Abstract
Objective:
Perovskia abrotanoides Karel, belongs to the family Lamiaceae and grows wild alongside the mountainous roads inarid and cold climate of Northern Iran. The anti-tumor activity of P. abrotanoides root extract has been shown previously. This study was designed to examine in vitro anti-proliferative and pro-apoptotic effects of flower extract of P. abrotanoides on MCF-7 and Hela cell lines.
Materials and Methods:
Cells were cultured in DMEM medium with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin and incubated with different concentrations of plant extracts. Cell viability was quantified by MTT assay. Apoptotic cells were determined using propidium iodide (PI) staining of DNA fragmentation by flow cytometry (sub-G1 peak).
Results:
P. abrotanoides extract inhibited the growth of malignant cells in a time and dose-dependent manner and 1000 µg/ml of extract following 48h of incubation was the most cytotoxic dose against Hela cell in comparison with other doses; however, in MCF-7 cells,1000 and 500 µg/ml PA induced toxicity at all time points but with different features.. Analysis of flowcytometry histogram of treated cells compared with control cells indicated that the cytotoxic effect is partly due toapoptosis induction.
Conclusion:
Hydro-alcoholic extract of P. abrotanoides flowers inhibits the growth of MCF-7 and HeLa cell lines, partly via inducing apoptosis. Their inhibitory effect was increased in a time and dose-dependent manner, especially in MCF7 cells. However, further studies are needed to reveal the mechanisms of P. abrotanoides extract-induced cell death.
Key Words: Perovskia abrotanoides, Breast cancer, Cervical cancer, Apoptosis, MCF-7, Hela, Cell death
Introduction
Cancer is regarded as a complex disease which is non-curable in most cases (Dalkic et al., 2010 ▶). Breast and cervical cancers are the most common cancers in women in Iran and worldwide. Breast cancer continues to be a major health problem for women. Cervical cancer is one of the most common cancers in Iran and worldwide and the second most common cancer in Iran (Farahmandbeigi et al., 2000 ▶). Unfortunately, this cancer is developing rapidly. Current systemic therapies for these cancers are often limited by their non-specific effects, normal tissues toxicity and short-term efficacy due to the emergence of drug resistance (Mousavi et al., 2009 ▶). The main strategy of chemotherapy protocols is a synergistic damaging effect of drugs on cancer cells with reduced toxicity to the host.
Currently, using medicinal plants is regarded as an effective strategy for treatment of various cancers. So, the application of some compounds that are derived from plants has been established in various cancer therapeutic regimens. However, there is still a great need to find new sources.
Perovskia abrotanoides (P. abrotanoides) is a medicinal plant which belongs to Lamiaceae family, grows wild in Iran, Afghanistan, Pakistan and Turkmenistan (SafaeiGhomi and Batooli, 2010 ▶). This perennial herb is distributed in North, East and Central regions of Iran. P. abrotanoides is a valuable medicinal species in northern Iran (Iran, North Khorasan). This plant is self-propelled, specially for cold and dry regions, and is used in traditional medicine (Mazandarani, 2010 ▶). This plant contains flavonoids, phenolics and anthocyanins (Mazandarani, 2010 ▶). Phytochemical study conducted on roots of P. abrotanoides in 2001 showed that the root contains diterpenoid 1,2-quinones which are called tanshinones (Sairafianpour et al., 2001 ▶).
Tanshinones have remarkably diverse biological activities that are important in medical sciences. For example, anti-microbial and fungal activity (Bolourian – Kashy, 2007 ▶), antioxidant (Zhou et al., 1999 ▶; Fan et al., 2009 ▶), anti-allergic, anti-inflammatory (Fan et al., 2009 ▶; Fu et al., 2007 ▶; Bai et al., 2008 ▶) and anti-diabetes (Kim et al., 2007 ▶) effects have been reported for them. They also protect human umbilical vein endothelial cells injured by hydrogen peroxide (Lin et al., 2006 ▶) and cardiac myocytes against oxidative stress (Fu et al., 2007 ▶). Also, protection against cardiotoxsicity (Jiang et al., 2009 ▶), anti-cardiomyocyte hypertrophia (Yang et al., 2007 ▶), reduction of cardiac fibrosis (Fang et al., 2010 ▶) and the expansion of the coronary artery (Bolourian – Kashy, 2007 ▶) are the other properties of this plant. Regulation of cytokine production (Choi et al., 2004 ▶) and increasing learning and memory (Kim et al., 2009 ▶) have also been attributed to these compounds. In recent years, studies have showed that tanshinones possess cytotoxic and apoptotic effects on some human cancer cell lines (Bolourian – Kashy, 2007 ▶; Fan et al., 2009 ▶; Aoyagi et al., 2006 ▶; Hazra et al., 2004 ▶). Considering the fact that flowers and roots have completely different compounds (Mazandarani, 2010 ▶) and also that apoptotic and anti-cancer effects of flower of this plant has not been investigated, this study was designed to examine in vitro anti-proliferative and proapoptotic effects of flower extract of P. abrotanoides on MCF-7 and Hela cell lines.
