Abstract
Objective:
Frankincense improves memory in different models of learning. However, its influence on models of Alzheimer's disease (AD) has not been studied widely. In the present study, the therapeutic effect of frankincense was evaluated in a model of AD induced by i.c.v administration of streptozotocin.
Materials and Methods:
Under stereotaxic surgery, two guide cannulas were implanted in the lateral ventricles of adult male Wistar rats weighing 230-270 g. One group received streptozotocin (1.5 mg/kg/2μl/side) bilaterally on the first and third day of surgery. Another group received artificial cerebro-spinal fluid. Fourteen days after surgery, learning was evaluated using the passive avoidance paradigm. Four other groups of animals received frankincense (50 mg/kg) or its solvent after establishment of AD for 21 or 42 consecutive days, and then, memory retrieval was assessed.
Results:
Streptozotocin increased the number of stimulations required for induction of short-term memory and decreased step-through latency on the test day, significantly (p<0.05). Chronic injection of the aqueous extract of frankincense for 21 days did not affect learning parameters, but injection of it for 42 days, significantly increased step-through latency (p<0.05), decreased the number of step-through into the dark compartment (p<0.01) and decreased the time spent in the dark compartment (p<0.05).
Conclusion:
The results indicate that chronic administration of frankincense has the potential to improve dementia type of AD induced by i.c.v injection of streptozotocin in a time-dependent manner.
Key Words: Streptozotocin, Alzheimer’s disease, Frankincense, Memory, Rat
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder which is characterized by severe memory loss and behavioral disturbances. The current symptomatic treatment of patients with mild-to-moderate AD is based on drugs such as donepezil, rivastigmine, galantamine and memantine which are associated with side effects (Mukherjee et al., 2007 ▶). These drugs are able to reduce the signs of the disease, but have not the potential to treat it. There is currently a high demand for natural therapies to treat AD and reduce the side effects of drugs used in the clinic. Frankincense is an oleo gum resin of trees of genus Boswellia. There is a growing body of evidence indicating that frankincense from different species of the Boswellia genus has the potential to improve memory in both normal brain (Farshchi et al., 2010 ▶; Hosseini-sharifabad and Esfandiari, 2014; Hosseini Sharifabad and Esfandiari, 2007 ▶; Hosseini Sharifabad and Esfandiari, 2011 ▶; Hosseinzadeh H, 2009 ▶; Mahmoudi et al., 2011 ▶) and impaired memory conditions (Hosseini et al., 2010 ▶; Hosseini et al., 2012 ▶; Jalili et al., 2014 ▶). For example, frankincense was shown to enhance the retention phase of spatial memory in the Morris water maze (Mahmoudi et al., 2011 ▶). Also, administration of the aqueous extract of frankincense remarkably improved learning deficits in kindled animals (Jalili et al., 2014 ▶). However, the effect of frankincense in models of AD has not been studied widely.
Streptozotocin (STZ) is a glucosamine derivative of nitrosourea. It was shown that intracerebroventricular (i.c.v) injection of STZ in a sub-diabetogenic dose, causes sustained impairment of brain glucose and energy metabolism, which is accompanied by impairment in learning and memory and reduces acetylcholine transferase levels in the hippocampus of rats. It is considered as an animal model of sporadic AD in humans (Sharma and Gupta, 2001 ▶).
We have previously indicated prophylactic effects of frankincense in an STZ model of AD (Zaker et al., 2015 ▶). In the present study, we assessed the probable therapeutic effect of aqueous extract of frankincense from Boswellia carteri in an STZ model of AD, using passive avoidance task (PAT).
Materials and Methods
Chemicals
Ketamine and xylazine were obtained from Alfasan (Netherland). Frankincense was a gift from Raha Pharmaceutical Co. (Iran). STZ was purchased from Sigma (U.S.A).
