Abstract
Objective:
Oxidative stress plays a key role in the pathophysiology of brain ischemia and neurodegenerative disorders. Previous studies indicated that Viola tricolor and Viola odorata are rich sources of antioxidants. This study aimed to determine whether these plants protect neurons against serum/glucose deprivation (SGD)-induced cell death in an in vitro model of ischemia and neurodegeneration.
Methods and Material:
The PC12 neuronal cells were pretreated for 4 hr with 1 to 50 µg/ml of V. odorata or V. tricolor hydroalcoholic extracts followed by 24 hr incubation under SGD condition. Cell viability was evaluated by 4,5-dimethyl-2-thiazolyl-2,5-diphenyl-2H-tetrazolium bromide assay. The level of intracellular reactive oxygen species (ROS) was quantitated by flow cytometry using 2',7'- dichlorofluorescin diacetate as a probe.
Results:
SGD condition led to significant decrease in cell viability (p<0.001). Pretreatment with both V. tricolor and V. odorata extracts reduced the SGD-induced cytotoxicity. SGD resulted in a significant increase in intracellular ROS production (p<0.001). Both extracts at concentrations of 25 and 50 µg/ml could reverse the increased ROS production (p<0.05).
Conclusion:
Results of the present study showed that V. tricolor and V. odorata protect neuronal cells against SGD-induced cell death, at least in part, by their antioxidant activities. Further studies on the possible application of these plants in prevention or treatment of cerebral ischemia and neurodegenerative diseases seem to be warranted.
Key Words: PC12, Reactive oxygen species, Viola tricolor, Viola odorata
Introduction
In spite of remarkable progress in prevention and treatment of cerebral ischemia, it still remains a leading cause of death and incapacitation among the aged population (Amantea et al., 2009 ▶). It has been well known that reactive oxygen species (ROS) are involved in ischemia-induced neuronal cell damage as well as neurodegenerative disorders (Amantea et al., 2009 ▶; Behl and Moosmann, 2002 ▶). Therefore, a promising approach to neuroprotection is the use of antioxidants, which suppress the effects of ROS (Ochiaia et al., 2004 ▶). Recently, there has been an increasing interest toward the use of herbal antioxidants in the prevention and treatment of ischemic and neurodegenerative cell damage.
Viola tricolor and Viola odorata (Family Violaceae) are common horticultural plants grown in Iran. V. tricolor has been reported to have a number of medicinal features including antioxidant (Piana et al., 2012 ▶; Vukics et al., 2008a ▶), anti-inflammatory (Toiu et al., 2009 ▶), anti-microbial (Witkowska-Banaszczak et al., 2005 ▶), sedative (Ghorbani et al., 2012 ▶), and anti-cancer (Mortazavian and Ghorbani, 2012 ▶; Mortazavian et al., 2012 ▶; Sadeghnia et al., 2014 ▶) activities. Traditionally, V. odorata has been used to treat anxiety (Keville, 1991 ▶), insomnia, and hypertension (Duke et al., 2002 ▶). Pharmacological studies have shown that this plant has also diuretic, laxative (Vishal et al., 2009 ▶), and antioxidant (Ebrahimzadeh et al., 2010 ▶) properties.
This study has attempted to determine whether V. tricolor and V. odorata extracts protect neurons against serum/glucose deprivation (SGD)-induced cell death. SGD condition is a suitable in vitro model for revealing molecular mechanisms involved in neuronal damage following ischemia and investigating neuroprotective agents for management of ischemia-induced brain injury (Hillion et al., 2005 ▶; Asadpour et al., 2014 ▶; Ghorbani et al., 2015 ▶; Woronowicz et al., 2007 ▶).
Materials and Methods
Cell line and Reagents
The rat pheochromocytoma cell line (PC12) was obtained from Pasteur Institute (Tehran, Iran). 2, 7-dichlorofluorescin diacetate (DCFH-DA) and 4,5-dimethyl-2-thiazolyl-2,5-diphenyl-2H-tetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO, USA). High glucose Dulbecco’s modified Eagle’s Medium (DMEM, 4.5 g/L), glucose-free DMEM, penicillin-streptomycin solution, and fetal bovine serum (FBS) were purchased from GIBCO (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO) was bought from Merck (Darmstadt, Germany).
