Abstract
The resilience of tropical corals to ocean acidification depends on their ability to regulate the pH within their calcifying fluid (pHcf). Recent work suggests pHcf homeostasis under short-term exposure to pCO2 conditions predicted for 2100, but it is still unclear if pHcf homeostasis can be maintained throughout a corals lifetime. At CO2 seeps in Papua New Guinea, massive Porites corals have grown along a natural seawater pH gradient for decades. This natural gradient, ranging from pH 8.1–7.4, provides an ideal platform to determine corals’ pHcf (using boron isotopes). Porites maintained a similar pHcf (~8.24) at both a control (pH 8.1) and seep-influenced site (pH 7.9). Internal pHcf was slightly reduced (8.12) at seawater pH 7.6, and decreased to 7.94 at a site with a seawater pH of 7.4. A growth response model based on pHcf mirrors the observed distribution patterns of this species in the field. We suggest Porites has the capacity to acclimate after long-time exposure to end-of-century reduced seawater pH conditions and that strong control over pHcf represents a key mechanism to persist in future oceans. Only beyond end-of-century pCO2 conditions do they face their current physiological limit of pH homeostasis and pHcf begins to decrease.
Tropical corals are the foundation species for coral reefs, the most diverse marine ecosystems in the world. The persistence of a species-rich reef-associated community will depend on the ability of corals to maintain net growth under future pCO2 conditions. To date our understanding of the fate of corals in the face of ocean acidification is based on controlled laboratory studies1,2, mesocosm studies mimicking coral community composition3,4,5, and field sites that function as natural ocean acidification analogues6,7,8. These efforts have provided strong evidence that many tropical corals will respond to future predicted pCO2 conditions with a decline in growth. However, corals actively establish a proton (H+) gradient by pumping protons out of the calicoblastic space between tissue and skeleton where calcification takes place, maintaining the pH of the calcifying fluid (pHcf) well above seawater pH (pHT)9,10. Therefore, the aragonite saturation state at the site of calcification is elevated relative to seawater, which likely fosters calcification.
The magnitude of the pHcf up-regulation can be derived indirectly by measuring the skeletal boron isotopic composition (δ11B) – an established pH proxy that appears to vary with pHcf at the calcification site9,11,12,13,14. Thus, it can be and is already used to determine the corals’ ability to elevate the pHcf at the site of calcification11,14,15,16,17. Culturing experiments have revealed that a reduction in seawater pHT is not directly reflected in the skeletal boron isotopic composition11,12,13, as the decline in skeletal δ11B, and hence internal pHcf, is less than the change in seawater pHT. At low seawater pHT, internal pHcf is still elevated compared to seawater pHT (up-regulation intensity, where ΔpH = pHcf − pHT18,19,20), but it does not reach those internal pHcf levels observed under control conditions11,18. Based on the observed relationship between internal pHcf and seawater pHT from laboratory studies, McCulloch et al.14 projected a continuous decline in growth under ocean acidification using their internal pH regulation and abiotic calcification (IpHRAC) model. The projected decline is species-specific, with massive Porites being regarded as a rather robust coral taxon. A recent short-term study, however, observed that corals exposed to reduced seawater pHT conditions in an in situ mesocosm experiment can maintain their internal pHcf irrespective of seawater pHT down to pHT 7.7416. While these data provide hope for coral persistence in the future, they cannot tell whether corals can maintain their internal pHcf in the long-term in their natural environment or if they are able to acclimate after long-term exposure to future ocean seawater pHT.
Volcanic carbon dioxide seeps in Milne Bay Province, Papua New Guinea (PNG) represent an ideal natural laboratory to investigate the effect of a seawater pHT gradient on coral skeletal δ11B and pHcf upregulation. A previous study found that at these seeps, massive Porites corals dominate coral reefs at seawater pHT levels projected for the end of the century (~7.8), and growth rates are similar compared to adjacent control sites6. At a seawater pHT of 7.7, reef formation ceases, and only a few scattered colonies of massive Porites are found close to a major seep site where seawater pHT is severely reduced (Supplementary Fig. S1). No corals are found below a seawater pHT of ~7.4, where seagrasses dominate the environment6. These distributional data contrast with projections based on the previously mentioned laboratory findings1, but allow testing of whether or not pHcf up-regulation is a key mechanism that allows Porites to dominate the PNG seeps and maintain pHcf homeostasis during their lifetime.
