Abstract
Objective
To examine whether exposure to general anesthesia for surgeries and procedures after the age of 40 is associated with incident mild cognitive impairment (MCI) in the elderly.
Patients and Methods
A population-based prospective cohort of Olmsted County, MN, residents ages 70-89 years on October 1, 2004, underwent baseline and 15-month interval evaluations that included the Clinical Dementia Rating scale, a neurologic evaluation, and neuropsychological testing. Anesthesia records after age 40 until last evaluation for MCI were abstracted. Proportional hazards regression, adjusting for other known MCI risk factors, was used to assess whether exposure to surgical general anesthesia after the age of 40 is associated with the incidence of MCI.
Results
Of 1,731 participants (mean age 79), 536 (31%) developed MCI during median 4.8 years follow-up. Anesthesia exposure was not associated with MCI when analyzed as a dichotomous variable (any vs. none, adjusted HR 1.07 [95% CI 0.83-1.37]; P=.61), the number of exposures (adjusted HR = 1.05 [0.78-1.42], 1.12 [0.86-1.47], and 1.02 [0.76-1.34] for 1, 2-3, and ≥4 exposures compared to no exposure as the reference; P=.73), or as the total cumulative duration of exposure (adjusted HR =1.00 [0.98-1.01] per 60 min increase, P=.83). In secondary sensitivity analyses anesthesia after age 60 was associated with incident MCI (adjusted HR =1.25 [1.02-1.55], P=.04), as was exposure in the previous 20 and 10 years.
Conclusion
We found no significant association between cumulative exposure to surgical anesthesia after age 40 and MCI. However, these data do not exclude the possibility that anesthetic exposures occurring later in life may be associated an increase in the rate of incident MCI.
Keywords: Anesthesia, aged humans, male, female, Alzheimer's dementia, dementia, procedures, surgery, mild cognitive impairment
Introduction
Exposure of the elderly to anesthesia and surgery can transiently affect postoperative cognitive function in some patients, a phenomenon referred to as postoperative cognitive dysfunction (POCD).1-3 Whether such exposures lead to more prolonged cognitive impairment remains unclear. Some preclinical studies suggest that exposure to volatile anesthetics cause neurohistological changes and subsequent memory impairment consistent with Alzheimer dementia (AD) or dementia generally.4-6 The majority of clinical research did not find an association between exposure to anesthesia and dementia.7-10 However, there are several challenges to conduct this type of studies. Cognitive changes sufficient to meet criteria for the diagnosis of dementia develop over many months or years, posing challenges to examine association between exposure and dementia. In particular, many studies have not been able to ascertain that persons without dementia do not have very mild, subtle, cognitive changes.7, 8 Furthermore, comorbidities necessitating surgery may themselves contribute to cognitive impairment,11, 12 confounding the interpretation of associations. Finally, the clinical diagnosis of dementia may not be the most sensitive measure of prolonged cognitive impairment. Mild cognitive impairment (MCI), defined as a stage of cognitive impairment between normal function and dementia, has been recently introduced as a useful clinical and research diagnostic category.13, 14 In this context, MCI diagnosis provides an additional opportunity to explore the association between anesthesia and cognitive function with potentially greater sensitivity to the effects of surgical procedures requiring general anesthesia.
The present study is based on a prospectively assembled cohort of Olmsted County, MN residents enrolled in the Mayo Clinic Study of Aging (MCSA).15 This long-standing study employs rigorous longitudinal assessment of cognitive function in a large population-based cohort. The primary aims of the MCSA are to examine the incidence, prevalence, and risk factors for cognitive decline.15-17 It has identified several factors, including age, education, sex, ApoE genotype, diabetes, depression, cardiovascular disease, and stroke as risk factors for incident MCI.18In the present study we combined the resources of Rochester Epidemiology Project (REP),19 which provides access to all medical records of residents of Olmsted County, MN, and the MCSA study data to test the hypothesis that exposure to general anesthesia after the age of 40 is associated with the incidence of MCI in the elderly. The study cohort included patients who were cognitively normal at the time of their enrollment in the MCSA so that the primary outcome of incident MCI could be assessed.
METHODS
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center, Rochester, MN. At the time of enrollment in the MCSA, all participants provided written informed consent for longitudinal assessment. This analysis included those MCSA participants who had also provided prior authorization for the use of their medical information in research (Minnesota Statute 144.335), such that their anesthesia and surgical records could be reviewed. Manuscript preparation was in accordance with the STROBE checklist for cohort studies (www.strobe-statement.org).
