Abstract
Background
In April 2014 we investigated the association of migration of a woman's husband with her high-risk human papillomavirus (HR-HPV) infection status and her abnormal cervical cytology status in the Achham district of rural Far-Western Nepal.
Methods
Women were surveyed and screened for HR-HPV during a health camp conducted by the Nepal Fertility Care Center. Univariate and multivariable statistical tests were performed to determine the association of a husband's migration status with HR-HPV infection and cervical cytology status.
Results
In 265 women, the prevalence of HR-HPV was 7.5% (20/265), while the prevalence of abnormal cervical cytology, defined using the Bethesda system as atypical glandular cells of undetermined significance or worse, was 7.6% (19/251). Half of the study participants (50.8%, 130/256) had husbands who had reported migrating for work at least once. Women aged ≤34 years were significantly less likely to test positive for HR-HPV than women aged >34 years (OR 0.22, 95% CI 0.07 to 0.71). HR-HPV infection and abnormal cervical cytology status were not directly associated with a husband's migration.
Conclusion
Older women were found to have a higher prevalence of HPV than younger women. It is possible that a husband's migration for work could be delaying HR-HPV infections in married women until an older age.
Keywords: Cervical cancer, HPV, Migration, Nepal
Introduction
In 2012, 528 000 new cases of cervical cancer were diagnosed and 266 000 women died of the disease, nearly 90% of them in low- and middle-income countries (LMICs).1 Barriers to cervical cancer screening, such as access to health care, low levels of screening knowledge, and a lack of perceived benefits from screening, impede early cervical cancer diagnosis in LMICs.2,3 In Nepal, where routine cervical screening is not widely available, cervical cancer is usually only detected when it is at an advanced stage which results in high mortality.
Due to a current scarcity of cervical screening and treatment programs, it is important to identify sub-populations of Nepali women who are at a greater risk for cervical cancer. In addition to risky sexual behaviors, social factors such as education, wealth and marital status are associated with abnormal cervical cytology outcomes.4–7 Due to lack of work during the agricultural off-season, a large proportion of men migrate to urban areas within Nepal, India or other countries for employment. Living away from a spouse potentially makes migrant workers vulnerable to STIs including high-risk human papillomavirus (HR-HPV) through extra-marital sexual contacts. In this regard, returning male migrants can also contribute to the risk of transmitting HPV to their wives who may subsequently develop cervical cancer.
Over 2.2 million Nepalese are engaged in international and domestic labor migration; the Nepal Central Bureau of Statistics estimates that over half of Nepali households in the Far-Western development region of Nepal have at least one family member who participates in some form of migratory work.8 This large group of highly mobile Nepali migrant workers poses unique health challenges for Nepal.9 Male Nepali migrant labor has been associated with increased risk of STIs including HIV.10,11 In Far-Western Nepal approximately 41% of patients with HIV are seasonal migrant laborers.12 By analogy, it is therefore possible that male migrant work in Nepal could be a factor contributing to cervical cancer among Nepali women.
To date, studies of factors associated with HPV infection and cervical cancer morbidity in Nepal have been limited primarily to urban populations with only one study performed in rural Nepal, where labor migration is more common.2,13 The aim of this study therefore is to investigate the association between husbands' migration for employment, and HR-HPV infection and abnormal cervical cytology among their spouses, in the rural Far-West Achham District of Nepal.
Materials and methods
Study setting
This cross-sectional study was conducted within Sanfebagar Village Development Committee, a local Nepali governmental administrative division, in Achham District. The population of Achham has poor indicators of health compared to the general Nepali population. The life expectancy for both men and women in Achham during 2011 was 55 years and the maternal mortality rate was 950 per 100 000 live births, both of which are worse than the national life expectancy of 67 years, with a maternal mortality rate of 170 per 100 000 live births.14 Currently, there are only two hospitals in Achham; the government's hospital in the district capital of Mangalsen, and Bayalpata Hospital, a collaboration between the Nepali government and the non-profit organization Possible Health (formerly Nyaya Health).
Study population
Women were recruited from a Nepal Fertility Care Center reproductive health (RH) camp.15 The RH camp was advertised through local newspapers and female support groups two weeks prior to conducting the camp. Women who attended the RH camp received a wide array of free reproductive health services, including testing for STIs, family planning counseling, and liquid cytology testing. Women were eligible for the study if they were married, between the ages of 16 and 60, had a cervix, were not menstruating, and were not pregnant. Consent forms were distributed and read to each participant before they agreed to enroll in the study. Each form was signed and dated by the study participant, a witness, and the study coordinator. Any woman who opted not to participate in the research study was provided with the same clinical services during the RH camp as those participating in the research study. Trained health staff with several years of clinical experience performed pelvic examinations. Institutional review boards from the University of Alabama at Birmingham and the Nepal Health Research Council approved this study.
