Abstract
Background
Generalized HIV epidemics propagate to future generations according to the age patterns of transmission. We hypothesized that future generations could be protected from infection using age-targeted prevention, analogous to the ring-fencing strategies used to control the spread of smallpox.
Methods
We modeled age-targeted or cohort-targeted outreach with HIV treatment and/or prevention using EMOD-HIV v0·8, an individual-based network model of HIV transmission in South Africa.
Results
Targeting ages 20 to 30 with intensified outreach, linkage, and eligibility for antiretroviral therapy (ART) averted 45% as many infections as universal outreach for approximately one-fifth the cost beyond existing HIV services. Though cost-effective, targeting failed to eliminate all infections to those under 20 due to vertical and inter-generational transmission. Cost-effectiveness of optimal prevention strategies included US$6238 per infection averted targeting ages 10–30, US$5031 targeting 20–30, US$4279 targeting 22–27, and US$3967 targeting 25–27, compared to US$10 812 for full-population test-and-treat. Minimizing burden (disability-adjusted life years [DALYs]) rather than infections resulted in older target age ranges because older adults were more likely to receive a direct health benefit from treatment.
Conclusions
Age-targeted treatment for HIV prevention is unlikely to eliminate HIV epidemics, but is an efficient strategy for reducing new infections in generalized epidemics settings.
Keywords: Antiretroviral therapy, Cost-effectiveness, Epidemic modeling, HIV prevention, South Africa
Introduction
Strategies to interrupt transmission of HIV are urgently needed, especially in generalized epidemic settings with extremely high HIV prevalence in the general population,1,2 high HIV-related disease burden3,4 and limited resources to identify, engage and retain infected individuals in care.5 In the long term, serving populations at highest risk of transmitting or acquiring HIV is necessary to reduce the total number of people who will require lifelong treatment, helping to close the gap between global treatment targets and present-day, substantially lower levels of HIV diagnosis, treatment, and viral suppression.6 In generalized epidemic settings, there is not yet an accepted strategy for identifying these sub-populations, but the patterns of generalized HIV epidemics provide clues about their characteristics.
A distinct characteristic of generalized epidemics is sustained high HIV incidence in young women,2 leading epidemiologists to hypothesize that the age patterns of HIV transmission are central to fueling the epidemic and may be vulnerable to interventions that collapse the chains of HIV transmission. We have previously used mathematical modeling to investigate the age patterns of those most at risk of transmitting HIV to another individual, and further, those most likely to be part of an ongoing chain of transmission (as opposed to a ‘dead-end’ transmission that never spreads beyond the receiving partner).7 Building upon those investigations, the current study explores whether age-targeted intensification of primary and/or secondary HIV prevention could collapse chains of transmission in generalized epidemic settings.
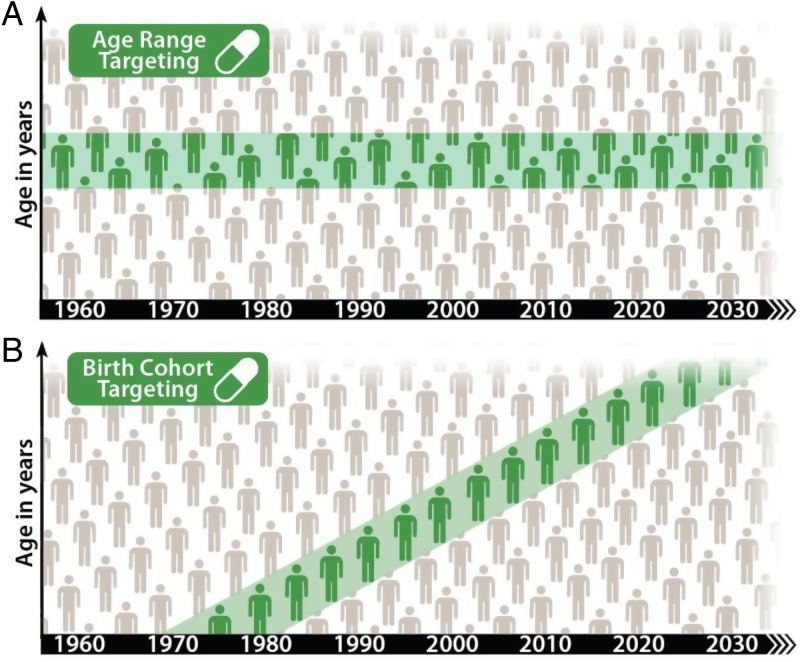
The targeting approach explored here draws inspiration from the epidemiological ring-fencing methods used to control the spread of smallpox since the 1870s8 up until the final eradication activities in the 1970s.9 Rather than the quarantine and vaccination approaches used to create a ‘shield’ to prevent the spread of smallpox, intensified HIV treatment and prevention services would be used to create a longer-term demographic ‘shield,’ preventing the spread of HIV from older to younger generations. We explore a strategy of intensified HIV services for a specific age range, forming a static age band that separates older from younger individuals (Figure 1A). Alternatively, we explore intensification to an aging birth cohort consisting of individuals born between two specified dates. If begun when these individuals are mostly HIV-unexposed, such a band could sweep through the population, potentially leaving behind a future generation protected from HIV.
