Abstract
Objective:
Curcumin is extracted from Curcuma longa and regulates the intracellular signal pathways which control the growth of cancerous cell, inflammation, invasion and apoptosis. Curcumin molecules have special intrinsic features that can target the intracellular enzymes, genome (DNA) and messengers (RNA). A wide range of studies have been conducted on the physicochemical traits and pharmacological effects of curcumin on different diseases like cardiovascular diseases, diabetes, cancer, rheumatoid arthritis, Alzheimer’s, inflammatory bowel disease (IBD), and even it has wound healing. Oral bioavailability of curcumin is rather poor, which would certainly put some boundaries in the employment of this drug.
Materials and Methods:
Bibliographical searches were performed using MEDLINE/ScienceDirect/OVID up to February 2015 using the following keywords (all fields): (“Curcumin” OR “Curcuma longa”) AND [(nanoparticles) OR (Nanomicelles) OR (micro emulsions) OR (liposome) OR (phospholipid).
Results:
Consequently, for any developments of curcumin in the future, analogues of curcumin that have better bioavailability or substitute formulations are needed crucially.
Conclusion:
These studies indicated that nanotechnology can formulate curcumin effectively, and this nano-formulated curcumin with a potent ability against various cancer cells, were represented to have better efficacy and bioavailability under in vivo conditions.
Key Words: Curcumin, Curcumin Nano formulations, Nano micelle
Introduction
Nanotechnology is the science which has revolutionized agriculture and food production systems (Roco, 2003 ▶). Studies of unusual characteristics of materials at Nano-metric scale (10-9 m) have led to their extensive applications in different industries. Nano sciences have been used in different forms since many years ago. These applications have been experiencing an ascending trend since the third and fourth centuries when different metal salts have been used for producing a diversity of colors up to emergence of modern scientific theories by scientists such as Richard Smalley, who primarily produced fullerenes followed by other theories developed by Eric Drexler, who designed molecular engines at non-metric scale (Smalley, 2003 ▶). Different polymer generations including ceramics and coatings as the first generation of which were commonplace between 2000 and 2005; the second generation was comprised of active Nano-structures with application of slow-releasing medicines during 2005-2010, and the third generation was composed of Nano-structures such as evolutionary and robotic systems which represent an ensemble of Nano-structures with the same performance, but partial intelligence; these applications were proceeded since 2010 up to now and finally, the fourth generation pertaining the Nano-systems molecular consisted of molecular devices with atomic design having novel functions. The examples include Nanomems which seem to act as the future outlook of Nano-systems (Oskuee et al., 2009 ▶). There are two means of building nano devices, one is to etch or mold bigger molecules or materials which are called the top-down approach, and the other is to build them by putting atoms or molecules together, which is called the bottom-up approach (Yih and Wei 2005 ▶).
With regard to this introduction, it can be demonstrated that not only applications of nano-systems are expanding in all industries, but also they have created new horizons. From many pharmacokinetic studies, it can be deduced that oral bioavailability of curcumin is rather poor, which would certainly put some boundaries in the employment of this chemical. Consequently, for any developments of curcumin in future, analogues of curcumin which have better bioavailability or substituted formulations are crucial.
Curcumin could be helpful in the treatment of different diseases such as cancers of pancreas or colon or even multiple myeloma, psoriasis or myelodysplastic syndromes (Goel et al., 2008 ▶., Liu et al., 2013 ▶). Recently, science is in pursuit of developing and building gears, apparatus or substances that are extremely small to the extent of being the same size as big biomolecular, for instance, enzymes or receptors, which are considered to be 100 to 10,000 times smaller than the human body cells (Gao et al., 2010 ▶; He et al., 2010 ▶; O'Shea et al., 2010 ▶). This field is called Nanotechnology which allows us to produce nano-scale apparatus. These devices, can effortlessly go through blood vessel walls, or even insert themselves into most of body cells when they are smaller than 20 nm or 50 nm, respectively, which makes them a perfect choice for customizable drugs to be used in targeted delivery of ample doses of chemotherapeutic drugs or to deliver therapeutic genes only to affected cancerous cells avoiding any unnecessary involvement or damage of surrounding healthy tissues(Bansal et al., 2011 ▶; Pandey et al., 2011 ▶; Setthacheewakul et al., 2010 ▶)
Therapeutic uses of curcumin
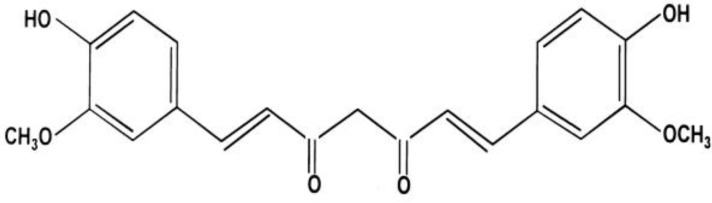
The origin of the usages of curcumin dates back to about 5000 years ago, which is around the prime time of “science of long life”, also known as Ayurveda (Garodia et al., 2007 ▶) (Figure 1).
Figure 1.
Chemical structure of Curcumin (Rahimi and Oskuee 2014
It has been found that the yellowish color of turmeric is due to curcumin (Braga et al., 2003 ▶). Curcumin was isolated from turmeric about 200 years ago, and in 1910 its structure was discovered, which gives rise to the name diferuloylmethane (Balogun et al.,2003 ▶; Soni and Kuttan 1992 ▶., Vareed et al., 2008 ▶).
Turmeric is found to be useful in various illnesses and disorders and is believed to have many medicinal effects on diseases of pulmonary, gastrointestinal tract and skin disease and can be helpful in the cases of aches and pains, sprains, wounds or even disorders related to liver. In the last few decades many researchers have focused on this matter, proving that curcumin is responsible for most of these effects (Garodia et al., 2007 ▶; Liu et al., 2013 ▶; Soni and Kuttan, 1992 ▶).
Even in countries that are famous for their traditional medicine such as China and India, curcumin which has a hydrophobic polyphenol structure, has been used extensively (Venkatesha et al., 2011 ▶). A wide range of studies have been conducted on the physicochemical traits and pharmacological effects of curcumin on different diseases, like cardiovascular diseases, diabetes, cancer, rheumatoid arthritis, Alzheimer’s, inflammatory bowel disease (IBD), or even wound healing, due to its minor toxicity(Garodia et al., 2007 ▶; Liu et al., 2013 ▶; Soni and Kuttan 1992 ▶).Curcumin has also been interesting because of many reasons, one of which is its effectiveness as a chemo-preventative agent, and also it is a chemo-/radio-sensitizer for tumorous cells, while acts as a chemo-/radio-protector for normal functioning organs (Garodia et al., 2007 ▶; Lao et al., 2006 ▶; Rushworth et al., 2006 ▶; Soni and Kuttan 1992 ▶; Yih and Wei 2005 ▶).On the other hand, low solubility in water and inadequate oral bioavailability have been the reasons for its restricted use in clinical trials(Kumar et al., 2000 ▶; Vareed et al., 2008 ▶).