To investigate the corals’ ability to regulate their internal pHcf in situ along a seawater pHT gradient, we studied skeletal samples of massive Porites colonies from the CO2 seeps in PNG. Fourteen corals were sampled from four sites, namely a control site (8.1 pHT), intermediate site (7.9 pHT), low pHT site (7.6 pHT), and extreme site (7.4 pHT; Supplementary Fig. S1, Table S1,3). We tested whether the IpHRAC model14 can be used to reproduce the observed pattern in Porites distribution and growth by inferring the internal pHcf from the skeletal boron isotopic signature. We used high-resolution boron measurements to address whether strong natural variations in seawater pHT (as observed at the seep sites) are reflected in the skeletal boron isotopic signature. This has important implication for the use of δ11B to distinguish between sites of high seawater pHT variability (e.g. internal wave influenced reefs, upwelling) and sites with more stable conditions21,22.
Results and Discussion
We derived the first δ11B-pHT relationship for tropical corals collected along a natural pHT gradient (Fig. 1A). The observed relationship of δ11B against the mean seawater pHT values recorded at the four sites (Supplementary Figs S3 and 4, Table S3) differs from previous laboratory findings (Fig. 1A). The δ11B values of the five corals from the control site agreed well, but could not be distinguished from those corals collected at the intermediate site, while those from the low pHT site were lower than and significantly different from the control site (mean of all colonies per site ± s.e.m.: control site 20.91‰ ± 0.26 and low pH site 19.48‰ ± 0.40, respectively, Supplementary Table S5). At the extreme site the δ11B values were significantly lower than δ11B at all other sites (Fig. 1A, Supplementary Tables S4,5). In contrast to laboratory studies (using the same genus Porites and two other genuses namely Acropora and Stylophora, Fig. 1A), the here observed trend does not allow the reconstruction of seawater pH. This is similarly to a recent study16 (see also Supplementary Material: “Average boron isotopic signature, variability and corresponding internal calcifying conditions”). Also our study and this recent work16 show that variations between individuals are often greater than the effect of external environmental conditions on the boron isotopic composition (e.g. individual differences at control = 1‰ and intermediate site = 1.09‰, compared to an average difference of 0.34‰). Corresponding pHcf values suggest that the corals’ internal pHcf remained within a narrow range, with mean values ranging from 8.30 to 7.83, while the seawater pHT changed from 8.1 to 7.4 (Fig. 1B), as confirmed by direct pHcf measurements9. This underlines a strong physiological control on their internal pHcf irrespective of seawater pHT. The pH up-regulation (∆pH) effort was significantly higher at all seep-influenced sites compared to the control site (mean of all colonies per site ± s.e.m.: control site 0.14 ± 0.02 pH units, intermediate site 0.31 ± 0.04, low pHT site 0.52 ± 0.04 and extreme site 0.54 ± 0.05, respectively; Supplementary Table S4,5). The highest mean ΔpH up-regulation observed in any of the studied colonies was 0.68 ± 0.03 (Fig. 2).
Figure 1. Boron isotopic signature and corresponding internal pHcf of Porites corals from the Papua New Guinea (PNG) pCO2 seeps.