Design Overview
This is a prospective population-based cohort designed to study the factors associated with decline in cognitive function. Details of the study design have been previously reported.15 MCSA procedures are summarized as follows.16, 17
Study Participants
Residents of Olmsted County were eligible for enrollment in the MCSA. Determination of residency status and medical record information was provided by the REP,20-23 a medical records linkage system that takes advantage of the fact that most Olmsted County residents seek medical care from 2 providers, the Mayo Clinic and Olmsted Medical Center. Both sites use a unit medical record which includes all outpatient and inpatient information for each patient, with an extensive indexing system based on surgical and medical diagnoses maintained by Mayo Clinic. Olmsted County residents who were between 70 and 89 years old on October 1, 2004 were identified, randomly selected and invited to participate in the study.15
Upon enrollment in the MCSA, participants completed a nurse evaluation including questions about memory and risk factor assessment (based on family and medical history), a neurological evaluation and a neuropsychological and laboratory (ApoE genotype biomarker) evaluation. Self-reported medical history was corroborated using information abstracted from the medical records of the records-linkage system. The neurologic evaluation included the Short Test of Mental Status,24 a modified Hachinski Scale,25, 26 a modified Unified Parkinson's Disease Rating Scale,27 and a questionnaire developed to elicit neurologic conditions that could influence cognition. The neuropsychological evaluation included subtests of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) and Wechsler Memory Scale-Revised,28 and assessed performance in 4 cognitive domains: memory, executive function, language, and visuospatial skills. A study partner (informant) completed the Clinical Dementia Rating (CDR) Scale29 and a Functional Activities Questionnaire.30 In addition, the functional status of each subject was elicited from the study partner also using the Functional Assessment Questionnaire.30 In the rare instances where there was no informant, subjective cognitive impairment was based solely upon information obtained from the participant.
Outcome Measures: Diagnosis of normal cognition, MCI and dementia
Subsequent to the evaluations, an expert consensus panel consisting of the physician and nurse or study coordinator who evaluated the participant, and a neuropsychologist reviewed and discussed all the data for each subject.15 The diagnosis of MCI was based on published criteria: 1) impairment in one of the four cognitive domains; 2) cognitive concerns by the subject, informant, examining nurse, or physician; 3) essentially normal functional activities, and; 4) absence of dementia (based on published criteria).13, 14, 31 Subjects who performed within the normal range and did not meet criteria for MCI or dementia were considered as cognitively normal. Each diagnosis was made by a consensus decision between the physician, neuropsychologist, and nurse or study coordinator.15 Only subjects who were cognitively normal at the baseline evaluation and had at least one follow-up evaluation to assess incident events were included in the current analysis. Participants were evaluated at 15-month intervals to assess changes in neurocognitive status and to detect incident MCI or dementia using the same protocol used at the baseline evaluation. Since surgery may affect cognition, participants were evaluated at least 1 month or longer following an acute illness, surgery or procedure.
Data abstraction of anesthesia for surgical and nonsurgical procedures
For all participants medical records were abstracted for each episode of general anesthesia between age 40 and the index date of MCI diagnosis or last follow-up in those who did not develop MCI. This age was chosen to provide a relatively long exposure history useful to perform sensitivity analyses (see Statistical analysis) and was available in the medical records for most individuals. For individuals whose medical records began after the age of 40, anesthetic exposure information was abstracted back to the date their medical records started. For each exposure to anesthesia the agents used for induction and maintenance of anesthesia, the type of surgery or procedure, and the duration of anesthesia were recorded. Episodes of anesthesia with regional block only, as well as those who received sedation and subcutaneous injection of local anesthetic, were excluded. All data were entered manually into the web-based Research Electronic Data Capture (REDCap®) system (Version 3.6.7, Vanderbilt University, Nashville, Tennessee).32 Anesthetic exposures were retrieved from paper medical records, surgical reports, electronic medical records, and all were cross checked with the electronic anesthesia database and Data Mart, a Microsoft SQL relational data warehouse that provides direct access to electronic medical records data for patients across Mayo Clinic Rochester hospitals. Exposures to anesthesia in Olmsted County hospitals were identified via REP procedure databases, with detailed information retrieved from scanned information, microfilms, and paper medical charts. Surgical anesthetics or procedures that were performed outside the Olmsted County and were self-reported in medical records were not included as exposures in our analysis.