Figure 1.
Schematic of age-based targeting and cohort-based targeting of outreach. Teal regions show the age range of the (A) age or (B) birth cohort receiving intensified HIV services. People can age in and out of the target group with age targeting, but not with birth cohort targeting.
The concept of a demographic ‘shield’ to protect future generations is relatively unexplored even among available age-structured models of HIV transmission. Because the penetration and effectiveness of a hypothetical intervention cannot be predicted in silico, the study presented here is exploratory in nature, scanning over a range of model assumptions and hypothetical combinations of novel or highly improved interventions to estimate the maximum potential impact of a given strategy.
Methods
We used EMOD-HIV v0.8, an age-structured, individual-based network model of HIV in South Africa, to model the impact of intensifying primary and/or secondary HIV prevention services for a specified age range (Figure 1A) or birth cohort (Figure 1B). Primary prevention is defined as a service targeted to an uninfected individual to prevent acquisition of disease, whereas secondary prevention is targeted to an infected individual to prevent transmission of disease.
The parameters, projections, and sensitivities of the baseline model projections (to which the interventions are compared) have been described previously7,10,11 and a detailed model description, user tutorials, model installer, and source code are available for download at http://idmod.org/software. Briefly, EMOD-HIV is an individual-based model that simulates transmission using an explicitly defined network of relationships that are formed according to preference patterns and dissolve according to age-dependent durations (younger individuals tending to form shorter-term relationships). The age patterns of sexual mixing were configured to match those observed in a rural, HIV-hyperendemic region of Kwazulu-Natal, South Africa.12 Recently, a validation study showed that self-reported partner ages in this setting to be relatively accurate, with 72% of self-reported estimates falling within two years of the partner's actual date of birth.13
The model replicates the demographic patterns of South Africa, explicitly simulating 1/200th of the South African population with age-dependent fertility and age/gender-dependent mortality. To ensure that the configured relationship preference patterns are realized given the underlying demographics, the rates of relationship formation are adjusted by a feed-forward algorithm to match the desired age patterns between couples.14 Transmission rates within relationships depend on HIV disease stage, male circumcision, condom usage, co-infections, and antiretroviral therapy–the latter causing the transmission rate to decline linearly over the first 6 months of therapy until viral suppression is achieved.15,16 Viral suppression is assumed to reduce transmission by 92% in our more conservative scenarios–an estimate based on observational data in which outside partnerships could have contributed to HIV acquisition17–or by as much as 100% in our most optimistic scenarios.
The model includes a configurable health care system module, which we have configured to follow trends in antiretroviral therapy (ART) expansion in South Africa. Treatment begins with voluntary counseling and testing (VCT), antenatal and infant testing, symptom-driven testing, and low level of couples testing. The model includes loss to follow-up between diagnosis and staging, between staging and linkage to ART or pre-ART care, and during ART or pre-ART care.18 The model's projections of baseline treatment expansion in South Africa predict gradual incidence declines without elimination, so that HIV remains endemic through 2050.19 The cost and HIV burden in the targeted treatment scenarios were compared to the baseline scenario using a unit cost and disability-adjusted life year (DALY) model developed by the HIV Modelling Consortium for the 2013 WHO revision of HIV treatment guidelines.19
Primary prevention was implemented as a decrease in the probability that an individual becomes infected, up to a 100% decrease corresponding to full protection. Secondary prevention was assumed to consist of HIV testing at a rate of once per year, with ART) initiation regardless of CD4 count for those testing positive. The intensified HIV testing was assumed to replace VCT for those in the target group. These assumptions about the target group match those of a recent collaborative analysis of hypothetical universal test-and-treat for the entire population of South Africa.19 In our analysis, we provide the full-population test-and-treat scenario as a reference point to compare to age-targeted test-and-treat. This provides a hypothetical maximum for cost and impact, and also provides a point of reference where our model has been compared to 11 other independently developed mathematical models.
Unlike birth cohort targeting, age range targeting would allow people to enter and leave the target group as they cross the lower and upper age thresholds. Before entering and after leaving the target group, individuals exhibited eligibility, testing, and linkage rates identical to those used for the baseline scenario. However, those aging out of the target group continued ART if they were initiated as part of the targeting intervention. VCT was continued at baseline rates after aging out of the target group. Presentation for antenatal, infant, couples, or symptom-driven testing was assumed to occur regardless of outreach, although the need for symptom-driven late presentation would decline for those who initiated care earlier than the onset of AIDS symptoms.