Laboratory analysis
Study protocols on sample collection and testing have been described previously.13 Briefly, health camp auxiliary nurse midwives collected cervical specimens using ThinPrep PreservCyt medium (Hologic/Gen-Probe Inc. San Diego, CA, USA). Cervical specimens were transported to the Hologic/Gen-Probe Inc. laboratory in San Diego for HPV testing. Laboratory testing of HPV was performed using the APTIMA HPV assay (Hologic/Gen-Probe Inc.), which detects the presence of mRNA from the E6/E7 oncogenes for 14 types of HR-HPV (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68).16 Cervical cytology was assessed for research purposes using clinician-collected samples in ThinPrep PreservCyt medium (Hologic/Gen-Probe Inc.), with results classified according to the Bethesda criteria.17 Women with an abnormal cervical cytology were referred to local medical centers for additional testing and follow-up.
Demographic and health risk survey
Women consenting to participate in the study completed a demographic and health risk survey, the details of which have been described previously.13 Briefly, survey questions were translated from English into Nepali, and then back-translated to English for quality control. The local Nepali dialect called Dotyali is sometimes spoken in the district of Achham and does not have a written form, and thus the survey could not be translated into the local Achham dialect.18,19 However, our interviewers were from Achham and were able to speak both Nepali as well as Dotyali.
Independent and dependent variables
We analyzed two separate outcomes of interest: HR-HPV infection (positive for at least one of the following types: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) and abnormal cervical cytology classified according to the Bethesda criteria. Cervical cytology was categorized as being either ‘Normal’ (benign cellular changes, results within normal limits, atypical squamous cells of undetermined significance [ASCUS], or actinomycosis) or ‘Abnormal’ (atypical glandular cells of undetermined significance [AGUS], or low-grade squamous intraepithelial lesion [LSIL], high-grade SIL [HSIL], squamous cell carcinoma [SCC], or atypical squamous cell-cannot exclude high-grade SIL [ASC-H]).
Demographic and socioeconomic variables included age, average number of children per woman, if a husband had ever migrated for work, if a migrating husband had returned home within the last 3 months, and the geographic location visited by a migrant husband. Age was categorized as being either ≤34 years or younger or >34 years in order to better represent the average age of women whose husbands had migrated for work. The geographic location of the husband's migration was categorized as ‘Migrated within Nepal for work’, ‘Migrated outside of Nepal for work’, or ‘Has never migrated for work’. Additional variables included STI awareness, cervical cancer awareness, and type of current contraception use. Type of contraception currently used was categorized in descriptive tables as hormonal, condom use, other (defined as IUD and withdrawal) and none. In multivariate models, type of contraception currently used was categorized as hormonal, condom use/other, and none due to a small number of women reporting condom use. Hormonal contraception was kept as a separate category because past studies have reported an association between abnormal cervical cytology and hormonal contraception use.20,21 Hormonal contraception use included the use of birth control pills, hormonal implants, and Depo-Provera injections (Pfizer Inc, New York, NY, USA). Self-reported health was categorized as ‘Excellent’, ‘Very Good’, ‘Good’, and ‘Fair/Poor’.
Statistical analysis
All statistical analyses were limited to data from married women. Cross-tabulations were used to describe the frequencies of potential risk factors associated with HR-HPV infection and the potential risk factors associated with an abnormal cytology outcome. ANOVA and Chi-square statistical tests were used to determine the univariate associations of continuous and categorical variables, respectively, with HR-HPV and cervical cytology status.
Multivariable logistic regression models were used to generate odds ratios and 95% CI describing factors associated with HR-HPV infection. Based on 1. a review of HPV risk factor literature from developing countries; 2. a p-value of less 0.1 in the univariate analyses; or 3. if they were of primary interest for the study, final models included the following covariates: age, cervical cancer awareness, type of current contraception used, a husband's migratory status. Cervical cancer awareness was included in the final models because it has been found to be associated with abnormal Pap smears in previous studies.22 The type of contraception used was included in the final models because it has been associated with abnormal cervical cytology in previous studies.23,24 Multivariable logistic regression models were also used to determine odds ratios and 95% CI associated with an abnormal cervical cytology.