Statistical analyses
Each individual was considered at risk for MCI from the date of enrollment in the MCSA. Proportional hazards regression, with age as the time scale, was used to assess whether exposure to surgeries and procedures requiring general anesthesia after the age of 40 was a risk factor for development of MCI. Anesthesia exposure was quantified as any vs. none; number of exposures (no exposure, 1, 2-3, ≥4), or cumulative exposure in separate analyses. To accommodate the surgeries that occurred after enrollment, time dependent variables were used when quantifying anesthesia exposure (see below). For the primary analyses the initial values for these variables were those calculated using all surgeries from age 40 up to the time of enrollment. The values of the variables were then updated to reflect any surgical procedures performed following enrollment. Both unadjusted and adjusted analyses were performed. For the adjusted analyses, sex, education, marital status, smoking status, alcohol, ApoE ε4 genotype, midlife diabetes, midlife hypertension, midlife dyslipidemia, atrial fibrillation, congestive heart failure, history of stroke, and coronary artery disease were included as covariates, factors found in prior analysis to predict MCI. In all cases, separate models were used to evaluate the different anesthesia exposure variables with results summarized using hazard ratio estimates and corresponding 95% confidence intervals.
Additional secondary sensitivity analyses were planned a priori. First, to explore the concept that risk produced by exposure to anesthesia and procedures increases with age, the primary analyses were repeated including only exposures to anesthesia after age 60 (i.e., not including exposures prior to age 60). In addition, using a reversible binary time dependent variables separate analyses were performed for any exposure to anesthesia in 5, 10, and 20 year intervals prior to developing MCI or last follow-up visit. For these analyses the time-dependent indicator variable that was set to 1 at the time of exposure was reset to 0 after the given time was elapsed with no further exposure. For example, if a patient who enrolled in the MCSA at age 70 was exposed to their first anesthetic on their 53rd birthday, and had no additional exposures prior to developing MCI (or last follow-up) at the age of 75; the binary variable for any exposure in the preceding 20 years would have value of 1 from the time of enrollment until the patient's 73rd birthday, at which point it would be reset to 0. Similar analyses were performed using time-dependent variables representing the number of exposures and cumulative duration of exposure in the 5 and 10 year intervals prior to developing MCI or last follow-up. To explore whether type of surgery is associated with MCI, separate proportional hazards regressions were performed using ten categories of primary types of surgeries or procedures. In addition, since ApoE genetic testing was performed prospectively, and its presence is a known risk for AD,33 we performed exploratory analyses to assess whether ApoE ε4 allele status (carrier or non-carrier) is a potential effect modifier of exposure effect. Analyses were performed using SAS statistical software (Version 9.3, SAS Institute, Inc, Cary, NC).
RESULTS
Population
A total of 1,813 MCSA participants had normal cognitive function at enrollment and returned for at least one follow-up visit. Of these 82 had denied research authorization to review their medical records. Thus, the present report includes a total of 1,731 participants (865 male and 866 females). For the 1,731 participants, the median duration of follow-up was 4.8 years, and the median number of MCSA follow-up visits was 4 (range 1 to 7). The mean age at the time of enrollment was 79.3 years. Additional baseline demographic and medical history characteristics of participants are presented in Table 1.
Table 1.
Demographic and clinical characteristics
| Characteristics | Overall (N=1,731) |
|---|---|
| Age at enrollment, years | 79.3 ± 5.1 |
| Sex | |
| Male | 865 (50) |
| Female | 866 (50) |
| Education, years | |
| <12 (less than high-school graduate)a,b | 145 (8) |
| 12 (high-school graduate) | 575 (33) |
| 13-15 (some college, but not 4 year degree) | 438 (25) |
| ≥16 (4-year college and more) | 573 (33) |
| Body mass index, kg/m2 (n=1,701) | 27.8 ± 5.0 |
| Body mass index ≥30 kg/m2 (n=1,701) | 462 (27) |
| Smoking status | |
| Never | 902 (52) |
| Former | 763 (44) |
| Currenta | 66 (4) |
| Marital status | |
| Married | 1,087 (63) |
| Widowedb | 479 (28) |
| Singleb | 165 (10) |
| ApoE4 genotype (n=1,726) | 430 (25) |
| Ever diagnosed alcohol problem (n=1,725)a,b | 66 (4) |
| Midlife diabetesa,c | 90 (5) |
| Midlife hypertension a | 617 (36) |
| Midlife dyslipidemia a | 764 (44) |
| Atrial fibrillation a,b | 282 (16) |
| Congestive heart failure | 181 (10) |
| Stroke a,b | 86 (5) |
| Coronary artery disease | 663 (38) |
Values are mean ± SD or N (%). Predictors for MCI in the clinical risk model for women and mem (a,b), for women only(a), for men only(b)[16].