Materials and Methods
Cell line and substances
Hela and MCF-7 cell lines were obtained from Pasteur institute (Iran, Tehran). 4, 5-dimethylthiazole-2-yl, 2, 5-diphenyl tetrazolium (MTT) and Dulbecco’s phosphate-buffered saline (PBS) were purchased from Sigma (St Louis, MO, USA). Glucose-high Dulbecco’s modified Eagle’s medium (DMEM), glucose free DMEM, fetal bovine serum (FBS) and penicillin streptomycin were purchased from Gibco (Grand Island, NY). Dimethyl sulfoxide (DMSO) was purchased from Merck (Germany). Propidium iodide (PI), sodium citrate and Triton X-100 were purchased from Sigma (St. Louis, MO, USA).
Cell culture and treatment
Cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin. Cells were then plated and incubated with various concentrations of extract for 24, 48 and 72 h. For MTT assay, cells were seeded at 7000/well on to 96-well culture plates. For each concentration and time course, there was a control sample which remained untreated and received the equal volume of medium. For assay of apoptosis, cells were seeded at 1×105/well onto a 24-well plate (Mahdian et al., 2014). All different treatments were carried out in triplicate.
Preparation of P. abrotanoides flower extraction
P. abrotanoides flowers were collected from Dargaz mountains (Khorasan Razavi,Iran ). Flowers were dried at room temperature, powdered with a grinder, wrapped in cloths and then placed in soxhlet apparatus for one week and extracted with hydro-alcoholic extraction (ethanol 70% W/W). The extract was then dried on a water bath and (yield 24% w/w) dissolved in DMSO.
Cell viability
The cell viability was determined using a modified 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium (MTT) assay (Mahdian et al., 2014). Briefly, cells were seeded at 7×103/well in flat-bottom 96-well culture plates and allowed to grow for 24 , 48 and 72h followed by treatment with extract at different concentrations (1-1000 µg/ml). After removing the medium, cells were incubated with MTT solution (5 mg/ml in PBS) for 4 h and the resulting formazan was solubilized with DMSO (100 µl). The absorption was measured at 570 nm (620 nm as a reference) using an ELISA reader.
All treatments were carried out in triplicate.
Cell apoptosis assay
Apoptotic cells were detected using PI staining of small DNA fragments followed by flow cytometry. It has been reported that a sub-G1 peak which is the reflective of DNA fragmentation can be observed following the incubation of cells with a hypotonic phosphate-citrate buffer containing a quantitative DNA-binding dye, such as PI. Apoptotic cells that have lost DNA take up less stain and appear on the left side of the G1 peak in the histogram. Briefly, Hela cells were seeded in a 24-well plate and treated with P. abrotanoides flower extract at different concentrations (10, 50, 100, 500, 1000µg/ml) for 24, 48 and 72h. Floating and adherent cells were then harvested and incubated with 750 μl of a hypotonic buffer (50 mg/ml PI in 0.1% sodium citrate with 0.1% Triton X-100) at 4 °C overnight in the dark. Next, flow cytometry was carried out using a FACScan flow cytometer (Becton Dickinson). A total of 1×104 events were acquired with FACS. All treatments were carried out in triplicate.
Statistical analysis
One-way analysis of variance (ANOVA) and the Bonferroni post-hoc test were used for data analysis. All the results are expressed as the mean ± SEM, and p values below 0.05 were considered statistically significant.