Results
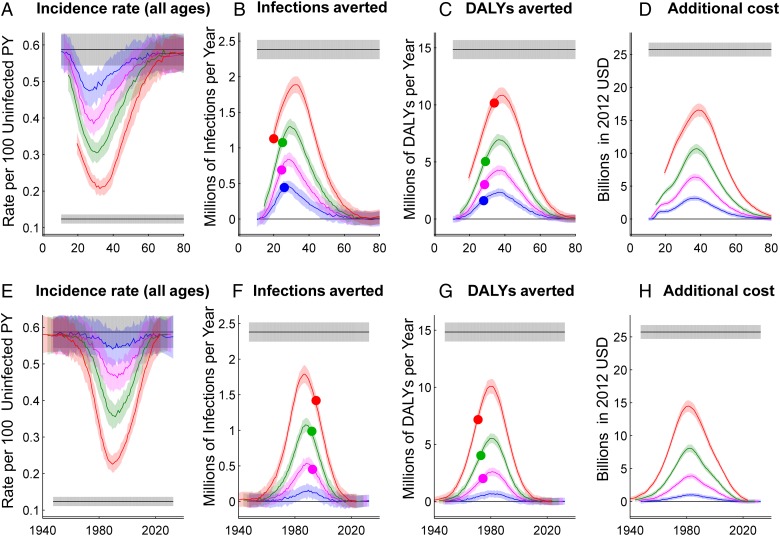
A broad view of the model results is presented by scanning over starting and ending ages or years for a hypothetical age range (Figure 1A) or birth cohort (Figure 1B), maintaining a range of two, five, 10 or 20 years from the youngest to the oldest age included in the target group. Figure 2 shows the HIV disease burden relative to baseline, infections averted relative to baseline, incidence rate in the total population, and cost relative to baseline, assuming 80% penetration of outreach to the target group. Infections, cost, and burden were accumulated over the first 20 years with a 3% annual discount rate, while incidence was reported for the 20th year (2035). Figure 2 also shows a maximum-impact scenario in which the whole population received the intensified testing and treatment intervention, providing a reference point for a population-wide intervention without age-specific targeting.
Figure 2.
Effect of age-targeted treatment expansion on HIV incidence, cumulative infections averted, cumulative disability-adjusted life years (DALYs) averted, and cumulative program cost. Effects of the interventions are represented on the y-axis, with x-axes showing the middle of the age group being targeted (panels A–D) or middle birthdate of the birth cohort being targeted (panels E–H). The inclusion criteria of the targeted groups span 20 (red), 10 (green), 5 (magenta) or 2 (blue) years. Shaded areas show one standard deviation of 20 stochastic simulations. Incidence is shown at 20 years after the intervention begins, and other outcomes are accumulated over the first 20 years with a 3% annual discount rate. At the left and right extremes of each plot, the curves converge to black lines showing the baseline (no intervention) scenario, which projects current guidelines and trends in HIV treatment. The opposite black line shows universal treatment expansion with 80% coverage of annual testing for all individuals. Dots in panels B and F show the strategy with the minimum cost per infection averted over 20 years. Dots in panels C and G show the strategy with the mimumum cost per DALY averted.
For interventions involving the scale-up of treatment, we used a unit cost model19 to approximate the cost and cost-effectiveness of universal and targeted treatment for prevention. The modeled universal treatment expansion intervention produced an additional five-fold reduction in incidence over what would be achieved with current trends in treatment, at an additional discounted cost of US$26 billion over twenty years (Figure 2A and 2E, gray lines). As expected, whole-population targeting was the most costly and highest-impact strategy, followed by 20-year age ranges spanning the highest-prevalence age groups.
Focusing on maximizing the cumulative infections averted, the dots in Figure 2B show the most cost-effective ranges to target: ages 10–30 at US$6238 per infection averted, ages 20–30 at US$5031 per infection averted, ages 22–27 at US$4279 per infection averted, and ages 25–27 at US$3967 per infection averted. Similarly, for infections averted by birth cohort targeting shown in Figure 2F, the most cost-effective birth year ranges are marked at 1985–2005 at US$6826 per infection averted, 1987–1997 at US$5856 per infection averted, and 1990–1995 at US$5686 per infection averted. Universal expansion to all ages/cohorts cost US$10 812 per infection averted.
Compared to universal expansion, targeting the 10-year age range of 20–30 cost US$5.4 billion over 20 years and provided more than double the number of infections averted per US$ invested. However, the cumulative infections averted by targeting the 20–30 age group was only 45% of the number possible with universal expansion.
For cumulative DALYs averted by age targeting shown in Figure 2C, dots show the most cost-effective ranges to target: ages 24–44 at US$1489 per DALY averted, 24–34 at US$1441 per DALY averted, 26–31 at US$1372 per DALY averted, and 27–29 at US$1285 per DALY averted. Similarly, for birth cohort targeting in Figure 2G, the most cost-effective birth year ranges are marked at 1961–1981 at US$1396 per infection averted, 1968–1978 at US$1374 per infection averted, and 1972–1977 at US$1373 per infection averted. Universal expansion to all ages/cohorts resulted in US$1735 per infection averted.