Preparation of the aqueous extract of frankincense
The plants used to obtain frankincense were identified by an expert botanist. An appropriate amount of the resin of Boswellia carteri was pulverized and soaked in distilled water. After 24 hr, it was warm-heated on a 50°C water bath for 60 min and filtered before injection. The volume of the extract for gavage injection (1 ml/kg) was calculated using the following formula:
The dose of frankincense (50 mg/kg; P.O) was selected according to previous reports indicating its effectiveness on cognitive functions (Yassin et al., 2013 ▶; Zaker et al., 2015 ▶).
Animals
Adult male Wistar rats (230–270 g) were obtained from the breeding colony of Department of Biology, University of Isfahan, Isfahan, Iran. Rats were housed four per cage in a temperature (24±1 °C)-controlled room with a 12hr:12hr light cycle (lights on at 07:00 a.m.). Rats had unrestricted access to food and water in their cage. After the surgery for cannula implantation, rats were housed individually in standard cages. The ethical aspects of the project were approved by the graduate studies committee of the Department of Biology, University of Isfahan and were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) in such a way to minimize the number of animals and their suffering.
Surgical procedures
Rats were anesthetized with a mixture of ketamine (100 mg/kg, i.p) and xylazine (10 mg/kg, i.p) and were bilaterally implanted with guide cannula (22-gauge) aimed at 1 mm dorsal to the lateral ventricles (AP: −0.9 mm from bregma, ML: ±1.4 mm from midline, and DV: −2.5 mm from dura) according to the atlas of Paxinos and Watson (Paxinos, 2007 ▶). One screw was inserted into the skull and cannulas were fixed with dental cement. The cannulas were closed with stainless steel stylets smeared with mineral oil to prevent clogging with blood.
Microinjection procedure
Intracerebroventricular injections were made via guide cannula with injection needles (27-gauge) that were connected by polyethylene tubing (PE20, Stoelting) to a 2μl Hamilton microsyringe. The injections (4 μl total volume) were delivered over 4 min bilaterally (2 μl each side), and the injection needles (extending 1 mm from the end of the guide cannula) were left in place for an additional 1 min before they were slowly withdrawn.
Passive avoidance task
The step-through passive avoidance paradigm was performed as previously described (Beheshti et al., 2014 ▶).
Briefly, each rat was placed in the white chamber of the PAT apparatus facing the sliding door. After 5 sec, the door was opened. When the animal stepped into the dark chamber with all four paws, the door was closed and the rat remained there for 20 sec. Then, the animal was removed and placed in a temporary cage. Thirty min later, the rat was again placed in the white chamber for 5 sec. Then, the door was raised to let the animal enter the dark chamber and following entrance, the door was closed, but this time a controlled electrical shock of 0.3 mA lasting for 1 sec, was delivered. After 20 sec, the rat was placed into a temporary cage. Two minutes later, the same testing procedure was repeated. When the rat remained in the white compartment for a 2-min period, the training was terminated. On the second day, a retrieval test was performed to evaluate long-term memory. Each animal was placed in the white start chamber for 20 sec, then, the door was raised and the step-through latency (STL), the number of step-through into the dark compartment (NST) and the time spent in the dark compartment (TDC) were recorded, up to 600 sec.
Experiments
Eight animals were used in each experimental group.
Experiment 1
In this experiment, the effect of i.c.v bilateral injection of STZ was evaluated on memory retrieval. Animals received STZ (1.5 mg/kg) on the first and third day after surgery. The control group received ACSF (124 mM NaCl, 4.4 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 1.25 mM KH2PO4, 25 mM NaHCO3, 10 mM Glucose; pH=7.2). Fourteen days after the first injection, memory retrieval was assessed, using PAT.
