Abstract
CKD is highly prevalent in the U.S. and throughout the world,1 with approximately 13% of adults affected.2 In addition, according to recent estimates, almost half of patients with CKD stage 3-5 are 70 years of age or older.2 In the United States, the number of prevalent ESRD cases continues to rise in patients aged over 65. In light of the demographic characteristics of patients with CKD and ESRD, there has been considerable focus on associations between CKD and cardiovascular outcomes.3 Until recently, less attention had been paid to other consequences of CKD in general and among older individuals with CKD in particular, but there is now solid evidence linking CKD with impairments of physical function, cognitive function, and emotional function and quality of life. This review will summarize available literature on these topics, focusing specifically on physical functioning and frailty; cognitive function; emotional health, including depression and anxiety; and health-related quality of life.
Keywords: Chronic kidney disease, physical function, frailty, cognitive function, depression, health-related quality of life
Physical Functioning and Frailty in CKD
It has been clear for some time that patients with ESRD4,5 and CKD not requiring dialysis6,7 have impaired maximal oxygen consumption during maximal exercise testing, and more recent studies have also examined other tests of physical performance. One of the larger studies of patients with CKD found that Vo2peak was 54 ± 20 % of sedentary age-predicted values among 32 patients with eGFR 30 ± 17 ml/min/1.73m2. These individuals also performed poorly on several tests of performance of day-to-day activities, with maximal gait speed over a short distance that was approximately 85% of population norms and sit-to-stand performance 79% of expected.8 A Swedish study of physical fitness in CKD patients found that the mean peak values of handgrip strength were 78.0 ± 24.8% of the age- and sex- matched reference values for men and 84.0 ± 22.2% for women. The proportion of patients who failed to rise from a chair without using their arms was higher among individuals with lower eGFR, and no patients with eGFR <12 ml/min/1.73m2 were able to perform the maneuver.9
Data from community cohorts have been analyzed to address individuals with earlier stages of CKD. In the prospective University of Alabama at Birmingham Study of Aging, community-dwelling older adults with CKD, defined as eGFR <60 ml/min/1.73m2 by the MDRD formula, had higher adjusted odds of decline in both basic (OR 2.46; 95% CI 1.19 to 5.12) and instrumental activities of daily living (ADL) (OR 1.83; 95% CI 1.06 to 3.17) compared to those without CKD.10 In data from the Health, Aging, and Body Composition Study, eGFR less than 60 ml/min/1.73m2 was independently associated with the development of difficulty in walking one-quarter of a mile or climbing 10 steps.11 In a follow-up to that study, participants in the highest quartile of cystatin C concentration (≥1.13 mg/L) experienced a significantly higher risk of developing functional limitation (HR 1.70; 95% CI 1.40 to 2.07) than those in the lowest quartile (<0.86 mg/L).11
It was not surprising that investigators in the Heart and Soul Study12 found that exercise capacity, defined as metabolic equivalents (METs) achieved at peak exercise, was reduced in patients with eGFR <60 ml/min/1.73m2 (OR 6.7; 95%CI 3.8 to 11.8 for low exercise capacity, defined as <5 METs) compared to those with eGFR >90 ml/min/1.73m2. However, more novel was their finding that participants with eGFR of 60-90 ml/min/1.73m2 were also more likely to have low exercise capacity (OR 2.3; 95% CI 1.4 to 3.8), suggesting that maximal exercise tolerance may become impaired even in the early stages of CKD. Furthermore, the proportion of participants with poor exercise capacity was 45% among those with cystatin C concentration in the highest quartile (>1.3 mg/L) compared with 12% among those in the lowest quartile (<0.92mg/L, adjusted OR 3.2; 95%CI 1.6 to 6.5, p=0.001).13 Leikis and colleagues also demonstrated14 that as renal function declined over 2 years, there was a corresponding decrease in exercise performance even when hemoglobin was maintained.
More recent studies have focused on frailty, which is described in the geriatric literature as a multidimentional construct characterized as a state of reduced functional capacity in the elderly that predicted risk of disability, hospitalization, institutionalization, and death.15 The most widely cited index used to define the frailty phenotype includes unintentional weight loss, muscle weakness (grip strength), self-reported fatigue or exhaustion, low physical activity, and slow walking speed.16 Analysis from the Cardiovascular Health Study, in which the measure was developed, showed that community-dwelling elderly individuals with CKD, defined as a serum creatinine level of ≥1.3 mg/dL in women and ≥1.5 mg/dL in men, were three times as likely to be frail compared to those with normal renal function.17 The frailty phenotype was associated with 2.5-fold (95% CI 1.4 to 4.4) higher risk of death or dialysis therapy when compared with nonfrail CKD participants followed for 2.5 year in the Seattle Kidney Study.18 Using a modification of previously validated frailty criteria, investigators reported that the prevalence of frailty was 20% among participants in the Third National Health and Nutrition Examination Survey (NHANES) with eGFR less than 45 ml/min/1.73m2 compared with 1.5% in those without CKD. The likelihood of frailty was significantly higher among individuals at all stages of CKD. Participants with more severe CKD were more likely to be frail, and both frailty and CKD were independent predictors of mortality.19 After dialysis initiation, two-thirds of the incident dialysis patients participating in the United States Renal Data Systems (USRDS) Dialysis Morbidity and Mortality Study were frail.20 Older patients, women, and patients with diabetes mellitus were more likely to be frail, and frailty was associated with 2-3 fold higher risk of death.
A few studies have examined functional outcomes after the start of dialysis among the elderly. Using a national registry of nursing home residents, Kurella Tamura and colleagues21 examined the effect of dialysis initiation on functional status, measured by the degree of dependence in activities of daily living (ADL) on the Minimum Data Set-Activities of Daily Living (MDS-ADL). By 12 months after initiation of dialysis, 58% had died, and predialysis functional status had been maintained in only 13%. Initiation of dialysis was associated with a sharp decline in functional status indicated by an increase of 2.8 points (95% CI 2.5 to 3.0) in the MDS-ADL score independent of age, sex, race, and functional status before the initiation of dialysis. Jassal et al.22 also demonstrated a loss of independent function 1 year after initiation of long-term dialysis in ESRD patients aged over 80 years with multiple coexisting conditions (mean modified Charlson score of 5.1±2.5). At the start of dialysis, 78% of the cohort was independent (residing in the home without assistance), but after 1 year, this percentage had decreased to 23%. Moreover, within the first 6 months, more than 30% of patients had functional loss requiring community or private-caregiver support, or transfer to a nursing home.
Although the extent to which initiation of dialysis directly affects frailty status remains unclear from the aforementioned studies, it does not appear that frailty and disability improve after the start of dialysis. Rather, available evidence suggests that physical functioning begins to decline in the early stages of CKD and continues unabated if not specifically addressed. Exercise interventions can improve physical functioning,23, 24 and rehabilitation programs can improve disability among patients with ESRD,25 but these programs have not been widely implemented in the U.S.
