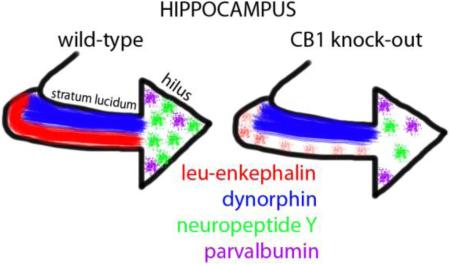
Abstract
Drug addiction requires learning and memory processes that are facilitated by activation of cannabinoid-1 (CB1) and opioid receptors in the hippocampus. This involves activity-dependent synaptic plasticity that is partially regulated by endogenous opioid (enkephalin and dynorphin) and non-opioid peptides, specifically cholecystokinin, parvalbumin and neuropeptide Y, the neuropeptides present in inhibitory interneurons that co-express CB1 or selective opioid receptors. We tested the hypothesis that CB1 receptor expression is a determinant of the availability of one or more of these peptide modulators in the hippocampus. This was achieved by quantitatively analyzing the immunoperoxidase labeling for each of these neuropeptide in the dorsal hippocampus of female wild-type (CB1+/+) and cannabinoid receptor 1 knockout (CB1−/−) C57/BL6 mice. The levels of Leu5-enkephalin-immunoreactivity were significantly reduced in the hilus of the dentate gyrus and in stratum lucidum of CA3 in CB1−/− mice. Moreover, the numbers of neuropeptide Y-immunoreactive interneurons in the dentate hilus were significantly lower in the CB1−/− compared to wild-type mice. However, CB1+/+ and CB1−/− mice did not significantly differ in expression levels of either dynorphin or cholecystokinin, and showed no differences in numbers of parvalbumin-containing interneurons. These findings suggest that the cannabinoid and opioid systems have a nuanced, regulatory relationship that could affect the balance of excitation and inhibition in the hippocampus and thus processes such as learning that rely on this balance.
Keywords: Opioid, Cannabinoid, Female mice, Neuropeptide Y, Parvalbumin, hippocampus, dentate gyrus, cholecystokinin, dynorphin
Graphical Abstract
Introduction
In the hippocampus, the opioid and cannabinoid systems are involved in spatial and episodic memory processes that are important for learning relevant to drug addiction [13;23;47]. Opioid peptides and endogenous cannabinoids (i.e., endocannabinoids) promote hippocampal long-term potentiation (LTP) and synaptic plasticity that allow these processes to occur [3;8]. Both opioids and endocannabinoids increase hippocampal net-excitability by disinhibiting their respective systems, which rely on different interneuronal circuitry [3;8]. Because of these similarities, more specifically defining the largely unknown relationship between cannabinoid and opioid systems in the hippocampus is essential for understanding their collaborative ability to promote associative learning.
In the rodent hippocampus, the opioid and cannabinoid systems have overlapping anatomical distributions. The opioid peptides Leu5-enkephalin (L-ENK) and dynorphin (DYN) are found in the mossy fiber pathway within the hilus of the dentate gyrus and stratum lucidum of CA3 [8]. Enkephalins are the endogenous ligands for mu-opioid receptors (MORs) and delta-opioid receptors (DORs), whereas dynorphins are the endogenous ligands for kappa-opioid receptors (KORs) but can bind MORs [8]. The opioid-containing mossy fiber pathway overlaps with parvalbumin-immunoreactive interneurons that express MORs and neuropeptide Y (NPY)-immunoreactive interneurons that express DORs [8]. (Few KORs are found in the mouse hippocampus [37]). Moreover, the mossy fiber pathway overlaps with cholecystokinin (CCK)-immunoreactive interneurons that are known to express cannabinoid-1 (CB1) receptors, particularly on their terminals in the dentate molecular layer [25;32;42]. These receptors are also present in axon terminals that are located in and around the CA3 pyramidal cell bodies as well as those in the stratum lucidum CB1 receptors [42]. Interneurons containing MORs, DORs and CB1 receptors also form networks with each other [21;24;36] suggesting another avenue by which the opioid and cannabinoid systems intersect. Thus, to help elucidate the relationship between these two independent yet linked systems, the present study quantitatively examined opioid system markers in mice lacking CB1 receptors compared to wild-type mice.