These negative traits can be surpassed by the promising platform of the nanofield and its advantages on the efficiency of delivering anti-cancer drugs (Hansel et al., 2009 ▶; Kunnumakkara et al., 2008 ▶; Safavy et al., 2007 ▶; Yih and Wei 2005 ▶). Mustards, spindle poisons, anti-metabolites, alkylating agents, and DNA binders and cutters are chemotherapeutic agents that reduce tumor size while they relatively are not effective in tumors’ destruction or prevention of recurrence(Das et al., 2010 ▶; Hansel et al., 2009 ▶; Mukerjee and Vishwanatha, 2009 ▶; Yallapu et al., 2012 ▶). One of the problems of repeated application of these agents is chemotherapies resistance (Yih and Wei 2005 ▶).
Thus, it is critical to detect some natural products with distinctive properties that eradicate tumor cells and impress the intercellular signals without targeting healthy tissues (Gangwar et al., 2013 ▶; Liu et al., 2013 ▶).Curcumin is one of the species in the family of Zingiberaceae that is isolated from the rhizomes of Curcuma longa Linn, and is a natural polyphenolic phyto-constituent (Kim et al., 2001 ▶). Some of curcumin properties such as its poor solubility in neutral or acidic environments and instability under alkaline condition restrict its usage (Tønnesen et al., 2002 ▶). Curcumin decomposes into feruloyl methane and ferulic acid in alkaline pH (Rachmawati et al., 2013a ▶; Tønnesen et al., 2002 ▶)
In addition to cancers, curcumin can be used as a significant treatment for malaria, cystic fibrosis, Alzheimer’s and anti-inflammatory diseases (Maheshwari et al., 2006 ▶). Targeting the DNA, RNA and intracellular enzymes are due to pleiotropic trait of curcumin molecules which happensconsecutively or concurrently (Garodia et al., 2007 ▶; Kakarala et al., 2010 ▶; Thamake et al., 2011 ▶). Exclusively, pleiotropic properties of the curcumin, unlike other chemotherapeutic agents can regulate the nuclear factor-kappaB (NF-kB), mitogen-activated protein kinase (MAPK), transcription factor activator protein-1 (AP-1), serine/threonine protein kinase (AKT) signaling pathways, nuclear b-catenin signaling and tumor protein 53 (p53) (Garodiaet al., 2007 ▶; Hatcher et al., 2008 ▶; Venkatesha et al., 2011 ▶). It has been indicated that curcumin suppresses the expression of some growth factors like estrogen receptors and epidermal growth receptor which are associated with cancers (Hatcher et al., 2008 ▶).Curcumin could down regulate the expression of TNF α, IL-1, IL-6, IL-8, adhesion molecules (ICAM, VCAM), C-reactive protein (Farzadniaet al., 2013 ▶; Ganjali et al., 2014 ▶; Shamsara et al., 2009 ▶).
During the last three decades, there have been consequential developments in discovering the molecular targets in modern biology (Goel et al., 2008 ▶; Kakarala et al., 2010 ▶). The examination of curcumin on these targets has revealed that curcumin is involved in modulation of several various transcription factors, kinases, growth factors, cytokines, and other enzymes (Kakarala et al., 2010 ▶; Thamake et al., 2011 ▶; Venkatesha et al., 2011 ▶). Since deregulation of inflammation is important in a great number of diseases, it is essentially important to find an effective and safe anti-inflammatory agent in modern medicine. Steroids are probably the most well-known anti-inflammatory agents.
Another important study in the field of nano-formulated curcumin was done by Aggarwal et al in 2007 (Garodia et al., 2007 ▶). In a study, curcumin was encapsulated with more than 97.5% potency in PLGA and PEG. It has been proved that nano-formulated curcumin represents more efficacy and faster cellular uptake than free curcumin in vitro. Nano-formulated curcumin also had at least the same potential (or was perhaps more potent) as curcumin in suppressing the proliferation of different cancer cell lines and in leukemic cells apoptosis (Garodia et al., 2007 ▶). The examination of electrophoretic gel shift mobility revealed that curcumin nanoparticles were more active than curcumin in suppression of NF-kB regulated proteins involved in tissues invasion (MMP-9), angiogenesis (VEGF), and cell proliferation (cyclin D1) and in inhibition of TNF-induced NF-kB activation (Garodiaet al., 2007 ▶; Goel et al., 2008 ▶).
The NF-E2-related factor 2 (Nrf2) is the transcription factor which is usually available in an inactive state as a result of binding to a cytoskeleton-associated protein Keap1, and redox-dependent stimuli can activate it (Balogunet al., 2003 ▶; Pandey et al., 2011 ▶). Conversion of the Nrf2–Keap1 interaction makes Nrf2 to translocate to the nucleus, bind to the antioxidant-responsive element (ARE), and begin the transcription of genes coding for detoxifying enzymes and cyto-protective proteins. Cellular antioxidants are activated by the Nrf2/ARE signaling pathway. These antioxidants consist of NADPH quinone oxidoreductase-1 (NQO1), heme oxygenase-1(HO-1), and GSH. A class of electrophilic composition considering curcumin can also trigger this reaction (Balogun et al., 2003 ▶; Pandey et al., 2011 ▶).
Rushworth et al., have illustrated that ARE-mediated gene expressions are activated by curcumin in human monocytes through upstream of p38, PKC delta, and Nrf2 (Rushworth et al., 2006 ▶).
Gastrointestinal glutathione peroxidase (GI-GPx) has been considered as a barrier against hydrogen peroxide absorption and has also been involved in the control of malignant growth and inflammation (Rushworth et al., 2006 ▶). The up-regulation of the selenoprotein GI-GPx by activating the Nrf2/Keap1 system leads to anti-carcinogenic and anti-inflammatory effect of curcumin (Balogun et al., 2003 ▶; Pandey et al., 2011 ▶).
This was verified by a major increase in the protein levels of Smad2, p27, Smad4 and decrease of c-Jun by 5 µM conjugate. An increased cytotoxicity in tumor cells is obtained by emerging the products of PEG–curcumin (PEG–CUR) nano-conjugates (Safavy et al., 2007 ▶).
Molecular weight and the sort of linker chain terminal functionality have an influence on the synergist activity.
Curcumin receptors
Receptors are the molecular structures which bind to a particular substance followed by a specific response. Although any veritable receptors for curcumin are not known, several molecules which bind to curcumin have been detected. These consist of serum albumin, xanthine oxidase, 5-LOX (lipooxygenase), COX-2(cyclooxygenase 2), thioredoxin reductase, IKK (IkB kinase), iron , p-glycoprotein, PKA(protein kinase A), GST(glutathione-S–transferase), PKC(protein kinase C), PhK , cPK, auto-phosphorylation-activated protein kinase, Ca2+-dependent protein kinase (CDPK), inositol 1,4,5-triphosphate receptor, pp60c-src tyrosine kinase, Ca2+-ATPase of sarcoplasmic reticulum, rat river cytochrome p450s, aryl hydrocarbon receptor, Topo II isomerase and glutathione (Shishodia et al., 2005 ▶; Wang et al., 2008 ▶).Several researchers have tried to enhance the bioavailability, solubility and pharmacokinetic traits of curcumin trough different micro-formulation or nano-formulations (Gangwar et al., 2013 ▶; Garodia et al., 2007 ▶; He et al., 2010 ▶).