Number (%) of patients with diabetes mellitus at study inclusion was n=314 (18%).
Primary Analysis of Association between Surgical Anesthesia and MCI
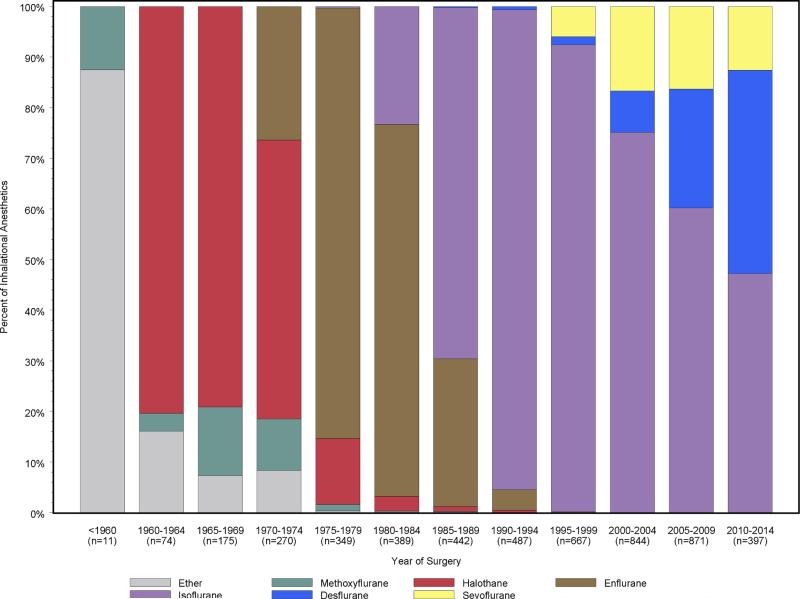
The median [25th, 75th percentiles] age at the time of the first medical record entry was 30 [23, 38] years. From review of these medical records 1,467 participants (85% of the cohort) received 1 or more surgeries or procedures requiring general anesthesia after the age of 40 (a total of 4,976 surgeries). Of these, 4,026 were performed prior to enrollment into the MCSA, and 950 were performed after enrollment but prior to development of MCI or last follow-up. There were 264 (15%) participants who did not undergo surgery after age 40 years, 333 (19%) who had a single surgery, 604 (35%) who had 2 or 3 surgeries and 530 (31%) who had 4 or more surgeries. Most surgeries (89%) included the use of halogenated inhalational agents with the specific agent used reflective of the era when the procedure was conducted (Figure 1). For the 4,976 surgeries the median duration of anesthesia was 129 [77, 195] minutes. Anesthesia induction was most frequently performed with sodium thiopental (58%) or propofol (40%), and the maintenance anesthetic typically included the use of nitrous oxide (75%). The most frequent surgeries were general surgery, orthopedic, obstetrics/gynecology and urologic procedures (Table 2).
Figure 1.
Volatile anesthetic agents used during the study period (the use of cyclopropane and nitrous oxide not shown). The numbers in parentheses represent the total number of surgeries and procedures performed during the given calendar period. Each color in a column denotes a partition of specific inhalational agent use during the respective year range.
Table 2.