The efficiency gained by age targeting was more modest for DALYs than for infections averted. The most cost-effective 10-year age range for infections averted (20–30) more than halved the cost per DALY averted, whereas the most cost-effective 10-year age range for DALYs averted (24–34) reduced the cost per DALY averted by only 17%. The most efficient age range for averting DALYs was older than for averting infections, because DALYs are averted by preventing new infections in the long run, but also by providing a direct health benefit to those receiving treatment in the short run. The health benefit is greatest for those who are most likely to progress quickly from asymptomatic HIV infection to symptomatic AIDS: older individuals, who experience faster disease progression20 and are more likely to have been infected for a longer period of time.
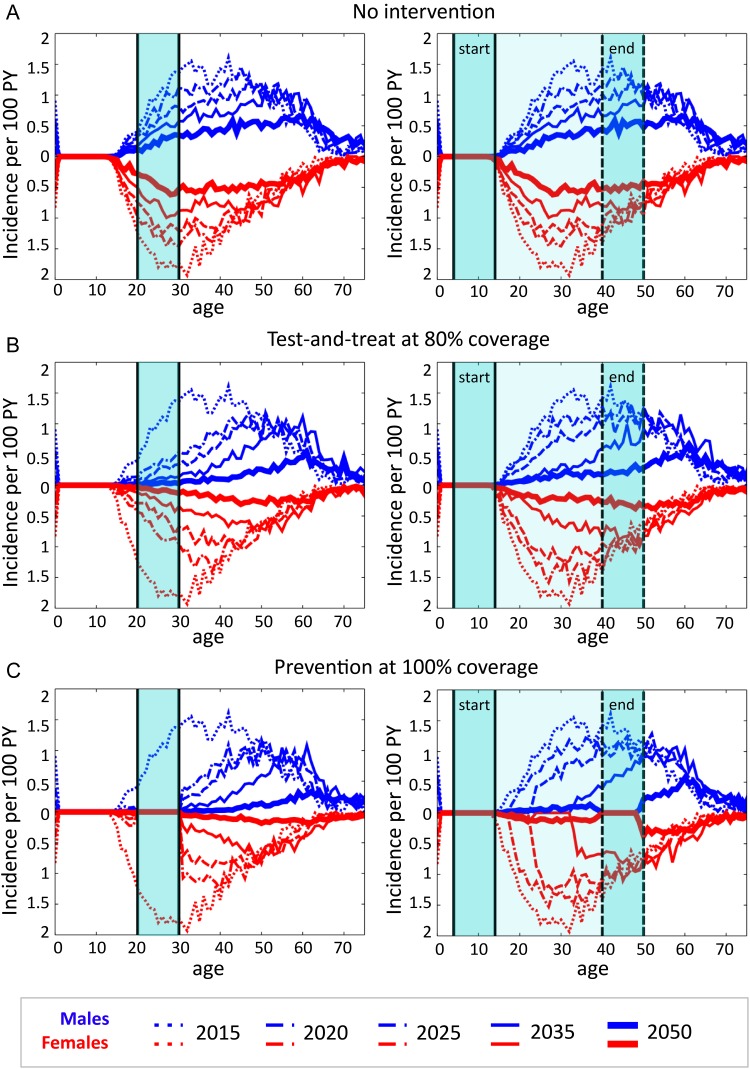
Having identified the 20–30 age group as an efficient target for prevention, we then selected this age range as a hypothetical target group, and examined the age pattern of HIV incidence in the broader population to determine whether efficient targeting of treatment expansion to ages 20–30 had the potential to interrupt HIV transmission to younger individuals. Figure 3A shows the age pattern of incidence for baseline trends in HIV treatment. Our model predicted a slow decline and slight aging of the pattern of HIV incidence, with HIV remaining endemic in the year 2050.
Figure 3.
Age distribution of HIV incidence for the baseline simulation (A), targeted treatment outreach (B), and targeted complete prevention of HIV infection (C). Incidence is shown just before implementation of the intervention, and at 5, 10, 20 and 35 years after implementation of the intervention. Shaded areas show the age range of the target group throughout the intervention (left) or for a birth cohort in the year of implementation (‘start’) and 35 years after implementation (‘end’). Age-dependent incidence per 100 uninfected person-years (PY) was averaged across 20 stochastic simulation runs.
When individuals aged 20–30 were targeted with HIV treatment, even at an optimistic penetration level of 80% for outreach with testing and linkage to care, incidence declined across all age groups but fell short of completely eliminating HIV by 2050 (Figure 3B, left panel). By 2030, incidence in the overall population was 37% lower than that of the baseline simulation, while incidence in those under age 20 was 60% lower than in the baseline scenario. By 2050, the overall and under-20 incidence rates were 59% and 78% lower than the baseline scenario, respectively.
One possible explanation for reduced but continuing incidence among teenagers is self-sustaining transmission among youth. Thus, we next tested a strategy of birth cohort targeting for to those born in the years 2000 through 2010. The targeted individuals would begin reaching sexual debut as the intervention was launched, and the 10-year targeted band would age through the population, with the intention of using a ring-fence like approach to ‘sweep out’ HIV, leaving behind an HIV-free generation.