On pharmacological assessment, curcumin has specific properties such as anti-oxidant, anti-cancer, anti-inflammatory, anti-bacterial, wound-healing, lipid-lowering, and hepato-protective activities (Bansal et al., 2011 ▶; Kunnumakkara et al., 2008 ▶; Rachmawati et al., 2013a ▶). In spite of curcumin’s therapeutic effects, its efficacy has not yet been proved. Curcumin has noticeable pharmacological effects which can be used for treatment and prevention of various diseases(Bansal et al., 2011 ▶). Moreover, as it has been studied inhuman and animal models, the oral consumption of this agent is safe even at high doses (Soni and Kuttan 1992 ▶).
Curcumin bioavailability
The poor oral bioavailability of curcumin (1% in rat) is one of the main problems which has limited its application. The gastrointestinal tract slightly absorbs curcumin with the solubility at its maximum level of 11ng/ml in plain aqueous buffer pH5.0 (Anand et al., 2007 ▶; Yang et al., 2007 ▶). Some factors like Piperine, liposomes, nanoparticles, and phospholipids or modified structure of curcumin analogues can improve the bioavailability of this agent (Anand et al., 2007 ▶; Yang et al., 2007 ▶).
Curcumin’s solubility in water is about 0.0004 mg/ml at pH 7.3 which is really insignificant and also the molecules which are dissolved at this physiological pH are intensely sensitive (Mulik et al., 2010 ▶; Yallapu et al., 2012 ▶).Low bioavailability of curcuminis demonstrated by several studies inrats, mice and humans(Shaikh et al., 2009 ▶). After oral administration of 10 or 12 g/ml of curcumin, only almost 50 ng/ml is detected in serum which has a very low therapeutic effect (Garodia et al., 2007 ▶).
Numerous examinations proved that in the presence of an adjuvant like piperine, bioavailability and tissue distribution are improved and deliver the effective agent to the damaged cells to achieve good results (Kakarala et al., 2010 ▶; Lao et al., 2006 ▶; Pathak and Khandelwal 2008 ▶).
Bioavailability improvement, minimizing the degradation during the metabolism of curcumin, and increasing delivery capacity to tumors are the important purposes that could eliminate the limits of curcumin usage. When nanoparticles (NPs) are used as an adjuvant, they can deliver the active curcumin to cancer cells such as solid lipid NPs, polymer NPs, polymeric micelles, Nano/micro emulsions, Nano gels, liposome/phospholipid, self-assemblies, polymer conjugates, etc. (Anand et al., 2007 ▶; Muqbil et al., 2011 ▶; Yang et al., 2007 ▶).
Development of curcumin nanocrystals and conjugates
In pharmaceutical products, the most effective process for stabilizing and improving bioavailability curcumin are nanocrystal conjugates which have a significant role in treatment of diseases as a potential natural therapeutic agent (Yallapu et al., 2012 ▶).
All drugs with low solubility like curcumin can be approached by nanocrystal methods, so this limitation factor for bioavailability or absorption of the agents can be solved. High-pressure homogenization (HPH) and pearl milling are the chief techniques for producing nanocrystal drug, as for curcumin nanocrystal (Jantarat, 2013 ▶).
The minimum possible size and physical stability which are crucial factors in stabilization are affected by the ability of stabilizers. Since the dissolution rate of combined nanocrystals decreases, the nanocrystal physical stability gets really acceptable (Rachmawati et al., 2013a ▶).
Throughout the last decade, major progress has happened in the formulation of nanocrystal drugs which is converted to an advanced drug development method resulting in the production of five products on the market, recently (Jantarat 2013 ▶). Higher dissolution rate is the significant property of these systems which causes bioavailability improvement after oral consumption (Ravichandran 2013 ▶). Successful enhancement of nanocrystal formulation by any synthesis procedures of nanoparticles from molecular or atomic species through chemical reactions or physical assembly depends upon the stabilization process (Rachmawati et al., 2013b ▶). Curcumin crystal arrangement takes 90 min to dissolve in alcohol and water which is a time-dependent process. After this, curcumin crystals aggregate and precipitate the timing (Jantarat 2013 ▶; Rachmawati et al., 2013b ▶; Ravichandran 2013). Sodium dodecyl sulfate, Tween 80, pluronic polymers, cetyltrimethylammonium bromide (CTAB) and Triton X-100 are the detergent that arranges micelles at a critical micelle concentration and impress the stabilizing of curcumin molecules. However, cationic micelles are preferred because of their exclusive characteristics including better stability which is provided to curcumin even at raised pH and their medicinal implications (Jantarat 2013 ▶; Rachmawati et al., 2013b ▶; Ravichandran 2013). In addition, curcumin by attaching to plasma proteins carriers to other part.
Rachmawati et al. suggested that curcumin nanoparticles enhance the solubility of curcumin by improving bioavailability more than 5 times from simple curcumin powder (Rachmawati et al., 2013b ▶). In this study, high-pressure homogenization method is used for increasing curcumin solubility. Various stabilizers [polyvinyl alcohol (PVA), carboxymethylcellulose sodium salt, D-α tocopheryl polyethylene glycol 1000 succinate (TPGS), polyvinyl pyrrolidone (PVP), sodium dodecyl sulfate (SDS)] exert their effects via different stabilization mechanism. However, the most impressive polymer that stabilizes curcumin nanoparticle is PVP (Rachmawati et al., 2013b ▶).
Compared to other methods, high pressure homogenization (HPH) technique possesses more benefits for producing curcumin nanocrystals (Hatcher et al., 2008 ▶; Mukerjee and Vishwanatha 2009 ▶; Safavy et al., 2007 ▶). This procedure is an appropriate method for diminishing bulk curcumin into nanoparticles. Curcumin crystals are produced under optimized condition which is defined by elevated dispersions, with ten cycles of HPH, and an applied pressure of 150 MPa, at 28ºC temperature (Hatcher et al., 2008 ▶; Mukerjee and Vishwanatha 2009 ▶; Safavy et al., 2007 ▶).
This formulation causes an increase in dissolution rate, saturation solubility and stability of nanocrystal-loaded drug capsule (Yallapu et al., 2012 ▶). In comparison to commercial product, the dissolution behavior has improved significantly which has been an examined in many experiments (Tsai et al., 2011 ▶; Yallapu et al., 2012 ▶). The enhanced bioavailability of drugs with poor solubility property is obtained by elevated solubility behavior in nanocrystal drug-loaded solid dosage types (Ravichandran 2013 ▶; Tsai et al., 2011 ▶; Yallapu et al., 2012 ▶).
After intravenous administration tomice and rabbits, D-α tocopheryl polyethylene glycol 1000 succinate (TPGS) stabilized curcuminnanosuspension (CUR-NS), crystal formulation has been measured for the curcuminbio-distribution and pharmacokinetics(Tsai et al., 2011 ▶; Yallapu et al., 2012 ▶).