Experiment 2
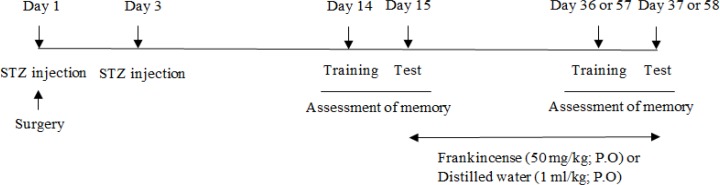
In this experiment, the effect of the aqueous extract of frankincense was evaluated in animals treated with STZ. Animals were divided into four groups. All groups received STZ (1.5 mg/kg; i.c.v) bilaterally on the first and third day after surgery. Fourteen days after the first injection, memory retrieval was assessed to confirm the impairment of memory. Following assessment of memory, groups 1 and 2 received the aqueous extract of frankincense (50 mg/kg; P.O) for 21 or 42 consecutive days and groups 3 and 4 received distilled water (1 ml/kg; P.O) for 21 or 42 consecutive days, respectively. Following completion of the treatment regime, memory retrieval was assessed using PAT (Figure 1).
Figure 1.
Diagram illustrating experimental design
Statistical analysis
The data are presented as mean ± SEM Unpaired and paired t-test was performed for data analysis. In all the experiments, p<0.05 was considered statistically significant.
Results
The effect of STZ on memory retrieval
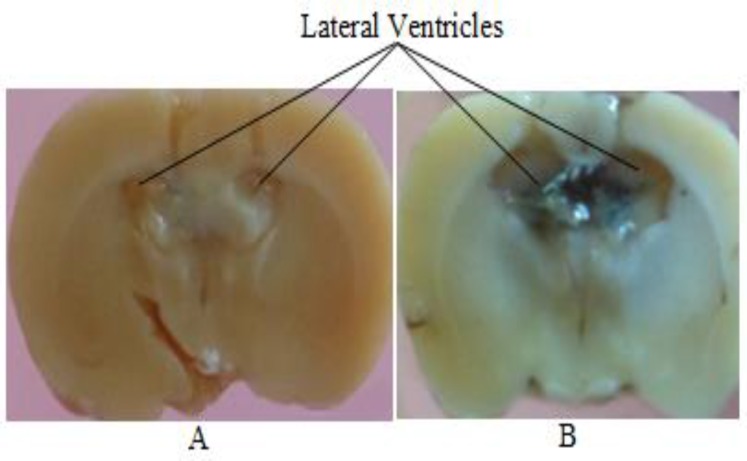
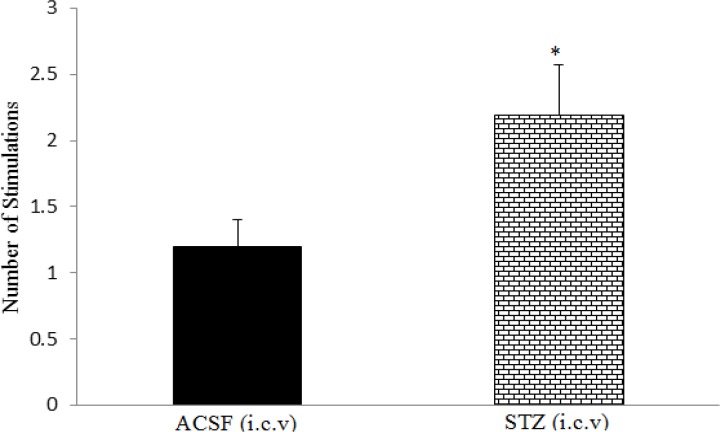
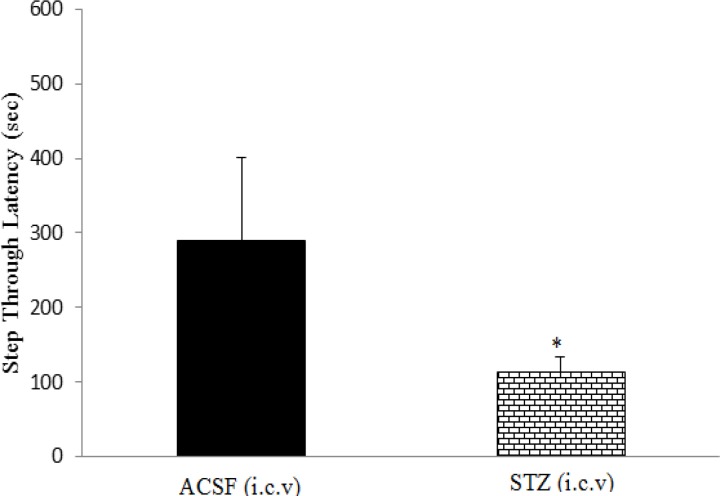
In the passive avoidance task, decrease in step-through latency and increase in the time spent in the dark compartment or the number of step-through into the dark compartment indicate loss of memory. Intracerebroventricular injection of STZ profoundly enlarged lateral ventricles (Figure 2), and significantly increased the number of stimulations required for induction of short-term memory on the training day and decreased step-through latency (STL) 24 hr later on the test day, compared to the control group (p<0.05; Figures 3 and 4).
