%) at room temperature. The Solvent used for extraction was completely removed by drying under reduced pressure at 40 ºC in a rotary evaporator. The samples were stored at 4 °C until use (3 g, 6% yield).
The effect of Z. tenuior extract on protoscolices
In the present study, six concentrations of Z. tenuior extract (3, 5, 10, 25, 50, and 100 mg/ml) were used for 10, 20, 30, 40, 50, and 60 min. Hypertonic saline (20%) was used as positive control. For the preparation of Z. tenuior solution at concentrations mentioned above, 0.03, 0.05, 0.1, 0.25, 0.5, and 1 g of dried extract was dissolved in 10 ml of normal saline, respectively. In each experiment, 2 ml of each concentration of Z. tenuior was placed in a test tube, to which a drop of protoscolex-rich sediment (containing at least 700 protoscolices) was added. The content of each tube was gently mixed and incubated for 10, 20, 30, 40, 50 and 60 min. At the end of each incubation time, 10 ml of normal saline was added to each tube, centrifuged for 1 min at 300 rpm and the supernatant was discarded. The same procedure was repeated 3 times. Two milliliter of 0.1% eosin stain was then added to the remaining settled protoscolices, mixed gently, and incubated for 15 min, and was followed by careful removal of the upper portion. Later, the remaining protoscolices pellet was smeared on a manually scaled glass slide, covered with a cover glass, and examined under a light microscope. The percentages of viable and dead protoscolices were determined by counting an average of 700 protoscolices. Non-treated protoscolices were used as negative control. All experiments were performed in triplicate (Moazeni and Nazer, 2010 ▶; Moazeni et al., 2012 ▶).
Statistical analysis
Statistical analyses were performed by one-way analysis of variance (ANOVA), Tukey’s test and t-test to express the difference among the groups. All analyses were accomplished using SPSS software version 16. Data were considered statistically significant at p<0.05.
Results
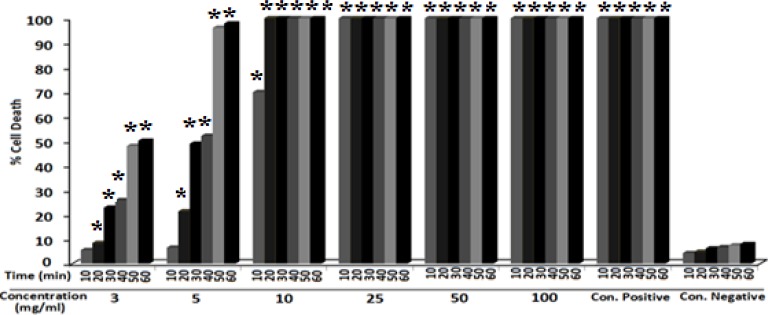
The scolicidal effects of Z. tenuior extract are summarized in Figure 1. Scolicidal activity of Z. tenuior extract at concentration of 3 mg/ml was 5.63%, 8.51%, 22.83%, 25.97%, 47.7%, and 50.1% after 10, 20, 30, 40, 50, and 60 min respectively. Moreover, the scolicidal effect of this extract at concentration of 5 mg/ml was 6.63%, 21.38%, 48.99%, 52.17%, 96.29%, and 97.91% after 10, 20, 30, 40, 50, and 60 min respectively. As shown in Figure 1, the extract revealed significant scolicidal effect at 3 and 5 mg/ml at exposure times above 10 min. In addition, the scolicidal effect of all concentrations of the extract was significant (p<0.05) compared with the control group at all exposure times.
Figure 1.
Protoscolicidal effects of various concentrations of Z. tenuior L.extract in different exposure times.
*p value < 0.05 (significant comparing to negative control)
Our findings indicated that, Z. tenuior extract at concentration of 10 mg/ml killed all protoscolices (100%) after 20 min. However, as shown in Figures 2 and 3, this medicinal plant at concentration of 25 mg/ml destroyed all protoscolices (100%) in a shorter exposure time (10 min). Therefore, the scolicidal activity of the extract at 10 and 25 mg/ml concentrations were strongly effective at lower concentrations and shorter exposure times. The results of our study showed that the ethanolic extract of Z. tenuior had a high scolicidal activity under in vitro condition.