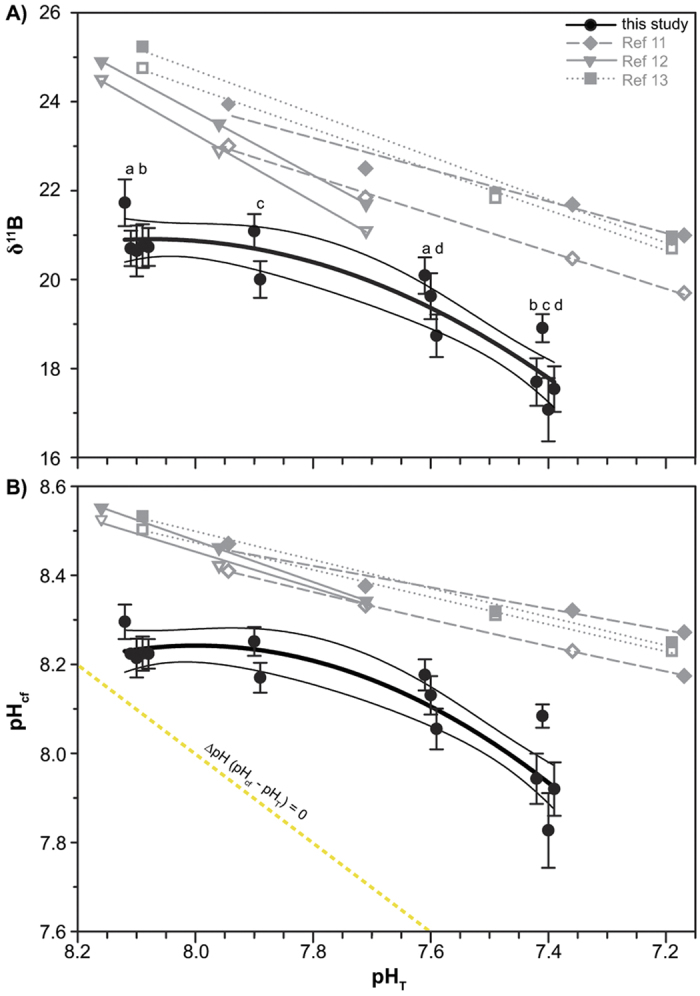
(A) Average δ11B values measured in 14 massive Porites coral skeletons collected along a seawater pHT (pH in total scale) gradient at the PNG seeps. (B) Coral skeletal δ11B signature translated into internal calcifying fluid pH (pHcf). Black filled circles and error bars are means ±1 SE per colony (n = 15–20 samples per colony). Individual colonies at each site are offset horizontally for clarity. Black lines: Regression analysis following a second-order polynomial fit (thick black line) with 95% confidence interval (thin black lines). Grey symbols and lines represent literature data of laboratory findings for tropical corals11,12,13 (11open symbol: Stylophora pistillata lateral growth and filled symbol: Stylophora pistillata apical growth, 12open symbol: Acropora nobilis and filled symbol: Porites cylindrica, 13open symbol: Stylophora pistillata and filled symbol: massive Porites sp.). Letters (a, b, c and d) indicate results of the post hoc test when there was a significant site effect (p < 0.01). Statistical test can be found in the supplements Table S4. Yellow dashed lines indicate internal pH conditions when organisms are not up-regulating pHcf.
Figure 2. Massive Porites corals pH up-regulation.
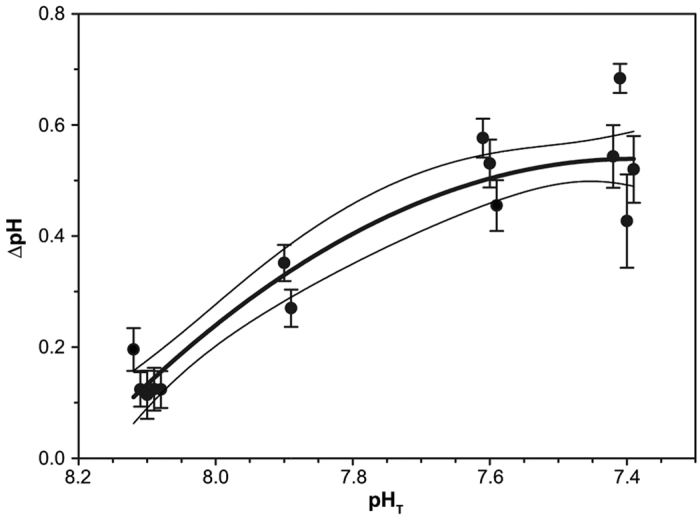
Internal pH up-regulation intensity of corals collected (ΔpH) along a natural seawater pH (pHT in total scale) gradient. Symbols display mean ± SE values for each coral colony collected at four sites with known pHT conditions. Solid black lines indicate regression analysis following a second-order polynomial fit (thick black line) with 95% confidence interval (thin black lines).
The corals in this study were growing within a few hundred meters distance from each other, under similar physicochemical settings excluding pCO2 (e.g. similar water flow, salinity, temperature, nutrient levels, total alkalinity)6,23. Under long-term exposure to these natural environmental conditions, the corals showed the ability to compensate for reduced external seawater pHT by increasing internal pHcf up-regulation. Combined with results from a recent study16, our results suggest corals can maintain pHcf homeostasis and highlight that even corals exposed to pCO2 conditions predicted for the end of the century for their entire lifetime can maintain internal pHcf. Only beyond this threshold do they face their current physiological limit, where pHcf begins to decrease.