Curcumin is less bioavailable mostly due to its low aqueous solubility. In this study, Gangwar et al. revealed that curcumin can conjugate with silica nanoparticles enhance ingitssolubility in water and bioavailability (Gao et al., 2010 ▶). As technology developed, the loading and conjugation of curcumin could be examined with silica nanoparticles with thermogravimetric analyzer and transmission electron microscope (TEM)(Gao et al., 2010 ▶; Tsai et al., 2011 ▶; Yallapu et al., 2012 ▶). Cytotoxicity analysis represents that synthesized silica-curcumin conjugate operates against both HeLa cell lines and normal fibroblast cell lines. This study demonstrates that the silica-curcumin conjugate has antitumor activity (Gao et al., 2010 ▶).
Biomacromolecules can conjugate with potential sites of curcumin which are active methylene groups and two phenolic rings. The nanoscale family represents the conjugate polymer–drug as an alternative treatment (Gangwar et al., 2013 ▶; Kumar et al., 2000 ▶).
Nucleoside curcumin bioconjugates are designed to obtain high levels of glucuronide and sulfate curcumin conjugates in healthy human volunteers (Vareed et al., 2008 ▶). A recent study based on a luteinizing hormone-releasing hormone (LHRH)–curcumin conjugate synthesized by fluorenylmethoxycarbonyl (Fmoc) solid phase has indicated that anticancer effect in xenograft models of pancreatic cancer has improved(Vareedet al., 2008 ▶).
The base label protecting group for amine groups or LHRH is Fmoc (Hansel et al., 2009 ▶). Solution phase synthesis by using urethane chemistry developed the curcumin monoester and diesterbioconjugates (Hansel et al., 2009 ▶).
In cell culture media, the glycoside formation with curcumin was improved by salicylic acid and methyl jasmonate (curcumin-40, 40- O-beta-D-digentiobioside) and the solubility of curcumin increased to approximately 0.65 mmol/m (Dubey et al., 2008 ▶; Kumar et al., 2000 ▶).
Likewise, in the presence of human glutathione S-transferase (GST), canalization of monoglutathionylcurcumin conjugates can be obtained in biological systems (Kaminaga et al., 2003 ▶).The excretion of these curcumin conjugates to the intestinal lumen achieved the better biological effects and bioavailability (Usta et al., 2007 ▶).
Pandey et al. demonstrated that antioxidant defense system can be regulated by new PEGylated curcumin analogs nuclear factor erythroid-2 related factor 2 (Nrf2) activators can modify inflammatory diseases (Pandey et al., 2011 ▶). The expression of Nrf2 in renal epithelial cells is dependent on time and concentration which is stimulated by curcumin (Dubey et al., 2008 ▶; Kumar et al., 2000 ▶; Pandey et al., 2011 ▶; Safavy et al., 2007 ▶).This consequence is associated with a considerable increase in hemoxygenase activity and HO-1 protein expression. As a result of higherinactivation of the Nrf2–Keap1 complex, Nrf2 binding to the present ho-1 AREs isincreased, therefore, ho-1 gene activity is stimulated by curcumin(Dubey et al., 2008 ▶; Kumar et al., 2000 ▶; Pandey et al., 2011 ▶; Safavy et al., 2007 ▶).
A cationic poly (vinyl pyrrolidone)–curcumin (PVP–CUR) conjugate formulation with constant particle size and z potential with a pH range of 3–9 has been considered by MTT assay to be more powerful against L929 fibroblast cells over free curcumin(Manju and Sreenivasan 2011 ▶). The condensation polymerization of anhydrides and curcumin synthesized the polycatocol–curcumin conjugates (Tang et al., 2010 ▶). Intercellular uptake conveyed the polyamine conjugates of curcumin analogs to mitochondria; Simoniet al., 2010 ▶).Curcumin molecules directly conjugate to carboxylic acid groups of a hyaluronic acid (HA) polymer and create the nano size micelle in aqueous solution via hydrophobic interactions (Manju and Sreenivasan 2011 ▶; Simoni et al., 2010 ▶) .
Curcumin was extremely potent in therapeutics almost 13 µg of curcumin in conjugate, which was able to kill 80% of the L929 cells. Some cell-specific surface markers including CD44 can exclusively target the HA–curcumin conjugates (Manju and Sreenivasan 2011 ▶). One of the human body’s innate carriers for hydrophobic molecules is albumin. Despite a good water solubility, it is easily dissolved in ethanol with these properties it improves the curcumin solubility by a natural pathway (Manju and Sreenivasan 2011 ▶; Simoni et al., 2010 ▶). By using albumin bound technology, unique curcumin-loaded human serum albumin (HSA) nanoparticles (CCM-HSA-NPs) administered intravenously (Manju and Sreenivasan 2011 ▶; Simoni et al., 2010 ▶). This type of CCM-HAS-NPS is stronger in both vivo anti-cancer activity and aqueous solubility (300-fold) also has a restricted size range (130-150 nm). Also, 10 or 20 mg/kg of CCM-HSA-NPs can inhibit tumor growth (50% or 66% therapeutic effect) better than curcumin (18% inhibition) in tumor xenograft HCT116 models without inducing toxicity (Liu et al., 2013 ▶).
Development of curcumin emulsions, phospholipids and micelles formulations
There are many types of curcumin nano formulations (Table 1). Microemulsions are stable solutions with isotropic nanostructure containing water, oil and surfactant(s) (Liu and Chang 2011 ▶; Liu et al., 2011 ▶). Intreatment of some skin conditions including psoriasis, scleroderma and skin cancer, curcumin-based microemulsions are used to enhance curcumin delivery through transdermal and local paths. Eucalyptol- based curcuminmicroemulsions have an average curcumin solubility with excessive flux and permeability in comparison with many esteem oil- and oleic acid-based microemulsions (Liu and Chang 2011 ▶; Liu et al., 2011 ▶).
Table 1.