Results
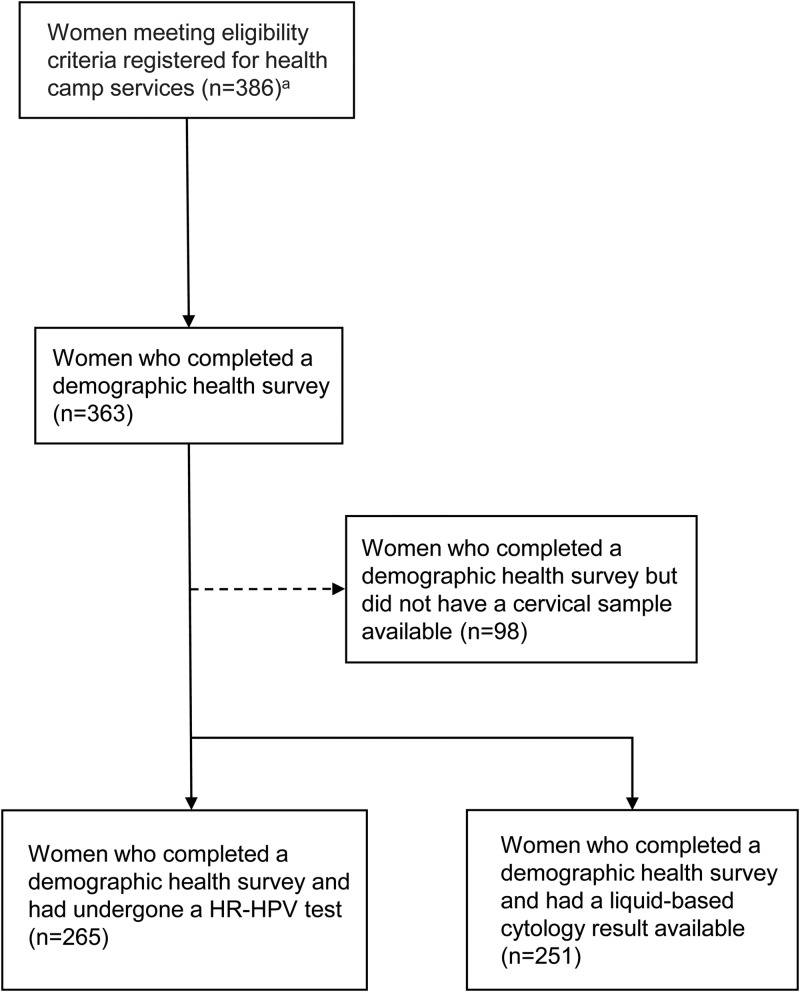
Out of 386 women who registered for the RH camp and met our eligibility criteria of being married, being between the ages of 16 and 60, having a cervix, were not menstruating, and were not pregnant, 363 women completed a demographic health survey. Of these 363 women, 73.0% (265/363) had a HR-HPV test, 69.1% (251/363) had a valid liquid-based cervical cytology reading (Figure 1). Twenty women (7.5%, [20/265]) tested positive for HR-HPV (Table 1) and 7.6% (19/251) of women had abnormal liquid-based cervical cytology results (Table 2). The mean age of study participants was 33.9 (SD 8.8) years. The percentage of women who reported their health to be ‘Excellent’ was 26.2% (69/263), 31.6% (83/263) of women reported their health to be ‘Very good’, 32.7% (86/263) of women reported their health to be ‘Good’, and 9.5% (25/263) of women reported their health to be ‘Fair/poor’ (Table 1).
Figure 1.
Study design flow chart illustrating the selection procedure and sample size of women attending a one-day reproductive health camp in Achham, Nepal. HR-HPV: high-risk human papillomavirus.
a Women were eligible for the study if they were married, between the ages of 16 and 60, had a cervix, were not menstruating, and were not pregnant.
Table 1.
Descriptive characteristics and multivariable logistic regression model assessing risk factors associated with high-risk human papillomavirus (HR-HPV) infection in women attending a Nepal Fertility Care Center health camp in the Far-West region of Nepal
| Variablesa | Total number of women who have undergone a HR-HPV test |
HR-HPV negative |
HR-HPV positive |
p-valueb | Adjusted OR (95%CI)c | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Age (n=262) | 0.02 | |||||||
| ≤34 years | 137 | 52.3 | 131 | 54.1 | 6 | 30.0 | 0.22 (0.07 to 0.71) | |
| >34 years | 125 | 47.7 | 111 | 45.9 | 14 | 70.0 | Ref | |
| Number of children (n=257) | NS | |||||||
| 0 | 9 | 3.5 | 8 | 3. 4 | 1 | 5.0 | ||
| 1–2 | 85 | 33.1 | 81 | 34.2 | 4 | 20.0 | ||
| 3–4 | 56 | 21.8 | 51 | 21.5 | 5 | 25.0 | ||
| 4+ | 107 | 41.6 | 97 | 40.9 | 10 | 50.0 | ||
| Cervical cytology (n=251) | 0.001 | |||||||
| Normal (WNL/BCC/ACTINO/ASCUS) | 232 | 81.7 | 224 | 96.9 | 8 | 40.0 | ||
| Abnormal (HSIL/SCC/ASC-H/AGUS/LSIL) | 19 | 12.8 | 7 | 3.1 | 12 | 60.0 | ||
| Self-reported health (n=263) | NS | |||||||
| Excellent | 69 | 26.2 | 64 | 26.3 | 5 | 25.0 | ||
| Very good | 83 | 31.6 | 74 | 30.5 | 9 | 45.0 | ||
| Good | 86 | 32.7 | 81 | 33.3 | 5 | 25.0 | ||
| Fair/poor | 25 | 9.5 | 24 | 9.8 | 1 | 5.0 | ||
| Husband's migration status (n=256) | NS | |||||||
| Migrated within Nepal | 20 | 7.8 | 19 | 8.0 | 1 | 5.3 | 1.05 (0.11 to 10.09) | |
| Migrated outside of Nepal | 110 | 42.9 | 100 | 42.2 | 10 | 52.6 | 1.81 (0.64 to 5.07) | |
| Husband never migrated | 126 | 49.2 | 118 | 49.8 | 8 | 42.1 | Ref | |
| Husband returned from working away from home in the past 3 months (n=126) | NS | |||||||
| Yes | 98 | 77.8 | 88 | 75.9 | 10 | 100.0 | ||
| No | 28 | 22.2 | 28 | 24.1 | 0 | 0 | ||
| Awareness of cervical cancer (n=253) | NS | |||||||
| Yes | 20 | 7.9 | 131 | 56.2 | 11 | 55.0 | 1.15 (0.41 to 3.19) | |
| No | 233 | 92.1 | 102 | 43.8 | 9 | 45.0 | Ref | |
| Type of current contraception use (n=255)d,e | NS | |||||||
| Hormonal | 69 | 27.1 | 62 | 26.4 | 7 | 35.0 | 2.14 (0.65 to 7.12) | |
| Condom use | 66 | 25.9 | 62 | 26.4 | 4 | 20.0 | 1.07 (0.30 to 3.80) | |
| Other | 15 | 5.9 | 13 | 5.5 | 2 | 10.0 | ||
| Does not use contraception | 105 | 41.2 | 98 | 41.7 | 7 | 35.0 | Ref | |
| Awareness of STIs (n=255) | NS | |||||||
| Yes | 144 | 56.5 | 134 | 56.8 | 10 | 52.6 | ||
| No | 111 | 43.5 | 102 | 43.2 | 9 | 47.4 | ||