Results
Effect of hydro-alcoholic extract of P. abrotanoides on Hela cell viability
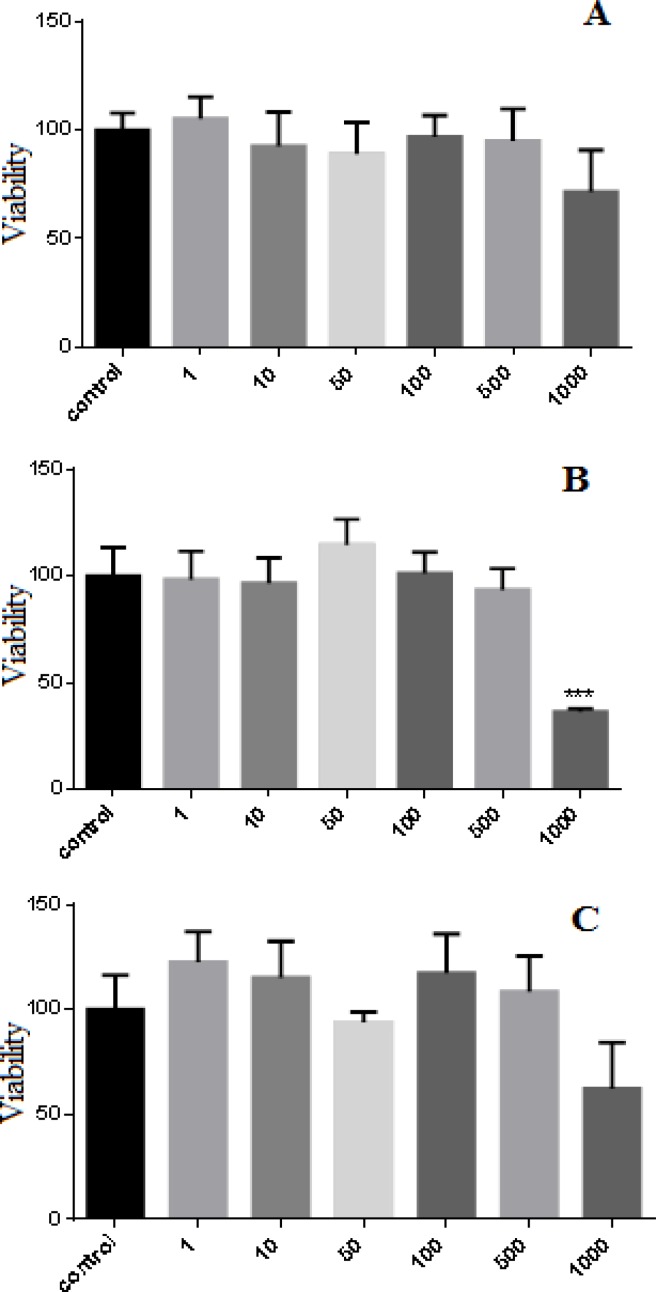
Hela cells were incubated with P. abrotanoides extract (1-1000 µg/ml) for 24, 48 and 72 h. Results showed that P. abrotanoides extract decreased cell viability in a time and dose-dependent manner (Figure 1). After 24 and 72h, P. abrotanoides extract had no effect on Hela cell viability but After 48h, P. abrotanoides diminished cell viability at doses of 1000µg/ml (p<0.001).
Figure1.