Cognitive dysfunction
Patients with CKD and ESRD have a higher prevalence of cognitive dysfunction than the general population even when accounting for age, diabetes, cardiovascular risk, and other comorbidities.26,27 Furthermore, several studies have established that dementia and mild cognitive impairment are associated with higher mortality among patients with ESRD. Kurella et al. reported a RR of mortality of 1.48 (95% CI 1.32 to 1.66) among HD patients with dementia compared to those without dementia.28 A separate prospective cohort study in patients treated with dialysis without a history of dementia or prior stroke also showed an adjusted HR for all-cause mortality of 2.53 (95% CI 1.03 to 6.22) at 7 years comparing mild to moderate cognitive impairment with no deficits.29
Although cognitive testing is not routinely performed among patients with CKD and ESRD, the prevalence of cognitive impairment is estimated to be 30-70% in this population.30,31 In a cross-sectional study of 338 patients treated with hemodialysis aged 55 years or older conducted by Murray et al., only 2.9% had a prior history of cognitive impairment. However, when tested for memory, executive function, and language performance, 13.1% had mild impairment, 36.1% moderate impairment, and 37.3% severe impairment; only 12.7% had normal function.32
Although the prevalence of dementia in hemodialysis patients has been known to be high for years,33 the prevalence and importance of mild cognitive impairment (MCI) has been less appreciated. Studies in the CKD population have varied according to which neuropsychological tests they used, with the Mini Mental State Examination (MMSE),34 the Modified Mini-Mental State (3MS), the Trailmaking Test B (or Trails B), and the Montreal Cognitive Assessment (MoCA) being most common. The 3MS measures global cognitive function and is more sensitive for MCI than the MMSE.35 The Trails B measures executive function, visuospatial ability, and concentration.36 Apart from type of testing, studies also vary with regard to adjustment for depression, vascular risk factors, and prior CVA,37 as well as to the timing of cognitive testing during, before, or after dialysis.38-40 Timing of cognitive testing relative to dialysis has been shown to affect scores, with better results either on the day after dialysis or immediately before dialysis and lower test scores during and within one hour after dialysis sessions.41 In a study of 51 male participants, 24 with CKD and 27 receiving HD, although all 51 patients scored >28 on the MMSE (implying normal cognition), 76% were diagnosed with MCI based on more comprehensive neuropsychological testing.42 In a hemodialysis-specific study, the optimal MoCA cut-off score, determined using receiver operating characteristics (ROC) analysis, was ≤24, which had 77% sensitivity and 79% specificity for mild cognitive impairment, whereas an MMSE score of ≤28, used in the general population, had 55% sensitivity and 75% specificity.43
Association of GFR and albuminuria with cognitive dysfunction
The majority of studies point to a strong association between eGFR and cognitive dysfunction.44 Cognitive impairment is more prevalent and more severe among individuals with lower eGFR, and the burden and severity are higher in ESRD as compared to earlier stages of CKD.31,45 A cross-sectional study of 923 patients with CKD without prior dementia or ESRD showed that eGFR of <60 ml/min/1.73 m2 (by MDRD equation) was related to lower cognitive performance even after adjusting for cardiovascular disease risk factors.46 In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study, which involved 23,405 participants with CKD, each 10 ml/min/1.73 m2 decrement in GFR was associated with an 11% higher risk of cognitive impairment (OR 1.11, 95% CI 1.0-1.2).47
In the Chronic Renal Insufficiency Cohort Cognitive Study (CRIC COG), comprising 825 adults aged 55 years and older enrolled in the CRIC, those with eGFR ≥60 ml/min/1.73 m2 had significantly lower odds of having a cognitive testing score 1 SD or more below the mean on a battery of tests as compared to those with eGFR from 45-59 ml/min/1.73 m2. Those with eGFR 30-44 ml/min/1.73m2 performed worse, and those with eGFR <30 ml/min/1.73 m2 had the highest odds of poor performance. After adjustment for age, sex, race, education, DM, HTN, BMI, and depression, the graded association between eGFR and cognitive function was preserved in all tested domains except verbal fluency.26
Individuals with lower eGFR are also at higher risk of decline in cognitive function over time, and cognitive impairment can also become apparent relatively early in the course of CKD if patients undergo cognitive testing. In the Northern Manhattan Study,48 a population-based, prospective cohort study of patients without prior stroke, those with a Cockroft-Gault estimated creatinine clearance (eCrCl) of <60 ml/min performed worse than those with eCrCl from 60-90 ml/min on the Telephone Interview for Cognitive Status Score (TICS-m). Patients in both categories of CKD performed worse than those with a eCrCl of >90 ml/min. Furthermore, each 0.1 mg/dL higher serum creatinine concentration corresponded to an average decline of 0.04 in the TICS-m score per year over a mean follow-up of 2.9 years. By way of comparison, each year of age was associated with a decline of 0.023 per year over the same follow-up, such that patients with eCrCl of 60-90 ml/min declined approximately three times faster than those without CKD.48
In the Intervention Project on Cerebrovascular Diseases and Dementia in the Community of Ebersberg (INVADE), there was no difference in baseline cognitive impairment according to eGFR category (>60, 45-60, and < 45 ml/min/1.73 m2) among 3679 elderly men and women. However, the incidence of new cognitive impairment was 5.8, 9.9, and 21.5% respectively, in these groups, suggesting that worse CKD was associated with more rapid decline in cognitive function.49
Davey et al. conducted a 5-year longitudinal study with 17 separate cognitive tests among community-dwelling individuals (mean age 62.1 years) without any history of dementia, stroke, or ESRD. More rapid decline in eGFR was associated with more rapid decline in cognition, including global cognitive functioning, visual memory, and abstract reasoning domains. A decline in eGFR of ≥6 ml/min/1.73 m2 per year corresponded to approximately the same decline in cognition over 5 years as might be expected with over 11 to 12 years in the absence of a decline in eGFR.50 The Health, Aging, and Body Composition Study showed a similar graded association between eGFR and cognition at baseline as well as risk for decline in cognition over time. Compared to patients with eGFR ≥60 ml/min/1.73m2, the odds ratio for developing cognitive impairment was 1.32 (95% CI 1.03 – 1.69) for those with eGFR 45-59 ml/min/1.73 m2 and 2.43 (95% CI 1.38 – 4.29) for those with eGFR <45 ml/min/1.73 m2.51
The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes (ACCORD-MIND) substudy examined the association of albuminuria (≥30 mg albumin/g Cr in spot urine) and four indices of cognitive function, including executive function among 2,977 patients with HbA1c >7.5%. Participants with albuminuria were more likely to perform poorly on the SDST and on a verbal memory test, with scores equivalent to ~3.6 years of aging.52 Barzilay et al. followed ACCORD-MIND participants and characterized them as having no, persistent, progressive (development of new albuminuria), or remitting albuminuria (resolution of albuminuria).53 Patients with persistent albuminuria had the poorest performance on cognitive tests at baseline, whereas those with no albuminuria performed best.53 Over an average of 3.3 years of follow-up, the largest change in cognitive function testing was in the SDST, and persistent and progressive albuminuria were associated with 7.2 and 3.2 years of cognitive aging per year of actual aging, respectively, independently of change in eGFR.
Association of dialysis modality and dose with cognitive function
The association of dialysis modality with cognitive function is not clear. One study found less cognitive impairment among patients treated with peritoneal dialysis than among those on hemodialysis based on neuropsychological testing.54 Another study using EEG-based parameters related to attention and cognition55 reported that EEG responses were worse among patients on hemodialysis than those on PD before the dialysis session but not after dialysis, suggesting that dialysis may correct metabolic factors affecting cognition.56 A more recent study comparing matched HD and PD patients did not find statistically significant differences in processing speed.57 A larger study that included 145 prevalent dialysis patients, of whom 17% were treated with home hemodialysis patients and 47% with PD, did not find any association between dialysis modality and cognitive function on a battery of neuropsychological tests. Of note, home hemodialysis patients were analyzed in the same group as in-center hemodialysis patients, which may have biased towards a healthier HD group.29 In general, the possibility of selection bias based on modality choice in these observational studies, with more cognitively intact patients selecting PD, cannot be discounted.58
Dose of dialysis has not been directly associated with cognitive function. In cross-sectional data from the Frequent Hemodialysis Network (FHN) Trials,40 there were no independent associations among urea clearance, dietary protein intake, nutritional markers, hemodynamic measures, or other dialysis therapy associated markers and global cognition or executive function. In the interventional parts of the studies, frequent hemodialysis did not improve cognitive dysfunction using the Trails B, the MMSE, and the 3MS, or other tests compared to standard dialysis. However, the studies were not blinded, and the sample sizes relatively small.59 Another study examining dialysis adequacy showed no association between dialysis dose (single pool Kt/V) and cognitive decline, but the range of spKt/V was relatively narrow, from 1.25 to 1.79.39 Interestingly, a longitudinal study of patients receiving renal allografts did show that patients with impaired cognitive function prior to transplant improved after transplantation such that post-transplant test scores were not appreciably different from healthy norms.60 Although we are not including children in this review, we note that the presence of neurocognitive deficits even in children61, 62 also supports a possible effect of CKD or its sequelae on neurologic function.
Etiology of cognitive dysfunction
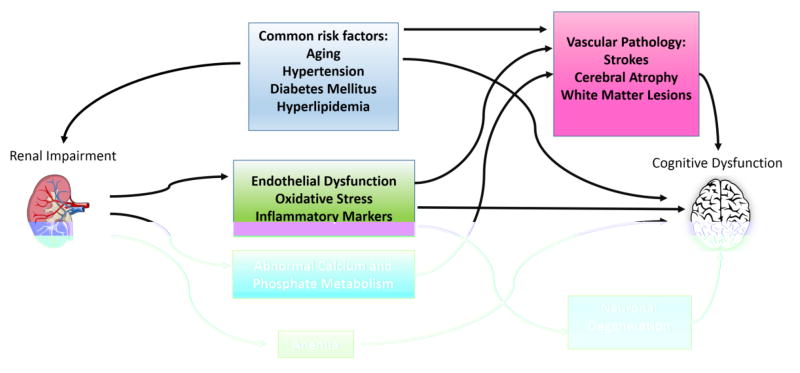
The pathophysiology of dementia and cognitive impairment in CKD has not been fully elucidated, but is likely multifactorial (Figure). In addition to possible toxic/metabolic causes of cognitive dysfunction, cerebrovascular disease (CVD) may contribute. CVD is more common in all stages of CKD relative to the general population, particularly among patients receiving maintenance dialysis.37, 63, 64 The relative risk of stroke among dialysis patients compared to the general population ranges from 4.4 – 9.7 after adjusting for age.65 Although some of the higher risk can be accounted for by shared risk factors, higher prevalence of traditional risk factors such as diabetes, hypertension, cardiovascular disease, and prior stroke, does not fully explain the inordinately high risk of stroke in CKD and ESRD patients. In addition to stroke, cerebral atrophy and other white matter lesions have also been encountered on brain MRI of patients treated with dialysis, and white matter lesions have been linked to cognitive dysfunction, even without a history of stroke.66
Figure.