Materials and Methods
Animals
Animal procedures were approved by the Weill Cornell Medicine Institutional Animal Care and Use Committee and were in accordance with the NIH guide for the Care and Use of Laboratory Animals. Homozygous CB1 receptor knockout (CB1−/−) mice used in this study were bred in-house at Weill Cornell Medicine from heterozygous (CB1−/+) C57/BL6 male and female mice. These mice were generously provided by Dr. Matthew N. Hill in Bruce McEwen's laboratory at The Rockefeller University, New York, NY. Genotyping was performed by Transnetyx, Inc. (Cordova, TN). The 2-4 month old adult female CB1+/+ (wild-type) and CB1−/− mice (N = 5/group) used in this study were age-matched littermates from three different litters. The wild-type and mutant mice were housed together in common litters of 3–5 mice under a 12-hour light/dark cycle. Food and water were available ad libitum. All mice were in estrus, as assessed by vaginal smear cytology [43], at the time of euthanasia.
Tissue preparation
Mice were overdosed with sodium pentobarbital (150 mg/kg, I.P.) and their brains were perfusion fixed with 5 mL of 2% heparin-saline followed by 30 mL of 3.75% acrolein and 2% paraformaldehyde in 0.1% phosphate buffer (PB; pH 7.4). Brains were removed from the skull, postfixed in 2% acrolein and 2% paraformaldehyde for 30 minutes and then placed into PB. Coronal sections (40 μm thick) through the hippocampus were cut on a Vibratome (Leica, Deerfield, IL) into PB. Sections were stored at −20°C in cryoprotectant solution until use [29].
Antisera
The mouse monoclonal antibody to L-ENK (Sera Labs, Crawley Down, UK) has been characterized using preadsorption controls and immunoblots where it recognizes L-ENK and to a lesser extent DYN (1-13, 1-17) and Met5-ENK, but not α, β and γ endorphin [7;27]. The guinea pig polyclonal antibody against DYN B was purchased from Peninsula Laboratories (Belmont, CA) and has been characterized for specificity using preadsorption [31;40]. Previous tests [44] determined the dilution of the L-ENK and DYN antibodies that yielded optimal detection of intensity variations.
The mouse monoclonal antibody to parvalbumin (Sigma, St. Louis, MO) has been characterized using Western blot, double immuno-diffusion methods and preadsorption controls [4;21]. The rabbit polyclonal antibody against NPY (Peninsula Laboratories) has been characterized using immunodot blots [28]. The rabbit polyclonal antibody to cholecystokinin (#8988; supplied by Dr. John Walsh, Veterans Administration, CA) has been shown to be specific using immunodot blots and preadsorption controls [5]. The optimal dilution of the CCK antibody for detecting intensity variations was determined using previously described methods [6].
Immunocytochemistry
To ensure identical labeling conditions [35], dorsal hippocampal sections from each animal were matched with regard to rostrocaudal level (−2.00 to −2.70 mm from Bregma [18]) and labeled with identifying hole punches in the cortex. Tissue sections from CB1+/+ and CB1−/− mice then were pooled into a single container and processed together in the immunocytochemical labeling procedure.
Immunolabeling for each of the peptides was preceded by incubation of tissue sections in 1% sodium borohydride in PB to remove reactive aldehydes then rinsed in PB until the gaseous bubbles disappeared. These sections were processed for detection of L-ENK, DYN, parvalbumin, NPY or CCK using the immunoperoxidase method [29]. For this, the sections were washed in Tris-Saline (TS; pH 7.6), blocked in 0.5% bovine serum albumin (BSA) in TS for 30 min and then placed in 0.1% BSA and 0.25% Triton-X 100 in TS containing the primary antisera. Primary antibody dilutions were: LENK, 1:1000; DYN, 1:4000; parvalbumin, 1:8000; NPY, 1:5000; CCK, 1:40,000. Sections were incubated in the primary antisera for 24 hours at room temperature and 2 days at 4°C. Sections were rinsed in TS and then incubated in either biotinylated anti-mouse immunoglobulin (L-ENK, parvalbumin), biotinylated anti-guinea pig immunoglobulin (DYN) or biotinylated anti-rabbit immunoglobulin (NPY, CCK) for 30 minutes in 0.1% BSA/TS (1:400; all from Jackson Immunoresearch, West Grove, PA). Sections were rinsed with TS and then incubated in avidin-biotin complex (ABC) in TS for 30 minutes. Following TS rinses, sections were placed in 3, 3’-diaminobenzidine (Sigma Aldrich, Milwaukee, WI) with H2O2 in TS for 6 minutes. The sections were rinsed in PB, mounted on gelatin-coated slides, dehydrated, and coverslipped in DPX (Sigma Aldrich).