Effect of hydro-alcholic extract of P. abrotanoides on viability of Hela cell. (A) Hela cells were treated with different concentrations of P. abrotanoides extract (1-1000 µg/ml) for 24h. (B) Hela cells were treated with different concentrations of P. abrotanoides extract (1-1000 µg/ml) for 48h. (C) Hela cells were treated with different concentrations of P. abrotanoides extract (1-1000 µg/ml) for 72 h. Viability was measured by MTT assay. Results are Mean ± SEM (n = 3). *p <0.05 , **p < 0.01 , ***p < 0.001
Effect of hydro-alcoholic extract of P. abrotanoides on MCF-7 cell viability
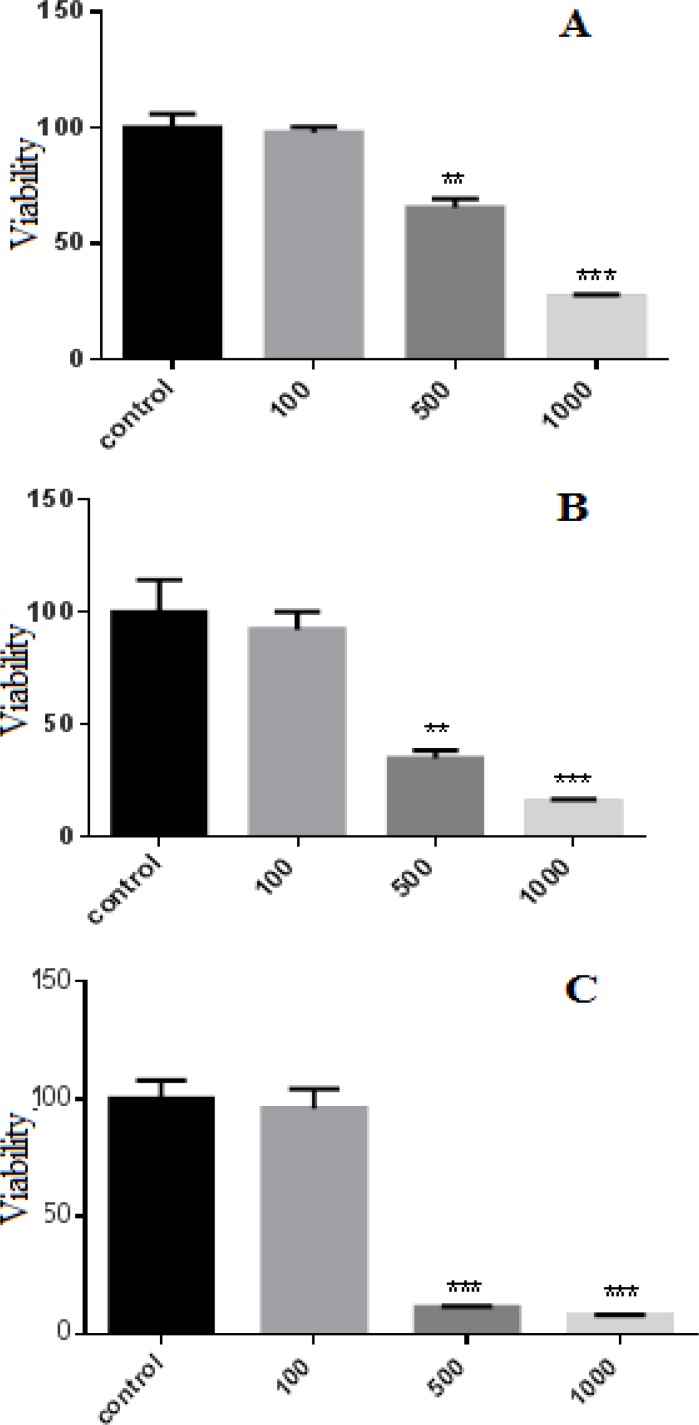
MCF-7 cells were incubated with P. abrotanoides extract (100-1000 µg/ml) for 24, 48 and 72 h. The cell viability was quantified by MTT assay. The results showed that P. abrotanoides extract decreased cell viability in a time and dose-dependent manner (Figure 2). After 24, 48 and 72 h, P. abrotanoides extract decreased cell viability significantly at concentration of 500 (p<0.01) and 1000 µg/ml (p<0.001).
Figure 2.