Preparation of extracts
The aerial parts of V. tricolor and V. odorata were separately dried, powdered, and extracted with 70% ethanol in a Soxhlet apparatus for 48 hr. The hydroalcoholic extracts were then concentrated on water bath and kept at -20 ºC until use.
Cell culture
PC12 cells were cultured in high-glucose DMEM containing 10% (v/v) FBS, 100 units/ml penicillin, and 100 µg/ml streptomycin under a humidified atmosphere containing 5% CO2 at 37ºC. For cell viability assay, the cells were seeded in 96-well culture plates overnight and then were pretreated with different concentrations (1-50 µg/ml) of V. odorata or V. tricolor extracts for 4 hr. Thereafter, the cells were exposed to SGD condition for 24 hr by replacing the standard culture medium with a glucose-and FBS-free DMEM containing antibiotic.
MTT assay
Cell viability was determined by MTT assay as described previously (Hajzadeh et al., 2007 ▶; Ghorbani et al., 2014 ▶). In brief, at the end of incubation under SGD condition, the MTT dye was added to the cell media at final concentration of 0.5 mg/ml. Then, the cells were incubated in a humidified atmosphere containing 5% CO2 at 37ºC. After 2 hr, the resulting formazan was solubilized using DMSO and the absorbance was measured at 545 nm.
Measurement of Reactive Oxygen Species
The level of intracellular ROS was determined as described previously with minor modifications (Wang et al., 1999 ▶; Ochiaia et al., 2004 ▶). Cells were seeded into 24-well culture plate (105 cells/well) and pretreated with extracts as described above. After 4 hr of SGD insult, the cells were incubated with 10 μM DCFH-DA at 37ºC for 30 min in the dark. The fluorescence intensity of 2', 7’- dichlorofluorescein (the oxidation product of DCFH-DA) was measured by flow cytometry using 485-nm excitation and 530-nm emission wavelengths. Flow cytometry Analysis was performed using WinMDI software.
Statistics
One-way analysis of variance and Bonferroni’s post hoc test were used for data analysis. All results were expressed as mean ± SEM. p<0.05 was considered statistically significant.
Results
Effects of V. tricolor and V. odorata extracts on cell viability
SGD condition significantly reduced cell viability as compared to control condition (100 ± 8.2% vs 10±2.4%, p<0.001). Pretreatment with 5, 25, and 50 µg/ml of V. tricolorr significantly increased the percentage of viable cells to 36.2 ± 1.2% (p<0.05), 44.4 ± 5.8% (p<0.01), and 45.8 ± 7.6% (p<0.01), respectively (Figure 1). Similarly, V. odorata at concentrations of 5, 25, and 50 µg/ml was able to increase the percentage of viable cells to 32.4 ± 5.3% (p<0.05), 34.5 ± 1.8% (p<0.01), and 36.7 ± 3.7% (p<0.01), respectively (Figure 2).
Figure 1.
Effect of Viola tricolor on viability of PC12 cells under serum/glucose deprivation (SGD) condition. The cells were pretreated for 4 hr with V. tricolor extract and then exposed to SGD for an additional 24 hr. The cell viability was expressed as the percentage of cells cultured in high-glucose medium (control). The data presented are means ± SEM of three independent experiments (n = 3). *** p<0.001 SGD compared to control. # p<0.05, ## p<0.01, and ### p<0.001 compared to concentration of 0 µg/ml in SGD condition
Figure 2.