ACTINO: actinomycosis; AGUS: atypical glandular cells of undetermined significance; ASC-H: atypical squamous cell-cannot exclude HSIL; ASCUS: atypical squamous cells of undetermined significance; BCC: benign cellular changes; HR-HPV: high-risk human papillomavirus; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; Ref: reference group; SCC; squamous cell carcinoma; WNL: within normal limits.
a The value for each variable may vary due to missing survey data.
b NS = not significant at alpha equal to 0.05. P-value >0.05.
c Models adjusted for: age, awareness of cervical cancer, type of current contraception use, husband's migration status. Multivariable logistic regression models were used to generate odds ratios and 95% confidence intervals (CI) describing factors associated with HR-HPV infection based on 1. a review of HPV risk factor literature from developing countries; 2. a p-value of less than 0.1 in the univariate analyses; or 3. if they were of primary interest for the study. Final models included the following covariates: age, cervical cancer awareness, type of current contraception used, a husband's migratory status.
d Hormonal contraception was defined as the use of birth control pills, hormonal implants, and Depo-Provera injections.
e The category ‘Other’ was collapsed with ‘Condom use’ in the multivariate analysis.
Table 2.
Descriptive characteristics and multivariable logistic regression model of cervical cytology in women attending a Nepal Fertility Care Center health camp in the Far-West region of Nepal
| Variablesa | Total number of women with a liquid-based cytology result available |
Normal (WNL/BCC/ACTINO/ ASCUS) |
Abnormal (HSIL/SCC/ASC-H/AGUS/LSIL) |
p-valueb | Adjusted OR (95% CI)c | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Age (n=248) | NS | |||||||
| ≤34 years | 132 | 53.2 | 125 | 54.6 | 7 | 36.8 | 0.31 (0.11 to 0.93) | |
| >34 years | 116 | 46.8 | 104 | 45.4 | 12 | 63.2 | Ref | |
| Number of children (n=244) | NS | |||||||
| 0 | 8 | 3.3 | 7 | 3.0 | 1 | 7.7 | ||
| 1–2 | 81 | 33.2 | 78 | 33.8 | 3 | 23.1 | ||
| 3–4 | 51 | 20.9 | 48 | 20.8 | 3 | 23.1 | ||
| 4+ | 104 | 42.6 | 98 | 42.4 | 6 | 46.2 | ||
| HR-HPV statusd (n=251) | 0.001 | |||||||
| Positive | 20 | 7.9 | 8 | 3.4 | 12 | 63.2 | ||
| Negative | 231 | 92.0 | 224 | 96.6 | 7 | 36.8 | ||
| Self-reported health (n=250) | NS | |||||||
| Excellent | 64 | 25.6 | 57 | 24.7 | 7 | 36.8 | ||
| Very good | 82 | 32.8 | 75 | 32.5 | 7 | 36.8 | ||
| Good | 83 | 33.2 | 80 | 34.6 | 3 | 15.8 | ||
| Fair/poor | 21 | 8.4 | 19 | 8.2 | 2 | 10.5 | ||
| Husband's migration status (n=243) | NS | |||||||
| Migrated within Nepal | 19 | 7.8 | 17 | 7.6 | 2 | 10.5 | 1.24 (0.22 to 6.99) | |
| Migrated outside of Nepal | 107 | 44.0 | 102 | 45.5 | 5 | 26.3 | 0.49 (0.16 to 1.53) | |
| Husband never migrated | 117 | 48.2 | 105 | 46.9 | 12 | 63.2 | Ref | |
| Husband returned from working away from home in the past 3 months (n=122) | NS | |||||||
| Yes | 94 | 77.1 | 88 | 75.9 | 6 | 100.0 | ||
| No | 28 | 22.9 | 28 | 24.1 | 0 | 0 | ||
| Awareness of cervical cancer (n=243) | NS | |||||||
| Yes | 106 | 43.6 | 96 | 42.9 | 10 | 52.6 | 1.86 (0.67 to 5.24) | |
| No | 137 | 56.4 | 128 | 57.1 | 9 | 47.4 | Ref | |
| Type of current contraception use (n=242)e,f | NS | |||||||
| Hormonal | 66 | 27.3 | 57 | 25.6 | 9 | 47.4 | 3.55 (0.98 to 12.85) | |
| Condom Use | 14 | 5.8 | 13 | 5.8 | 1 | 5.3 | 1.47 (0.36 to 5.92) | |
| Other | 63 | 26.0 | 59 | 26.5 | 4 | 21.1 | ||
| Does not use contraception | 99 | 40.9 | 94 | 42.2 | 5 | 26.3 | Ref | |
| Awareness of STIs (n=251) | NS | |||||||
| Yes | 137 | 54.6 | 124 | 53.4 | 13 | 68.4 | ||
| No | 114 | 45.4 | 108 | 46.6 | 6 | 31.6 | ||
ACTINO: actinomycosis; AGUS: atypical glandular cells of undetermined significance; ASC-H: atypical squamous cell-cannot exclude HSIL; ASCUS: atypical squamous cells of undetermined significance; BCC: benign cellular changes; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; Ref: reference group; SCC: squamous cell carcinoma; WNL: within normal limits.