Surgeries and procedures performed under general anesthesiaa
| Type of surgery/procedure | N | (%) |
|---|---|---|
| General surgery | ||
| Colorectal | 138 | (9) |
| Hepatobiliary | 24 | (2) |
| Other general | 627 | (43) |
| Orthopedic surgery | 568 | (39) |
| Genitourinary/reproductive surgery | ||
| Obstetrics / gynecology | 402 | (27) |
| Urological | 322 | (22) |
| Cardiac surgery with bypass | 211 | (14) |
| Vascular surgery without bypass | ||
| Vascular | 148 | (10) |
| Cardiac without bypass | 41 | (3) |
| Neurosurgery | 176 | (12) |
| Thoracic surgery | 65 | (4) |
| Head and neck surgery | ||
| Ear, nose and throat | 174 | (12) |
| Oral and maxillofacial | 114 | (8) |
| Plastic surgery | ||
| Breast | 158 | (11) |
| Plastic reconstructive | 37 | (3) |
| Miscellaneous | 95 | (6) |
| Surgeries | ||
| Ophthalmologic surgery | 56 | (4) |
| Dermatologic surgery | 8 | (1) |
| Procedures | ||
| Endoscopy | 10 | (1) |
| Electroconvulsive therapy | 8 | (1) |
| Interventional radiology | 3 | (<1) |
| Angiogram, aortogram, arteriogram | 2 | (<1) |
1,467 individuals underwent 4,976 surgeries or procedures after the age of 40, but prior to the development of MCI or last follow-up visit. The data presented correspond to the number (%) of individuals who underwent at least one surgery in the given category (i.e., patients who underwent multiple surgeries of the same type are counted only once). For this reason, the sum across categories does not equal the total number of surgeries performed.
During follow-up (median 4.8 years), 536 participants (31%) developed MCI (63.5 per 1000 person-years). When exposure to surgery utilizing general anesthesia was assessed as a dichotomous variable (any vs. none), anesthesia was not associated with incident MCI (Table 3). In addition, no association was found between the number of exposures or the total cumulative duration of exposure and incident MCI (Table 3). In the planned secondary sensitivity analyses examining exposure over more restricted time periods, any exposure to anesthesia after the age of 60, exposures within 20 years prior to MCI diagnosis, and exposures within 10 years were associated with an increased risk for incident MCI (Table 3). However, when analyzed according to the number of exposures, the observed associations were not consistent with a causal dose-response effect. For example, for each analysis hazard ratio values for 4 or more exposures were less than for single exposures and their 95% CI included unity. When exposure was quantified as the cumulative duration of anesthesia, none of the associations were statistically significant.
Table 3.
Analyses for association between MCI and exposure to general anesthesia for surgeries and procedures after age 40 and 60, and during 20, 10 and 5 years prior to MCI diagnosis or last follow-up.
| Anesthetic Characteristics | Exposure to general anesthesiaa | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After age 40 | After age 60 | Within 20 years | Within 10 years | Within 5 years | |||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Any anesthetic | 1.07 | 0.83-1.37 | .61 | 1.25 | 1.02-1.55 | .04 | 1.33 | 1.08-1.63 | .006 | 1.23 | 1.04-1.47 | .02 | 1.02 | 0.85-1.22 | .86 |
| Anesthetic exposures (n) | .73 | .03 | .01 | .07 | .98 | ||||||||||
| 0 | Reference | Reference | Reference | Reference | Reference | ||||||||||
| 1 | 1.05 | 0.78-1.42 | 1.28 | 1.00-1.63 | 1.43 | 1.14-1.81 | 1.31 | 1.07-1.60 | 1.03 | 0.84-1.27 | |||||
| 2-3 | 1.12 | 0.86-1.47 | 1.34 | 1.06-1.70 | 1.33 | 1.05-1.69 | 1.14 | 0.90-1.45 | 0.99 | 0.74-1.33 | |||||
| 4 or more | 1.00 | 0.76-1.34 | 1.02 | 0.75-1.37 | 1.06 | 0.77-1.46 | 1.14 | 0.76-1.70 | 0.93 | 0.46-1.89 | |||||
| Cumulative anesthesia (min)b | 1.00 | 0.98-1.01 | .83 | 1.00 | 0.99-1.02 | .63 | 1.01 | 0.99-1.03 | .27 | 1.01 | 0.99-1.04 | .18 | 1.01 | 0.98-1.04 | .70 |
Hazard ratios (HR) and P-values are from proportional hazards regression with surgical anesthesia exposure analyzed using time dependent variables. HR greater than 1 indicates an increased risk for developing MCI. Separate models were used to assess anesthesia exposure quantified as any anesthetic, number of exposures (0, 1, 2-3, ≥4) and cumulative duration of exposure in minutes. In all cases the model includes sex, education, marital status, smoking status, alcohol, APOE ε4 genotype, midlife diabetes, midlife hypertension, midlife dyslipidemia, atrial fibrillation, history of congestive heart failure, stroke, and coronary artery disease as covariates.