Analysis
To insure unbiased assessment of the results, an experimenter (S.A.R.) blinded to genotype performed all data collection and analysis. The density of L-ENK, dynorphin and CCK were determined using densitometric methods [35]. A low-magnification (4x) photograph was taken of the hippocampus using a Dage MTI CCD-72 camera on a Nikon Eclipse 80i microscope that was converted to a grayscale using MicroComputer Imaging Device software. Using ImageJ64 software (NIH), mean gray value (of 256 levels) was selected for different subregions of the mossy fiber pathway (circles, Fig. 1A). The average pixel density from a region without labeling was subtracted from each measurement to control for background staining variations. For parvalbumin and NPY, only cells with defined nuclei were counted. The area of the hilus was determined using ImageJ software and a mouse atlas [18]. The total number of cells per unit area of the hilus was then calculated. Statistical significance (p<0.5) was determined using a student's t-test with a Holm correction [19]. Graphs were prepared using GraphPad Prism6 (V6.0b, GraphPad Software, Inc.).
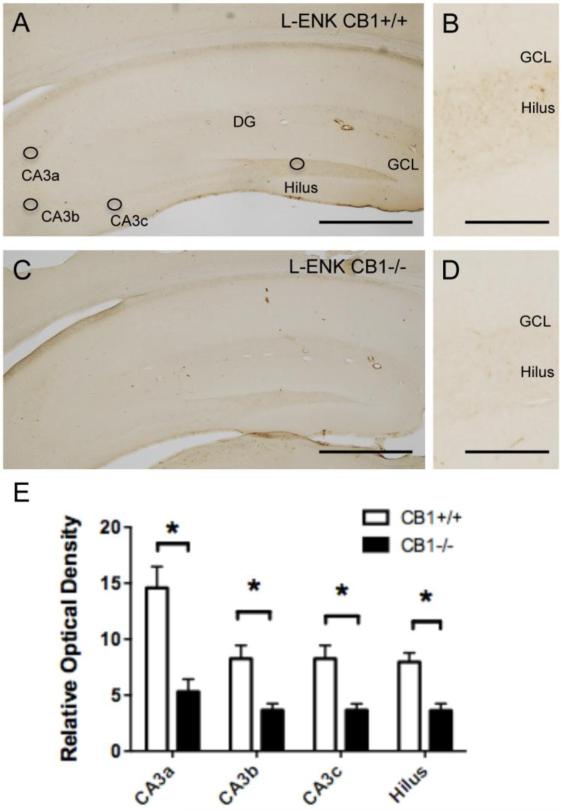
Fig. 1. Localization of L-ENK-ir in the mossy fiber pathway.