We calculated relative rates of calcification based on our internal pHcf values using the IpHRAC model14: G = k*(Ωcf − 1)n (see McCulloch et al.14, and Materials and Methods for more details). Fabricius et al.6 only measured growth at vent sites with seawater pHT levels not lower than 7.75 (expected seawater pHT values for the end of the century), not covering the seawater pHT range of this study. We used their measured growth ratio and compared it to the relative growth rate calculated by the IpHRAC model based on our derived pHcf. The similarity in growth (G) between our intermediate site and the control site (Gintermediate/Gcontrol = 1.23 to 0.91; Supplementary Table S6) corroborates the lack of calcification response observed in a previous study6. Hence, we used the model to extrapolate growth for the other two sites (low pHT and extreme site). Calcification rates at the low pHT site are still similar to present day rates and become reduced at the extreme site. Overall, our modelled growth response mirrors the coral distribution observed at the PNG sites.
The IpHRAC model from McCulloch et al.14 for Porites (Fig. 3) suggests a continuous decline in growth and contrasts to the model derived using our data from the PNG seeps. Here we found a similar growth rate for control, intermediate and low pHT site before growth rates decrease to the extreme site (Fig. 3). The ΔpH up-regulation intensity potentially reaches a physiological limit and becomes energetically expensive at the extreme site. This site corresponds to the limit of Porites occurrence at the PNG seeps, beyond which the coral do not grow. Corals at seawater pHT levels of 7.9 potentially acclimate to these pCO2 conditions by enhancing internal pHcf up-regulation. Laboratory studies have also shown a curvilinear growth response9,24, even with similar growth rates at a pCO2 of 2553 ppm (pHT = 7.32) compared to pre-industrial levels (Fig. 324). In the latter study24, they did not test whether internal pHcf was similarly elevated at pCO2 324 and 2553 ppm. Our study and a recent pHcf homeostasis hypothesis16 would indicate that pHcf in corals exposed to both treatments should be similarly elevated, but this still needs to be validated for the experiment by Castillo et al.24. Considering the short duration (95 days) of their experiment, it is questionable whether the corals would be able to maintain calcification at 2553 ppm CO2 for longer periods of time, considering the expected increase in energy demand at ecological time scales. Our calcification model (Fig. 3) suggests that even the very robust Porites corals would have reduced rates of calcification when exposed to levels that are far beyond those projected for the end of the century for a lifetime. Calcification is an energy expensive process and hence, the increased up-regulation at the seep sites requires more energy that must be provided in order for the corals to acclimate. The expected increase in seawater dissolved inorganic carbon in future oceans may enhance photosynthesis, and consequently provide more energy to the corals at the intermediate and low pHT sites to cover their increased daily budget without negative consequence24,25. However, the increase in photosynthesis might not be sufficient to maintain a high pHcf. Furthermore, it is not known what other physiological and metabolic trade-offs the corals may face, potentially affecting the calcification response and also their viability. Here we support previous findings that internal pHcf up-regulation mitigates ocean acidification9. Thus, pH up-regulation represents a mechanism that can make corals more resilient to future pCO2 conditions14. Venn et al.9 cultured corals under various pCO2 levels and observed a similar calcification response as modelled here. However, in contrast to our observations their directly measured internal pHcf decreased with external seawater pH changes. They explored two potential models (extended models of McCulloch et al.14) and tested whether they can explain their observed calcification rates based on their internal pHcf values. One model assumes constant energy investment and proton removal rate, and the second model is based on a variable proton removal rates. Their second model more closely represents their corals’ response with an initial increase and then a decrease in proton removal rate. We do not have independent measurements of calcification rates for the low pHT and extreme sites. Such data would allow to test whether the modelled growth values (based on mainly the boron derived internal pHcf) also match measured growth data for these sites as they did for the intermediate site. What we wanted to point out here is that while for our intermediate site boron derived internal pHcf and modelled growth agree, internal pHcf is likely not the only determining factor for calcification rates. Hence, the variable proton removal model revealed a very important aspect: to fully understand the calcification response we need to constrain more than just internal pHcf and calcification rates. Studies investigating gene regulation variation as a consequence of increasing pCO2 conditions26,27 indicated that full suite of processes are potentially affected by ocean acidification and can affect calcification rates. For instance, after short-term exposure to near-future seawater pHT conditions, corals responded with an up-regulation of genes involved in ion transport (in particular Ca2+-transporters like Ca-ATPase that also affects internal pHcf, but also bicarbonate transporters)26. Such a response might help to maintain the internal pHcf and calcification rate. In the same study26, short-term exposure of corals to pHT 7.2 resulted in a down-regulation of ion transporters and potentially can explain physiological limits in growth. Ocean acidification also can affect a wide range of cellular processes that are not directly linked to biomineralization27. These studies indicate the need for a more comprehensive approach combining physiological and transcriptomic investigations with ecological and geochemical studies.