Since anesthesia exposure was analyzed using a time-dependent covariate the number of participants in the various exposure categories differs over the duration of follow-up. If the 1731 participants were defined according to the maximum number of exposures they experienced, the number with 0, 1, 2-3 and ≥4 exposures was 264, 333, 604 and 530 after the age of 40; 436, 444 and 560, 291 after the age of 60; 409, 471, 573, and 278 during a prior 20 year interval; 623, 515, 442 and 151 during a prior 10 year interval; and 839, 513, 309 and 70 during a prior 5 year interval.
When cumulative duration of anesthesia is analyzed the HR is for a 60-min increase.
To explore whether type of surgery is associated with MCI, separate proportional hazards regressions for exposure to 10 categories of primary procedures were performed (Table 4). No significant associations were found between exposure to various types of surgeries under general anesthesia and incident MCI after age 40. However, when examining exposures over more restricted time periods, undergoing non-bypass cardiac/vascular surgery was associated with incident MCI; no other significant associations were found. Finally, in a secondary analysis to assess whether individuals with the ApoE ε4 allele are more susceptible to potential effects of anesthesia, the primary analyses were repeated with the anesthesia exposure by ApoE ε4 status interaction included in the model. In all cases no interactions were detected (all P>.21 data not shown).
Table 4.
Analyses for association between MCI and exposure to various types of surgeries under general anesthesia after age 40 and 60, and during 20, 10 and 5 years prior to MCI diagnosis or last follow-up.
| Type of surgery/procedure | Exposure to specific type of surgery/procedure | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After age 40 | After age 60 | Within 20 years | Within 10 years | Within 5 years | |||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| General | 0.88 | 0.74-1.06 | .18 | 1.02 | 0.84-1.23 | .86 | 0.98 | 0.81-1.19 | .84 | 1.25 | 1.00-1.56 | .05 | 1.21 | 0.92-1.59 | .18 |
| Orthopedic | 1.05 | 0.87-1.26 | .63 | 1.07 | 0.87-1.31 | .53 | 1.19 | 0.97-1.45 | .10 | 1.06 | 0.85-1.33 | .61 | 0.95 | 0.72-1.24 | .70 |
| Genitourinary/reproductive | 0.95 | 0.79-1.13 | .54 | 0.97 | 0.79-1.19 | .78 | 1.00 | 0.81-1.24 | .97 | 0.85 | 0.63-1.13 | .27 | 0.68 | 0.45-1.04 | .07 |
| Cardiac with bypass | 0.80 | 0.59-1.08 | .14 | 0.75 | 0.55-1.02 | .06 | 0.86 | 0.63-1.17 | .34 | 0.88 | 0.60-1.28 | .50 | 0.89 | 0.52-1.53 | .69 |
| Cardiac without bypass/Vascular | 1.21 | 0.92-1.59 | .17 | 1.55 | 1.12-2.14 | .009 | 1.61 | 1.16-2.24 | .005 | 1.78 | 1.23-2.57 | .002 | 2.15 | 1.37-3.37 | .001 |
| Neurosurgery | 0.96 | 0.71-1.29 | .78 | 1.00 | 0.72-1.37 | .97 | 1.00 | 0.72-1.39 | .99 | 1.22 | 0.83-1.78 | .31 | 0.94 | 0.53-1.68 | .84 |
| Thoracic | 1.04 | 0.64-1.70 | .88 | 1.11 | 0.67-1.85) | .68 | 1.13 | 0.67-1.91 | .64 | 0.89 | 0.47-1.67 | .71 | 0.67 | 0.27-1.62 | .37 |
| Head and neck | 1.15 | 0.92-1.45 | .23 | 1.02 | 0.76-1.37 | .88 | 1.11 | 0.82-1.51 | .49 | 1.24 | 0.86-1.78 | .25 | 1.26 | 0.77-2.05 | .36 |
| Plastics | 0.98 | 0.74-1.31 | .89 | 0.98 | 0.72-1.35 | .91 | 0.98 | 0.70-1.39 | .93 | 0.88 | 0.53-1.44 | .60 | 0.67 | 0.32-1.43 | .30 |
| Miscellaneousa | 1.20 | 0.90-1.60 | .22 | 1.20 | 0.88-1.63 | .25 | 1.23 | 0.90-1.69 | .19 | 1.15 | 0.81-1.64 | .44 | 1.08 | 0.68-1.72 | .75 |
Miscellaneous category includes minor surgeries (e.g. dermatology) and various procedures performed in cardiac catheterization lab, gastroenterology procedures, electroconvulsive therapy, all under general anesthesia. Analysis was performed using proportional hazard regression with time varying binary variables included to indicate any exposure to the specific type of surgery after the age of 40 and in the previous 20, 10 and 5 years. In addition to these surgery variables, the model includes covariates in order to adjust for sex, education, marital status, smoking status, alcohol, APOE ε4 genotype, midlife diabetes, midlife hypertension, midlife dyslipidemia, atrial fibrillation, history of congestive heart failure, stroke, and coronary artery disease.