Potential causes of cognitive dysfunction in CKD.
Patients with ESRD appear to have altered remodeling of vasculature and increased arterial stiffness, which could be related to volume overload and abnormalities in calcium and phosphate metabolism.67 Hemodynamic variability experienced by HD patients may be an exacerbating factor, leading to perfusion mismatches and in turn to higher risk of stroke or other forms of brain injury.68,69
Fazekas et al. performed a study in which magnetic resonance imaging (MRI) and neuropsychological testing were performed on 30 patients on HD and 30 patients matched for age, sex, and presence of HTN, CAD, and DM.70 HD patients had significantly worse cognitive function than controls, as well as higher grades of brain atrophy and a higher prevalence of all types of vascular lesions than the controls. Marked cognitive impairment was associated with atrophy, but not with vascular lesions in the dialysis group.
In a cross-sectional study of 45 maintenance dialysis patients and 67 controls without known kidney disease, Drew and colleagues found that hemodialysis patients had more white matter disease and cerebral atrophy compared with controls, as well as a high prevalence of unrecognized infarcts.66 A study in 335 elders in Boston receiving home health care found that albuminuria was associated with worse executive function when controlling for traditional cardiovascular risk factors and eGFR, and was also associated with higher volume of white matter hyperintensity, suggesting a role for microvascular disease in the cognitive dysfunction and MRI abnormalities of individuals with CKD.71 A study of 600 older individuals with vascular risk factors found that CKD was associated with a higher risk of incident dementia even after adjusting for MR findings, including cerebral atrophy, lacunar infarction, and white matter hyperintensities, implying that there may be damage not visible with traditional MRI techniques.72 In fact, studies using more sensitive MRI techniques have shown that patients with ESRD have increased axonal demyelination compared to healthy, age-matched controls.73 Taken together, these studies demonstrate correlations between anatomic abnormalities and cognitive dysfunction in the dialysis population. Few studies have addressed treatment of cognitive dysfunction in the CKD population, so management of metabolic disturbances and dementia-specific treatments used in the general population are the rule.
Impact of CKD on Emotional Functioning and Quality of Life
Patients with CKD are at risk for developing or worsening preexisting psychological illness such as depression and anxiety. They face stressors that can affect their emotional state, such as adjustment to strict dietary and fluid restriction and concern about initiation of dialysis, as well as fear of burdening caregivers. The heavy burden of symptoms among patients with CKD and ESRD, including pain, fatigue, and impaired well-being, may also contribute.74, 75
Depression
Magnitude of the Problem
Depression is one of the most common psychiatric illnesses in the CKD population. Although the actual prevalence of depressive symptoms among CKD patients is presumably underestimated, it has been reported to be nearly three times higher than the 2-10% commonly reported in the general population76 and also higher than among patients with other chronic diseases such as diabetes mellitus77 and congestive heart failure.78 The reported prevalence clearly depends on the screening techniques used to identify depression. Data from the Chronic Renal Insufficiency Cohort (CRIC) study revealed that the prevalence of depressive symptoms varied according to level of kidney function, with lower eGFR associated with higher odds of depression.79 In contrast, Hedayati and colleagues found that the point prevalence of major depression was approximately 20% and did not vary significantly among different stages of CKD.80 Palmer and co-workers81 conducted a meta-analysis of depressive symptoms determined by clinician-administered questionnaire across all stages of CKD and reported that dialysis patients experienced the highest rate of depressive symptoms (39.3%). The prevalence of depressive symptoms in patients with CKD (a mix of stages 1-5) and kidney transplant recipients were 26.5% and 26.6%, respectively. The prevalence of depression in CKD patients was markedly lower, 20.3%, when assessed by specified diagnostic criteria using structured clinical interview.81
In addition to the negative experience of having depressive symptoms, they are also associated with adverse medical outcomes. CKD patients with depression have worse overall quality of life, significantly faster eGFR decline with rapid progression to ESRD82, as well as higher risk of hospitalization and death.83-86 Longitudinal data from chronic hemodialysis patients observed that worse scores on the Patient Health Questionnaire (PHQ)-9 depression instrument were related to reductions of 1.09, 4.52, and 0.64 points in quality of life evaluated by the physical and mental component summary of Short form 12, and Global Quality of life score, respectively.87 Using data collected from participants of the Symptom Management Involving End-Stage Renal Disease (SMILE) Trial, depressive symptoms were independently associated with both missed and shortened dialysis sessions, with incidence rate ratios [IRR] of 1.21; 95% CI 1.10 to1.33 and 1.08; 95% CI 1.03 to 1.14, respectively, as well as with more emergency department visits (IRR 1.24; 95% CI 1.12 to 1.37).88 Indeed, patients receiving hemodialysis who have a clinical diagnosis of depression have a higher hazard of death or hospitalization (HR 2.07, 95% CI 1.10 to 3.90) compared with those without depression after adjustment for age, gender, race, dialysis vintage, and comorbidities.89 Among patients treated with PD, the median survival was significantly shorter among patients with depressive symptoms compared to non-depressed patients.90
Screening and diagnostic tools
Various screening questionnaires have been validated against Diagnostic and Statistical Manual of Mental Disorders (DSM)-based structured interviews in CKD and dialysis populations (Table 1). Most of these screening tools are not time-consuming and could be easily used in CKD clinics and dialysis facilities. Therefore it has been suggested that screening for depression should be incorporated into routine clinical practice in these settings. The Beck Depression Inventory (BDI) has been extensively studied, and Hedayati and colleagues suggested that the optimal cutoff score for diagnosis of depression was ≥14 (sensitivity 62%, specificity 81%) in patients receiving hemodialysis,91 whereas they suggested a lower BDI cutoff value ≥11 (sensitivity 89%, specificity 88%) in stage 2 to 5 CKD.92 The higher cutoff values for the ESRD population are necessary because of overlapping somatic symptoms of uremia and depression: loss of energy, fatigue, decreased appetite, and difficulty concentrating. Importantly, in order to affirm the diagnosis of depression and grade the disease severity in patients who screen positive using questionnaire-based criteria, particularly among ESRD patients, a physician-administered interview such as the Structured Clinical Interview for DSM criteria (SCID)93 with the Mini International Neuropsychiatric Interview (MINI)92 is suggested before initiating treatment.
Table 1.
Commonly used questionnaires for depression screening in CKD and ESRD.