Light microscopic images of representative L-ENK immunolabeling in the dorsal hippocampus of CB1+/+ (A) and CB1 −/− (C) mice. Diffuse L-ENK-ir is seen in the hilus and in the stratum lucidum of CA3. Circles indicate where densitometry measurements were taken in each subregion. Higher power micrographs show examples of the density of L-ENK-ir in the hilus of the dentate gyrus from the CB1 +/+ (B) and CB1−/− (D) mice. E. Bar graph showing that the relative optical density of L-ENK-ir is significantly lower (p < 0.05) in all regions of the CA3 and dentate gyrus from the CB1 −/− compared to the CB1 +/+ mice. CA, cornu ammonis; DG, dentate gyrus; GCL, granule cell layer. N = 5/group, Bar A,C = 50 μm; B, D = 10 μm
Results
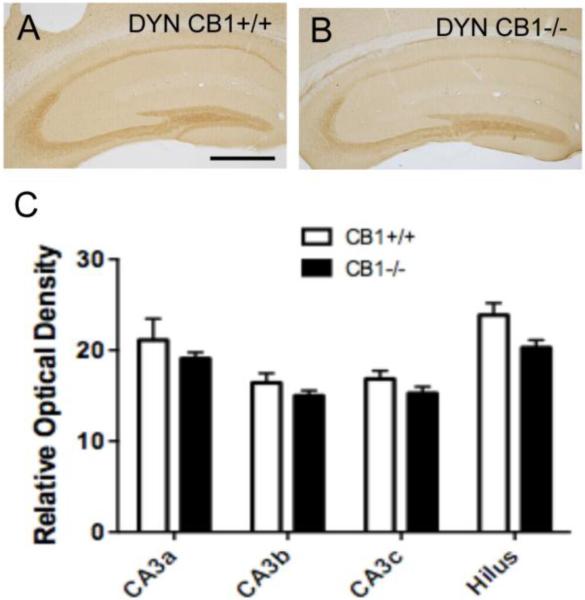
Consistent with previous studies [15], L-ENK- and DYN-immunoreactivities were found in the mossy fiber pathway in the dorsal hippocampus. Diffuse L-ENK-immunoreactivity (ir) was concentrated in the hilus of the dentate gyrus and in stratum lucidum of CA3a,b (Fig. 1A). DYN-ir was similarly distributed in the mossy fiber pathway, but comparatively denser in all subregions (Fig. 2A). Qualitatively, the density of LENK-ir was less in CB−/− mice compared to CB1+/+ mice (Fig. 1B,D). Quantitatively, CB1−/− mice compared to CB1+/+ mice showed a significantly lower relative optical density of L-ENK-ir in all CA3 regions and in the hilus (CA3a: t(10)=4.202, P=0.00672; CA3b: t(10)=3.468, P= 0.0118; CA3c: t(10)=3.482, P=0.0118; Hilus: t(10)=4.253, P=0.00672) (Fig. 1E). Conversely, there were qualitative differences in the density of DYN-ir in the mossy fiber pathway (Fig. 2A,B). Quantitative analysis showed no significant differences (P > 0.05) in the relative optical density of DYN-ir in any subregion of mossy fiber pathway in CB1−/− mice and CB1+/+ mice (Fig. 2C).
Fig. 2. Localization of DYN-ir in the mossy fiber pathway.
Light micrographs of representative DYN immunolabeling in the dorsal hippocampus of CB1+/+ (A) and CB1 −/− (B) mice. Diffuse DYN-ir is observed in the hilus and in stratum lucidum of CA3. C. Bar graphs show that the relative optical density of DYN-ir in the CA3 regions and hilus (see figure 1) is not significantly different in the CB1 −/− compared to CB1+/+ mice. N = 5/group. Bar = 50 μm
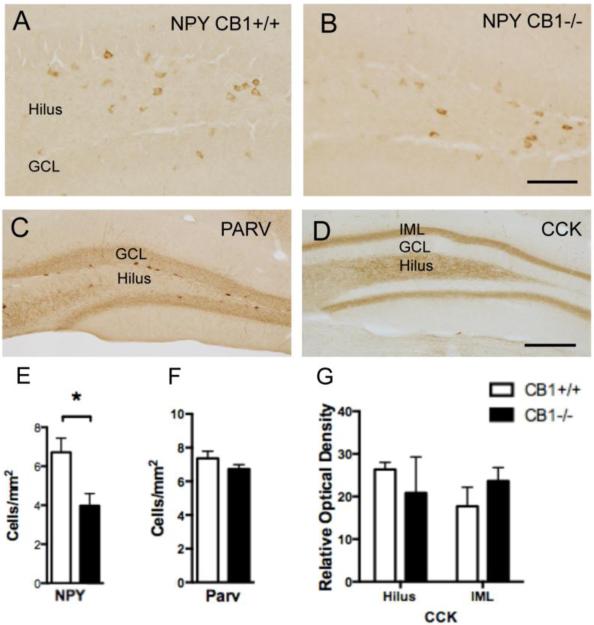
NPY-immunoreactive neurons were distributed in the central hilus (Fig. 3A) whereas parvalbumin-immunoreactive neurons were found in the subgranular hilus of the dentate gyrus (Fig. 3C). Qualitatively, NPY-immunoreactive neurons were fewer in CB1−/− mice compared to CB1+/+ mice (Fig. 3A,B). The number of NPY-immunoreactive neurons in CB1−/− mice was significantly lower compared to the CB1+/+ mice (t(5)=5.238, P=.0034) (Fig. 3E). Conversely, CB1−/− and CB1+/+ mice did not significantly differ (P > 0.05) in the number of parvalbumin-immunoreactive neurons (Fig. 3F).