a The value for each variable may vary due to missing survey data.
b NS = not significant at alpha equal to 0.05. P-value >0.05.
c Models adjusted for: age, awareness of cervical cancer, type of current contraception use, husband's migration status. Multivariable logistic regression models were used to generate odds ratios and 95% confidence intervals (CI) describing factors associated with HR-HPV infection based on 1. a review of HPV risk factor literature from developing countries; 2. a p-value of less than 0.1 in the univariate analyses; or 3. if they were of primary interest for the study. Final models included the following covariates: age, cervical cancer awareness, type of current contraception used, a husband's migratory status.
d HR-HPV status determined using APTIMA HR-HPV mRNA Assay (Hologic/Gen-Probe, Inc. San Diego, CA, USA).
e Hormonal contraception was defined as the use of birth control pills, hormonal implants, and Depo-Provera injections.
f The category ‘Other’ was collapsed with ‘Condom use’ in the multivariate analysis.
Women whose husbands had migrated within Nepal were significantly younger than both women whose husbands migrated outside of Nepal for employment and women whose husbands had never migrated for work (average age [SD]: ‘migrated within Nepal’, 28.8 [7.25], ‘migrated outside Nepal’, 33.7 [9.3], ‘never migrated’, 34.9 [8.5], p≤0.01). Unadjusted odds ratios assessing the association between age and the migration status of a woman's husband indicated that women whose husbands migrated within Nepal for work were over three times more likely to be aged 34 years or younger than women whose husbands never migrated for work (unadjusted OR [95% CI]: 3.68 [1.15 to 11.78]).
High Risk HPV (HR-HPV)
HR-HPV infected women were more likely to have an abnormal cervical cytology than women who were HR-HPV negative (HR-HPV infected 60.0% [12/20] vs uninfected 3.1% [7/224]) (Table 1).
Multivariable logistic regression models using HR-HPV infection as the outcome determined that women aged ≤34 years were significantly less likely to be infected with HR-HPV than women who were aged >34 years, after adjustment for current type of contraception used, awareness of cervical cancer, and husband's migration status (OR 0.22, 95% CI 0.07 to 0.71, p=0.02) (Table 1). A husband's migratory status was not directly associated with HR-HPV infection (‘migrated within Nepal’ OR 1.05, 95% CI 0.11 to 10.09; ‘migrated outside of Nepal’ OR 1.81, 95% CI 0.64 to 5.07; ‘never migrated’ was the reference group).
Cervical cytology
Sixty percent of women infected with HR-HPV were categorized as having an abnormal cervical cytology (12 women with either AGUS, LSIL, ASC-H, HSIL or SCC / 20 women with HR-HPV), while 87.5% of women with squamous cell carcinoma or high-grade SIL also tested positive for HR-HPV (7 women with HR-HPV and HSIL or SCC / 8 women with HSIL or SCC) (Table 3). Multivariable logistic regression models using abnormal cervical cytology status as the outcome with normal cervical cytology as the reference category determined that women aged ≤34 years were less likely to have an abnormal cervical cytology than women aged >34 years (OR 0.31, 95% CI 0.11 to 0.93, p≤0.01), after adjusting for current type of contraception used, awareness of cervical cancer, and husband's migration status (Table 2). Abnormal cervical cytology was not directly associated with a husband's migratory status (‘migrated within Nepal’ OR 1.24, 95% CI 0.22 to 6.99; ‘migrated outside of Nepal’ OR 0.49, 95% CI 0.16 to 1.53; ‘never migrated’ was the reference group).