Figure 3. Growth response modelled for massive Porites corals at the Papua New Guinea seeps.
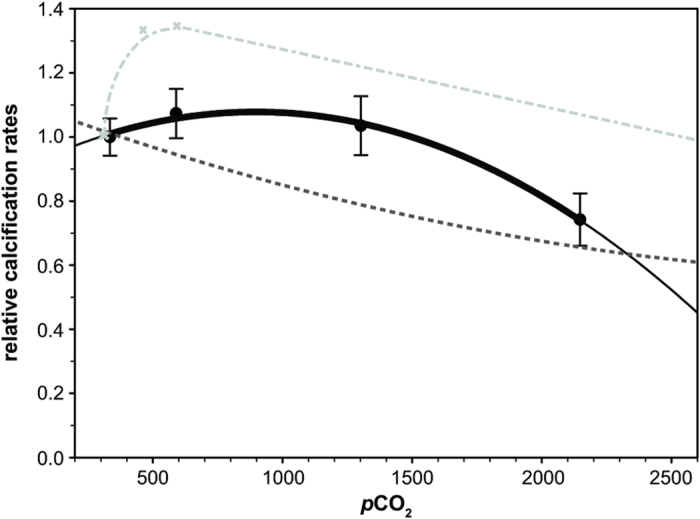
The modelled growth response displays relative changes in calcification rate (relative calcification rate = mean control/mean site). Black circles and error bars represent means ±1 SE per site and the black solid line indicates a second-order polynomial fit for the growth model in this study. The growth response curve is compared to published growth responses: McCulloch et al.19 (dark grey dashed line) and Castillo et al.24 (light grey dashed line).
Natural analogues to ocean acidification, such as the PNG seeps, provide unique opportunities for studying the potential effects of elevated pCO2 on coral reefs, but they also have limitations, e.g. strong fluctuations in pHT/pCO2 and close connectivity to undisturbed areas that supply propagules. The physiological consequences of such strong pHT fluctuations are still not fully understood. Recent studies have shown that growth in coral recruits was higher under fluctuating pCO2 conditions than under constant reduced pHT28, and that exposure to strong temperature variations resulted in an improved stress-resistance29,30 and faster acclimation31 in corals. Similarly, the hypothesized pH homeostasis observed during the free ocean carbon enrichment (FOCE) experiment, argues that the seasonal seawater pHT variations the corals are facing are a driver for stronger control on their internal pHcf environment16. Thus, the fluctuating conditions could potentially foster acclimation to low seawater pHT. Daily swings in pCO2 or strong fluctuating seawater pHT conditions are not unusual in coral reefs21,22,32,33. At the CO2 seeps in PNG, Porites is able to cope with the projected near-future increase in pCO2, in contrast to most other coral species, including the structurally complex species that form the habitat for many reef-associated organisms34. Responses to pCO2 also vary between regions with naturally reduced seawater pHT7,35,36, as changes in seawater pHT are not the only factor in the field and act in concert with the full suite of environmental variability (e.g. seasonality, differences in current regimes, etc.). In addition, boron isotopic composition is highly variable at high spatial resolution15,17, and in our study, irrespective of seawater pHT variability. This agrees with the conclusion that such δ11B variations reflect the effect of biological processes on skeletal isotopic composition rather than external seawater pHT variations15. In particular, since the control site corals (where the seawater pH is stable) showed the same skeletal variations. Several factors are thought to contribute to these internal variations in pHcf, but they are not yet fully understood (see also Supplementary Material: Average boron isotopic signature, variability and corresponding internal calcifying conditions).