Figure 2.
Coronal sections of the rat brain showing the effect of i.c.v injection of streptozotocin (1.5 mg/kg) on enlargement of the lateral ventricles. (A) ACSF-treated brain and (B) STZ-treated brain
Figure 3.
The effect of i.c.v injection of streptozotocin (1.5 mg/kg) on the number of stimulations required for induction of short-term memory. Data are shown as mean±SEM. *p<0.05
Figure 4.
The effect of i.c.v injection of streptozotocin (1.5 mg/kg) on STL. Data are shown as means ±SEM. *p<0.05.
The effect of aqueous extract of frankincense on memory retrieval in STZ-treated rats
Treatment of animals with AD with aqueous extract of frankincense (50 mg/kg; P.O) for 21 days did not affect STL, TDC or NST compared to the control group (Tables 1, 2 and 3). Paired t-test indicated that treatment of rats with AD with frankincense (50 mg/kg; P.O) for 42 days, significantly increased STL (p<0.05) and decreased NST (p<0.01) and TDC (p<0.05), compared to the control group (Tables 1, 2 and 3).
Table 1.
The effect of aqueous extract of frankincense on STL in STZ-treated rats
| 15 days after STZ injection | 37 days after surgery | 15 days after STZ injection | 58 days after surgery | ||
|---|---|---|---|---|---|
|
Group 1
(Frankincense for 21 days) |
140±35 | 269±113 | Group 2 (Frankincense for 42 days) |
122±38 | 339±103* |
|
Group 3
(Distilled water for 21 days) |
146±42 | 145±40 | Group 4 (Distilled water for 42 days) |
130±22 | 147±23 |
Data are shown as mean ± SEM.
p<0.05
Table 2.
The effect of aqueous extract of frankincense on NST in STZ-treated rats
| 15 days after STZ injection | 37 days after surgery | 15 days after STZ injection | 58 days after surgery | ||
|---|---|---|---|---|---|
|
Group 1
(Frankincense for 21 days) |
11.5±3.5 | 11.2±3.1 | Group 2 (Frankincense for 42 days) |
14.0±3.2 | 7.0±3.9** |
|
Group 3
(Distilled water for 21 days) |
12.4±4 | 11.0±4.3 | Group 4 (Distilled water for 42 days) |
16.0±1.4 | 20.0±3.3 |
Data are shown as mean ±SEM.
p<0.01.
Table 3.
The effect of aqueous extract of frankincense on TDC in STZ-treated rats
| 15 days after STZ injection | 37 days after surgery | 15 days after STZ injection | 58 days after surgery | ||
|---|---|---|---|---|---|
|
Group 1
(Frankincense for 21 days) |
250±45 | 260±54 | Group 2 (Frankincense for 42 days) |
286±34 | 131±65* |
|
Group 3
(Distilled water for 21 days) |
240±30 | 256±29 | Group 4 (Distilled water for 42 days) |
209±72 | 285±35 |
Data are shown as mean ±SEM.
p<0.05.
Verification of cannulas placements
After completion of the experimental sessions, each animal was euthanized with an overdose of ether. The brains were removed and fixed in a 10% formalin solution 5 days before sectioning. Sections were examined to determine the location of the cannulas aimed for the lateral ventricles. The cannula placements were verified using the atlas of Paxinos and Watson (Paxinos, 2007). Data from the animals with the injection sites located outside the lateral ventricles were not included in the results.