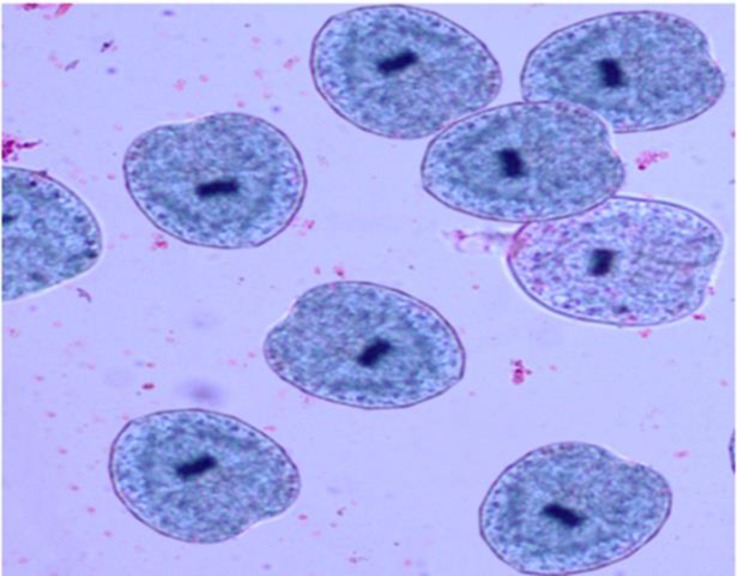
Figure 2.
Live protoscolices after staining with 0.1% eosin
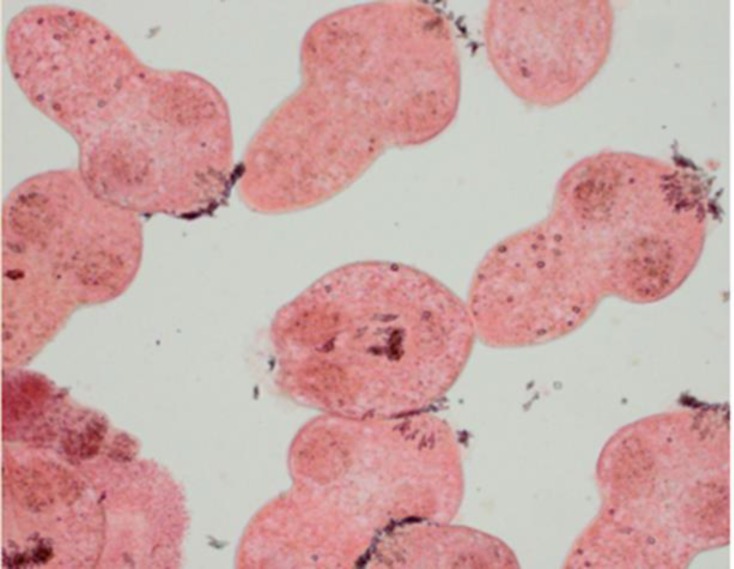
Figure 3.