Effect of hydro-alcholic extract of P. abrotanoides on viability of MCF-7 cells. (A) MCF-7 cells were treated with different concentrations of P. abrotanoides extract (100-1000 µg/ml) for 24h. (B) MCF-7 cells were treated with different concentrations of PA extract (100-1000 µg/ml) for 48h. (C) MCF-7 cells were treated with different concentrations of P. abrotanoides extract (100-1000 µg/ml) for 72h. Viability was measured by MTT assay. Results are Mean ± SEM (n = 3). *p <0.05, **p < 0.01 , ***p < 0.001
Apoptosis induction using the hydro-alcoholic extraction of PA
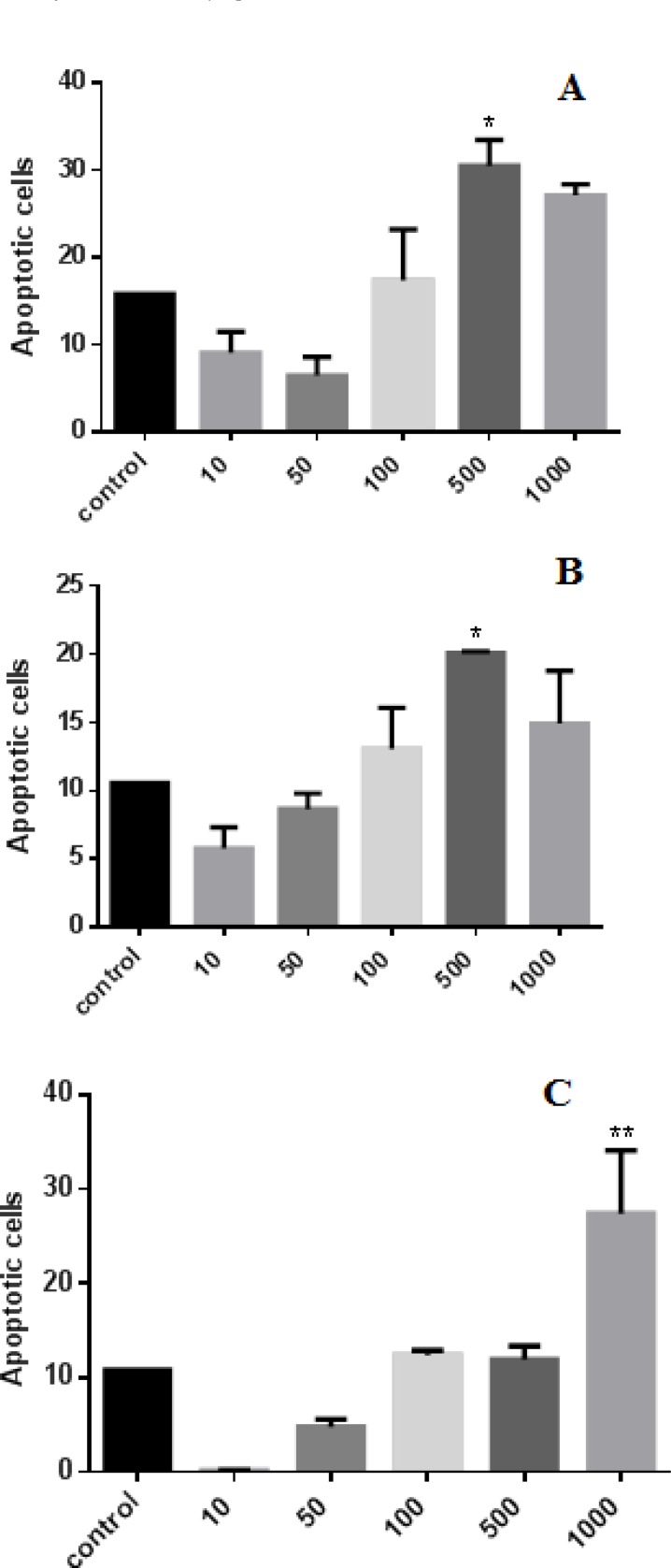
Apoptosis in MCF-7 cell line was detected with flow cytometry using PI staining. Cells were incubated for 24, 48 and 72h with various concentrations (10, 50, 100, 500 and 1000 µg/ml) of P. abrotanoides extract. Analysis of the sub-G1 peak in flow cytometry histograms revealed the induction of apoptosis in treated cells when compared with untreated control cells (Figure 3 and 4). As shown in Figure 3, 24 and 48h treatment with P. abrotanoides extract 500 µg/ml (p<0.05) induced apoptosis significantly in MCF7 cell line even though at 72h, P. abrotanoides extract induced apoptosis only at 1000µg/ml.
Figure 3.
Role of apoptosis in P. abrotanoides extract induced toxicity in MCF7 cells. (A) MCF7 cells treated with DMEM medium as control and 10-1000 µg/ml of hydro-alcholic extract of P. abrotanoides after 24h. (B) MCF7 cells treated with DMEM medium as control and 10-1000 µg/ml of hydro-alcholic of P. abrotanoides extract after 48h. (C) MCF7 cells treated with DMEM medium as control and 10-1000 µg/ml of hydro-alcholic of P. abrotanoides extract after 72h.
Bars: SEM. (n=6), *p<0.05, **p<0.01 , ***p<0.001.