Effect of Viola odorata on viability of PC12 cells under serum/glucose deprivation (SGD) condition. The cells were pretreated with V. odorata extract for 4 hr and then exposed to SGD for an additional 24 hr. The cell viability was expressed as the percentage of cells cultured in high-glucose medium (control). The data presented are means ± SEM of three independent experiments (n = 3). *** p<0.001 SGD compared to control. # p<0.05, ## p<0.01, and ### p<0.001 compared to concentration of 0 µg/ml in SGD condition
Effects of V. tricolor and V. odorata extracts on ROS production
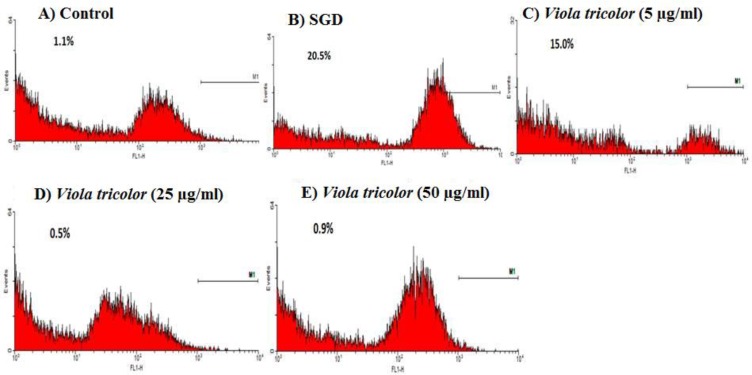
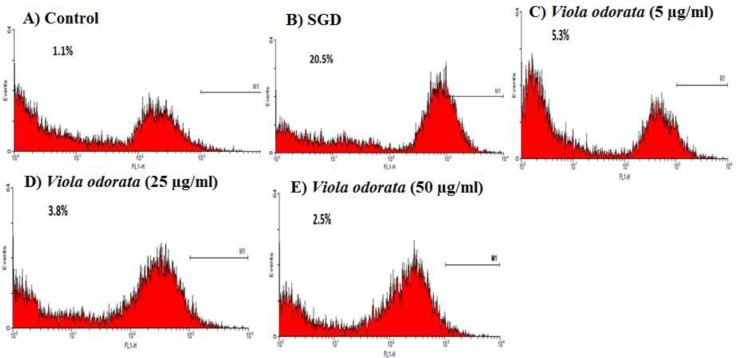
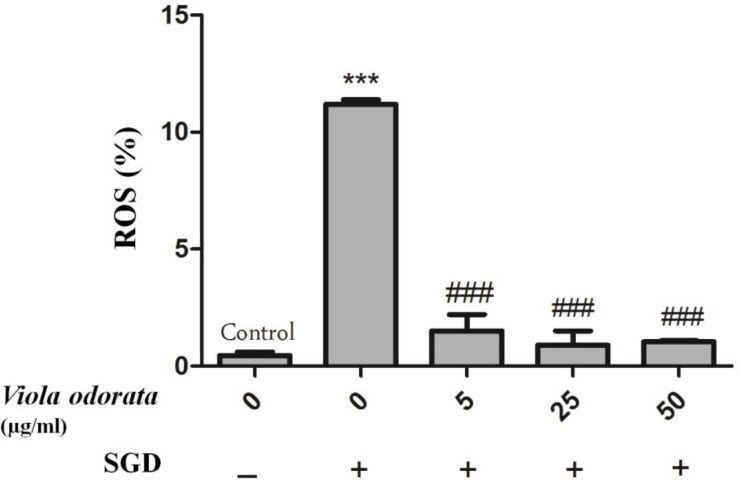
Production of intracellular ROS in PC12 cells was significantly increased after 4 hr of SGD insult, as compared to that in cells cultured under control condition (11.2 ± 0.2% versus 0.45 ± 0.15%, p<0.001). Pre-incubation with 25 and 50 μg/ml of V. tricolor extract significantly decreased the SGD-induced ROS accumulation to 6.65 ± 0.65% (p<0.05), and 1.05 ± 0.75% (p<0.01), respectively (Figures 3 and 4). Similarly, the presence of 5, 25 and 50 μg/ml of V. odorata extract in cell medium reduced the ROS content from 11.2 ± 0.2% to 1.5 ± 0.7% (p<0.001), 0.9 ± 0.6% (p<0.001) and 1.05 ± 0.05 (p<0.001), respectively (Figures 5 and 6).
Figure 3.
Flow cytometry for measuring ROS production in PC12 cells pretreated with Viola tricolor. A: control cells; B: Cells under serum/glucose deprivation (SGD) condition; C-E: pretreatment with 5, 25, and 50 µg/ml Viola tricolor, respectively, followed by 4 hr exposure to SGD
Figure 4.
Effect of Viola tricolor on the level of intracellular reactive oxygen species (ROS) in PC12 cells under serum/glucose deprivation (SGD) condition. The cells were pretreated with V. tricolor extract for 4 hr and then exposed to SGD for an additional 24 hr. The values represent 5 independent experiments. *** p<0.001 SGD compared to control; # p<0.05 and ## p<0.01 compared to concentration of 0 µg/ml in SGD condition
Figure 5.