Fig. 3. Localization of NPY, parvalbumin and CCK in the hilus of the dentate gyrus.
Light micrographs of representative NPY immunolabeled interneurons in the hilus of CB1+/+ (A) and CB1 −/− (B) mice. C. Example of parvalbumin-labeled interneurons. D. Example shows diffuse CCK-ir in the hilus and inner molecular layer (IML) of the dentate gyrus. E. Bar graphs showing that the number of NPY-labeled interneurons in the dentate hilus is significantly less (p < 0.05) in CB1 −/− mice compared to CB1+/+ mice. F and G. Bar graphs respectively showing that neither the number of parvalbumin immunolabeled interneurons nor the density CCK immunolabeling in the hilus and intermolecular layer is significantly different in CB1+/+ and CB1 −/− mice. N = 5/group. Bar B = 25 μm; D = 50 μm
In agreement with previous studies in mice [20], dense CCK-ir was observed in the mossy fiber pathway (Fig. 3D). Unfortunately, this dense labeling obscured the discrimination of CCK-labeled perikarya that were also seen in this region. Diffuse CCK-ir was detected in the inner molecular layer of the dentate gyrus (Fig. 3D), where CCK-immunoreactive axon terminals arising from CCK-containing hilar interneurons have been previously demonstrated [36]. No significant differences (P > 0.05) were observed in density of CCK-ir between CB1−/− mice and CB1+/+ mice in either the hilus or inner molecular layer (Fig. 3G).
Discussion
This study provides the first microscopic evidence that constitutive deletion of the CB1 receptor gene decreases the expression of selective opioid and non-opioid peptides located in interneurons that are differentially enriched in opioid receptors [8]. We have specifically shown that L-ENK levels in the mossy fiber pathway and number of NPY-labeled hilar interneurons are decreased in the CB1−/− mice. Conversely, DYN and CCK levels as well as the number of parvalbumin interneurons were unaffected by CB1 receptor gene deletion. These results have important implications for understanding the regulatory mechanisms by which cannabinoids control hippocampal opioid systems involved in memory and learning.
The L-ENK containing mossy fibers arise from granule cells [8]. In the dentate gyrus, CB1 receptors are located in the inner molecular layer on GABAergic terminals that synapse upon granule cell dendrites [22]. Activation of presynaptic CB1 receptors can suppress neurotransmitter release [3;15]. Thus, the decrease of mossy fiber L-ENK levels in the CB1−/− mice may be a result of the unrestrained inhibition coming from the GABAergic interneurons that normally have CB1 receptors. The decrease in mossy fiber L-ENK levels could impact opioid-dependent synaptic plasticity, including long-term potentiation [16].
Several possibilities could contribute to the selective effects of CB1 knockout on L-ENK compared to DYN in the mossy fibers. For instance, as the levels of L-ENK in the mossy fibers are much less than those of DYN [15], changes in granule cell activity following knockout of the CB1 receptors may have a more noticeable effect on L-ENK. Alternatively, the significant reduction in L-ENK, but not DYN-ir in mice deficient in CB1 receptors may specifically relate to their respective activation of MOR/DOR and KOR [8;33]. Mossy fibers contain MORs [1;2], the preferred receptors for enkephalins rather than dynorphins [4], and previous studies have shown that MORs and CB1 receptors can reciprocally interact in the hippocampus [13]. Moreover, as the granule cells that release L-ENK also receive GABAergic transmission from terminals with CB1 receptors [22], it seems likely that CB1 receptor activation could regulate L-ENK activity. Activation of CB1 receptors has been directly correlated to the up-regulation of the L-ENK precursor pro-enkephalin [9]. Thus, removing CB1 receptors could directly down-regulate the production of L-ENK or otherwise alter MOR signaling.
NPY-containing neurons in the hilus of the dentate gyrus express enkephalin-activating DORs, but do not express CB1 receptors in their cell bodies [46][16]. Thus, the decrease in the number of NPY-immunoreactive neurons seen in the hilus of CB1−/− may be an indirect reflection of the reduced L-ENK in mossy fibers in these mutant mice. However, the levels of NPY might also be reduced indirectly through retrograde signals, since NPY containing neurons synapse on granule cell dendrites [45] as well as on CB1 receptor containing interneurons [24]. While one or both these indirect mechanisms seem likely to account for cannabinoid regulated NPY expression in hippocampal interneurons, we cannot exclude the possibility for a direct action involving yet undisclosed CB1 receptors on NPY containing axon terminals in the hilus.