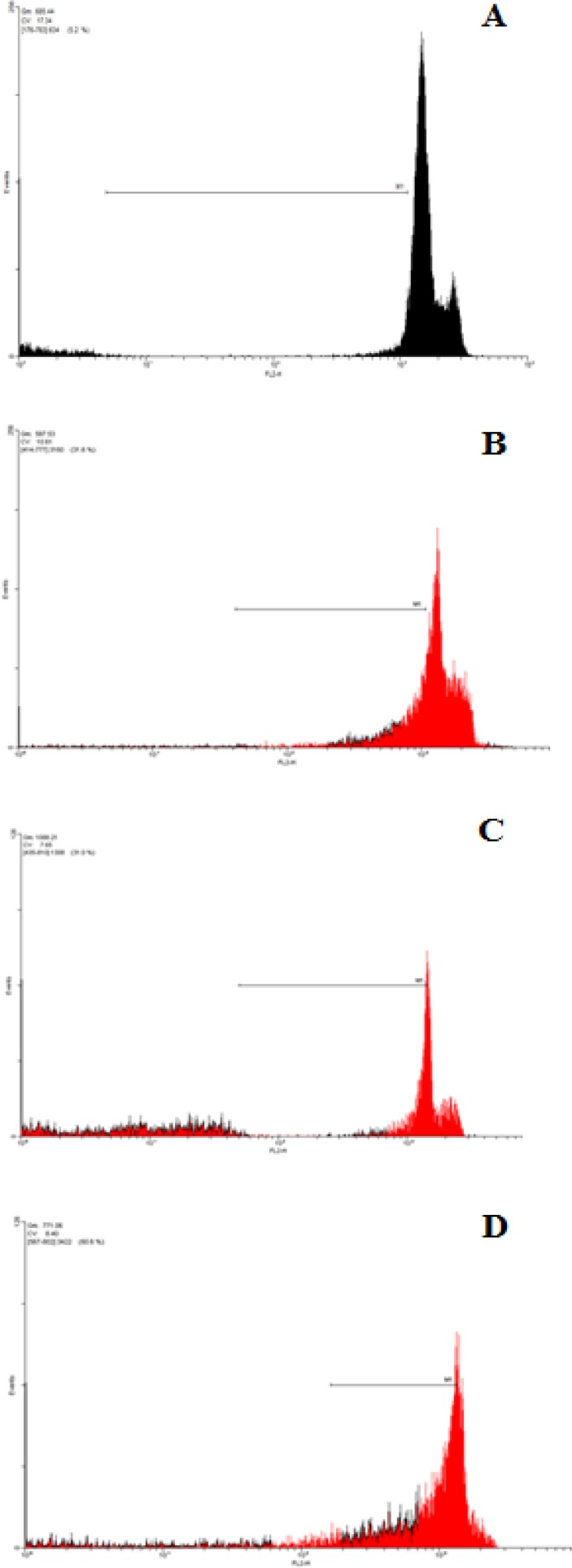
Figure 4.
Role of apoptosis in P. abrotanoides extract-induced toxicity in MCF7 cells. (A) flow cytometry histograms of PI-stained MCF7 cells treated with DMEM medium (B) Flow cytometry histograms of PI-stained MCF7 cells treated with 500µg/ml of P. abrotanoidesextract for 24h. (C) Flow cytometry histograms of PI-stained MCF7 cells treated with 500µg/ml of P. abrotanoides extract for 48h.(D) Flow cytometry histograms of PI-stained MCF7 cells treated with 1000µg/ml of P. abrotanoides extract for 72h. Sub-G1 peak as an indicative of apoptotic cells, was induced in P. abrotanoides extract-treated but not in control cells
Discussion
Cancer is a growing health problem around the world. Considering the safety and efficacy of phytochemicals, molecular mechanistic research has focused on plants with cancer prevention potentials (Shu et al., 2010 ▶). Changes in cell proliferation and cell death are basic determining factors in the pathogenesis of cancer. Anti-cancer phytochemicals can act either as cell cycle and apoptosis regulators, or as anti-oxidative stress and anti-inflammatory agents (Hanahan and Weinberg, 2000 ▶). Various studies have revealed that essential oil extracted from P. abrotanoides stem and leaves could be a promising source of natural products with potential antioxidant activities, compared to fixed oil (Ashraf et al., 2014 ▶). Anti-inflammatory and anti-pain effects of P. abrotenoides is have been mentioned in traditional medicine, as proved in modern animal model researches. According to reports, villagers in the Isfahan province of Iran applied a poultice made of crushed roots of the plant, water, sesame oil and wax, on lesions caused by cutaneous leishmaniasis. In Golestan, North Iran, P. abrotenoides is used to treat leishmaniasis and dermal problems. Aerial branches of Pabrotenoides have anti-inflammatory and anti-pain effects. In another study, the anti-plasmodium property of floral aerial of P. abrotanoides against Plasmodium falciparum and cytotoxic activity has been shown (Beikmohammadi, 2012 ▶). Researchers indicate that ethanolic extracts of salvia tribola and salvia dominica S. triloba and S. dominica (lamiaceae family) may be useful in breast cancer management/treatment via pro-apoptotic cytotoxic mechanisms (Abu-Dahab et al., 2015 ▶).