| Number of items | Cutoff in the general population | Cut-off in CKD | Cut-off in ESRD | |
|---|---|---|---|---|
|
| ||||
| BDI91, 92, 144-146 | 21 | ≥10 | ≥11 | ≥17144 |
| ≥16145, 146 | ||||
| ≥1491, 92 | ||||
|
| ||||
| CES-D91, 147 | 20 | ≥16 | ≥16 | ≥18 |
|
| ||||
| PHQ-9145 | 9 | ≥10 | ≥10 | |
|
| ||||
| QIDS-SR1692 | 16 | ≥10 | ≥10 | |
|
| ||||
| MHI-5148, 149 | 5 | ≤60-76 | ≤70 | |
|
| ||||
| HDRS150 | 21 | ≥8 | ≥8 | |
|
| ||||
| Zung SDS151-153 | 20 | ≥50 or SDS index ≥0.63 | ≥40 or SDS index ≥0.50 | |
Abbreviations: BDI, Beck Depression Inventory; CES-D, Center for Epidemiological Study of Depression; HDRS, Hamilton Depression Rating Scale; MHI-5, 5 items Mental Health Inventory (subscale of the 36-item Short-Form Health Survey Questionnaire); QIDS-SR16, 16 items Quick Inventory of Depressive Symptomatology Self-Report; PHQ-9, 9 items Patient Health Questionnaire; SDS, Self-Rated Depression Scale
Management
A multidisciplinary team approach including psychiatrists, psychologists or social workers should be taken in the treatment of depression. Combined pharmacological and nonpharmacological treatment (psychological counseling) is the mainstay of treatment of depression in patients with CKD and ESRD. A randomized trial of 12 weekly sessions of cognitive behavioral therapy (CBT) in 85 depressed hemodialysis patients demonstrated that the intervention group experienced significant improvement compared to usual treatment in mean BDI scores (24.2±9.7 to 14.1±8.7 vs 27.3±10.7 to 21.2±9.7, p<0.001, respectively).94 Cukor and colleagues conducted a randomized crossover trial in 59 ESRD patients comparing chairside CBT during dialysis treatment versus a wait-list control group that received usual standard of care, including formal psychological or psychopharmacologic treatment by their primary nephrologists and found that CBT intervention significantly reduced the rate of diagnosis of depression by SCID (89.5% of participants were considered non-depressed at the end of treatment compared with 37.5% in the wait-list group, p=0.01) and subjective depression scores (mean BDI score change -11.7±1.5 vs -4.8±1.4, p<0.001, respectively).95 Exercise training programs may also be an option for reducing depressive symptoms in dialysis populations, as long as the intervention is of sufficient duration – typically at least six months96-98. Social support has also been shown to decrease depressive symptoms, specifically increasing optimism and self-esteem among ESRD patients.99
In pharmacologic treatment of depression among patients with advanced CKD and ESRD, consideration is needed regarding the appropriate medication dose, potential adverse effects, and drug-drug interactions. Despite the high prevalence of depression, a majority of patients are left untreated100, perhaps as a result of medication-related concerns of patients or providers. In a cross-sectional analysis of the African American Study of Kidney Disease and Hypertension (AASK) cohort study, only 20% of patients with depressive symptoms diagnosed by BDI scores over 14 were prescribed antidepressants.101
Most antidepressant medications are highly protein bound and are not effectively removed by HD or PD.102 These medications commonly undergo hepatic metabolism, but the active metabolites are excreted via the kidney. Hence the dosing should be cautiously adjusted according to eGFR level, with careful monitoring for potential adverse drug effects (Table 2). Use of tricyclic antidepressants, serotonin modulators, and monoamine oxidase inhibitors should be limited because of cardiac and central nervous system side effects, including prolonged QTc, arrhythmia, anticholinergic effects, and orthostatic hypotension.103 Selective serotonin re-uptake inhibitors (SSRIs) are considered the first-line agents for patients with stage 3-5 CKD if treatment with an antidepressant is indicated.104, 105 There have been several studies of these agents in patients with ESRD103 , but most were of short duration and had small sample sizes. Blumenfield et al.106 studied the efficacy and safety of fluoxetine in hemodialysis patients with major depression by randomly assigning patients to fluoxetine vs placebo for an eight-week period. The treatment group experienced a significant improvement in depression at 4 weeks that was not sustained at 8 weeks. All side effects reported were minor, and no patients discontinued study drugs due to adverse events. Koo and coworkers107 reported that treatment with paroxetine 10 mg/day plus concurrent psychotherapy for 8 weeks in ESRD patients decreased the severity of depressive symptoms assessed by Hamilton Depression Rating Scale (from 16.6±7.0 to 15.1±6.6, p<0.01). Citalopram is frequently prescribed, and there is minimal change in pharmacokinetics of this drug in patients with ESRD.108 Treatment with 50 mg/day of sertraline (another SSRI) for 12 weeks was associated with a decrease in BDI score from 22.4 to 15.7, p<0.001 in patients on PD.109 However, well-designed studies are limited regarding the efficacy and safety profile of antidepressant medications in patients with CKD not requiring dialysis. The Chronic Kidney Disease Antidepressant Sertraline Trial (CAST) study110 is an ongoing double-blinded placebo-controlled trial that aims to recruit 200 participants to investigate whether treatment with sertraline improves severity of depression, overall ability to work and maintain close relationships, and quality of life in patients with stage 3-5 CKD. The results of this trial will generate novel information regarding the efficacy and safety of antidepressant agents in the treatment of depression in patients with advanced CKD.
Table 2.
| Medication class | Dosing in normal GFR | Dosing in CKD and ESRD | Potential class adverse effects | Comments |
|---|---|---|---|---|
| Selective serotonin re- uptake inhibitors | Sertraline 50- 200 mg/day Paroxetine - IR 20-50 mg/day - ER 25-62.5 mg/day Fluoxetine 20-80 mg/day Citalopram 10-60 mg/day Escitalopram 10-20 mg/day | No dose adjustment recommended, but active metabolites excreted via kidney | Increased risk of bleeding, GI side effects (e.g. nausea and vomiting), suicidal ideation | Higher citalopram doses associated with QTc prolongation in severe renal impairment |
| Dopamine/norepinephrine re-uptake inhibitors | Bupropion 200-450 mg/d | Use with caution with maximal dose 150 mg/day | Cardiac dysrhythmia, wide QRS complex, nausea, insomnia | Increased risk for seizures in ESRD. Drug interaction with MAOIs. |
| Noradrenergic and serotonergic agonist | Mirtazapine 7.5-22.5 mg/day | Dosage reduced by 50% in ESRD | somnolence, hallucination, weight gain | Drug interaction with MAOIs |
| Tricyclics and tetracyclics | Amitriptyline 75-150 mg/day Desipramine 100-300 mg/day Doxepin 25-300 mg/day Nortriptyline 25-75 mg/day | No dose adjustment recommended, but | QTc prolongation, orthostatic hypotension, anti- cholinergic effects | Avoid in CKD if possible given cardiac side effects |
| Serotonin/norepinephrine re-uptake inhibitors | Venlafaxine - IR 75-225 mg/day - ER 37.5-225 mg/day | Reduce dose by 25-50% | Hypertension, sexual dysfunction | Not removed by dialysis |
| Serotonin modulators | Nefazodone 100-600 mg/day Trazodone 150-600 mg/day | No dose adjustment needed | Cardiac dysrhythmia, serotonin syndrome | Avoid in cardiovascular and liver disease |
| Monoamine oxidase inhibitors | Phenelzine 45-90 mg/day Selegiline 5-10 mg/day | Generally avoid in CKD | Orthostatic hypotension | Significant drug-drug interactions |
Abbreviations: CAPD, continuous ambulatory peritoneal dialysis; ER extended-release;IR, immediate-release; HD, hemodialysis; MAOIs, monoamine oxidase inhibitors
Other emotional disorders
Anxiety is also commonly encountered among CKD and ESRD patients, but this problem has been substantially less well studied than depression. In fact, anxiety has a negative impact on quality of life and several physical conditions (e.g., migraine headache, allergic conditions, respiratory diseases).111 In a cross-sectional study, Lee et al. reported that the prevalence of anxiety was 24.8%, 29.9%, and 34.3% in CKD stage 3, 4, and 5, respectively, and did not differ significantly across CKD stages.112 (By comparison, the prevalence of anxiety in the general population is estimated to be approximately 18%.113) A systematic review of the prevalence of symptoms of anxiety in maintenance dialysis patients reported that approximately 38% (range 12% to 52%) of patients were affected.114 Although screening for depression is increasing, data on screening for anxiety are scarce. Cukor and colleagues115 found that 71% of hemodialysis patients were diagnosed as having anxiety disorder using the gold standard SCID, whereas only 45.7% of participants met a criterion of anxiety by the Hospital Anxiety and Depression Scale (HADS). These findings suggested that there is poor agreement for anxiety diagnosis between questionnaires and formal interviews. More accurate screening tools are necessary to improve the diagnosis before comparing medical outcomes after treatment of anxiety disorder in patients with ESRD.
Benzodiazepines have been commonly prescribed for treatment of acute episodes of anxiety.116 No dose adjustment is required for renal impairment because benzodiazepines are metabolized mainly by the liver. However, this drug class should be avoided due to high rates of side effects, such as prolonged sedation, delusions, and hallucinations. Alprazolam and diazepam have higher free serum drug levels in patients with ESRD. Lorazepam is also not recommended for use in patients with CKD.108
Impact of CKD on Quality of Life
Quality of life is a broad, multidimentional concept that includes an individual’s perception of subjective general well-being, in the context of the culture and value systems in relation to their goals, expectations, standards, and concerns.117 Health-related quality of life (HRQoL) is defined by the Centers for Disease Control and Prevention as quality of life and its relationship with health – either physical or mental – comprising health risks and conditions, functional status, social support, and socioeconomic status.118 HRQoL is often compromised in patients with CKD.119
There are numerous valid and reliable tools for evaluating HRQoL. Many experts recommend using disease-specific tools in conjunction with established generic instruments. The most widely used instrument in ESRD clinical practice and research is the Kidney Disease Quality of Life (KDQoL), which incorporates generic and disease-specific scores.120 The Medical Outcomes Survey 36 item-Short Form (SF-36) is a generic instrument developed for use in many populations with 8 dimensions of HRQoL that can be summarized into Physical Component Summary (PCS) and Mental Health Component Summary (MCS) scores. The KDQoL also contains disease-specific scales including symptoms/problems, effects of kidney disease on daily life, burden of kidney disease, cognitive function, work status, sexual function, quality of social interaction, and sleep. Multi-item measures of social support, dialysis staff encouragement and patient satisfaction and a single-item overall rating of health are also included.121 Lower scores for the three major components of KDQoL (PCS, MCS, and the kidney disease component summary [KDCS]) were associated with significantly higher risk of death (adjusted RR per 10-point lower score were 1.13 for MCS [95% CI 1.09 to 1.17], 1.25 for PCS [95% CI 1.20 to 1.30], and 1.11 for KDCS [95% CI 1.08 to 1.13]) and first hospitalization (adjusted RR 1.06 [95% CI 1.04 to 1.08], 1.15 [95% CI 1.12 to 1.39], and 1.07 [95% CI 1.05 to 1.09] for MCS, PCS, and KDCS, respectively) among over seven thousand dialysis patients recruited into the Dialysis Outcomes and Practice Pattern Study (DOPPS).122
HRQoL in patients with CKD
Although there is growing evidence of an association between HRQoL and mortality risk in ESRD patients, few studies have examined HRQoL in patients with earlier stages of CKD. A cross-sectional study in 535 patients with CKD stage 2-5 observed that HRQoL, assessed using the SF-36, was significantly impaired compared to matched controls, and there was a graded association between higher stage of CKD and worse HRQoL.123 However, this study should be interpreted with caution because there were relatively few patients with early stages of CKD (eGFR >50 ml/min/1.73m2) and no patients with eGFR between 30 and 50 ml/min/1.73m2. A prospective large-scale study by Mujais and colleagues showed that HRQoL using the KDQoL-SF™ was proportionally lower with greater severity of CKD stage. Further, HRQoL significantly declined over time with the maximal decrement in Physical Function score, which fell by a mean of -3.23 ± 0.64 points per year, p<0.0001.124 Female gender, presence of diabetes, and cardiovascular co-morbidities were associated with lower HRQoL. Another study by Molsted et al.125 also documented that CKD patients had lower SF-36 scores than the general population and that the most pronounced impairment was in the Physical Function and Role Physical scales.