Summarized some types of Curcumin nanoparticles
| Type of nanoparticles | Form | Size(nm) | Used models | Methods | Results |
|---|---|---|---|---|---|
| Liposome | Globular | 25-205 | Breast cancer Melanoma Renal Ischemia Malaria |
In vitro In vivo (Dog and mice) |
Increased solubility, Tissue distribution and stability Enhanced anti tumor and anti angiogenesis effects Showed anti melanoma anti inflammatory and anti malarial effects |
| Micelle | Spherical | 10-100 | Lung tumor Breast cancer |
In vitro In vivo(mice) |
Increased solubility and bioavailability Improved anti oxidative and anti tumor effects Prolong circulation time Enhanced fluorescence effects |
| Noisome | Lamellar | 190-1140 | Albino rat skin Cancerous cells |
In vitro In vivo(Snake and mice) |
Increased skin penetration Prolonged delivery system Anti Infection and Anti cancer effects Enhanced fluorescence intensity |
| Cyclodextrin | Cyclic | 150-500 | Bowel disease Breast, Lung, pancreatic and prostate cancer |
In vitro In vivo(mice) |
Improved solubility Enhanced anti proliferative effects Increased anti cancer and anti inflammatory effects Developed bioavailability |
| Dedrimer | Globular Polymer |
15-150 | Breast cancer Colon cancer |
In vitro In vivo(mice) |
Improved stability Increased anti tumor and anti proliferative effects |
| Nanogel | Cross- linked polymer network | 10-200 | Melanoma Breast and pancreatic cancer cells |
In vitro | Increased solubility Enhanced fluorescence effects Developed bioavailability Improved anti cancer effects Get better controlled released Prolonged half-life Enhanced treatment of melanoma |
| Chitosan | Linear polysaccharide composed | 100-250 | Wound Melanoma tumors |
In vitro In vivo (rat and mice) |
Improved chemical stability Showed wound healing effects Increased anti tumor effects Improved antioxidant effects Prolonged blood circulation |
| Gold | Globular | 200-250 | Cancerous cells | In vitro | Improved solubility Enhanced anti oxidative and anti cancer effects |
| Silver | Film layer | ~15 | Infection Skin wounds |
In vitro | Showed anti microbial effects Improved wound healing Increased antiviral and anti cancer effects |
| Solid lipid | Spherical | 50-1000 | Cerebral ischemia Colitis Allergy Breast cancer |
In vitro In vivo (rat and mice) |
Prolonged blood circulation Increased anti inflammatory effects Improved brain delivery |
Nanoemulsion stability intimated a huge surface tension, and thus a significant surface energy (which is the interfacial tension times the surface area)(Liu and Chang 2011 ▶; Liu et al., 2011 ▶). Although many nano-emulsionsare unstable thermodynamically, they may have high kinetic stability because of their specific size (Liu and Chang 2011 ▶; Liu et al., 2011 ▶; Yallapu et al., 2012 ▶). Since the small creaming rate induced by gravity is lower than the Brownian motion, nanoemulsionsdo not sediment (Liu and Chang 2011 ▶; Liu et al., 2011 ▶; Yallapu et al., 2012 ▶). A perfect reservoir for phytochemicals that need Transportation and protection is supplied by the internal phases of nanoemulsions (Liu and Chang 2011 ▶; Liu et al., 2011 ▶; Yallapu et al., 2012 ▶). The nano sizes of emulsions show higher stability and bioavailability than the encapsulated phytochemicals. On the whole, either low or high energy emulsifications can prepare the nanoemulsions. The conventional emulsions are in the range of 1 to 100 μm that are much larger than that of nanoemulsions (50 to 200 nm) (Liu and Chang, 2011 ▶; Liu et al., 2011 ▶; Yallapu et al., 2012 ▶).
Nanoemulsion bioavailability, preparation, and features have been considered, wherein curcumin nanoemulsions inhibit 85% of TPA-induced mouse ear inflammation and inhibit the expression of cyclin D1, while compared to the conventional DBM emulsion, dibenzoylmethane (DBM) nanoemulsion shows approximately 3-folds increase in oral bioavailability(Liu and Chang 2011 ▶; Liu et al., 2011 ▶; Yallapu et al., 2012 ▶). Biopolymer micelles have indicated extremely improved water solubility/dispensability and in vitro antitumor activity of phytochemicals (He et al., 2010 ▶).
Curcumin with characteristics including stable self-emulsifying liquid formulations, approximately 99% curcumin loading and particles size of about 30 nm have been developed (Setthacheewakul et al., 2010 ▶). These studies proposed the effective strategies which were obtained by the new self-microemulsifying systems in liquid and pellet form for the formulation of lipophilic compounds with low solubility and poor oral bioavailability (Setthacheewakul et al., 2010 ▶). The examination of these formulations in male Wistar-strain rats revealed that they have a 10 to 14-folds greater absorption compared to the same oral dose of free curcumin (oral treatment of 50 mg/kg) (Setthacheewakul et al., 2010 ▶).
Moreover, the other compositions that increase curcumin absorption in rats after oral consumption are curcumin–phospholipid complexes or curcumin–phosphatidylcholine complexes (Belcaro et al., 2010 ▶).Curcumin levels in the liver and plasma werealmost5-folds higher ascompared to the control animals (Esmaili et al., 2011 ▶).
Tiyaboonchai et al indicated that a solid lipid nanoparticle (SLN) was spherical in shape and was characterized with the diameter of 10 to 1000 nanometers (Tiyaboonchai et al., 2007 ▶). SLNs can improve curcumin stability and solubilize it with a solid lipid core matrix (Tiyaboonchai et al., 2007 ▶). Lyophilized curcuminoids-loaded SLNs are considered to be spherical with a polydispersity index of 0.4 and an average particle size of 450nm. Curcuminoids possess the incorporation efficacy of up to 70% (w/w) (Tiyaboonchaiet al., 2007 ▶). This experiment demonstrated the improvement of curcuminoids stability. SLNs werestored in the dark (in the absence of sunlight) for 6 months, then it was shown that the percentages of the remaining curcumin, demethoxycurcumin and bisdemethoxycurcumin were approximately 91%, 88%, and 96%, respectively (Liu et al., 2013 ▶; Tiyaboonchai et al., 2007 ▶). Another assay has used the micro-emulsification technique to prepare curcumin-loaded solid lipid nanoparticles (C-SLNs) with a mean particle size of 135nm and an overall drug entrapment of 92.33±1.63%. The particles were generally spherical, with high drug content of 81.9% drug loading (Kakkar et al., 2011 ▶). Considerable progress (at p<0.05) in bioavailability was revealed compared to the free curcumin by in vivo pharmacokinetics (39 times at 50 mg/kg; 155 times at 1mg/kg; and, 59 times at 12.5 mg/kg, and 32 times at 25mg/kg) (Kakkaret al., 2011 ▶).
Raveendran et al. recommended that curcumin-loaded micelles are prepared based on amphiphilic Pluronic/Polycaprolactone (Pluronic/PCL) block copolymers (Ravindran et al., 2009 ▶). This nano-preparation with the size smaller than200 nm was enhancing curcumin’s water solubility; in addition, its encapsulation efficiency was between 72.1%-96.6 % (Ravindran et al., 2009 ▶). Colorectal adenocarcinoma cells demonstrated the cellular uptake of the curcumin-loaded micelles and in vitro cytotoxicity (Ravindran et al., 2009 ▶).
Nanomicelles containing curcumin is a registered curcumin product (SinaCurcumin®) for oral use which has been developed in Nanotechnology Research Center of Mashhad University of Medical Science and marketed by Exir Nano SinaCompany in Tehran-Iran. Each soft gel of SinaCurcumin® contains 80 mg of curcumin as a nano micelle. These nanomicelles are prepared from GRAS (generally recognized as safe) pharmaceutical excipients and C3-complex form of curcumin. The percentage of encapsulation of curcumin in this nano micelle is near to 100% and their sizes are around 10 nm. SinaCurcumin® has a significantly higher bioavailability after oral use compared to a simple powder form of curcumin with the following mechanisms.
An intact layer of water is on the surface of intestinal epithelial cells (unstirred water layer), so any medication should pass this barrier (Smithson et al., 1981 ▶), this is a great barrier for a lipophilic molecule such as curcumin. Bile salts help the absorption of many kinds of molecules like fat-soluble vitamins, lipids, fatty acids, cholesterol, etc. (Howles, 2010 ▶).