Throughout the present study, different concentrations of hydro-alcoholic extract of P. abrotenoides flowers were evaluated for possible cytotoxic/apoptotic activity. In addition, the extract was evaluated for apoptotic potential. According to our findings, the extract increased DNA fragmentation in MCF-7 cells as shown by the low fluorescent intensity of PI-stained cells in the flow cytometry histogram. These data suggest that the extract induced apoptosis in MCF-7 cells. Apoptotic cell populations (sub-G1 peak) of cells treated with the extract (500 and 1000 µg/ml) were increased in a time and concentration-dependent manner.
As shown in Hela cells, P. abrotenoides extract just following 48h incubation could inhibit the growth of cells. This finding might imply that during 72h incubation, compensatory mechanisms are activated, which in turn leads to cell growth in the presence of P. abrotenoides extract, and also diminishes the toxic effect of P. abrotenoides extract on cell growth.
The use of crude plant extracts in biological assays makes the synergistic and antagonistic evaluation of the phytochemicals possible. Moreover, biological evaluation of single components from plants eliminates the recognized inconsistencies found in extract preparations (Bandaranayake, 2006 ▶).
References
- Abu-Dahab R, Abdallah MR, Kasabrik V, Mhaida NM, Afifi FU. Mechanistic studies of antiproliferative effects of Salvia triloba and Salvia dominica (Lamiaceae) on breast cancer cell lines (MCF7 and T47D) Z Naturforsch. 2015;69:443–451. doi: 10.5560/znc.2013-0147. [DOI] [PubMed] [Google Scholar]
- Aoyagia Y, Takahashit Y, Satake Y, Takeya K, Aiyama R, Matsuzaki T, Hashimoto S, Kurihara T. Cytotoxicity of abietane diterpenoids from Perovskia abrotanoides and of their semisynthetic analogues. Bioorg Med Chem. 2006;14:5285–5291. doi: 10.1016/j.bmc.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Ashraf S N, Zubair M, Rizwan K, Tareen R B, Rasool N, Zia-Ul-Haq M, Ercisli S. Compositional studies and biological activities of Perovskia abrotanoides Kar. oils. Biol Res. 2014;47:1–9. doi: 10.1186/0717-6287-47-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai A, Lu N, Guo Y, Fan X. Tanshinone IIA ameliorates trinitrobenzene sulfonic acid (TNBS)-induced murine colitis. Dig Dis Sci. 2008;53:421–428. doi: 10.1007/s10620-007-9863-8. [DOI] [PubMed] [Google Scholar]
- Bandaranayake W M. Quality control, screening, toxicity, and regulation of herbal drugs. Modern phytomedicine: turning medicinal plants into drugs. 2006:25–58. [Google Scholar]
- Beikmohammadi M. The evaluation of medicinal properties of Perovskia abrotanoides Karel. Middle-East J Sci Res. 2012;11:189–193. [Google Scholar]
- Bolourian–Kashy M. Extraction, isolation, purification and structure elucidation of tanshinones from the dried roots of Perovskia abrotanoides Karel. Using spectroscopic methods and evaluation of their biological activity. pharmacy signaling. Denmark: university of Copenhagen; 2007. [Google Scholar]
- Choi MS, Cho DL, Choi HK, Im S Y, Ryu SY, Kim KM. Molecular mechanisms of inhibitory activities of tanshinones on Lipopolysaccharide-lnduced nitric oxide generation in RAW 264.7 cells. Arch Pharm Res. 2004;27:1233–1237. doi: 10.1007/BF02975887. [DOI] [PubMed] [Google Scholar]