Our study shows that massive Porites will be able to persist in the oceans of 2100, due to observed similar growth rates to present day conditions6,25, enhanced photosynthesis25 and also its ability to maintain a high internal pHcf. All of these factors contribute to Porites’ dominance at the Papua New Guinea CO2 seeps. Enhanced pHcf up-regulation enables them to sustain their present day calcification rate up to pCO2 levels projected for the end of the century. From massive Porites at the PNG seeps, we have observed that this species has the potential to adjust and maintain their internal pHcf even after lifetime exposure to increased pCO2. For other more sensitive corals, it needs to be elucidated whether or not they are able to maintain their internal pHcf. Our study underlines that conclusions projected from laboratory studies alone need to be treated with caution, and should be complemented by results from field studies. Together with a recent study we emphasize that seawater pH reconstruction from Porites need to be taken with caution. Both studies underline this genus ability to exert strong physiological control. Such local acclimations represent one possibility for resisting future changes. It is thus essential to understand what allows corals in a certain environment to acclimate, and whether other species in other regions have the same capacity to adjust to future changes.
Material and Methods
Site description and coral core collection
Fourteen coral cores were collected during three research cruises from four sites that differed in their seeping intensity: an extreme site, a low pHT site, an intermediate site, and a control site (Supplementary Material: sites and coral sample overview, Table S1, Fig. S1). The seawater pHT adjacent to the coral colonies was recorded with data loggers and total alkalinity (TA) measured in discrete water samples. The carbonate chemistry was calculated from seawater pHT and TA for the four sites (Supplementary Material: Seawater pHT characterization at the collection sites and seawater carbonate chemistry, Figs S3-4, Table S3).
Sample preparation and analyses
Coral skeletons were bleached for 24 h, thoroughly washed with milli-Q and dried overnight at 50 °C. Subsequently, they were embedded in resin, cut along the growth axis, ground and polished. From long cores, a piece approx. 5 mm wide and 1 cm long oriented along the growth axis was prepared for boron analysis and carefully ground and polished. The δ11B composition was measured with a laser ablation multi collector inductive coupled plasma mass spectrometer (Thermo Fisher MC-ICP-MS AXIOM, connected to a UP193fx laser ablation system of New Wave Research, equipped with an excimer 193 nm laser) following the method by Fietzke et al.37 (Supplementary Material: Boron isotopic signature, Table S2).
Data analyses
The data reduction followed Fietzke et al.37. This yielded one δ11B value per sample and session with an average precision of 1‰ (1 SD) for approx. 2.5 μg of carbonate sample. A minimum of 15 individual values of δ11B spread over the core surface from the upper few mm of each coral colony were measured to obtain a representative data set per sample. The data set reflects the high variability in δ11B for a single colony. For each individual δ11B value the internal pHcf and ∆pH was calculated. Individual values per colony were averaged to yield values that reflect the average δ11B value, the average internal pHcf and ∆pH (see below).
Each individual δ11B value was translated into internal pHcf following equation (1) with a seawater δ11Bsw of 39.61‰38, a fractionation factor (αB) of 1.027239 and pK*B of 8.5640.
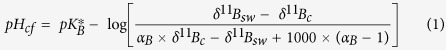 |
Following the method in Trotter et al.18, the superimposed physiological pH control was calculated using the equation:
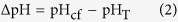 |
and related to the seawater pHT to quantify the extent of the physiological control on the internal pHcf.
Calcification rate (G) was calculated following the McCulloch et al.14 IpHRAC model: G = k*(Ωcf − 1)n. Seawater dissolved inorganic carbon concentration [DIC]sw was calculated by the R package seacarb41 using the external seawater pHT and the average total alkalinity (2272 μmol kg−1), salinity (34.5 ppm) and temperature (28.5 °C) measured at the sites2 (and Supplementary Table S3). Carbonate saturation state at the site of calcification (Ωcf) was calculated using seacarb by setting [DIC]cf equivalent to 2*[DIC]sw114 assuming an elevated [Ca]2+ concentration of 11 mmol kg−1 and the average salinity and temperature. The modelled calcification response was calculated for constant temperature and with the temperature-dependent rate law constant k = 42.42 and reaction order constant n = 1.89 (applying the equations given in McCulloch et al.14: k = −0.0177*T2 + 1.47*T + 14.9 and n = 0.0628*T + 0.0985).