Discussion
The results of the present study indicated that i.c.v administration of a sub-diabetogenic dose of STZ induced memory impairment in rats as shown by the increasing number of stimulations required to induce short-term memory on the training day and decreasing STL 24 hr after training on the test day. These data reflect poorer acquisition and/or retrieval of memory after STZ injection. STZ also enlarged the lateral ventricles, profoundly. These findings are in accordance with earlier reports (Sharma and Gupta, 2001 ▶; Shoham et al., 2003 ▶; Sodhi and Singh, 2013 ▶).
The main outcome of our study is that chronic administration of aqueous extract of frankincense improves memory in rats receiving STZ i.c.v, in a time-dependent manner.
There is accumulating evidence indicating the effectiveness of frankincense on improvement of memory both in normal brain and impaired memory conditions. Frankincense dramatically increased the number of neuronal processes in CA1 region and improved passive-avoidance learning ability in both normal and pentylene tetrazole -kindled animals (Jalili et al., 2014 ▶). It also facilitated spatial learning and memory in the Morris water maze (Farshchi et al., 2010 ▶; Mahmoudi et al., 2011 ▶). Chronic maternal administration of Boswellia gum resin in gestational period induced significant volumetric alteration in the layers of the CA3 hippocampal field in young male rats (Hosseini Sharifabad and Esfandiari, 2007 ▶). Frankincense could also prevent hypothyroidism-induced spatial memory impairment in rats (Hosseini et al., 2010 ▶). It induced significant improvement in visuospatial memory in multiple sclerosis patients (Sedighi et al., 2014 ▶). Long-term administration of frankincense could attenuate age-related dendritic regression in CA1 pyramidal cells in rat hippocampus (Hosseini-sharifabad and Esfandiari, 2015 ▶). Based on the effects of frankincense on structural and functional changes in the brain which lead to memory improvement, it is probable that these effects might be involved in the therapeutic effects of frankincense in the i.c.v STZ model of AD.
In all forms of dementia of the Alzheimer’s type, there are severe abnormalities in glucose metabolism. Brain glucose utilization and brain tissue levels of energy-rich phosphates are reduced in patients with AD (Nitsch and Hoyer, 1991 ▶). Oxidative stress is involved in the inhibition of energy metabolism. In the brain of STZ-treated rats, there is an increase in lipid peroxidation showing an increase in free radical production as well as a decrease in the antioxidant glutathione, showing a decrease in the scavenging capacity that proves a state of oxidative stress (Sharma and Gupta, 2001 ▶). In this regard, studies have shown that frankincense possesses powerful antioxidant activity. It was indicated that the aqueous extract of B. serrata has persuasive anti-oxidant activity in removing free radicals in a concentration-dependent manner (Sharma, 2011 ▶).
Neuro-inflammation that is associated with AD contributes to AD pathogenesis (Heppner et al., 2015 ▶). In the i.c.v STZ model of AD, there is also a neuro-inflammatory condition (Nazem et al., 2015 ▶). In this regard, the putative anti-inflammatory activity of frankincense has been approved by various investigations. Boswellic acids are the main active components of the resin of Boswellia carteri which participate in its ethno-medicinal use for the treatment of inflammatory diseases (Banno et al., 2006 ▶). Boswellic acids were shown to specifically inhibit the synthesis of pro-inflammatory enzyme, 5-lipooxygenase (5-LO). 5-LO generates inflammatory leukotrienes. Leukotrienes cause inflammation by promoting free radical damage, cell adhesion and migration of inflammation-producing cells to the inflamed zone. Incensole acetate, another active component of frankincense, was also shown to inhibit activation of nuclear factor-kappa B (NF-кB) (Moussaieff et al., 2008 ▶). Although we did not evaluate inflammatory mediators, it is likely that an important part of the therapeutic effect of frankincense in the STZ model of AD might be due to its anti-inflammatory properties, which needs additional investigations.