The model results showed the cohort-based targeting approach to be less effective than age targeting (Figure 3B, right panel). By the year 2030 (the year in which the target group reached 20–30 years of age), incidence among those under 20 years of age was only 29% lower than in the baseline scenario, and incidence in the overall population was only 11% lower than in the baseline scenario. We further augmented the simulations of age-targeted treatment with a hypothetical prevention intervention to the same target group (Figure 3C). The extreme assumption of 100% coverage with perfect HIV prevention for those aged 20–30 still did not eliminate HIV infections among those younger than age 20, although incidence was greatly diminished. Even complete HIV prevention on the 2000–2010 birth cohort failed to ‘sweep out’ HIV from the population (Figure 3C, right panel). By the year 2030, incidence emerged in young females at a rate of 0·05–0·15% per year, and similar levels later emerged in young males.
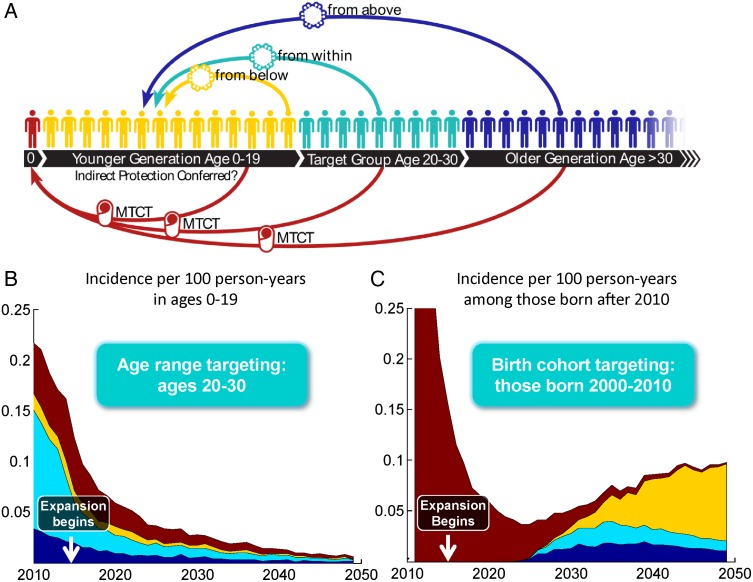
Finally we addressed the question of how HIV the epidemic reached youth, even with a complete one-decade gap in HIV transmission. We considered four possible routes by which HIV could have entered the younger generation, illustrated in Figure 4A. First, individuals older than the target group could infect those younger than the target group, essentially ‘hopping the fence’ that was meant to protect younger generations. Second, individuals in the target group could transmit to younger generations due to imperfect treatment coverage or efficacy. Even when coupled with aggressive prevention interventions, some individuals in the target group could already be infected at the start of the intervention. Third, the younger generation could sustain an epidemic on its own, unfettered by treatment or prevention in older generations. Fourth, imperfect coverage and effectiveness of interventions to prevent mother-to-child transmission could permit some children to become infected and, with ART, survive until sexual maturity. In Figure 4A, these modalities are illustrated in blue, green, yellow, and red, respectively.
Figure 4.
Sources of new HIV infections in the generation younger than the target group. The diagram in panel (A) shows how infections can enter the younger generation from those older than the target group (blue), those in the target group (teal), those already infected in the younger generation itself (yellow), or through mother-to-child transmission from any age group (maroon). The contribution of each of these sources to incidence in those younger than the target group is shown in panel (B) for age targeting and panel (C) for birth cohort targeting. The denominator for incidence in (B) is the uninfected population ages 0 through 19. In (C), the denominator is the population of uninfected individuals with a birthdate after 2010, which grows over time as new individuals are born.
The relative contribution from each of these four mechanisms was estimated by categorizing transmission events in the model according to their origin: infection by a sex partner older than, in, or younger than the target group, or mother-to-child transmission. Figures 4B and 4C illustrate the relative contribution of each component over time in the targeted treatment scenarios. The baseline scenario (without any targeted intervention), the dominant source of infections was proposed the target group. Age or cohort targeting of HIV treatment greatly reduced the target group's contribution to transmission.
In the case of cohort targeting, the age range of those younger than the target group expanded as the group aged. In 2015, the younger generation consisted of children under age five, and mother-to-child transmission was the only source of new infections. After reaching sexual maturity, they became infected by individuals in or older than the target group, before eventually reaching an age and prevalence level at which transmission within the generation became dominant. This explains the time-dependent sources of incidence in younger generations, shown in Figure 4C.