Data analysis and visualisation was done with R Studio version 3.0.1 (R Development Core Team, 2015). The regression analysis and growth model fit were done using a generalized linear model. An AIC criterion was used to find the best-fit comparing linear vs polynomial (2nd and 3rd order) fits. The software package visreg (2.0–4) was used to visualize the best fit.
Additional Information
How to cite this article: Wall, M. et al. Internal pH regulation facilitates in situ long-term acclimation of massive corals to end-of-century carbon dioxide conditions. Sci. Rep. 6, 30688; doi: 10.1038/srep30688 (2016).
Supplementary Material
Acknowledgments
We thank the families of Upa-Upasina for allowing us to study their coral reefs. Many thanks also to the National Research Institute of Papua New Guinea, the crew of the MV Chertan, and QantasLink, for logistic support. The study was funded by German Federal Ministry for Education and Research project BIOACID II (Consortium 3: Natural CO2-rich reefs as windows into the future: Acclimatization of marine life to long-term ocean acidification and consequences for biogeochemical cycle, WP 3.5: Structural and Chemical Changes in biogenic carbonates Grant number: 03F0655A), the Great Barrier Reef Foundation’s “Resilient Coral Reefs Successfully Adapting to Climate Change” research and development programme in collaboration with the Australian Government, and the Australian Institute of Marine Science.
Footnotes
Author Contributions M.W., J.F. and K.E.F. designed the experiments. M.W., L.C.H., A.F., D.d.B. and K.E.F. performed field research. M.W. and J.F. analysed the samples. A.F. and D.d.B. provided pH measurements. M.W., K.F. and A.F. analysed data. M.W., J.F., G.M.S., L.C.H., A.F., D.d.B. and K.E.F. were involved in the preparation of the manuscript
References
- Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S. & Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 105, 17442–17446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq N., Gattuso J.-P. & Jaubert J. CO2 partial pressure controls the calcification rate of a coral community. Glob. Chang. Biol. 6, 329–334 (2000). [Google Scholar]
- Langdon C. et al. Effect of calcium carbonate saturation state on calcifcation rate of an experimental coral reef. Global Biogeochem. Cycles 14, 639–654 (2000). [Google Scholar]
- Jokiel P. L. et al. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483 (2008). [Google Scholar]
- Dove S. G. et al. Future reef decalcification under a business-as-usual CO2 emission scenario. Proc. Natl. Acad. Sci. USA 110, 15342–15347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 1, 165–169 (2011). [Google Scholar]
- Crook E. D., Cohen A. L., Rebolledo-vieyra M., Hernandez L. & Paytan A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 110, 11044–11049 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamberger K. E. F. et al. Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys. Res. Lett. 41, 499–504 (2014). [Google Scholar]
- Venn A. A. et al. Impact of seawater acidification on pH at the tissue – skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. USA 110, 1634–1639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Horani F., Al-Moghrabi S. M. & de Beer D. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: active internal carbon cycle. J. Exp. Mar. Bio. Ecol. 288, 1–15 (2003). [Google Scholar]
- Holcomb M. et al. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, 5207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S. et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta 74, 4988–5001 (2010). [Google Scholar]
- Hönisch B. et al. Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects. Geochim. Cosmochim. Acta 68, 3675–3685 (2004). [Google Scholar]
- McCulloch M., Falter J., Trotter J. & Montagna P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2, 623–627 (2012). [Google Scholar]
- Allison N., Finch A. & EIMF A. δ11B, Sr, Mg and B in a modern Porites coral: the relationship between calcification site pH and skeletal chemistry. Geochim. Cosmochim. Acta 74, 1790–1800 (2010). [Google Scholar]
- Georgiou L. et al. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl. Acad. Sci. USA 112, 13219–13224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollion-Bard C., Chaussidon M. & France-Lanord C. pH control on oxygen isotopic composition of symbiotic corals. Earth Planet. Sci. Lett. 215, 275–288 (2003). [Google Scholar]