Flow cytometry for measuring ROS production in PC12 cells pretreated with Viola odorata. A: control cells; B: Cells under serum/glucose deprivation (SGD) condition; C-E: pretreatment with 5, 25, and 50 µg/ml Viola odorata, respectively, followed by 4 hr exposure to SGD
Figure 6.
Effect of Viola odorata on the level of intracellular reactive oxygen species (ROS) of PC12 cells under serum/glucose deprivation (SGD) condition. The cells were pretreated with V. odorata extract for 4 hr and then exposed to SGD for an additional 24 hr. The values represent 5 independent experiments. *** p<0.001 SGD compared to control; ### p<0.001 compared to concentration of 0 µg/ml in SGD condition
Discussion
Oxidative stress is a key deleterious factor in neuronal cell damage during brain ischemia which is also involved in other neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, and traumatic brain injury (Manzanero et al., 2013 ▶; Navarro-Yepes et al., 2014 ▶). In acute ischemia, the increased level of ROS can cause oxidative damage to cellular macromolecules including lipids, proteins, and nucleic acids. Polyunsaturated fatty acids of lipid membranes are especially vulnerable to ROS-induced lipid peroxidation. The oxidation of these fatty acids rises the hydrophilic nature of the membrane resulting in alteration in fluidity and permeability. Also, the function of membrane bound receptors and enzymes is inhibited (Fisher et al., 2001 ▶; Manzanero et al., 2013 ▶). Therefore, utilization of novel antioxidant agents might be a good therapeutic approach against neuronal damage during brain ischemia (Gilgun-Sherki et al., 2002 ▶; Love et al., 1999 ▶; Navarro-Yepes et al., 2014 ▶). In ischemia, restriction of blood flow results in deficiency of oxygen, glucose and serum growth factors leading to neuronal damage (Broughton et al., 2009 ▶). Deprivation of cultured neurons from serum and glucose provides a reliable in vitro method for studying pathological process of cerebral ischemia and for development of new agents for management of ischemia (Hillion et al., 2005 ▶; Asadpour et al., 2014 ▶; Ghorbani et al., 2015 ▶; Woronowicz et al., 2007 ▶). In this study, we utilized the SGD-induced insult in PC12 cells and showed that hydroalcoholic extracts of V. tricolor and V. odorata are able to inhibit neuronal damage under SGD condition. SGD condition led to a 90% decrease in cell viability, which was comparable to that observed previously (70-90% cell death) (Alinejad et al., 2013 ▶; Sadeghnia et al., 2012 ▶; Forouzanfar et al., 2013 ▶; Mousavi et al., 2010 ▶). Also, consistent with previous reports, SGD condition resulted in an enhancement of intracellular ROS level (Ghorbani et al., 2015 ▶; Forouzanfar et al., 2013 ▶). Pretreatment with V. tricolor and V. odorata effectively blocked the SGD-induced ROS production, indicating that an inhibition of intracellular ROS generation might be involved in the neuroprotective effects of V. tricolor and V. odorata. Consistent with our findings, Ebrahimzadeh et al. (2010) ▶ evaluated the Fe2+-chelating ability and 1,1-diphenyl-2-picryl hydrazyl radical-scavenging activity of V. odorata methanolic extract and demonstrated that it has antioxidant and free radical scavenging properties. Also, it has been reported that the crude extract of V. tricolor flowers shows much better antioxidant capacity than ascorbic acid (Piana et al., 2012 ▶). The antioxidant effect of these plants may be due to their flavonoid compounds such as rutin and violantin (Piana et al., 2012 ▶; Vukics et al., 2008a ▶; Vukics et al., 2008b ▶; Ebrahimzadeh et al., 2010 ▶; Gonçalves et al., 2012 ▶). For example, the antioxidant properties of rutin have been demonstrated in both in vitro and in vivo studies (Yang et al., 2008 ▶; Sadeghnia et al., 2013 ▶). Also, in a middle cerebral artery occlusion animal model of brain ischemia, it has been shown that pretreatment with rutin attenuates ischemic neural apoptosis by increasing endogenous antioxidant enzymatic activities (Khan et al., 2009 ▶).
In conclusion, the results of the present study showed that V. tricolor and V. odorata protect neuronal cells against SGD-induced cell death through their antioxidant mechanisms. Further studies on the possible application of these plants in prevention and/or treatment of cerebral ischemia and neurodegenerative diseases seem to be warranted.