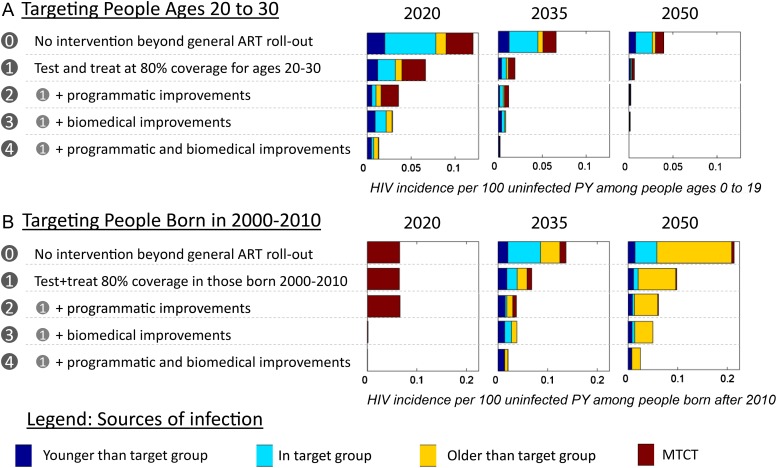
Finally, we examined how hypothetical improvements to HIV programs might influence the ability to isolate HIV from future generations using the age targeting approach. In Figure 5 and Supplementary Figure 1, the incidence rates among individuals younger than the target group are shown in response to a variety of improvements to age-targeted or cohort-targeted treatment for prevention. To test the extent to which tools must be improved, we pushed the assumptions to their extreme, assuming that it would be possible to eliminate imperfections in already high-efficacy and high-coverage interventions.
Figure 5.
Impact of biomedical or programmatic improvements on the sources of new HIV infections in the generation younger than the target group. Stacked bar charts show the rate of new HIV infections in future generations, defined as those younger than the targeted (A) age range or (B) birth cohort. New infections are color-coded by their source, with possible contributors being those older than the target group (blue), those in the target group (teal), those already infected in the younger generation itself (yellow), and mother-to-child transmission from any age group (maroon). The projections are shown for general ART roll-out without any specific intervention for the target group (0), achieving 80% coverage of annual testing and ART for all new diagnoses in the target group (1), further programmatic improvements in treatment as prevention the target group (2), further biomedical improvements (3), and a combination of programmatic and biomedical improvements (4). The individual components of programmatic and biomedical improvements are not shown here, but are compared in Supplementary Figure 1.
For improved biomedical technologies, we first examined increasing the sensitivity of HIV diagnostic testing from 98 to 100%. It had a negligible impact on incidence reduction in younger generations. We then examined increasing the effectiveness of antivirals for prevention of mother-to-child transmission from 90 to 100%. This reduced, but did not eliminate, mother-to-child transmission due to imperfect coverage. Finally, we examined increasing the effectiveness of ART at preventing transmission in couples from 92 to 100%. Of all technological improvements, the prevention efficacy of ART had the largest effect of protecting younger generations. As expected, the combination of improved testing and antiviral drugs had a greater impact than any intervention alone.
We next examined hypothetical programmatic improvements. Increasing the penetration of outreach in the target group from 80 to 100% reduced incidence in youth, as did eliminating delays between infection and treatment initiation. As with technological improvements, the two together reduced incidence in the younger generation more than each one did alone.
No single improvement to a technology or program was sufficient to fully interrupt transmission in youth, nor were combinations of purely programmatic or purely technological solutions sufficient. The combination of all of the interventions discussed, both biomedical and programmatic, enabled the elimination of HIV in young generations by 2050 when targeting an age range of 20–30 year olds.
Discussion
Despite vigorous policy debate about whether an AIDS-free generation is within reach21,22 and the availability of models that include age patterns of HIV transmission,19,23,24 the concept of a demographic ‘shield’ to protect future generations is relatively unexplored. We have used a network model to demonstrate how age-based targeting could as much as double cost-effectiveness of treatment for prevention, but would be insufficient to prevent HIV transmission to future generations until biomedical tools and programs are dramatically and simultaneously improved.
The efficiency of targeting individuals in their twenties was consistent with our prior analysis of HIV transmission chains,7 which showed that this age group is vital for transmitting HIV from older to younger subsets of the HIV transmission network.
Exploring the limits of age-based targeting yielded two surprising conclusions. First, targeting a birth cohort to ‘sweep out’ HIV is less effective than simply shutting down transmission in a high-incidence age band. Second, treatment as prevention is perhaps an even more powerful tool than primary HIV prevention for interrupting transmission, if used optimally. In Figure 3, we saw that perfect prevention in the 20–30 age range was insufficient to protect those under age 20 from acquiring HIV. In contrast, in Figure 5 we showed how an idealized treatment intervention would be able to do so, in large part because prevention alone would allow some transmissions to occur from individuals who were already infected when entering the target group.
Outreach programs already have the potential to reach populations with diverse age distributions. These include school-based programs,25 workplace wellness programs,26–28 youth centers29 and programs leveraging health care utilization such as antenatal care30 and sexually transmitted disease treatment.31 Age targeting considerations could potentially help to prioritize and justify the expansion of outreach methods that can reach young adults.