One approach to improving HRQoL among patients with CKD has been treatment of anemia using erythropoietin stimulating agents (ESA). A large multi-center cross-sectional study in stage 3-5 CKD by Finkelstein and co-workers126 showed that higher hemoglobin levels were associated with significantly higher scores on the SF-36 PCS and general health score of the KDQoL-SF™. The maximal increase in HRQoL per incremental increase in hemoglobin appears to occur in the range of 10-12g/dL, with blunted improvements in HRQoL above this level. The most improved domains are physical symptoms, vitality, energy, and performance.127 An open-label study among patients with CKD naïve to ESA with a baseline hemoglobin of 9.1±0.1 g/dL also demonstrated a mean improvement in the KDQoL subscales of burden of kidney disease (+9.8 ± 3.2 points), symptoms (+4.2 ± 1.6), and overall health (+9.8±3.3, all p<0.05) after anemia correction to a hemoglobin of 11.3 g/dL after 8 weeks.128 However, the decision to initiate ESA therapy should be individualized based on the risks related to ESA therapy, the presence of anemic symptoms, the rate of fall of hemoglobin concentration, and prior response to iron therapy.129
Since there is strong evidence to suggest that emotional problems such as depressive symptoms or anxiety affect HRQoL, it is logical to consider that treatment of these conditions might improve HRQoL. However, it is unclear whether this is the case.101, 130, 131
HRQoL in dialysis patients
A national, prospective cohort study, the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study132 examined the difference in self-reported HRQoL and overall health status between patients on HD and PD using the CHOICE Health Experience Questionnaire (CHEQ). This study included 928 incident dialysis patients and reported that HD and PD patients did not differ with respect to the unadjusted or adjusted change in overall health status by CHEQ after 1 year of dialysis. HD patients had better improvement in two general SF-36 domains: Physical Function (5.1 points higher, p<0.05) and General Health (4.1 points higher, p<0.05) than PD patients. However, the results were mixed for ESRD specific domains. PD was significantly better than HD (8.1 points greater) for finances, whereas HD was better than PD for sleep (8.1 points higher) and for overall quality of life (4.3 points higher, all p<0.05). One small randomized trial conducted to compare mean quality-adjusted life-year (QALY) and survival among incident HD and PD patients reported statistically equivalent mean QALY scores in the first two years after the start of dialysis.133 Liem et al134 conducted a meta-analysis to compare SF-36 scores among patients on renal replacement therapy and reported that the unadjusted scores of all SF-36 dimensions did not significantly differ between patients on HD and PD. Recently, a systematic review by Purnell et al.135 concluded that patients receiving HD and PD experienced similar rates of life participation outcomes, including physical functioning, social and recreational activities, freedom, travel, as well as ability to work.
Previous studies have demonstrated that interventions that may improve HRQoL in dialysis patients include correction of anemia,136, 137 treatment of depression,109 structured exercise programs,97, 138 and alterations in dialysis regimens. The Frequent Hemodialysis Network (FHN) Trial evaluated changes in HRQoL in patients undergoing HD six times per week compared to patients who continued on a conventional dialysis regimen and showed a significant improvement of the PCS score of SF-36 with frequent dialysis.139 However, no dimensions of the SF-36 were changed in patients receiving nocturnal HD performed three times per week for 7-8 hours a night, whereas they deteriorated in the conventional group (bodily pain from 64.9± 29.6 to 57.1±28.8, p=0.03; mental health from 69.2±23.9 to 65.1±23.1, p=0.04; vitality from 68.7±24.3 to 64.4±25.2, p=0.01).140 In the London Daily/Nocturnal Hemodialysis Study141, quotidian HD patients reported significant improvement in renal disease-specific QOL indicators including the numbers of dialysis symptoms, recovery time from dialysis, as well as the General Health and Vitality subscales of SF-36. One of the most striking changes in patients undergoing daily or nocturnal HD was the faster time to recovery after dialysis sessions, which dropped from 327 to 16 minutes after 12 months of short daily HD and 2 minutes after 15-month of nocturnal HD.142 In PD patients, however, data regarding the change of dialysis regimen yielded an opposing result. HRQoL assessed by KDQoL-SF™ did not significantly improve after increasing small solute peritoneal clearance in the Adequacy of Peritoneal Dialysis in Mexico study.143
Conclusions
Overall, CKD and ESRD are associated with impairments in physical function, cognitive function, emotional health, and health-related quality of life. In general, these issues seem to worsen as kidney disease progresses despite management of kidney disease and initiation of dialysis. These disorders may respond to traditional therapy, but in some cases adjustments are needed in the CKD. Routine screening for depression, as is recommended in the general population, could lead to more treatment and better HRQoL among patients with CKD. In addition, although vigorous exercise training has not been a sustainable model among patients with advanced CKD and ESRD, more research is needed to determine whether increasing physical activity through less vigorous means could improve physical function, depression, and cognitive function in this population – or, short of improvement, whether the seemingly inevitable decline can be mitigated.
Acknowledgments
Financial disclosure and conflict of interest: This work has been made possible in part by an International Society of Nephrology funded Fellowship to Dr. Kittiskulnam. Dr. Johansen’s effort was supported in part by a grant (K24 DK085153) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Dr. Sheshadri is supported by fellowship funding from the Veterans Health Administration. Dr. Johansen serves on the Steering Committee for the GlaxoSmithKline Prolyl Hydroxylase Inhibitor Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.USRDS 2015 Annual Data Report. Atlas of Chronic Disease and End Stage Renal Disease in the United States. [November 19, 2015] Available from: http://www.usrds.org/2015/view/v2_01.aspx.