Acknowledgment
This study has been financially supported by a grant (No. 921057) from Research Affairs of Mashhad University of Medical Sciences, Mashhad, Iran. This study was a part of an MSc thesis. The authors would like to appreciate Ms. Aghaee for her assistance in the preparation of V. tricolor and V. odorata extracts. Also, we are grateful to Mr. Malaeke for his assistance in flow cytometry.
Conflict of interest
The authors have no conflict of interests to declare.
References
- Alinejad B, Ghorbani A, Sadeghnia HR. Effects of combinations of curcumin, linalool, rutin, safranal, and thymoquinone on glucose/serum deprivation-induced cell death. Avicenna J Phytomed. 2013;3:321–328. [PMC free article] [PubMed] [Google Scholar]
- Amantea D, Marrone MC, Nistico R, Federici M, Bagetta G, Bernardi G, Mercuri NB. Oxidative stress in stroke pathophysiology; Validation of hydrogen peroxide metabolism as a pharmacological target to afford neuroprotection. Int Rev Neurobiol. 2009;85:363–374. doi: 10.1016/S0074-7742(09)85025-3. [DOI] [PubMed] [Google Scholar]
- Asadpour E, Ghorbani A, Sadeghnia HR. Water-soluble compounds of lettuce inhibit DNA damage and lipid peroxidation induced by glucose/serum deprivation in N2a cells. Acta Pol Pharm. 2014;71:409–413. [PubMed] [Google Scholar]
- Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med. 2002;33:182–191. doi: 10.1016/s0891-5849(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Broughton BRS, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Duke JA, Bogenschutz-Godwin MJ, Ducelliar J, Duke PAK. Sweet Violet (Viola odorata L) Handbook of Medicinal Herbs. 2nd edition . Boca Raton: CRC Press; 2002. 715 pp. [Google Scholar]
- Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR. Antioxidant and free radical scavenging activity of H officinalis L var angustifolius, V odorata, B hyrcana and C speciosum. Pak J Pharm Sci. 2010;23:29–34. [PubMed] [Google Scholar]
- Fisher M. Stroke therapy. 2nd edn. Boston: Butter worth-Heinemann; 2001. pp. 25–50. [Google Scholar]
- Forouzanfar F, Goli AA, Asadpour E, Ghorbani A, Sadeghnia HR. Protective effect of Punica granatum L against serum/glucose deprivation-induced PC12 cells injury. Evid Based Complement Alternat Med. 2013:7167730. doi: 10.1155/2013/716730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani A, Hadjzadeh MR, Rajaei Z, Zendehbad SB. Effects of fenugreek seeds on adipogenesis and lipolysis in normal and diabetic rat. Pak J Biol Sci. 2014;17:523–528. doi: 10.3923/pjbs.2014.523.528. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Youssofabad N. 2012. Effect of Viola tricolor on pentobarbital-induced sleep in mice. Afr J Pharm Pharmacol. J, and Rakhshandeh H;6:2503–2509. [Google Scholar]
- Ghorbani A, Asadpour E, Sadeghnia HR. Mechanism of protective effect of lettuce against glucose/serum deprivation-induced neurotoxicity. Nutr Neurosci. 2015;18:103–109. doi: 10.1179/1476830513Y.0000000107. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury Current state. Pharmacol Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- Gonçalves AFK, Friedrich RB, Boligon AA, Piana M, Beck RCR, Athayde ML. Anti-oxidant capacity, total phenolic contents and HPLC determination of rutin in Viola tricolor (L) flowers. Free Radicals Antioxid. 2012;2:32–37. [Google Scholar]
- Hajzadeh MA, Afshari JT, Ghorbani A, Shakeri MT. Antiproliferative property of aqueous extract of garlic on human larynx tumour and non-tumour mouse fibroblast cell lines. Australian J Med Herbalism. 2007;19:33–37. [Google Scholar]
- Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25:154–162. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keville K. Viola odorata L. In: Rosart S, editor. 1991. New York: Michael Friedman publishing group, Inc; 207 pp. [Google Scholar]