An important limitation of this modeling exercise is the assumption that the age- or birthdate-based targeting intervention had a precise cutoff date for eligibility, whereas realistic programs that use age to prioritize outreach would of undoubtedly have spillover effects into other age groups. We would therefore expect that the realistic efficiency gains from age targeting would be more modest than what is predicted by a model of ideal age targeting.
An additional shortcoming of the model used for this exercise is the lack of consideration for outreach cost, which could differ for age-based targeting compared to universal expansion of treatment. This too would depend on the program used to access the age group of interest. Future modeling studies could potentially explore the tradeoff between coverage, precision of targeting, and cost-effectiveness of outreach by different modalities.
Lastly, it is important to consider the broader uncertainty of model projects of HIV epidemics. The underlying drivers of generalized epidemics–what causes HIV to spread in the general population in some settings and not others–are still not well-understood. There is evidence of spatial and sociodemographic heterogeneities in HIV risk, implying hidden sub-epidemics embedded within generalized epidemics. These are still poorly characterized, and thus unlikely to be appropriately captured in existing mathematical models. Data on past trends in partnership age gaps are limited and not necessarily predictive of future behavior. The model scenarios presented here were scaled to represent the demographics and baseline treatment program in South Africa, but the age patterns represented in the model were measured in a small, rural hyperendemic region of Kwazulu-Natal,12 and not necessarily representative of mixing patterns at the national level. Although a recent validation study found the self-reported mixing patterns to be relatively accurate,13 there may be types of relationships that are less likely to be reported and more difficult to validate through demographic surveillance.
Further, trends in past HIV incidence and self-reported partner choice do not necessarily predict future trends in partner choice. If age patterns of partnerships shift in the future, so too would the optimum age groups to target with intensified HIV services. More broadly, over the time horizons discussed in this analysis, model projections will need to be updated to reflect changes in the arrival of new biomedical tools and new information about the course of the HIV epidemic.
Conclusions
As uninfected adolescents reach sexual maturity, primary HIV prevention for adolescents could be compounded with early treatment targeted to the most likely sources of future infections. Our modeling exploration revealed that age-based targeting could double the cost-effectiveness of outreach with HIV treatment as prevention, provided that the cost to access the age groups of interest would be similar to that of reaching the general population. However, age-targeted outreach with current tools would be insufficient to fully protect future generations from HIV infection.
Dramatic but isolated improvements, such as reliable treatment regimens, full coverage in age groups of interest, or rapid detection of new HIV infections, would be insufficient to protect future generations from acquiring HIV. A combination of biomedical and programmatic improvements, applied consistently for more than three decades, would be required to interrupt transmission.
These findings highlight the need for diverse investments in both quality and coverage of HIV treatment, as well as potential epidemiological efficiencies of programs targeting diverse populations, such as school-based programs,25 workplace wellness programs,26–28 youth centers29 and programs leveraging health care utilization such as antenatal care30 and treatment clinics for sexually transmitted diseases.31
Supplementary Material
Acknowledgments
Authors' contributions: AB, DJK, and PAE conceived of the study. AB and DJK carried out the analysis. AB drafted the manuscript. AB, DJK, and PAE critically revised the manuscript. All authors read and approved the final manuscript. AB is the guarantor of the paper.
Acknowledgements: The authors thank Bill and Melinda Gates for their active support of this work and their sponsorship through the Global Good Fund. The HIV Modelling Consortium provided feedback during the development of EMOD-HIV and developed the cost model used in this analysis. Samantha Zimmerman, an illustrator of biological textbooks, illustrated Figure 1.
Funding: This work is supported by Bill and Melinda Gates through the Global Good Fund. Funding to pay the Open Access publication charges for this article was provided by Bill and Melinda Gates through the Global Good Fund.
Competing interests: None declared.
Ethics approval: Not required.