Dead protoscolices after exposure to Z. tenuior L. extract and staining with 0.1% eosin
Discussion
Hydatidosis continues to be a significant cause of morbidity and mortality in many parts of the world (Da Silva, 2010 ▶). The surgical operation is considered as the most efficient method for treatment. So far, several chemical agents with scolicidal effect have been used to solve this problem, but many of these substances may cause unwanted side effects that limit their usage (Moazeni and Nazer, 2010 ▶; Albi et al., 2002 ▶; Caglar et al., 2008 ▶; Besim et al., 1998 ▶; Erzurumlu et al., 1998 ▶; Paksoy et al., 2005 ▶; Puryan et al., 2005 ▶; Shahnazi et al., 2014 ▶). In this regard, several studies have shown that the hypertonic saline at 20% concentration has the ability to kill all protoscolices (100%) after 6, 15, and 45 min exposure times (Caglar et al., 2008 ▶; Besim et al., 1998 ▶; Erzurumlu et al., 1998 ▶; Kayaalp et al., 2001 ▶). Moreover, the scolicidal activity of 1.5% cetrimide has been reported to destroy 86.9% and 92.6% of protoscolices after 5 and 10 min exposure times, respectively (Khan et al., 2010 ▶). In other studies, ethyl alcohol (95%) and silver nitrate (20%) were found to have the potential to completely destroy protoscolices after 15 and 20 min (Caglar et al., 2008 ▶; Besim et al., 1998 ▶). Furthermore, the in vitro scolicidal activity of 10% providine iodine was unsatisfactory after 5 and 10 min (Besim et al., 1998 ▶) whereas the application of H2o2 (3%) and mannitol (20%) produced excellent results (100%) over killing of protoscolices after 15 and 45 min, respectively (Caglar et al., 2008 ▶; Rajabi, 2009 ▶). Erzurumlu et al. showed that albendazole sulfoxide was effective on protoscolices at 50 and 100 µg/ml concentrations with 50% and 100% efficacy, respectively. Our pervious study on the protoscolicidal effects of hypertonic glucose at different concentrations (10-50%) and exposure times (1-60 min) showed that glucose at 10%, 15%, 20%, 25%, and 30% concentrations has little effect on protoscolices after 1-30 min exposure times. The scolicidal effect of glucose at 40% concentration after 20, 30, and 40 min was 98.8%, 99.6%, and 100%, respectively. Also, the scolicidal activity of glucose with 50% concentration after 6, 7, 8, 9, 10, and 20 min exposure times was 63.9%, 67.2%, 83.4%, 95.3%, 97.4%, and 100%, respectively (Shahnazi et al., 2014 ▶).
On the other hand, a fast and complete scolicidal effect with no side effect and low cost is considered as part of the properties of an ideal scolicidal solution. However, no ideal scolicidal solution and agents have been described yet (Adas, et al., 2009 ▶; Rouhani et al., 2013 ▶).
Ziziphora, as an herbal medicine, has been used in the treatment of gastrointestinal disorders and infectious diseases for a long times (Naeini et al., 2009 ▶; Naghibi et al., 2005 ▶; Zargari, 1987 ▶). In the present study, the effectiveness of ethanolic extract of Z. tenuior was investigated on protoscolices. Based on our results, Z. tenuior extract had the ability to effectively destroy all scolices at lower concentrations (10 and 25 mg/ml) and shorter exposure times (20 and 10 min), respectively.
Also, the findings of the current study showed that the ethanolic extract of Z. tenuior L. contains an efficient protoscolicidal agent which may be used in surgery of hydatidosis. Moreover, previous studies had demonstrated that the Ziziphora species are rich in thymol and pulegone compounds and also suggested that the antioxidant (Admassu Shimelis and Kumar Rakshit, 2005 ▶; Tian et al., 2011 ▶), antifungal (Shokri et al., 2012 ▶), antibacterial (Salehi et al., 2005 ▶; Sarac and Ugur, 2009 ▶), and antiprotozoal (Rota et al., 2004 ▶) activities of this plant may be associated with the presence of these compounds. Although it seems that these compounds are the major substances responsible for the scolicidal effect on hydatid cyst, nevertheless, the scolicidal effects of Z. tenuior L. involved in this activity need further investigation. The current study is the first report on the protoscolicidal activity of Z. tenuior L. Therefore, it may be used as an appropriate and effective scolicidal agent in the surgery of hydatidosis following more in-depth, in vivo investigations.
Acknowledgments
We would like to thank the Research Vice-Chancellor of Qazvin University of Medical Sciences for financial support and also the Medicinal Plants Research Center, Institute of Medicinal Plants, (ACECR), Karaj, Iran. This manuscript was part of a thesis accomplished for receiving an MSc degree in medical parasitology in Qazvin University of Medical Sciences, Qazvin, Iran.