After oral administration, the soft gels of SinaCurcumin® open and pass through the small intestine in less than 15 minutes in the stomach. These nano micelles after reaching the small intestinecould bedissolved in unstirred water layer. Although curcumin is insoluble in water, it could be water-soluble as nano micelles and absorbed in GI tract.
Development of curcumin-encapsulated as NPs
In production of various biomedical devices, poly (lactic-co-glycolic acid) (PLGA) with biocompatibility and biodegradability properties is an excellent choice (Mukerjee and Vishwanatha 2009 ▶; Thamake et al., 2011 ▶). Several types of curcumin-encapsulated PLGA NPs have been discovered as a safe carrier (Mukerjee and Vishwanatha 2009 ▶) (Table 1). A simple solid–oil–water solvent evaporation technique has been used for the preparation of PLGA NP for curcumin encapsulation sonication time and the surfactant concentration can also be controlled by the particle size (Mukerjee and Vishwanatha 2009 ▶). Therefore, the solvent evaporation method can regulate the curcumin-encapsulated PLGA NPs via a lower particle size, improved intracellular uptake and antibody conjugation characteristics (Mukerjee and Vishwanatha 2009 ▶; Thamake et al., 2011 ▶).
Surface functionalization of curcumin-loaded PLGA NPs by a bis (sulfosuccinimidyl) suberate (BS3) can conjugate the annexin A2 and induce an effective target therapy of curcumin to annexin A2- positive MDA-MB-231 tumor cells(Thamake et al., 2011 ▶). Furthermore, in another study, two poly (lactide-co-glycolide) (PLGA) combinations of curcumin nanoparticle formulations were compared (50:50 and 75:25 lactide: glycolide ratio) and the result revealed that nano-curcumin 50:50 formulation is more efficient from many aspects including it releases curcumin faster in molecular dispersion form, it possesses the smaller size with higher encapsulation efficiency, and in aqueous media, it has better anticancer activity (Mukerjee and Vishwanatha 2009 ▶; Thamake et al., 2011 ▶).
Das et al., represented three biocompatible nanoparticles — chitosan (CS), pluronic, alginate (ALG)—by ionotropic pre-gelation followed by polycationic cross-linking (Das et al., 2010 ▶). Toimprovethe solubility of curcumin in the ALG-CS NPs used Pluronic F127. ALG-CS-PF127 composite NPs could be deemed as a potential agent in the delivery of nanoformulated hydrophobic drugs to tumor cells (Das et al., 2010 ▶). All these composite NPs—namely ALG, CS, and PF127 —have been reported to be biocompatible (Das et al., 2010 ▶). Again, ALG and CS are biodegradable, while PF127 is a US Food and Drug Administration which is the appropriate polymer for drug delivery because of its nontoxicity (Das et al., 2010 ▶).
Liposomes contain artificial phospholipid vesicles that can protect drugs from external stimuli, and have some important features such as biological safety and biocompatibility. It has been shown that dissolving complexing or mixing curcumin with various types of phospholipids can enhance its absorption capacity. Souet al., have illustrated that a curcumin lipid formulation has been prepared successfully by using an anionic amphiphile, 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), N-(3-carboxy-1-oxopropyl)-,1,5-dihexadecyl ester (SA) ,and L-glutamic acid (Souet al., 2008 ▶). After administration of this formulation intravenously torats, no acute response in blood cells circulation observed, and a large amount of the curcumin was accumulated in spleen tissues and bone marrow (Sou et al., 2008 ▶).
For the purpose of intravenous administration, Li et al., encapsulated curcumin in a liposomal delivery system, and using human pancreatic carcinoma cells, they studied in vivo and in vitro effects of this composite on proliferation, signaling, apoptosis and angiogenesis (Li et al., 2005 ▶). NF-kB was generally active in all human pancreatic carcinoma cell lines evaluated (Li et al., 2005 ▶). Electrophoretic mobility gel shift assay proved that liposomal curcumin persistently suppressed NF-kB binding and decreased the expression of NF-kB-regulated gene products which have been involved in tumor invasiveness or growth (including interleukin-8 [Enzyme-linked immunoassay] and cyclooxygenase- 2 [Immunoblot])(Li et al., 2005 ▶). These in vitro changes totally appertained to concentration and time-dependent anti-proliferative activity (3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide assay [MTT assay]) and pro-apoptotic effects (Annexin V/Propidium iodide staining fluorescence activated cell sorting] and polyadenosine-5-diphosphate-ribose-polymerase cleavage)(Li et al., 2005 ▶). Liposomal curcumin had an equal or better activity than that of free curcumin at the same concentration. In vivo, the angiogenesis of the tumor and pancreatic carcinoma growth in murine xenograft models were suppressed by curcumin (Li et al., 2005 ▶). On the other side, in vitro liposomal curcumin induced apoptosis of human pancreatic cells, down-regulated the NF-kB machinery, and suppressed growth (Anand et al., 2007 ▶; Li et al., 2005 ▶). In vivo, the anti-angiogenesis and anti-tumor effects were the base of experiments that wereassociated with this nontoxic phytochemical encapsulated in liposomes for systemic delivery in order to treat pancreatic carcinoma (Li et al., 2005 ▶; Takahashi et al., 2009 ▶). Overall, this study indicates that both liposomal and free curcumin have the same potential to suppress COX-2, NF-κB activity, and IL-8 expression, as well as survival or cell proliferation of pancreatic carcinoma cells (Li et al., 2005 ▶). In vivo, liposomal curcumin has a potent anti-angiogenic response and these effects are accompanied by growth inhibition of pancreatic cell in murine xenograft models (Li et al., 2005 ▶). After the administration of maximum dosage to mice, no obvious host toxicity is attained (Li et al., 2005 ▶). In conclusion, observations recommended that liposomal curcumin should be examined in the clinical setting (Li et al., 2005 ▶; Takahashi et al., 2009 ▶).
Curcumin-loaded PLGA is designed by Tsai et al. Moreover; they proved that the retention time values of the hippocampus and the cerebral cortex were elevated to approximately 1.8- and 2.0-folds, respectively (Tsai et al., 2011 ▶). In addition, the half-life of curcumin in the hippocampus considerably increased from 7.56 to 16.7 min, likewise in cerebral cortex from 2.32 to 19.9 min (Tsai et al., 2011 ▶). This nano formulation causes slight growth in the levels of curcumin plasma (Tsai et al., 2011 ▶). The other biocompatible nano formulations which can be used for intravenous, oral, and controlled-delivery purposes are based on dextran sulfate–chitosan (Tsai et al., 2011 ▶).
Co-encapsulation of curcumin and doxorubicin in polymer NPs can be used for effective treatment of multidrug resistant cancer cells (K-562 cells)(Tsai et al., 2011 ▶). Down-regulation of MDR1 and BCL-2 expression and inhibition of nuclear efflux mechanism were performed by primary curcumin released from NPs (Tsai et al., 2011 ▶). Subsequently, cancer cell death was stimulated by the release of doxorubicin. This CUR–RUB complex possesses anti-cancer effect against the human breast, pancreatic, and colon cancer cell lines (Tsai et al., 2011 ▶). Additionally, it indicated that any degradation did not occur by stability under physiological conditions, and also no precipitation or clusters were observed (Tsai et al., 2011 ▶).