In AD, there is a consistent deficit in cholinergic neurotransmission, particularly affecting cholinergic neurons in the basal forebrain (Kása et al., 1997 ▶). Inhibition of the acetylcholinesterase, which is responsible for the hydrolysis of acetylcholine, is considered as a promising strategy for the treatment of neurological disorders such as AD (Ali et al., 2008 ▶). Acetylcholinesterase inhibitors (AChEIs) protect cells from free radical toxicity, β-amyloid-induced injury, and increased production of antioxidants. In addition, it was reported that AChEIs directly inhibit the release of cytokines from microglia and monocytes. These observations are supported by evidence showing a role for acetylcholine in suppression of cytokine release through a ‘cholinergic anti-inflammatory pathway’ (Tabet, 2006 ▶). So, it is believed that the effect of AChEIs on improvement of AD is not only due to increasing cholinergic synapses, but also depends on their anti-inflammatory properties. Therefore, it is probable that the therapeutic effect of frankincense in the STZ model of AD might be somehow due to its impact on improvement of the cholinergic system of the brain, which needs further studies.
In accordance with our results, protective and therapeutic effects of frankincense on aluminum chloride (AlCl3)-induced AD in rats, have been recently indicated (Yassin et al., 2013 ▶). Based on the results of that study and our study, it seems that chronic consumption of frankincense might have beneficial effects in patients with AD, which requires clinical investigations.
Taken together, we hypothesize that the antioxidant, anti-inflammatory and anti-acetylcholinesterase activities of frankincense, as well as its direct effect on improvement of cognitive functions might be involved in the observed results.
In conclusion, the results of our study indicate that chronic administration of frankincense has a time-dependent therapeutic property on the STZ model of AD. Further studies are required to investigate probable mechanisms involved in the observed results.
Acknowledgment
This work which was part of a M.Sc. thesis, was supported by a grant (grant No. 10325/92) from Vice-chancellorships for Research and Technology, University of Isfahan.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Ali NA, Wurster M, Arnold N, Teichert A, Schmidt J, Lindequist U, et al. Chemical composition and biological activities of essential oils from the oleogum resins of three endemic Soqotraen Boswellia species. Rec Nat Prod. 2008;2:6–12. [Google Scholar]
- Banno N, Akihisa T, Yasukawa K, Tokuda H, Tabata K, Nakamura Y, et al. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. J Ethnopharmacol. 2006;107:249–253. doi: 10.1016/j.jep.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Beheshti S, Hosseini SA, Noorbakhshnia M, Eivani M. Role of hippocampal CA1 area gap junction channels on morphine state-dependent learning. Eur J pharmacol. 2014;745:196–200. doi: 10.1016/j.ejphar.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Farshchi A, Ghiasi G, Farshchi S, Malek Khatabi P. Effects of boswellia papyrifera gum extract on learning and memory in mice and rats. Iran J Basic Med Sci. 2010;13:9–15. [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Hadjzadeh MA, Derakhshan M, Havakhah S, Rassouli FB, Rakhshandeh H, et al. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res. 2010;33:463–468. doi: 10.1007/s12272-010-0317-z. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Shafei MN, Safari V, Taiarani Z, Kafami Ladani m, Sadeghian R. The effects of olibanum administered to methimazole-treated dams during lactation on learning and memory of offspring rats. Nat Prod Res. 2012;26:1544–1548. doi: 10.1080/14786419.2011.566223. [DOI] [PubMed] [Google Scholar]