Conflict of interest
The authors declare no conflict of interests regarding the publication of this paper.
References
- Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study) World J Surg. 2009;15:112–116. doi: 10.3748/wjg.15.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admassu Shimelis E, Kumar Rakshit S. Antinutritional factors and in vitro protein digestibility of improved haricot bean (Phaseolus vulgaris L) varieties grown in Ethiopia. Int J Food Sci Nutr. 2005;56:377–387. doi: 10.1080/09637480500512930. [DOI] [PubMed] [Google Scholar]
- Albi A, Baudin F, Matmar M, Archambeau D, Ozier Y. Severe hypernatremia after hypertonic saline irrigation of hydatid cysts. Anesth Analg. 2002;95:1806–1808. doi: 10.1097/00000539-200212000-00062. [DOI] [PubMed] [Google Scholar]
- Ammann RW, Eckert JC. Echinococcus. Gastroenterol Clin North Am. 1996;25:655–689. doi: 10.1016/s0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- Besim H, Karayalçin K, Hamamci O, Güngör C, Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10:347–351. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglar R1, Yuzbasioglu MF, Bulbuloglu E, Gul M, Ezberci F, Kale IT. In vitro effectiveness of different chemical agents on scolices of hydatid cyst. J Invest Surg. 2008;21:71–75. doi: 10.1080/08941930701883640. [DOI] [PubMed] [Google Scholar]
- Da Silva AM. Human echinococcosis: a neglected disease. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/583297. Article ID 583297, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NC, Newland RC. The reporting of colorectal cancer: The Australian clinico-pathological staging system. Aust N Z J Surg. 1982;52:395–397. doi: 10.1111/j.1445-2197.1982.tb06017.x. [DOI] [PubMed] [Google Scholar]
- De Sousa DP, Júnior EV, Oliveira FS, de Almeida RN, Nunes XP, Barbosa-Filho JM. Antinociceptive activity of structural analogues of rotundifolone: structure-activity relationship. Z Naturforsch. 2007;C62:39–42. doi: 10.1515/znc-2007-1-207. [DOI] [PubMed] [Google Scholar]
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu K, Hokelek M, Baris S, Sahin M, Birinci A, Amanvermez R, Tac K. Effect of albendazole sulfoxide solution on the scolices and the hepatobiliary system. Eur Surg Res. 1998;30:433–438. doi: 10.1159/000008610. [DOI] [PubMed] [Google Scholar]
- Kasumov FY. Component compositions of the essential oils of some species of the Genus Thymus. Chem Nat Comp. 1987;23:637–638. [Google Scholar]
- Kayaalp C, Balkan M, Aydin C, Ozgurtas T, Tanyuksel M, Kirimlioglu V, Akoglu M, Oner K, Pekcan M. Hypertonic saline in hydatid disease. World J Surg. 2001;25:975–979. doi: 10.1007/s00268-001-0065-9. [DOI] [PubMed] [Google Scholar]
- Khan R, Zakir M, Afaq SH, Latif A, Khan AU. Activity of solvent extracts of Prosopis spicigera, Zingiber officinale and Trachys permumammi against multidrug resistant bacterial and fungal strains. J Infect Dev Ctries. (292) 2010;4 doi: 10.3855/jidc.621. [DOI] [PubMed] [Google Scholar]
- Lahmar S, Chéhida FB, Pétavy AF, Hammou A, Lahmar J, Ghannay A, Gharbi HA, Sarciron ME. Ultrasonographic screening for cystic echinococcosis in sheep in Tunisia. Vet Parasitol. 2007;143:42–49. doi: 10.1016/j.vetpar.2006.08.001. [DOI] [PubMed] [Google Scholar]
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- Moazeni M, Nazer A. In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J Surg. 2010;34:2677–2681. doi: 10.1007/s00268-010-0718-7. [DOI] [PubMed] [Google Scholar]