A cyclodextrin–curcumin self-assembly has presented much influence over curcumin in up-regulation of death receptors (DR4 and DR5) in KBM-5 tumor cells and inhibiting tumor necrosis factor (TNF)-induced expression of NF-kB regulated genes (VEGF, MMP-9 and cyclin D1)(Tsai et al., 2011 ▶).
Although there was not any proof in a 40-patient cohort that curcumin increased CD4 counts or reduced viral load, it has been considered as an antiviral agent for human immune-deficiency virus (HIV)(Tsai et al., 2011 ▶).
In a study, 10 healthy volunteers received 500 mg of curcumin per day for 7 days, and then the efficacy of curcumin in decreasing the serum levels of lipid peroxides and cholesterol was investigated (Hsu and Cheng 2007 ▶).
An increase in high-density lipoprotein cholesterol (29%), a major reduction in the level of serum lipid peroxides (33%) and total serum cholesterol (11.63%) was reported (Soni and Kuttan 1992 ▶).
In former clinical studies, curcumin preparations and combinations were heterogeneous making the comparison of the results from different studies difficult (Liu et al., 2013 ▶). Therefore, for future clinical studies, it is essential to use pure curcumin manufactured by a standardized method.
Conclusions
Although curcumin nanoparticles have numerous benefits in drug therapy, but one of the main problems is that the drugs are delivered to the healthy tissues around the tumor cell, as well as the cancer cells. Therefore, it is required to conduct a research for targeted drug delivery. Also, its recognition is evaluated in clinical examinations such as cancer.
For obtaining a better-targeted therapeutic method, it is required that the future studies combine curcumin delivery with other first-line anti-tumor chemotherapeutic agents or imaging-, contrast-, antibody- or peptide targeted delivery. To demonstrate the anti-cancer effect of curcumin, most of the studies were restricted to the small animal or in vitro studies. These studies indicated that nanotechnology can formulate curcumin effectively, and this nanoformulated curcumin with a potent ability against various cancer cells, were represented to have better efficacy and bioavailability under in vivo conditions. However, it is crucial to study more to take the research into clinical practice. Additionally, more studies are required to investigate the toxicity and efficacy of curcumin NP formulations in both large and small group of patients, also in patients with cancer in phase I or II clinical experiments. These studies would compare the free curcumin and curcumin NP formulations in terms of anti-cancer efficiency, and suggest whether it is possible to use curcumin NP formulations as a useful treatment for cancer.
Acknowledgements
Mashhad University of Medical Science Research Council supported this research. This paper was prepared based on data from PhD thesis of Hamid Reza Rahimi, MD, the PhD candidate of the Molecular Medicine of Mashhad University of Medical Science, Mashhad, Iran.
Conflicts of interest
The authors has nothing to disclose
References
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mole Pharma. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green C, Roberta A, Jawed M. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res. 2011;4:1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, Grossi MG, Togni S, Appendino G. Efficacy and safety of Meriva (R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. 2010;15:337–344. [PubMed] [Google Scholar]
- Braga ME, Leal PF, Carvalho JE, Meireles MAA. Comparison of yield, composition, and antioxidant activity of turmeric (Curcuma longa L) extracts obtained using various techniques. J Agric Food Chem. 2003;51:6604–6611. doi: 10.1021/jf0345550. [DOI] [PubMed] [Google Scholar]
- Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 2010;6:153–160. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Dubey SK, Sharma AK, Narain U, Misra K, Pati U. Design, synthesis and characterization of some bioactive conjugates of curcumin with glycine, glutamic acid, valine and demethylenated piperic acid and study of their antimicrobial and antiproliferative properties. Eur J Med Chem. 2008;43:1837–46. doi: 10.1016/j.ejmech.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Esmaili M, Ghaffari SM, Moosavi-Movahedi Z, et al. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT-Food Sci Tech. 2011;44:2166–2172. [Google Scholar]
- Farzadnia M, Ayatollahi H, Hasan-Zade M, Rahimi HR. A Comparative Study of Serum Level of Vascular Cell Adhesion Molecule-1 (sVCAM-1), Intercellular Adhesion Molecule-1(ICAM-1) and High Sensitive C - reactive protein (hs-CRP) in Normal and Pre-eclamptic Pregnancies. Iran J Basic Med Sci. 2013;16:689–93. [PMC free article] [PubMed] [Google Scholar]
- Gangwar RK, Tomar GB, Dhumale VA, Zinjarde S, Sharma RB, Datar S. Curcumin conjugated silica nanoparticles for improving bioavailability and its anticancer applications. J Agric Food Chem. 2013;61:9632–9637. doi: 10.1021/jf402894x. [DOI] [PubMed] [Google Scholar]
- Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S, Ferns G, Parizadeh SM, Ghayour-Mobarhan G. Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. Sci World J. 2014;2014:898361. doi: 10.1155/2014/898361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Li Z, Sun M, Li H, Guo C, Cui J, Li A, Cao F, Xi Y, Lou H, Zhai G. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm. 2010;36:1225–1234. doi: 10.3109/03639041003695139. [DOI] [PubMed] [Google Scholar]
- Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- Goel A, Jhurani S, Aggarwal BB. Multi‐targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- Hansel W, Aggarwal S, Hammer RP. Curcumin conjugates for treating and preventing cancers. Google Patents. 2009 [Google Scholar]
- Hatcher H, Planalp R, Cho J, Torti F, Torti S. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Huang Y, Cheng Y. Structure evolution of curcumin nanoprecipitation from a micromixer. Cryst. Growth Des. 2010;10:1021–1024. [Google Scholar]
- Howles PN. Cholesterol absorption and metabolism. Methods Mol Biol. 2010;602:157–179. doi: 10.1007/978-1-60761-058-8_10. [DOI] [PubMed] [Google Scholar]
- Hsu C-H, Cheng A-L. Clinical studies with curcumin The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; 2007. pp. 471–480. [Google Scholar]
- Jantarat C. Bioavailability enhancement techniques of herbal medicine: A case example of curcumin. Int J Pharm and Pharm Sci. 2013;5:493–500. [Google Scholar]
- Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2011;55:495–503. doi: 10.1002/mnfr.201000310. [DOI] [PubMed] [Google Scholar]
- Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, Maitani T, Mizukami H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555:311–316. doi: 10.1016/s0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