Table 3.
High-risk human papillomavirus (HR-HPV) test results and husband's migration status stratified by liquid-based cytology
| Liquid-based cytologya results of clinician-collected samples |
||||||||
|---|---|---|---|---|---|---|---|---|
| SCC | HSIL | ASC-H | LSIL | AGUS | ASCUS | WNL/BCC/ACTINO | Total | |
| Total cytology results | 1 | 7 | 6 | 4 | 1 | 27 | 205 | 251 |
| HR-HPVb | ||||||||
| Positive | 1 | 6 | 2 | 3 | 0 | 2 | 6 | 20 |
| Negative | 0 | 1 | 4 | 1 | 1 | 25 | 199 | 231 |
| Husband's migration statusc | ||||||||
| Migrated within Nepal | 0 | 0 | 1 | 1 | 0 | 2 | 15 | 19 |
| Migrated outside of Nepal | 1 | 2 | 1 | 1 | 0 | 8 | 94 | 107 |
| Never migrated for work | 0 | 5 | 4 | 2 | 1 | 16 | 89 | 117 |
ACTINO: actinomycosis; AGUS: atypical glandular cells of undetermined significance; ASC-H: atypical squamous cell-cannot exclude HSIL; ASCUS: atypical squamous cells of undetermined significance; BCC: benign cellular changes; HR-HPV: high-risk human papillomavirus; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; SCC: squamous cell carcinoma; WNL: within normal limits.
a Cytology analysed using ThinPrep PreservCyt medium (Hologic/Gen-Probe, Inc. San Diego, CA, USA).
b HR-HPV status determined using APTIMA HR-HPV mRNA Assay (Hologic/Gen-Probe, Inc.).
c The value for the variable ‘Husband's migration status’ is missing survey data for eight women.
Discussion
Younger age was found to be protective against HR-HPV infection and against having an abnormal cervical cytology. Additionally, women aged 34 years and younger were found to be significantly more likely than older women to have a husband who migrated. It is possible that married Nepali women are less likely to contract HPV when they are younger because their husbands are working away from home for extended periods of time during their younger years. Our study's inverse associations between younger age and HR-HPV infection contrasts with most reports that suggest younger women are more likely to test positive for HR-HPV than older women.25,26 Young women between the ages of 25 and 34 years had the highest prevalence of HPV of any age group in a study of 2501 women living in Kolkata, India.25 Women in younger age groups of the ATHENA study, a multi-center clinical trial of over 46 000 women, had higher percentages of HPV infection than women in older age groups.26 It is possible that the results from our study could indicate that age related cofactors, such as having a husband who has previously migrated for employment, could potentially delay HPV transmission among women.
HPV studies conducted in Indian provinces bordering Nepal have study populations with characteristics similar to our study population and show a similar prevalence of HPV infection to our study.27,28 Although it is almost impossible to know the exact numbers of Nepalese who migrate to India for work, due to the open border polices between the two countries, the Indian provinces bordering Nepal are known to have high rates of employed Nepali migrant workers.29,30 Since the characteristics of our study sample are similar to the characteristics of HPV studies conducted in North India and the most popular destination for migration in our study sample was India, it is possible that women from our study whose husbands have traveled to India for employment have similar cervical cytology and HPV infection rates as women from areas in India with a high prevalence of Nepali workers.
It is possible the results of our study are due to cohort effects which were unable to be measured using our data. Political strife originating from Nepal's 10-year-long civil war caused an increase in the number of labor migrants between 1996 and 2007.31 With migratory work proving to be profitable for many Nepali citizens, the increase in migration which began during the civil war period has continued to this day. It is possible that older women who were married before the civil war period had husbands who were more financially secure during the war-period and therefore did not have to migrate for work. In contrast, it is possible younger women who were married either during or after the conflict might be more likely to have husbands who migrated for work. This phenomenon could help explain why women who were aged ≤34 years were less likely to test positive for HR-HPV than older individuals.
Our study could potentially suffer a self-selection bias. While we are interpreting the results of our study as suggesting that it is husbands who are transmitting HPV to the women, this may not always be the case. It is possible that women are forming sexual relationships with other men while their husbands are away and are choosing not to participate in our study because of social stigma. This could explain why younger women, who are normally at a greater risk for testing positive for HR-HPV than older women, were less likely to test positive for HR-HPV in our study sample.