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Deo R, Fyr CL, Fried LF, Newman AB, Harris TB, Angleman S, et al. Kidney dysfunction and fatal cardiovascular disease--an association independent of atherosclerotic events: results from the Health, Aging, and Body Composition (Health ABC) study. Am Heart J. 2008;155:62–8. doi: 10.1016/j.ahj.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinidou E, Koukouvou G, Kouidi E, Deligiannis A, Tourkantonis A. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med. 2002;34:40–5. doi: 10.1080/165019702317242695. [DOI] [PubMed] [Google Scholar]
- 5.Storer TW, Casaburi R, Sawelson S, Kopple JD. Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol Dial Transplant. 2005;20:1429–37. doi: 10.1093/ndt/gfh784. [DOI] [PubMed] [Google Scholar]
- 6.Gregory SM, Headley SA, Germain M, Flyvbjerg A, Frystyk J, Coughlin MA, et al. Lack of circulating bioactive and immunoreactive IGF-I changes despite improved fitness in chronic kidney disease patients following 48 weeks of physical training. Growth Horm IGF Res. 2011;21:51–6. doi: 10.1016/j.ghir.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Mustata S, Groeneveld S, Davidson W, Ford G, Kiland K, Manns B. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol. 2011;43:1133–41. doi: 10.1007/s11255-010-9823-7. [DOI] [PubMed] [Google Scholar]
- 8.Padilla J, Krasnoff J, Da Silva M, Hsu CY, Frassetto L, Johansen KL, et al. Physical functioning in patients with chronic kidney disease. J Nephrol. 2008;21:550–9. [PubMed] [Google Scholar]
- 9.Brodin E, Ljungman S, Sunnerhagen KS. Rising from a chair: a simple screening test for physical function in predialysis patients. Scand J Urol Nephrol. 2008;42:293–300. doi: 10.1080/00365590701797556. [DOI] [PubMed] [Google Scholar]
- 10.Bowling CB, Sawyer P, Campbell RC, Ahmed A, Allman RM. Impact of chronic kidney disease on activities of daily living in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:689–94. doi: 10.1093/gerona/glr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006;54:750–6. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 12.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the heart and soul study. J Am Soc Nephrol. 2004;15:2908–15. doi: 10.1097/01.ASN.0000143743.78092.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McManus D, Shlipak M, Ix JH, Ali S, Whooley MA. Association of cystatin C with poor exercise capacity and heart rate recovery: data from the heart and soul study. Am J Kidney Dis. 2007;49:365–72. doi: 10.1053/j.ajkd.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leikis MJ, McKenna MJ, Petersen AC, Kent AB, Murphy KT, Leppik JA, et al. Exercise performance falls over time in patients with chronic kidney disease despite maintenance of hemoglobin concentration. Clin J Am Soc Nephrol. 2006;1:488–95. doi: 10.2215/CJN.01501005. [DOI] [PubMed] [Google Scholar]
- 15.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–7. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–21. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm-Leen ER, Hall YN, T MK, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–71.e2. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–7. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 21.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–47. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361:1612–3. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 23.Johansen KL. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18:1845–54. doi: 10.1681/ASN.2007010009. [DOI] [PubMed] [Google Scholar]
- 24.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59:126–34. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farragher J, Jassal SV. Rehabilitation of the geriatric dialysis patient. Semin Dial. 2012;25:649–56. doi: 10.1111/sdi.12014. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2010;58:338–45. doi: 10.1111/j.1532-5415.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2007;18:2205–13. doi: 10.1681/ASN.2006101165. [DOI] [PubMed] [Google Scholar]
- 28.Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2006;21:2543–8. doi: 10.1093/ndt/gfl275. [DOI] [PubMed] [Google Scholar]
- 29.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56:693–703. doi: 10.1053/j.ajkd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Murray AM, Knopman DS. Cognitive impairment in CKD: no longer an occult burden. Am J Kidney Dis. 2010;56:615–8. doi: 10.1053/j.ajkd.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurella MC, Glenn M, Luan Jennifer, Yaffe Kristine. Cognitive Impairment in Chronic Kidney Disease. J Am Geriatr Soc. 2004;52:1863–9. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 32.Murray AM, T DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AK, Kane RL. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–23. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 33.Sehgal A, G SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41–9. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” a Practical Method for Grading the Cognitive State of Patients for the Clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.McDowell I, K B, Hill GB, Hebert R. Community Screening for Dementia: The Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) Compared. J Clin Epidemiol. 1997;50:377–83. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 36.Corrigan JD, H MS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol. 1987;43:402–9. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353–63. doi: 10.1681/ASN.2012050536. [DOI] [PubMed] [Google Scholar]
- 38.Altmann P, Barnett ME, Finn WF. Group SPDLCS. Cognitive function in Stage 5 chronic kidney disease patients on hemodialysis: no adverse effects of lanthanum carbonate compared with standard phosphate-binder therapy. Kidney Int. 2007;71:252–9. doi: 10.1038/sj.ki.5001932. [DOI] [PubMed] [Google Scholar]
- 39.Giang LM, Weiner DE, Agganis BT, Scott T, Sorensen EP, Tighiouart H, et al. Cognitive function and dialysis adequacy: no clear relationship. Am J Nephrol. 2011;33:33–8. doi: 10.1159/000322611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol. 2010;5:1429–38. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray AM, Pederson SL, Tupper DE, Hochhalter AK, Miller WA, Li Q, et al. Acute variation in cognitive function in hemodialysis patients: a cohort study with repeated measures. Am J Kidney Dis. 2007;50:270–8. doi: 10.1053/j.ajkd.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Post JB, Jegede AB, Morin K, Spungen AM, Langhoff E, Sano M. Cognitive profile of chronic kidney disease and hemodialysis patients without dementia. Nephron Clin Pract. 2010;116:c247–55. doi: 10.1159/000317206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiffin-Richards FE, Costa AS, Holschbach B, Frank RD, Vassiliadou A, Kruger T, et al. The Montreal Cognitive Assessment (MoCA) - a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One. 2014;9:e106700. doi: 10.1371/journal.pone.0106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etgen T. Kidney disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7:29. doi: 10.1186/s13195-015-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madan P, Kalra OP, Agarwal S, Tandon OP. Cognitive impairment in chronic kidney disease. Nephrol Dial Transplant. 2007;22:440–4. doi: 10.1093/ndt/gfl572. [DOI] [PubMed] [Google Scholar]
- 46.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009;24:2446–52. doi: 10.1093/ndt/gfp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–34. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khatri M, Nickolas T, Moon YP, Paik MC, Rundek T, Elkind MS, et al. CKD associates with cognitive decline. J Am Soc Nephrol. 2009;20:2427–32. doi: 10.1681/ASN.2008101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etgen T, Sander D, Chonchol M, Briesenick C, Poppert H, Forstl H, et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrol Dial Transplant. 2009;24:3144–50. doi: 10.1093/ndt/gfp230. [DOI] [PubMed] [Google Scholar]
- 50.Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol Dial Transplant. 2013;28:1810–9. doi: 10.1093/ndt/gfs470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–33. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 52.Murray AMB, Joshua I, Lovato James F, Willliamson Jeff D, Miller Michael E, Marcovina Santica, Launer Lenore J. Biomarkers of Renal Function and Cognitive Impairment in Patients with Diabetes. Diabetes Care. 2011;34:1827–32. doi: 10.2337/dc11-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barzilay JI, Lovato JF, Murray AM, Williamson J, Ismail-Beigi F, Karl D, et al. Albuminuria and cognitive decline in people with diabetes and normal renal function. Clin J Am Soc Nephrol. 2013;8:1907–14. doi: 10.2215/CJN.11321112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolcott DL, W D, Marh JT, Schaeffer J, Landsverk J, Nissenson AR. Relationship of dialysis modality and other factors to cognitive function in chronic dialysis patients. Am J Kidney Dis. 1988;12:275–84. doi: 10.1016/s0272-6386(88)80220-8. [DOI] [PubMed] [Google Scholar]
- 55.Buoncristiani U, A A, Gubbiotti G, Gallai V, Quinaliani G, Gaburri M. Better preservation of cognitive faculty in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1993;13:S202–4. [PubMed] [Google Scholar]
- 56.Marsh JT, Brown WS, Wolcott D, Landsverk J, Nissenson AR. Electrophysiological indices of CNS function in hemodialysis and CAPD. Kidney Int. 1986;30:957–63. doi: 10.1038/ki.1986.279. [DOI] [PubMed] [Google Scholar]
- 57.Radic J, Ljutic D, Radic M, Kovacic V, Sain M, Dodig-Curkovic K. Is there differences in cognitive and motor functioning between hemodialysis and peritoneal dialysis patients? Ren Fail. 2011;33:641–9. doi: 10.3109/0886022X.2011.586480. [DOI] [PubMed] [Google Scholar]
- 58.Radic J, L D, Radic M, Kovacic V, Sain M, Dodig Curkovic K. The possible impact of dialysis modality on cognitive function in chronci dialysis patients. The Netherlands Journal of Medicine. 2010;68:153–7. [PubMed] [Google Scholar]
- 59.Kurella Tamura M, Unruh ML, Nissenson AR, Larive B, Eggers PW, Gassman J, et al. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. Am J Kidney Dis. 2013;61:228–37. doi: 10.1053/j.ajkd.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramer L, M C, Stokenhuber F, Yeganehfar W, Eisenhiber E, Derfler K, Lenz K, Schneider B, Grimm G. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int. 1996;49:833–8. doi: 10.1038/ki.1996.115. [DOI] [PubMed] [Google Scholar]
- 61.Ruebner RL, Laney N, Kim JY, Hartung EA, Hooper SR, Radcliffe J, et al. Neurocognitive Dysfunction in Children, Adolescents, and Young Adults With CKD. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 62.Mendley SR, Matheson MB, Shinnar S, Lande MB, Gerson AC, Butler RW, et al. Duration of chronic kidney disease reduces attention and executive function in pediatric patients. Kidney Int. 2015;87:800–6. doi: 10.1038/ki.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matta SMd, Moreira JM, Kummer AMe, Barbosa IG, Teixeira AL, Silva ACSe. Cognitive alterations in chronic kidney disease: an update. Jornal Brasileiro de Nefrologia. 2014;36:241–5. doi: 10.5935/0101-2800.20140035. [DOI] [PubMed] [Google Scholar]