The finding that NPY-containing neurons are sensitive to changes in their environment is supported by previous studies. For example, NPY-containing hilar interneurons are decreased following removal of the septohippocampal cholinergic inputs [30]. Conversely, after chronic unpredictable stress, the number of NPY-containing neurons increases in the hippocampus [17]. The decrease in the detectable number of NPY-containing neurons in the present study may reflect a reduction in peptide expression and/or a loss of NPY cells. Regardless, the decrease in NPY-containing cells could affect hippocampal function. Specifically, NPY-containing hilar neurons project to the outer dendrites of granule cells where they converge with entorhinal cortical afferents [45]. As NPY at entorhinal-granule cell synapses normally inhibits glutamate release and LTP [39], reduction of NPY would promote these processes.
The present finding that the number of hippocampal parvalbumin cells is not altered in CB1−/− mice was surprising as previous studies showed that parvalbumin cells are significantly reduced in the prefrontal and motor cortices as well as the striatum in male CB1−/− mice [14]. These differences could indicate that the effects of CB1 −/− are regionally selective. However, the lack of effect of CB1 receptor gene deletion on parvalbumin immunoreactive interneurons in the dentate gyrus of female mice used in the present study may also be the result of sexual dimorphism that is known to be influential in both the cannabinoid and opioid systems [26;34;46]. Alternatively, we cannot rule out the possibility of non-specific developmental changes in the CB1 −/− mice.
The lack of change in the density of CCK-ir in the inner molecular layer in CB1 −/− compared with wild-type mice is surprising given the normal prevalence of CB1 receptors in CCK-containing interneurons in the hippocampus [25;32;42]. This suggests that CB1 receptors in this brain region do not mediate the regulation of CCK production or CCK interneuronal networks. If so, this could have important functional consequences. In particular, GABAergic transmission in CCK-containing interneuron would occur with more strength in CB1−/− mice, as they have the same amount of cells as wild-type mice without the CB1 receptor mediated disinhibition. This idea also is consistent with the decrease in L-ENK in the mossy fibers seen in the CB1−/− mice, as this would result in decreased disinhibition [8].
Conclusions and Implications
Our results provide anatomical evidence that the presence of CB1 receptors is necessary for full expression of L-ENK in the mossy fibers and NPY in hilar interneurons. These results are consistent with the many behavioral studies that support a functional relationship between CB1 receptors and opioid systems in the hippocampus. CB1 receptor agonist-induced amnesic effects and memory consolidation are notably impaired by morphine activation of MORs, which are also the mediators of many of the central neural effects produced by enkephalin [10;48]. Moreover, cannabinoid abuse increases one's likelihood of developing opiate addiction and vice versa [11-13]. Prenatal and adolescent exposure to THC can also increase heroin-seeking tendencies in adults [9;38;41]. Our results further implicate CB1 receptor-dependent regulation of enkephalin and NPY in the underlying mechanisms that contribute to complementarity between cannabinoid and opioid systems in hippocampal learning and memory processes that promote addictive diseases.
Highlights.
Leu5-enkephalin levels are reduced in the mossy fiber pathway in CB1 knock-out mice
Neuropeptide Y interneurons in the dentate gyrus were less in CB1 knock-out mice
Dynorphin, cholecystokinin, and parvalbumin were unchanged in CB1 knock-out mice
Acknowledgements
Sophie Rogers is currently a student at Dalton High School, NY, NY. We thank her teacher, Dr. Jennifer Hackett, for her comments on the manuscript.
Supported by: NIH grants DA08259 & HL098351 (TAM), DA004600 (VMP)
Abbreviations
- BSA
bovine serum albumin
- CA
cornu ammonis
- CB1
cannabinoid 1 receptors
- CCK
cholecystokinin
- DG
dentate gyrus
- DOR
delta-opioid receptor
- DYN
dynorphin
- GABA
gamma amino butyric acid
- GCL
granule cell layer
- IML
inner molecular layer
- KOR
kappa-opioid receptor
- L-ENK
leu5-enkephalin
- LTP
long-term potentiation
- MOR
mu-opioid receptor
- NPY
neuropeptide Y
- PB
phosphate buffer
- TS
tris saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors declare no competing financial interests.