Our study was a cross-sectional survey conducted in one area of Achham district and it is possible our study did not adequately represent the factors associated with HR-HPV infection in all of Far-West Nepal. However, our study was conducted in Sanfebagar Village Development Committee, which is 25 km from the district capital of Mangalsen. The proximity of our health camp to the district capital helped ensure that a diverse population of women had access to the reproductive services we provided. Ninety eight women registered for reproductive health services and completed a survey but did not participate in screening, this could be for a variety of possible reasons. Women continuously came to the health camp throughout the day and women who registered towards the end of the camp might not have received screening services before the camp closed for the day. It is also possible that the wait time to be screened was too long and women left before completing the study. While several women completed a survey but did not provide a biological sample we are confident that the women who were not screened left the study at random and did not inject bias into the study. The small sample size of our study reduces the generalizability of our findings. While our study sample consisted of 265 women, only 20 women tested positive for HR-HPV and only 19 women had an abnormal cytology result. The small number of women with outcomes in our study restricted the number of covariates we could include in our multivariate models and could suggest that our study might be underpowered. However, while our sample size is small, we were able to include several covariates in our models that are considered to be HR-HPV risk factors in other studies. It is possible some questions from our survey were misrepresented when translated into the local dialect spoken in Achham. Given that the Nepali dialect spoken in the district of Achham does not have a written form, we had to rely on our interviewers to translate our survey into the local Achham dialect when needed. Despite this, our survey should not have suffered from difficulties in communication because the majority of people living in Achham are able to speak and understand Nepali and our interviewers were able to speak the local Achham dialect.
Conclusions
Contrary to similar reports on HPV infection, there seems to be an increasing prevalence of HR-HPV with age in our study population. Our results could have potentially important implications for reproductive health in Nepal. If older women are more likely to test positive for HR-HPV than younger women, cervical screening resources could be saved by shifting more attention to this population. Additionally, given that having a husband who has migrated for work is not associated with HR-HPV or cervical abnormalities, reproductive health resources can be saved by shifting cervical screening interventions away from this population. While our study has potential implications for reproductive health in Nepal, caution must be taken when assessing our results due to our study's small sample size. Future studies in a larger population are warranted to validate our findings and assess whether our observations are generalizable to spouses of migrant workers from other parts of Nepal.
Acknowledgments
Authors' contributions: DK, MB, MCK and EC conceived and designed the study; DJ, PL, PB, JS and SS contributed to acquiring the data; DJ, MB, MCK and SV carried out the analysis and interpretation of the data; SA and KR assisted with understanding Nepal's screening policy. DJ, PL, PB, SA, KR and SS drafted the manuscript; SV contributed to drafting the discussion; JC and EC helped edit the manuscript. DK is the guarantor of the manuscript. All authors read and approved the final manuscript.
Acknowledgements: We thank the study participants and the staff at Nepal Fertility Care Center (NFCC-International) with the data collection and assisting with the health camp in Achham. Hologic/GenProbe Inc. (San Diego) donated reagents and kits and provided testing of HPV, STI and liquid cytology.
Funding: The study was supported in part by the pilot fund from the Department of Epidemiology, UAB School of Public Health (EC and SS). Derek Johnson was supported by a National Institutes of Health Cancer Prevention and Control Training Grant [R25CA47888]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: None declared.
Ethical approval: The Institutional Review Board at the University of Alabama at Birmingham and Ethics Review Board at the Nepal Health Research Council approved this study.
References
- 1.Bruni L, Barrionuevo-Rosas L, Serrano B et al. Human papillomavirus and related diseases in Nepal ICO information centre on HPV and cancer (HPV Information Centre); 2014. http://www.hpvcentre.net/statistics/reports/XWX.pdf [accessed 22 February 2016].