- 64.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 65.Seliger SG, Daniel L, Longstreth WT, Jr, Kestenbaum Bryan, Stehman-Breen Catherine O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–9. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 66.Drew DA, B Rafeeque, Tighiouart Hocine, Bovak Vera, Scott Tammy M, Lou Kristina V, Shaffi Kamran, Weiner Daniel E, Sarnak Mark J. Anatomic Brain Disease in Hemodialysis Patients. A Cross-sectional Study Am J Kidney Dis. 2013;61:271–8. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.London GM, Sylvain J, Guerin Alain P, Metivier Fabien, Adda Hassan. Arterial structure and function in end-stage renal disease. Nephrol Dial Transplant. 2002;17:1713–24. doi: 10.1093/ndt/17.10.1713. [DOI] [PubMed] [Google Scholar]
- 68.Flythe JE, Inrig JK, Shafi T, Chang TI, Cape K, Dinesh K, et al. Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am J Kidney Dis. 2013;61:966–74. doi: 10.1053/j.ajkd.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pringle EPC, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bupitt CJ, Fagard RH. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–7. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 70.Fazekas G, F F, Schmidt R, Japeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134:83–8. doi: 10.1016/0022-510x(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 71.Weiner DE, Bartolomei K, Scott T, Price LL, Griffith JL, Rosenberg I, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009;53:438–47. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miwa K, Tanaka M, Okazaki S, Furukado S, Yagita Y, Sakaguchi M, et al. Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology. 2014;82:1051–7. doi: 10.1212/WNL.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 73.Chou MC, Hsieh TJ, Lin YL, Hsieh YT, Li WZ, Chang JM, et al. Widespread white matter alterations in patients with end-stage renal disease: a voxelwise diffusion tensor imaging study. AJNR Am J Neuroradiol. 2013;34:1945–51. doi: 10.3174/ajnr.A3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kimmel PL. Depression in patients with chronic renal disease: what we know and what we need to know. J Psychosom Res. 2002;53:951–6. doi: 10.1016/s0022-3999(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 75.Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage. 2010;39:477–85. doi: 10.1016/j.jpainsymman.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 77.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–73. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 78.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161:1849–56. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 79.Fischer MJ, Xie D, Jordan N, Kop WJ, Krousel-Wood M, Kurella Tamura M, et al. Factors associated with depressive symptoms and use of antidepressant medications among participants in the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic-CRIC Studies. Am J Kidney Dis. 2012;60:27–38. doi: 10.1053/j.ajkd.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Prevalence of major depressive episode in CKD. Am J Kidney Dis. 2009;54:424–32. doi: 10.1053/j.ajkd.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179–91. doi: 10.1038/ki.2013.77. [DOI] [PubMed] [Google Scholar]
- 82.Cukor D, Fruchter Y, Ver Halen N, Naidoo S, Patel A, Saggi SJ. A preliminary investigation of depression and kidney functioning in patients with chronic kidney disease. Nephron Clin Pract. 2012;122:139–45. doi: 10.1159/000349940. [DOI] [PubMed] [Google Scholar]
- 83.Tsai YC, Chiu YW, Hung CC, Hwang SJ, Tsai JC, Wang SL, et al. Association of symptoms of depression with progression of CKD. Am J Kidney Dis. 2012;60:54–61. doi: 10.1053/j.ajkd.2012.02.325. [DOI] [PubMed] [Google Scholar]
- 84.Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. Jama. 2010;303:1946–53. doi: 10.1001/jama.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kellerman QD, Christensen AJ, Baldwin AS, Lawton WJ. Association between depressive symptoms and mortality risk in chronic kidney disease. Health Psychol. 2010;29:594–600. doi: 10.1037/a0021235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiang HH, Guo HR, Livneh H, Lu MC, Yen ML, Tsai TY. Increased risk of progression to dialysis or death in CKD patients with depressive symptoms: A prospective 3-year follow-up cohort study. J Psychosom Res. 2015;79:228–32. doi: 10.1016/j.jpsychores.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 87.Belayev LY, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Longitudinal associations of depressive symptoms and pain with quality of life in patients receiving chronic hemodialysis. Hemodial Int. 2015;19:216–24. doi: 10.1111/hdi.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weisbord SD, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clin J Am Soc Nephrol. 2014;9:1594–602. doi: 10.2215/CJN.00220114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hedayati SS, Bosworth HB, Briley LP, Sloane RJ, Pieper CF, Kimmel PL, et al. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. 2008;74:930–6. doi: 10.1038/ki.2008.311. [DOI] [PubMed] [Google Scholar]
- 90.Einwohner R, Bernardini J, Fried L, Piraino B. The effect of depressive symptoms on survival in peritoneal dialysis patients. Perit Dial Int. 2004;24:256–63. [PubMed] [Google Scholar]
- 91.Hedayati SS, Bosworth HB, Kuchibhatla M, Kimmel PL, Szczech LA. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69:1662–8. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 92.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Validation of depression screening scales in patients with CKD. Am J Kidney Dis. 2009;54:433–9. doi: 10.1053/j.ajkd.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cukor D, Coplan J, Brown C, Friedman S, Cromwell-Smith A, Peterson RA, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:484–90. doi: 10.2215/CJN.00040107. [DOI] [PubMed] [Google Scholar]
- 94.Duarte PS, Miyazaki MC, Blay SL, Sesso R. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int. 2009;76:414–21. doi: 10.1038/ki.2009.156. [DOI] [PubMed] [Google Scholar]
- 95.Cukor D, Ver Halen N, Asher DR, Coplan JD, Weedon J, Wyka KE, et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol. 2014;25:196–206. doi: 10.1681/ASN.2012111134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kouidi E, Karagiannis V, Grekas D, Iakovides A, Kaprinis G, Tourkantonis A, et al. Depression, heart rate variability, and exercise training in dialysis patients. Eur J Cardiovasc Prev Rehabil. 2010;17:160–7. doi: 10.1097/HJR.0b013e32833188c4. [DOI] [PubMed] [Google Scholar]
- 97.Ouzouni S, Kouidi E, Sioulis A, Grekas D, Deligiannis A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil. 2009;23:53–63. doi: 10.1177/0269215508096760. [DOI] [PubMed] [Google Scholar]
- 98.Mitrou GI, Grigoriou SS, Konstantopoulou E, Theofilou P, Giannaki CD, Stefanidis I, et al. Exercise training and depression in ESRD: a review. Semin Dial. 2013;26:604–13. doi: 10.1111/sdi.12112. [DOI] [PubMed] [Google Scholar]
- 99.Symister P, Friend R. The influence of social support and problematic support on optimism and depression in chronic illness: a prospective study evaluating self-esteem as a mediator. Health Psychol. 2003;22:123–9. doi: 10.1037//0278-6133.22.2.123. [DOI] [PubMed] [Google Scholar]
- 100.Lopes AA, Albert JM, Young EW, Satayathum S, Pisoni RL, Andreucci VE, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–53. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 101.Fischer MJ, Kimmel PL, Greene T, Gassman JJ, Wang X, Brooks DH, et al. Sociodemographic factors contribute to the depressive affect among African Americans with chronic kidney disease. Kidney Int. 2010;77:1010–9. doi: 10.1038/ki.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cohen SD, Perkins V, Kimmel PL. Psychosocial issues in ESRD patients. In: Daugirdas J, Ing T, editors. Handbook of Dialysis. Fourth. Little Brown; Boston: 2007. pp. 455–61. [Google Scholar]
- 103.Cohen SD, Norris L, Acquaviva K, Peterson RA, Kimmel PL. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2:1332–42. doi: 10.2215/CJN.03951106. [DOI] [PubMed] [Google Scholar]
- 104.Nagler EV, Webster AC, Vanholder R, Zoccali C. Antidepressants for depression in stage 3-5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP) Nephrol Dial Transplant. 2012;27:3736–45. doi: 10.1093/ndt/gfs295. [DOI] [PubMed] [Google Scholar]
- 105.Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;71(Suppl E1):e04. doi: 10.4088/JCP.9058se1c.04gry. [DOI] [PubMed] [Google Scholar]
- 106.Blumenfield M, Levy NB, Spinowitz B, Charytan C, Beasley CM, Jr, Dubey AK, et al. Fluoxetine in depressed patients on dialysis. Int J Psychiatry Med. 1997;27:71–80. doi: 10.2190/WQ33-M54T-XN7L-V8MX. [DOI] [PubMed] [Google Scholar]
- 107.Koo JR, Yoon JY, Joo MH, Lee HS, Oh JE, Kim SG, et al. Treatment of depression and effect of antidepression treatment on nutritional status in chronic hemodialysis patients. Am J Med Sci. 2005;329:1–5. doi: 10.1097/00000441-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 108.Cohen LM, Tessier EG, Germain MJ, Levy NB. Update on psychotropic medication use in renal disease. Psychosomatics. 2004;45:34–48. doi: 10.1176/appi.psy.45.1.34. [DOI] [PubMed] [Google Scholar]
- 109.Atalay H, Solak Y, Biyik M, Biyik Z, Yeksan M, Uguz F, et al. Sertraline treatment is associated with an improvement in depression and health-related quality of life in chronic peritoneal dialysis patients. Int Urol Nephrol. 2010;42:527–36. doi: 10.1007/s11255-009-9686-y. [DOI] [PubMed] [Google Scholar]
- 110.Jain N, Trivedi MH, Rush AJ, Carmody T, Kurian B, Toto RD, et al. Rationale and design of the Chronic Kidney Disease Antidepressant Sertraline Trial (CAST) Contemp Clin Trials. 2013;34:136–44. doi: 10.1016/j.cct.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sareen J, Jacobi F, Cox BJ, Belik SL, Clara I, Stein MB. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109–16. doi: 10.1001/archinte.166.19.2109. [DOI] [PubMed] [Google Scholar]
- 112.Lee YJ, Kim MS, Cho S, Kim SR. Association of depression and anxiety with reduced quality of life in patients with predialysis chronic kidney disease. Int J Clin Pract. 2013;67:363–8. doi: 10.1111/ijcp.12020. [DOI] [PubMed] [Google Scholar]
- 113.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14:82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Cukor D, Coplan J, Brown C, Friedman S, Newville H, Safier M, et al. Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis. 2008;52:128–36. doi: 10.1053/j.ajkd.2008.02.300. [DOI] [PubMed] [Google Scholar]
- 116.Yeh CY, Chen CK, Hsu HJ, Wu IW, Sun CY, Chou CC, et al. Prescription of psychotropic drugs in patients with chronic renal failure on hemodialysis. Ren Fail. 2014;36:1545–9. doi: 10.3109/0886022X.2014.949762. [DOI] [PubMed] [Google Scholar]
- 117.The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–9. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 118.Center for Disease Control and Prevention. http://www.cdc.gov/hrqol/concept.htmCenter.