Author contributions: All authors designed experiments. S.A.R. and T.V.K. performed research and analyzed data. V.M.P. and T.A.M. obtained funding. S.A.R., T.A.M., and V.M.P. wrote the paper.
References Cited
- 1.Abbadie C, Pan YX, Drake CT, Pasternak GW. Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neurosci. 2000;100:141–153. doi: 10.1016/s0306-4522(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 2.Burstein SR, Williams TJ, Lane DA, Knudsen MG, Pickel VM, McEwen BS, Waters EM, Milner TA. The influences of reproductive status and acute stress on the levels of phosphorylated delta opioid receptor immunoreactivity in rat hippocampus. Brain Res. 2013;1518:71–81. doi: 10.1016/j.brainres.2013.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat. Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- 4.Celio MR, Heizmann CW. Calcium-binding protein parvalbumin as a neuronal marker. Nature. 1981;293:300–302. doi: 10.1038/293300a0. [DOI] [PubMed] [Google Scholar]
- 5.Chandy J, Pierce JP, Milner TA. Rat hippocampal mossy fibers contain cholecystokinin-like immunoreactivity. Anat. Rec. 1995;243:519–523. doi: 10.1002/ar.1092430415. [DOI] [PubMed] [Google Scholar]
- 6.Chang PC, Aicher SA, Drake CT. Kappa opioid receptors in rat spinal cord vary across the estrous cycle. Brain Res. 2000;861:168–172. doi: 10.1016/s0006-8993(99)02461-0. [DOI] [PubMed] [Google Scholar]
- 7.Commons KG, Milner TA. Ultrastructural localization of delta-opioid receptor immunoreactivity in the rat dentate gyrus. Analgesia. 1995;1:367–370. doi: 10.1016/s0006-8993(96)00774-3. [DOI] [PubMed] [Google Scholar]
- 8.Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog. Brain Res. 2007;163:245–814. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- 9.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 10.Farahmandfar M, Kadivar M, Naghdi N, Choopani S, Zarrindast MR. Influence of pre-exposure to morphine on cannabinoid-induced impairment of spatial memory in male rats. Behav. Brain Res. 2013;256:157–164. doi: 10.1016/j.bbr.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 11.Fattore L, Spano M, Melis V, Fadda P, Fratta W. Differential effect of opioid and cannabinoid receptor blockade on heroin-seeking reinstatement and cannabinoid substitution in heroin-abstinent rats. Br. J. Pharmacol. 2011;163:1550–1562. doi: 10.1111/j.1476-5381.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fattore L, Spano S, Cossu G, Deiana S, Fadda P, Fratta W. Cannabinoid CB(1) antagonist SR 141716A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacol. 2005;48:1097–1104. doi: 10.1016/j.neuropharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D. Bidirectional regulation of muopioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur. J. Neurosci. 2007;25:2191–2200. doi: 10.1111/j.1460-9568.2007.05470.x. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald ML, Lupica CR, Pickel VM. Decreased parvalbumin immunoreactivity in the cortex and striatum of mice lacking the CB1 receptor. Synapse. 2011;65:827–831. doi: 10.1002/syn.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 16.Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE. Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat. J. Neurosci. 2015;35:1723–1738. doi: 10.1523/JNEUROSCI.0820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley DF, Leasure JL. Region-specific response of the hippocampus to chronic unpredictable stress. Hippocampus. 2012;22:1338–1349. doi: 10.1002/hipo.20970. [DOI] [PubMed] [Google Scholar]
- 18.Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/SV mouse brains. Elsevier; Amsterdam: 2000. [Google Scholar]
- 19.Holm S. A simple sequentially rejective multiple testprocedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- 20.Jinno S, Kosaka T. Cellular architecture of the mouse hippocampus: a quantitative aspect of chemically defined GABAergic neurons with stereology. Neurosci. Res. 2006;56:229–245. doi: 10.1016/j.neures.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J. Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesner RP, Warthen DK. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–557. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- 24.Ledri M, Sorensen AT, Erdelyi F, Szabo G, Kokaia M. Tuning afferent synapses of hippocampal interneurons by neuropeptide Y. Hippocampus. 2011;21:198–211. doi: 10.1002/hipo.20740. [DOI] [PubMed] [Google Scholar]