- Trotter J. et al. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: Validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 303, 163–173 (2011). [Google Scholar]
- McCulloch M. et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim. Cosmochim. Acta 87, 21–34 (2012). [Google Scholar]
- Anagnostou E., Huang K., You C., Sikes E. L. & Sherrell R. M. Evaluation of boron isotope ratio as a pH proxy in the deep sea coral Desmophyllum dianthus: Evidence of physiological pH adjustment. Earth Planet. Sci. Lett. 349–350, 251–260 (2012). [Google Scholar]
- Schmidt G. M. et al. Coral community composition and reef development at the Similan Islands, Andaman Sea, in response to strong environmental variations. Mar. Ecol. Prog. Ser. 456, 113–126 (2012). [Google Scholar]
- Hofmann G. E. et al. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLos One 6, e28983 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthicke S., Momigliano P. & Fabricius K. E. High risk of extinction of benthic foraminifera in this century due to ocean acidification. Sci. Rep. 3, 1–5 (2013). [Google Scholar]
- Castillo K. D., Ries J. B., Bruno J. F. & Westfield I. T. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. R. Soc. B Biol. Sci. 281, doi: (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl J. et al. Physiological and ecological performance differs in four coral taxa at a volcanic carbon dioxide seep. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 184, 179–186 (2015). [DOI] [PubMed] [Google Scholar]
- Vidal-Dupiol J. et al. Genes related to ion-transport and energy production are upregulated in response to CO2-driven pH decrease in corals: new insights from transcriptome analysis. PLos One 8, e58652 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniewska P. et al. Major Cellular and Physiological Impacts of Ocean Acidification on a Reef Building Coral. PLos One 7, e34659 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufault A. M., Cumbo V. R., Fan T., Edmunds P. J. & Soc P. R. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B 279, 20112545 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M. et al. Large-amplitude internal waves benefit corals during thermal stress. Proc. R. Soc. B 282, 20140650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger P., Schmidt G. M., Wall M., Held C. & Richter C. Temperature tolerance of the coral Porites lutea exposed to simulated large amplitude internal waves (LAIW). J. Exp. Mar. Bio. Ecol. 471, 232–239 (2015). [Google Scholar]
- Oliver T. A. & Palumbi S. R. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30, 429–440 (2011). [Google Scholar]
- Price N. N., Martz T. R., Brainard R. E. & Smith J. E. Diel Variability in Seawater pH Relates to Calcification and Benthic Community Structure on Coral Reefs. PLos One 7, 1–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook E. D., Potts D., Rebolledo-Yieyra M., Hernandez L. & Paytan A. Calcifying coral abundance near low-pH springs: implications for future ocean acidification. Coral Reefs 31, 239–245 (2012). [Google Scholar]
- Fabricius K. E., De’ath G., Noonan S. & Uthicke S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc. R. Soc. B 281, 20132479 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley H. C. et al. Changes in coral reef communities across a natural gradient in seawater pH. Sci. Adv. 1, e1500328 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Kayanne H., Yamamoto S. & Kurihara H. Spatial community shift from hard to soft corals in acidified water. Nat. Clim. Chang. 3, 1–5 (2013). [Google Scholar]
- Fietzke J. et al. Boron isotope ratio determination in carbonates via LA-MC-ICP-MS using soda-lime glass standards as reference material. J. Anal. At. Spectrom. 25, 1953 (2010). [Google Scholar]
- Foster G. L. Seawater pH, pCO2 and [CO32−] variations in the Caribbean Sea over the last 130 kyr: A boron isotope and B/Ca study of planktic foraminifera. Earth Planet. Sci. Lett. 271, 254–266 (2008). [Google Scholar]
- Klochko K., Kaufman A. J., Yao W., Byrne R. H. & Tossell J. A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 276–285 (2006). [Google Scholar]
- Dickson A. G. Standard potential of the reaction: AgCl(s) + 1/2H2(g) = Ag(s) + HCl(aq) and the standard acidity constant of the ion HSO4− in synthetic seawater from 273/15 to 318.15K. J. Chem. Thermodyn. 22, 113–127 (1990). [Google Scholar]
- Gattuso J.-P. & Lavigne H. Technical Note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 6, 2121–2133 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