- Hosseini Sharifabad M, Esfandiari E. A morphometeric study on CA3 hippocampal field in young rats following maternal administration of Boswellia serrata resin during gestation. Iran J Basic Med Sci. 2007;10:176–82. [Google Scholar]
- Hosseini Sharifabad M, Esfandiari E. Effect of Boswellia serrata Triana & Planch. gum resin administration during lactation on morphology of pyramidal neurons in hippocampus of rat. JHD. 2011;2:45–52. [Google Scholar]
- Hosseini-sharifabad M, Esfandiari E. Effect of Boswellia serrata gum resin on the morphology of hippocampal CA1 pyramidal cells in aged rat. Anat Sci Int. 2015;90:47–53. doi: 10.1007/s12565-014-0228-z. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H RM, Akhtar Y, Ziaie ST. Evaluation of ethyl acetate and N-botanolic fractions of Frankincense on learning and memory of rats in the Morris water maze. J Med Plants. 2009;34:95–101. [Google Scholar]
- Jalili C, Salahshoor MR, Moradi S, Pourmotabbed A, Motaghi M. The therapeutic effect of the aqueous extract of boswellia serrata on the learning deficit in kindled rats. Int J Prev med. 2014;5:563–568. [PMC free article] [PubMed] [Google Scholar]
- Kása P, Rakonczay Z, Gulya K. The cholinergic system in Alzheimer's disease. Prog Neurobiol. 1997;52:511–535. doi: 10.1016/s0301-0082(97)00028-2. [DOI] [PubMed] [Google Scholar]
- Mahmoudi A, Hosseini-Sharifabad A, Monsef-Esfahani HR, Yazdinejad AR, Khanavi M, Roghani A, et al. Evaluation of systemic administration of Boswellia papyrifera extracts on spatial memory retention in male rats. J Nat Med. 2011;65:519–525. doi: 10.1007/s11418-011-0533-y. [DOI] [PubMed] [Google Scholar]
- Moussaieff A, Shein NA, Tsenter J, Grigoriadis S, Simeonidou C, Alexandrovich AG, et al. Incensole acetate: a novel neuroprotective agent isolated from Boswellia carterii. J Cereb Blood Flow Metab. 2008;28:1341–1352. doi: 10.1038/jcbfm.2008.28. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Nazem A, Sankowski R, Bacher M, Al-Abed Y. Rodent models of neuroinflammation for Alzheimer's disease. J Neuroinflamm. 2015;12:1–15. doi: 10.1186/s12974-015-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch R, Hoyer S. Local action of the diabetogenic drug, streptozotocin, on glucose and energy metabolism in rat brain cortex. Neurosci lett. 1991;128:199–202. doi: 10.1016/0304-3940(91)90260-z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Sedighi B, Pardakhty A, Kamali H, Shafiee K, Hasani BN. Effect of Boswellia papyrifera on cognitive impairment in multiple sclerosis. Iran J Neurol. 2014;13:149–153. [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Gupta Y. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–1029. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- Sharma AU, Jitendra; Jain, Amit; Kharya, M D; Namdeo, Ajay; Mahadik, K R. Antioxidant activity of aqueous extract of Boswellia serrata. J Chem Biol and Physic Sci. 2011;1:60–71. [Google Scholar]
- Shoham S, Bejar C, Kovalev E, Weinstock M. Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp Neurol. 2003;184:1043–1052. doi: 10.1016/j.expneurol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Sodhi RK, Singh N. All-trans retinoic acid rescues memory deficits and neuropathological changes in mouse model of streptozotocin-induced dementia of Alzheimer's type. Prog Neuro Psychoph. 2013;40:38–46. doi: 10.1016/j.pnpbp.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Tabet N. Acetylcholinesterase inhibitors for Alzheimer's disease: anti-inflammatories in acetylcholine clothing! Age Ageing. 2006;35:336–338. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- Yassin N, El-Shenawy S, Mahdy KA, Gouda N, Marrie A, Farrag A, et al. Effect of Boswellia serrata on Alzheimer’s disease induced in rats. J Arab Soc Med Res. 2013;8:1–11. [Google Scholar]
- Zaker S, Beheshti S, Aghaie R, Noorbakhshnia M. Effect of olibanum on a rat model of Alzheimer’s disease induced by intracerebroventricular injection of streptozotocin. Physiol Pharmacol. 2015;18:477–489. [Google Scholar]