- 25.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 26.Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, Williams TJ, Schierberl KC, Torres-Reveron A, Gonzales KL, McEwen BS, Waters EM. Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse. 2013;67:757–772. doi: 10.1002/syn.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milner TA, Pickel VM, Reis DJ. Ultrastructural basis for interactions between central opioids and catecholamines: I. Rostral ventrolateral medulla. J. Neurosci. 1989;9:2114–2130. doi: 10.1523/JNEUROSCI.09-06-02114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milner TA, Veznedaroglu E. Ultrastructural localization of neuropeptide Y-like immunoreactivity in the rat hippocampal formation. Hippocampus. 1992;2:107–126. doi: 10.1002/hipo.450020204. [DOI] [PubMed] [Google Scholar]
- 29.Milner TA, Waters EM, Robinson DC, Pierce JP. Degenerating processes identified by electron microscpic immunocytochemical methods. In: Manfredi G, Kawamata H, editors. Neurodegeneration, Methods and Protocols. Springer; New York: 2011. pp. 23–59. [DOI] [PubMed] [Google Scholar]
- 30.Milner TA, Wiley RG, Kurucz O, Prince SR, Pierce JP. Selective changes in hippocampal neuropeptide Y neurons following removal of the cholinergic septal inputs. J. Comp Neurol. 1997;386:46–59. [PubMed] [Google Scholar]
- 31.Neal CR, Jr., Newman SW. Prodynorphin peptide distribution in the forebrain of the Syrian hamster and rat: A comparative study with antisera against dynorphin A, dynorphin B, and the C-terminus of the prodynorphin precursor molecule. J. Comp. Neurol. 1989;288:353–386. doi: 10.1002/cne.902880302. [DOI] [PubMed] [Google Scholar]
- 32.Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J. Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol. Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA. Hippocampal mossy fiber leuenkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J. Chem. Neuroanat. 2014;55:9–17. doi: 10.1016/j.jchemneu.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierce JP, Kurucz O, Milner TA. The morphometry of a peptidergic transmitter system before and after seizure. I. Dynorphin B-like immunoreactivity in the hippocampal mossy fiber system. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Savanthrapadian S, Meyer T, Elgueta C, Booker SA, Vida I, Bartos M. Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J. Neurosci. 2014;34:8197–8209. doi: 10.1523/JNEUROSCI.5433-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slowe SJ, Simonin F, Kieffer B, Kitchen I. Quantitative autoradiography of m-, d- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res. 1999;818:335–345. doi: 10.1016/s0006-8993(98)01201-3. [DOI] [PubMed] [Google Scholar]
- 38.Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 39.Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog. Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- 40.Svingos AL, Colago EEO, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J. Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol. Psychiatry. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsou K, Mackie K, Sañudo-Peña MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing gabaergic interneurons in the rat hippocampal formation. Neurosci. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 43.Turner CD, Bagnara JT. General Endocrinology. W.B. Saunders; Philadelphia: 1971. [Google Scholar]
- 44.Van Kempen TA, Kahlid S, Gonzalez AD, Spencer-Segal JL, Tsuda MC, Ogawa S, McEwen BS, Waters EM, Milner TA. Sex and estrogen receptor expression influence opioid peptide levels in the mouse hippocampal mossy fiber pathway. Neurosci. Lett. 2013;552:66–70. doi: 10.1016/j.neulet.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veznedaroglu E, Milner TA. Elimination of artifactual labeling of hippocampal mossy fibers seen following pre-embedding immunogold-silver technique by pretreatment with zinc chelator. J. Microsc. Res. Tech. 1992;23:100–101. doi: 10.1002/jemt.1070230110. [DOI] [PubMed] [Google Scholar]
- 46.Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA. Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol. Learn. Mem. 2011;95:206–220. doi: 10.1016/j.nlm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav. Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Zarrindast MR, Navaeian M, Nasehi M. Influence of three-day morphine-treatment upon impairment of memory consolidation induced by cannabinoid infused into the dorsal hippocampus in rats. Neurosci. Res. 2011;69:51–59. doi: 10.1016/j.neures.2010.09.007. [DOI] [PubMed] [Google Scholar]