- Khan MM, Ahmad A, Ishrat T, Khuwaja G, Srivastawa P, Khan MB, Raza SS, Javed H, Vaibhav K, Khan A, Islam F. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res. 2009;1292:123–135. doi: 10.1016/j.brainres.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem Int. 2013;62:712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Mortazavian SM, Ghorbani A. Antiproliferative effect of viola tricolor on neuroblastoma cells in vitro. Australian J Herbal Med. 2012;24:93–96. [Google Scholar]
- Mortazavian SM, Ghorbani A, Hesari TG. Effect of hydro-alcoholic extract of Viola tricolor and its fractions on proliferation of uterine cervix carcinoma cells. Iran J Obstet Gynecol and Infertil. 2012;15:9–16. [Google Scholar]
- Mousavi SH, Asghari M, Tayarani-Najaran Z, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30:591–598. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Yepes J, Zavala-Floresa L, Anandhana A, Wangc F, Skotakc M, Chandrac N, Lid M, Pappag A, Martinez-Fongf D, Del Razoe LM, Quintanilla-Vegae B, Franco R. Antioxidant gene therapy against neuronal cell death. Pharmacol Ther. 2014;142:206–230. doi: 10.1016/j.pharmthera.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiaia T, Ohnoa S, Soedaa S, Tanakab H, Shoyamab Y, Shimenoa H. Crocin prevents the death of rat pheochromyctoma (PC12) cells by its antioxidant effects stronger than those of atocopherol. Neurosci Lett. 2004;362:61–64. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Piana M, Zadra M, de Brum TF, Boligon AA, Gonçalves AF, da Cruz RC, de Freitas RB, do Canto GS, Athayde ML. Analysis of rutin in the extract and gel of Viola tricolor. J Chromatogr Sci. 2013;51:406–411. doi: 10.1093/chromsci/bms155. [DOI] [PubMed] [Google Scholar]
- Sadeghnia HR, Farahmand SK, Asadpour E, Rakhshandeh H, Ghorbani A. Neuroprotective effect of Lactuca sativa on glucose/serum deprivation-induced cell death. Afr J Pharm Pharmacol. 2012;6:2464–2471. [Google Scholar]
- Sadeghnia HR, Ghorbani Hesari T, Mortazavian SM, Mousavi SH, Tayarani-Najaran Z, Ghorbani A. Viola tricolor induces apoptosis in cancer cells and exhibits antiangiogenic activity on chicken chorioallantoic membrane. BioMed Research International. 2014:625792. doi: 10.1155/2014/625792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghnia HR, Yousefsani BS, Rashidfar M, Boroushaki MT, Asadpour E, Ghorbani A. Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity. Ren Fail. 2013;35:1151–1155. doi: 10.3109/0886022X.2013.815546. [DOI] [PubMed] [Google Scholar]
- Toiu A, Muntean E, Oniga L, Vostinaru O, Tamas M. Pharmacognostic research on Viola tricolor L (Violaceae) Rev Med Chir Soc Med Nat Iasi. 2009;113:246–247. [PubMed] [Google Scholar]
- Vishal A, Parveen K, Pooja S, Kannappan N, Kumar S. Diuretic, laxative and toxicity Studies of Viola odorata aerial parts. Pharmacol online. 2009;1:739–748. [Google Scholar]
- Vukics V, Kery A, Guttman A. Analysis of polar antioxidants in Heartsease (Viola tricolor L) and Garden pansy (Viola x wittrockiana Gams) J Chromatogr Sci. 2008a;46:823–827. doi: 10.1093/chromsci/46.9.823. [DOI] [PubMed] [Google Scholar]
- Vukics V, Kery A, Bonn GK, Guttman A. Major flavonoid components of heartsease (Viola tricolor L) and their antioxidant activities. Anal Bioanal Chem. 2008b;390:1917–1925. doi: 10.1007/s00216-008-1885-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Witkowska-Banaszczak E, Bylka W, Matlawska I, Goslinska O, Muszynski Z. Antimicrobial activity of Viola tricolor herb. Fitoterapia. 2005;76:458–461. doi: 10.1016/j.fitote.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Woronowicz A, Amith SR, Davis VW, Jayanth P, De Vusser K, Laroy W, Contreras R, Meakin SO, Szewczuk MR. Trypanosome trans-sialidase mediates neuroprotection against oxidative stress, serum/glucose deprivation, and hypoxiainduced neurite retraction in Trk-expressing PC12 cells. Glycobiology. 2007;17:725–734. doi: 10.1093/glycob/cwm034. [DOI] [PubMed] [Google Scholar]
- Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT-Food Sci Tech. 2008;41:1060–1066. [Google Scholar]