References
- 1.Simbayi L, Shisana O, Rehle TM et al. South African National HIV Prevalence, Incidence, Behaviour Survey, 2012. Cape Town: HSRC Press; 2014. [Google Scholar]
- 2.Eaton JW, Rehle TM, Jooste S et al. Recent HIV prevalence trends among pregnant women and all women in sub-Saharan Africa: implications for HIV estimates. AIDS 2014;28:S507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Barber RM, Foreman KJ et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015;386:2145–91. http://linkinghub.elsevier.com/retrieve/pii/S014067361561340X [accessed 7 November 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granich R, Gupta S, Hersh B et al. Trends in AIDS deaths, new infections and ART coverage in the top 30 countries with the highest AIDS mortality burden;1990–2013. PLoS ONE 2015;10:e0131353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resch S, Ryckman T, Hecht R. Funding AIDS programmes in the era of shared responsibility: an analysis of domestic spending in 12 low-income and middle-income countries. Lancet Glob Health 2015;3:e52–61. [DOI] [PubMed] [Google Scholar]
- 6.Levi J, Raymond A, Vernazza P et al. Can the UNAIDS 90–90–90 target be achieved? Analysis of 12 national level HIV treatment cascades. Abstract Number MOAD0102 presented at the 8th IAS Conference on HIV Pathogenesis, Treatment, and Prevention. Vancouver, BC, Canada, July 19–22, 2015. [Google Scholar]
- 7.Bershteyn A, Klein DJ, Eckhoff PA. Age-dependent partnering and the HIV transmission chain: a microsimulation analysis. J R Soc Interface 2013;10:20130613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser SM. Leicester and smallpox: the Leicester method. Med Hist 1980;24:315–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson DA. The eradication of smallpox. Sci Am 1976;235:25–33. [DOI] [PubMed] [Google Scholar]
- 10.Bershteyn A, Klein DJ, Wenger E, Eckhoff PA. Description of the EMOD-HIV Model v0.7. ArXiv 12063720 2012. http://arxiv.org/pdf/1206.3720.pdf [accessed 26 january 2016]. [Google Scholar]
- 11.Klein DJ, Bershteyn A, Eckhoff PA. Dropout and re-enrollment: implications for epidemiological projections of treatment programs. AIDS 2014;28:S47–59. [DOI] [PubMed] [Google Scholar]
- 12.Ott MQ, Bärnighausen T, Tanser F et al. Age-gaps in sexual partnerships: seeing beyond ‘sugar daddies’. AIDS 2011;25:861–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harling G, Tanser F, Mutevedzi T, Bärnighausen T. Assessing the validity of respondents' reports of their partners' ages in a rural South African population-based cohort. BMJ Open 2015;5:e005638 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4360781/ [accessed 7 November 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein DJ. Relationship formation and flow control algorithms for generating age-structured networks in HIV modeling. 2012 IEEE 51st Annual Conference on Decision and Control (CDC). 2012, p: 1041–6. [Google Scholar]
- 15.Attia S, Egger M, Müller M et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS Lond Engl 2009;23:1397–404. [DOI] [PubMed] [Google Scholar]
- 16.Marconi VC, Grandits G, Okulicz JF et al. Cumulative viral load and virologic decay patterns after antiretroviral therapy in HIV-infected subjects influence CD4 recovery and AIDS. PLoS ONE 2011;6:e17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnell D, Baeten JM, Kiarie J et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010;375:2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen S, Fox MP. Retention in HIV Care between testing and treatment in Sub-Saharan Africa: a systematic review. PLoS Med 2011;8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton JW, Menzies NA, Stover J et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health 2014;2:e23–34. [DOI] [PubMed] [Google Scholar]
- 20.Todd J, Glynn JR, Marston M et al. Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy. AIDS 2007;21:S55–63 DOI:10.1097/01.aids.0000299411.75269.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauci AS, Folkers GK. Toward an AIDS-free generation. JAMA 2012;308:343–4. [DOI] [PubMed] [Google Scholar]
- 22.Karim SSA. An AIDS-free generation. Science 2012;337:133. [DOI] [PubMed] [Google Scholar]
- 23.Johnson LF, White PJ. A review of mathematical models of HIV/AIDS interventions and their implications for policy. Sex Transm Infect 2011;87:629–34. [DOI] [PubMed] [Google Scholar]
- 24.Mishra S, Steen R, Gerbase A et al. Impact of high-risk sex and focused interventions in heterosexual HIV epidemics: a systematic review of mathematical models. PLoS ONE 2012;7:e50691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jemmott LS, Jemmott JB, Ngwane Z et al. ‘Let Us Protect Our Future’ a culturally congruent evidenced-based HIV/STD risk-reduction intervention for young South African adolescents. Health Educ Res 2014;29:166–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhagwanjee A, Govender K, Akintola O et al. Patterns of disclosure and antiretroviral treatment adherence in a South African mining workplace programme and implications for HIV prevention. Afr J AIDS Res 2011;10:357–68. [DOI] [PubMed] [Google Scholar]
- 27.Pillay R, Terblanche L. Caring for South Africa's public sector employees in the workplace: a study of employee assistance and HIV/AIDS workplace programmes. J Hum Ecol 2012;39:229–39. [Google Scholar]
- 28.Houdmont J, Munir F, Grey M. Acceptance of repeat worksite HIV voluntary counselling and testing in a rural South African factory. AIDS Care 2013;25:1199–202. [DOI] [PubMed] [Google Scholar]
- 29.Black S, Wallace M, Middelkoop K et al. Improving HIV testing amongst adolescents through an integrated Youth Centre rewards program: Insights from South Africa. Child Youth Serv Rev 2014;45:98–105. [Google Scholar]
- 30.Hensen B, Baggaley R, Wong VJ et al. Universal voluntary HIV testing in antenatal care settings: a review of the contribution of provider-initiated testing and counselling. Trop Med Int Health 2012;17:59–70. [DOI] [PubMed] [Google Scholar]
- 31.Leon N, Naidoo P, Mathews C et al. The impact of provider-initiated (opt-out) HIV testing and counseling of patients with sexually transmitted infection in Cape Town, South Africa: a controlled trial. Implement Sc. 2010;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.