- 119.Fukuhara S, Yamazaki S, Hayashino Y, Green J. Measuring health-related quality of life in patients with end-stage renal disease: why and how. Nat Clin Pract Nephrol. 2007;3:352–3. doi: 10.1038/ncpneph0510. [DOI] [PubMed] [Google Scholar]
- 120.Center for Medicare and Medicaid Services. [November 18]; Available from: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/downloads/SCletter09-01.pdf.
- 121.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–38. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 122.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–49. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 123.Pagels AA, Soderkvist BK, Medin C, Hylander B, Heiwe S. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes. 2012;10:71. doi: 10.1186/1477-7525-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, et al. Health-related quality of life in CKD Patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Molsted S, Prescott L, Heaf J, Eidemak I. Assessment and clinical aspects of health-related quality of life in dialysis patients and patients with chronic kidney disease. Nephron Clin Pract. 2007;106:c24–33. doi: 10.1159/000101481. [DOI] [PubMed] [Google Scholar]
- 126.Finkelstein FO, Story K, Firanek C, Mendelssohn D, Barre P, Takano T, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:33–8. doi: 10.2215/CJN.00630208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leaf DE, Goldfarb DS. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int. 2009;75:15–24. doi: 10.1038/ki.2008.414. [DOI] [PubMed] [Google Scholar]
- 128.Alexander M, Kewalramani R, Agodoa I, Globe D. Association of anemia correction with health related quality of life in patients not on dialysis. Curr Med Res Opin. 2007;23:2997–3008. doi: 10.1185/030079907X242502. [DOI] [PubMed] [Google Scholar]
- 129.Kidney Disease: Improving Global Outcomes (KDIGO) Work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012;2:1–64. [Google Scholar]
- 130.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–64. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soni RK, Weisbord SD, Unruh ML. Health-related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:153–9. doi: 10.1097/MNH.0b013e328335f939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu AW, Fink NE, Marsh-Manzi JV, Meyer KB, Finkelstein FO, Chapman MM, et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol. 2004;15:743–53. doi: 10.1097/01.asn.0000113315.81448.ca. [DOI] [PubMed] [Google Scholar]
- 133.Korevaar JC, Feith GW, Dekker FW, van Manen JG, Boeschoten EW, Bossuyt PM, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int. 2003;64:2222–8. doi: 10.1046/j.1523-1755.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 134.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG. Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2007;10:390–7. doi: 10.1111/j.1524-4733.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 135.Purnell TS, Auguste P, Crews DC, Lamprea-Montealegre J, Olufade T, Greer R, et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am J Kidney Dis. 2013;62:953–73. doi: 10.1053/j.ajkd.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moreno F, Sanz-Guajardo D, Lopez-Gomez JM, Jofre R, Valderrabano F. Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialysis patients. Spanish Cooperative Renal Patients Quality of Life Study Group of the Spanish Society of Nephrology. J Am Soc Nephrol. 2000;11:335–42. doi: 10.1681/ASN.V112335. [DOI] [PubMed] [Google Scholar]
- 137.McMahon LP, Mason K, Skinner SL, Burge CM, Grigg LE, Becker GJ. Effects of haemoglobin normalization on quality of life and cardiovascular parameters in end-stage renal failure. Nephrol Dial Transplant. 2000;15:1425–30. doi: 10.1093/ndt/15.9.1425. [DOI] [PubMed] [Google Scholar]
- 138.Levendoglu F, Altintepe L, Okudan N, Ugurlu H, Gokbel H, Tonbul Z, et al. A twelve week exercise program improves the psychological status, quality of life and work capacity in hemodialysis patients. J Nephrol. 2004;17:826–32. [PubMed] [Google Scholar]
- 139.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ok E, Duman S, Asci G, Tumuklu M, Onen Sertoz O, Kayikcioglu M, et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dial Transplant. 2011;26:1287–96. doi: 10.1093/ndt/gfq724. [DOI] [PubMed] [Google Scholar]
- 141.Heidenheim AP, Muirhead N, Moist L, Lindsay RM. Patient quality of life on quotidian hemodialysis. Am J Kidney Dis. 2003;42:36–41. doi: 10.1016/s0272-6386(03)00536-5. [DOI] [PubMed] [Google Scholar]
- 142.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. 2006;1:952–9. doi: 10.2215/CJN.00040106. [DOI] [PubMed] [Google Scholar]
- 143.Paniagua R, Amato D, Vonesh E, Guo A, Mujais S. Health-related quality of life predicts outcomes but is not affected by peritoneal clearance: The ADEMEX trial. Kidney Int. 2005;67:1093–104. doi: 10.1111/j.1523-1755.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 144.Dervisoglu E, Kir HM, Kalender B, Eraldemir C, Caglayan C. Depressive symptoms and proinflammatory cytokine levels in chronic renal failure patients. Nephron Clin Pract. 2008;108:c272–7. doi: 10.1159/000126907. [DOI] [PubMed] [Google Scholar]
- 145.Watnick S, Wang PL, Demadura T, Ganzini L. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis. 2005;46:919–24. doi: 10.1053/j.ajkd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 146.Chilcot J, Wellsted D, Farrington K. Screening for depression while patients dialyse: an evaluation. Nephrol Dial Transplant. 2008;23:2653–9. doi: 10.1093/ndt/gfn105. [DOI] [PubMed] [Google Scholar]
- 147.Kop WJ, Seliger SL, Fink JC, Katz R, Odden MC, Fried LF, et al. Longitudinal association of depressive symptoms with rapid kidney function decline and adverse clinical renal disease outcomes. Clin J Am Soc Nephrol. 2011;6:834–44. doi: 10.2215/CJN.03840510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van den Beukel TO, Siegert CE, van Dijk S, Ter Wee PM, Dekker FW, Honig A. Comparison of the SF-36 Five-item Mental Health Inventory and Beck Depression Inventory for the screening of depressive symptoms in chronic dialysis patients. Nephrol Dial Transplant. 2012;27:4453–7. doi: 10.1093/ndt/gfs341. [DOI] [PubMed] [Google Scholar]
- 149.Kelly MJ, Dunstan FD, Lloyd K, Fone DL. Evaluating cutpoints for the MHI-5 and MCS using the GHQ-12: a comparison of five different methods. BMC Psychiatry. 2008;8:10. doi: 10.1186/1471-244X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Al Zaben F, Khalifa DA, Sehlo MG, Al Shohaib S, Shaheen F, Alhozali H, et al. Depression in patients with chronic kidney disease on dialysis in Saudi Arabia. Int Urol Nephrol. 2014;46:2393–402. doi: 10.1007/s11255-014-0802-2. [DOI] [PubMed] [Google Scholar]
- 151.Dong J, Pi HC, Xiong ZY, Liao JL, Hao L, Liu GL, et al. Depression and Cognitive Impairment in Peritoneal Dialysis: A Multicenter Cross-sectional Study. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 152.Zung WW. A self rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 153.World Health Organization 2015. http://www.who.int/substance_abuse/research_tools/zungdepressionscale/en/
- 154.Hedayati SS, Yalamanchili V, Finkelstein FO. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int. 2012;81:247–55. doi: 10.1038/ki.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]