- Dalkic E, Wang X, Wright N, Chan C. Cancer-drug associations: a complex system. PloS one. 2010;5:e10031. doi: 10.1371/journal.pone.0010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM, Su YF, Kang LY, Zhang BL. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol. 2009;113:275–280. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Fang J, Xu SW, Wang P, Tang FT, Zhou SG, Gao J, Chen JW, Huang HQ, Liu PQ. Tanshinone II-A attenuates cardiac fibrosis and modulates collagen metabolism in rats with renovascular hypertension. Phytomedicine. 2010;18:58–64. doi: 10.1016/j.phymed.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213–221. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg R A. The hallmarks of cancer. cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hazra B, Sarma M D Sanyal U. Separation methods of quinonoid constituents of plants used in Oriental traditional medicines. J Chromatogr. 2004;812:259–275. doi: 10.1016/j.jchromb.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Jiang B, Zhang L, Wang Y, Li M, Wu W, Guan S, Liu X, Yang M, Wang J, Guo DA. Tanshinone IIA sodium sulfonate protects against cardiotoxicity induced by doxorubicin in vitro and in vivo. Food and Chemical Toxicology(FCT) 2009;47:1538–1544. doi: 10.1016/j.fct.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Kim D H, Kim S, Jeon S J, Son , K H, Lee S, Yoon B H, Cheong J H, Ko K H Ryu J H. Tanshinone I enhances learning and memory, and ameliorates memory impairment in mice via the extracellular signal‐regulated kinase signalling pathway. Br J Pharmacol. 2009;158:1131–1142. doi: 10.1111/j.1476-5381.2009.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E J, Jung SN, Son K H, Kim S R, Ha T Y, Park M G, Jo I G, Park J G, Choe W, Kim SS. Antidiabetes and antiobesity effect of cryptotanshinone via activation of AMP-activated protein kinase. Mol Pharmacol. 2007;72:62–72. doi: 10.1124/mol.107.034447. [DOI] [PubMed] [Google Scholar]
- Lin R, Wang WR, Liu JT, Yang GD, Han CJ. Protective effect of tanshinone IIA on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanism. J Ethnopharmacol. 2006;108:217–222. doi: 10.1016/j.jep.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Farahmandbeigi M, Kadivar MR. The incidence rate of registered cancer in Fars province . Disease Control Unit. Iran: Shiraz University Press; 2000. [Google Scholar]
- Mazandarani M, Beykmohammadi M, Bayat H. Ethno pharmacology and investigation secondary metabolites of Perovskia abrotanoides Karel in two natural regions, North of Iran. J Plant Sci Res. 2010;4:69–77. [Google Scholar]
- Mousavi S H, Tavakkol-Afshari J, Brook A, Jafari-Anarkooli I. Direct toxicity of Rose Bengal in MCF-7 cell line: role of apoptosis. Food Chem Toxicol. 2009;47:855–859. doi: 10.1016/j.fct.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Safaeighomi J, Batooli H. Determination of bioac tive molecules from flowers, leaves, stems and roots of Perovskia abrotanoides Karel growing in central Iran by nano scale injection. DIG J NANOMATER BIOS. 2010;5:551–556. [Google Scholar]
- Sairafianpour M, Christensen J, Staerk D, Budnik B A, Kharazmi A, Bagherzadeh K, Jaroszewski J W. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1, 2-quinones from Perovskia abrotanoides: new source of tanshinones. J Nat Prod . 2001;64:1398–1403. doi: 10.1021/np010032f. [DOI] [PubMed] [Google Scholar]
- Shu L, Cheung KL, Khor T O, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer and Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- Yang L, Zou X, Ljang Q, Chen H, Feng J, Yan L, Wang Z, Zhou D, Li S, Yao S. Sodium tanshinone ⅡA sulfonate depresses angiotensin Ⅱ-induced cardiomyocyte hypertrophy through MEK/ERK pathway. EXP MOL MED. 2007;39:65–73. doi: 10.1038/emm.2007.8. [DOI] [PubMed] [Google Scholar]
- Zhou GY, Zhao BL, Hou JW, Ma , GE , Xin WJ. Protective efffects of sodium tanshinone IIA sulphate against adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacol Res. 1999;40:487–491. doi: 10.1006/phrs.1999.0545. [DOI] [PubMed] [Google Scholar]