- 2.Fort VK, Makin MS, Siegler AJ et al. Barriers to cervical cancer screening in Mulanje, Malawi: a qualitative study. Patient Prefer Adherence 2011;5:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyenwali D, Pariyar J, Onta SR. Factors associated with late diagnosis of cervical cancer in Nepal. Asian Pac J Cancer Prev 2013;14:4373–7. [DOI] [PubMed] [Google Scholar]
- 4.Waggaman C, Julian P, Niccolai LM. Interactive effects of individual and neighborhood race and ethnicity on rates of high-grade cervical lesions. Cancer epidemiology 2014;38:248–52. [DOI] [PubMed] [Google Scholar]
- 5.King CJ, Chen J, Garza MA, Thomas SB. Breast and cervical screening by race/ethnicity: comparative analyses before and during the Great Recession. Am J Prev Med 2014;46:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tornesello ML, Giorgi Rossi P, Buonaguro L et al. Human papillomavirus infection and cervical neoplasia among migrant women living in Italy. Front Oncol 2014;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogunsiji O, Wilkes L, Peters K, Jackson D. Knowledge, attitudes and usage of cancer screening among West African migrant women. J Clin Nurs 2013;22:1026–33. [DOI] [PubMed] [Google Scholar]
- 8.Piotrowski M. Mass media and rural out-migration in the context of social change: evidence from Nepal. Int Migr 2013;51:169–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkin DM, Khlat M. Studies of cancer in migrants: rationale and methodology. Eur J Cancer 1996;32A:761–71. [DOI] [PubMed] [Google Scholar]
- 10.Poudel KC, Okumura J, Sherchand JB et al. Mumbai disease in far western Nepal: HIV infection and syphilis among male migrant-returnees and non-migrants. Trop Med Int Health 2003;8:933–9. [DOI] [PubMed] [Google Scholar]
- 11.Poudel KC, Jimba M, Okumura J et al. Migrants' risky sexual behaviours in India and at home in far western Nepal. Trop Med Int Health 2004;9:897–903. [DOI] [PubMed] [Google Scholar]
- 12.Bam K, Thapa R, Newman MS et al. Sexual behavior and condom use among seasonal Dalit migrant laborers to India from Far West, Nepal: a qualitative study. PLoS One 2013;8:e74903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DC, Bhatta MP, Smith JS et al. Assessment of high-risk human papillomavirus infections using clinician- and self-collected cervical sampling methods in rural women from far Western Nepal. PLoS One 2014;9:e101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nepal Ministry of Health and Population. Nepal demographic and health survey 2011. Kathmandu, Nepal; New ERA and ICF International; 2012. http://dhsprogram.com/pubs/pdf/FR257/FR257%5B13April2012%5D.pdf [accessed 22 February 2016]. [Google Scholar]
- 15.Nepal Fertility Care Center. http://www.nfcc.org.np/home.html [accessed 22 November 2015].
- 16.APTIMA HPV Assay. San Diego, CA, USA; Hologic, Inc.; 2016. http://www.hologic.com/products/clinical-diagnostics-blood-screening/assays-and-tests/aptima-hpv-assay [accessed 22 February 2016]. [Google Scholar]
- 17.Apgar BS, Zoschnick L, Wright TC Jr. The 2001 Bethesda System terminology. Am Fam Physician 2003;68:1992–8. [PubMed] [Google Scholar]
- 18.Poertner E, Junginger M, Muller-Boker U. Migration in far west Nepal: intergenerational linkages between internal and international migration of rural-to-urban migrants. Crit Asian Stud 2011;43:23–47. [DOI] [PubMed] [Google Scholar]
- 19.Nepal National Planning Commission Secretariat, Central Bureau of Statistics. National Population and Housing Census 2011. Kathmand, Nepal; 2012. http://unstats.un.org/unsd/demographic/sources/census/2010_phc/Nepal/Nepal-Census-2011-Vol1.pdf [accessed 22 November 2015]. [Google Scholar]
- 20.Huchko MJ, Leslie H, Sneden J et al. Risk factors for cervical precancer detection among previously unscreened HIV-infected women in Western Kenya. Int J Cancer 2014;134:740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samir R, Asplund A, Tot T et al. Oral contraceptive and progestin-only use correlates to tissue tumor marker expression in women with cervical intraepithelial neoplasia. Contraception 2012;85:288–93. [DOI] [PubMed] [Google Scholar]
- 22.Tiwari A, Kishore J, Tiwari A. Perceptions and concerns of women undergoing Pap smear examination in a tertiary care hospital of India. Indian J Cancer 2011;48:477–82. [DOI] [PubMed] [Google Scholar]
- 23.McFarlane-Anderson N, Bazuaye PE, Jackson MD et al. Cervical dysplasia and cancer and the use of hormonal contraceptives in Jamaican women. BMC Womens Health 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chichareon SB, Tocharoenvanich S. Risk factors of having high-grade cervical intraepithelial neoplasia/invasive carcinoma in women with atypical glandular cells of undetermined significance smears. Int J Gynecol Cancer 2006;16:568–74. [DOI] [PubMed] [Google Scholar]
- 25.Dutta S, Begum R, Mazumder Indra D et al. Prevalence of human papillomavirus in women without cervical cancer: a population-based study in Eastern India. Int J Gynecol Pathol 2012;31:178–83. [DOI] [PubMed] [Google Scholar]
- 26.Stoler MH, Wright TC Jr., Sharma A et al. High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol 2011;135:468–75. [DOI] [PubMed] [Google Scholar]
- 27.Pandey S, Mishra M, Chandrawati. Human papillomavirus screening in north Indian women. Asian Pac J Cancer Prev 2012;13:2643–6. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava S, Gupta S, Roy JK. High prevalence of oncogenic HPV-16 in cervical smears of asymptomatic women of eastern Uttar Pradesh, India: a population-based study. J Biosci 2012;37:63–72. [DOI] [PubMed] [Google Scholar]
- 29.Nepal B. Population mobility and spread of HIV across the Indo-Nepal border. J Health Popul Nutr 2007;25:267–77. [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford G, Franceschi S, Diaz M et al. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006;24(Suppl 3):S26–S34. [DOI] [PubMed] [Google Scholar]
- 31.Sharma K. The political economy of civil war in Nepal. World Dev 2006;34:1237–53. [Google Scholar]