- Kim DS, Park S-Y, Kim J-Y. Curcuminoids from Curcuma longa L (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from βA (1–42) insult. Neurosci Lett. 2001;303:57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- Kumar S, Dubey KK, Tripathi S, Fujii M, Misra K. vol 44. Oxford Univ Press: Nucleic acids symposium series; 2000. Design and synthesis of curcumin-bioconjugates to improve systemic delivery; pp. 75–76. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Lao CD, Ruffin MT Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin. Cancer. 2005;104:1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- Liu C-H, Chang F-Y. Development and characterization of eucalyptol microemulsions for topic delivery of curcumin. Chem Pharm Bulletin, (Tokyo) 2011;59:172. doi: 10.1248/cpb.59.172. [DOI] [PubMed] [Google Scholar]
- Liu C-H, Chang F-Y, Hung D-K. Terpene microemulsions for transdermal curcumin delivery: effects of terpenes and cosurfactants. Colloids Surf B Biointerfaces. 2011;82:63–70. doi: 10.1016/j.colsurfb.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen S, Lv L, Song L, Guo S, Huang S. Recent progress in studying curcumin and its nano-preparations for cancer therapy. Curr Pharm Des. 2013;19:1974–1993. [PubMed] [Google Scholar]
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Manju S, Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J Colloid Interface Sci. 2011;359:318–325. doi: 10.1016/j.jcis.2011.03.071. [DOI] [PubMed] [Google Scholar]
- Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–387. [PubMed] [Google Scholar]
- Mulik RS, Mönkkönen J, Juvonen RO, Mahadik KR, Paradkar AR. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J Pharm. 2010;398:190–203. doi: 10.1016/j.ijpharm.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Muqbil I, Masood A, Sarkar FH, Mohammad RM, Azmi AS. Progress in nanotechnology based approaches to enhance the potential of chemopreventive agents. Cancers. 2011;3:428–445. doi: 10.3390/cancers3010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- Oskuee RK, Dehshahri A, Shier WT, Ramezani M. Alkylcarboxylate grafting to polyethylenimine: a simple approach to producing a DNA nanocarrier with low toxicity. J Gene Med. 2009;11:921–32. doi: 10.1002/jgm.1374. [DOI] [PubMed] [Google Scholar]
- Pandey MK, Kumar S, Thimmulappa RK, Parmar VS, Biswal S, Watterson AC. Design, synthesis and evaluation of novel PEGylated curcumin analogs as potent Nrf2 activators in human bronchial epithelial cells. Eur J Pharm Sci. 2011;43:16–24. doi: 10.1016/j.ejps.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Pathak N, Khandelwal S. Comparative efficacy of piperine, curcumin and picroliv against Cd immunotoxicity in mice. Biometals. 2008;21:649–661. doi: 10.1007/s10534-008-9150-y. [DOI] [PubMed] [Google Scholar]
- Rachmawati H, Al Shaal L, Muller RH, Keck CM. Development of curcumin nanocrystal: physical aspects. J Pharm Sci. 2013a;102:204–14. doi: 10.1002/jps.23335. [DOI] [PubMed] [Google Scholar]
- Rachmawati H, Shaal LA, Müller RH, Keck CM. Development of curcumin nanocrystal: physical aspects. J pharm sci. 2013b;102:204–214. doi: 10.1002/jps.23335. [DOI] [PubMed] [Google Scholar]
- Rahimi HR, Oskuee RK. Curcumin From Traditional Iranian Medicine to Molecular Medicine. Razavi Int J Med. 2014;2:e19982. [Google Scholar]
- Ravichandran R. Development of an Oral Curcumin Nanocrystal Formulation. J Nanotech Eng Med. 2013;3:041007–041007. [Google Scholar]
- Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roco MC. Nanotechnology: convergence with modern biology and medicine. Curr Opin Biotechnol. 2003;14:337–346. doi: 10.1016/s0958-1669(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Ogborne RM, Charalambos CA, O’Connell MA. Role of protein kinase C δ in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem Biophys Res Commun. 2006;341:1007–1016. doi: 10.1016/j.bbrc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Safavy A, Raisch KP, Mantena S, et al. Design and Development of Water-Soluble Curcumin Conjugates as Potential Anticancer Agents. J Med Chem. 2007;50:6284–6288. doi: 10.1021/jm700988f. [DOI] [PubMed] [Google Scholar]
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm. 2010;76:475–485. doi: 10.1016/j.ejpb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Shaikh J, Ankola D, Beniwal V, Singh D, Kumar MR. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Shamsara J, Ramezani M, Mohammadpour AH. Curcumin as a novel plaque stabilizing agent in prevention of acute coronary syndrome. Iran J Med Hypotheses Ideas. 2009;3:1–6. [Google Scholar]
- Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–21. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- Simoni E, Bergamini C, Fato R, et al. Polyamine Conjugation of Curcumin Analogues toward the Discovery of Mitochondria-Directe Neuroprotective Agents. J Med Chem. 2010;53:7264–7268. doi: 10.1021/jm100637k. [DOI] [PubMed] [Google Scholar]
- Smalley R. Nanotechnology. Chemical & Engineering News. 2003;81:37–42. [Google Scholar]
- Smithson KW, Millar DB, Jacobs LR, Gray GM. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981;214:1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- Soni K, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36:273–273. [PubMed] [Google Scholar]
- Sou K, Inenaga S, Takeoka S, Tsuchida E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm. 2008;352:287–293. doi: 10.1016/j.ijpharm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009;57:9141–9146. doi: 10.1021/jf9013923. [DOI] [PubMed] [Google Scholar]
- Tang H, Murphy CJ, Zhang B, et al. Curcumin polymers as anticancer conjugates. Biomaterials. 2010;31:7139–7149. doi: 10.1016/j.biomaterials.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Thamake S, Raut S, 2 , Ranjan A, Gryczynski Z, Vishwanatha J. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology. 2011;22:035101. doi: 10.1088/0957-4484/22/3/035101. [DOI] [PubMed] [Google Scholar]
- Tiyaboonchai W, Tungpradit W, Plianbangchang P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm. 2007;337:299–306. doi: 10.1016/j.ijpharm.2006.12.043. [DOI] [PubMed] [Google Scholar]
- Tønnesen HH, Másson M, Loftsson T. Studies of curcumin and curcuminoids XXVII Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002;244:127–135. doi: 10.1016/s0378-5173(02)00323-x. [DOI] [PubMed] [Google Scholar]
- Tsai Y-M, Chien C-F, Lin L-C, Tsai T-H. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int J Pharm. 2011;416:331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Usta M, Wortelboer HM, Vervoort J, et al. Human glutathione S-transferase-mediated glutathione conjugation of curcumin and efflux of these conjugates in Caco-2 cells. Chem Res Toxicol. 2007;20:1895–1902. doi: 10.1021/tx7002245. [DOI] [PubMed] [Google Scholar]
- Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha SH, Berman BM, Moudgil KD. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arthritis. Bioorg Med Chem. 2011;19:21–29. doi: 10.1016/j.bmc.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xu Y, Wu H-L, et al. The antidepressant effects of curcumin in the forced swimming test involve 5-HT 1 and 5-HT 2 receptors. Eur J Pharmacol. 2008;578:43–50. doi: 10.1016/j.ejphar.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17:71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K-Y, Lin L-C, Tseng T-Y, Wang S-C, Tsai T-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Yih TC, Wei C. Nanomedicine in cancer treatment. Nanomedicine: Nanotechnology, Biology and Medicine. 2005;1:191–192. doi: 10.1016/j.nano.2005.04.001. [DOI] [PubMed] [Google Scholar]