- Salehi P, Sonboli A, Eftekhar F, Nejad Ebrahimi S, Yousezadi M. In vitro scolicidal effect of Satureja khuzistanica (Jamzad) essential oil. Asian Pac J Trop biomed. 2012;2:616–620. doi: 10.1016/S2221-1691(12)60107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeini A, Khosravi AR, Chitsaz M, Shokri H, Kamlnejad M. Anti-Candida albicans activity of some Iranian plants used in traditional medicine. J Med Mycol. 2009;19:168–172. [Google Scholar]
- Naghibi F, Mosaddegh M, Mohammadi Motamed M, Ghorbani A. Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iran J Pharm Res. 2005;4:63–79. [Google Scholar]
- Paksoy Y, Odev K, Sahin M, Arslan A, Koç O. Percutaneous treatment of hydatid cysts: comparison of direct injection of albendazole and hypertonic saline solution. Am J Roentgenol. 2005;185:727–734. doi: 10.2214/ajr.185.3.01850727. [DOI] [PubMed] [Google Scholar]
- Puryan K, Karadayi K, Topcu O. Chlorhexidine gluconate: an ideal scolicidal agent in the treatment of intraperitoneal hydatidosis. World J Surg. 2005;29:227–230. doi: 10.1007/s00268-004-7587-x. [DOI] [PubMed] [Google Scholar]
- Rajabi MA. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surg Pract. 2009;13:2–7. [Google Scholar]
- Rota C, Carramiñana JJ, Burillo J, Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J Food Prot. 2004;67:1252–1256. doi: 10.4315/0362-028x-67.6.1252. [DOI] [PubMed] [Google Scholar]
- Rouhani S, Salehi N, Kamalinejad M, Zayeri F. Efficacy of Berberis vulgaris aqueous extract on viability of Echinococcus granulosus protoscolices. J Invest Surg. 2013;26:347–351. doi: 10.3109/08941939.2013.818746. [DOI] [PubMed] [Google Scholar]
- Salehi P, Sonboli A, Eftekhar F, Nejad Ebrahimi S, Yousezadi M. Essential oil composition, antibacterial and antioxidant activity of the oil and various extracts of Ziziphora clinopodioides subsp rigida (BOISS) RECH f from Iran. Biol Pharm Bull. 2005;28:1892–1896. doi: 10.1248/bpb.28.1892. [DOI] [PubMed] [Google Scholar]
- Sarac N, Ugur A. The in vitro antimicrobial activities of the essential oils of some Lamiaceae species from Turkey. J Med Food. 2009;12:902–907. doi: 10.1089/jmf.2008.0089. [DOI] [PubMed] [Google Scholar]
- Sezik E, Tümen G, Başer KHC. Ziziphora tenuior L, a new source of pulegone. Flavour Fragrance J. 1991;6:101–103. [Google Scholar]
- Shahnazi M, Badakhsh F, Azadmehr A, Saraei M, Alipour M, Shahnazi M, Jamshidi M. Study of protoscolicidal effects of hypertonic glucose on protoscolices of hydatid cyst at different concentrations and exposure times. International Scholary Research Notices. 2014 doi: 10.1155/2014/314502. Article ID 314502, 5 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri H, Sharifzadeh A, Ashrafi Tamai I. Anti-Candida zeylanoides activity of some Iranian plants used in traditional medicine. J Mycol Med. 2012;22:211–216. doi: 10.1016/j.mycmed.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Tian S, Shi Y, Zhou X, GeL , Upur H. Total polyphenolic content and antioxidant capacity of different Ziziphora clinopodioides Lam extracts. Pharmacogn mag. 2011;7:65–68. doi: 10.4103/0973-1296.75904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcu O, Aydin C, Arici S, Duman M, Koyuncu A, Sen M. The effects of various scolicidal agents on the hepatopancreatic biliary system. Chir Gastroenterol. 2006;22:185–190. [Google Scholar]
- Zargari A. Iranian medicinal plants. Tehran, IR Iran: Tehran university press; 1987. [Google Scholar]
- Zohrabi M. Investigation botany, microscopy, analysis and indicated Composition of Ziziphora clinopodioides subsp. Tehran: Tehran University; 2000. [Google Scholar]