DISCUSSION
The main finding of our study is that cumulative exposure to procedures requiring general anesthesia after the age 40 was not associated with the development of incident MCI in cognitively normal elderly participants. At the same time, our data do not exclude the possibility that anesthetic exposures occurring later in life may be associated an increase in the rate of incident MCI, especially in patients undergoing vascular surgery
Clinical experience and case series showed that postoperative transient cognitive dysfunction is not uncommon in elderly,1-3, 34, 35 and more so after cardiac surgery.36-38 Presently it is unclear whether permanent cognitive deficits may be directly associated with undergoing surgical procedures in the elderly,2 and a phenotype and consistent nomenclature for “postoperative cognitive decline” still remains to be defined. Even if surgical procedures under general anesthesia are associated with permanent cognitive deficits, causation is difficult to assess, with potential factors including major illnesses necessitating surgery, the considerable physiologic trespass caused by surgery itself (e.g., inflammatory responses),39 perioperative delirium,40 adverse events during anesthesia (hypotension, hypoxemia), and the anesthetics and adjuvant drugs themselves.41 Even nonsurgical hospitalization of elderly for acute care may be associated with higher likelihood for incident dementia.12 As recently reviewed,11 pre-clinical studies provide support for concept that inflammatory responses may affect long-term cognition, while the evidence to support an effect of anesthetic drugs themselves is more equivocal.
The multiplicity of factors that may determine cognitive function poses major challenges to observational clinical studies in this area, whose limitations have been extensively discussed.35 A meta-analysis of available studies concluded that there is little evidence that procedures requiring general anesthesia are associated with incident dementia.7 Consistent with this analysis, a subsequent case-control study of the Olmsted County, MN population found no association between exposure after the age of 45 and incident dementia.8 Indeed, dementia is the outcome of interest in most of the current literature.7, 8, 10 Avidan et al.9 examined long-term cognitive trajectories after noncardiac surgery, including 3 groups: cognitively normal individuals (N=214), those with very mild dementia (N=225 or MCI), and those with dementia (N=136). They found that long-term cognitive decline was not independently attributable to surgery or nonsurgical illness with hospitalization, and these events were not associated with accelerated progression of cognitive decline.9
The results of the primary analysis, examining exposure to anesthesia as a risk factor after adjusting for relevant covariates, are consistent with the bulk of prior work, including the study of Avidan et al,9 failing to demonstrate an association between procedures requiring anesthesia and sustained deleterious neurocognitive outcomes.7, 8, 10 This finding was robust irrespective of how exposure was quantified. However, the planned secondary sensitivity analyses, which considered exposures within periods of time more proximate to MCI diagnosis or end of follow-up, revealed potential complexities. Analysis of this more proximate exposure, which does not take into account exposures earlier in life, revealed significant associations between MCI and exposure to surgical anesthesia in some analyses. This finding would be consistent with the concept that the brain may be more vulnerable to exposures at a more advanced age, such that risk in these analyses would not be diluted by exposures at younger age that may not pose risk. However, some of our findings may be at odds with this interpretation. First, if exposure to surgical anesthesia caused MCI (or is a factor that may unveil MCI in those already at risk), a dose-response relationship might be expected with more exposure associated with increased hazard ratios. However, this was not observed; the confidence interval for the hazard ratios for the greatest number of anesthetic exposures included unity, and there was no association when exposure was quantified as the cumulative duration of anesthesia. This could imply a threshold effect, beyond which greater exposure does not confer greater risk. Second, associations were not observed for exposures within the most recent period analyzed (within 5 years), a finding not consistent with age-related vulnerability. However, it is possible that if exposure is causative, it may take more than five years for MCI to develop. Finally, when analyzing exposures to specific types of procedures we found that only vascular surgery after age 60 was associated with incident MCI. This raises the question of whether a general anesthesia and surgery, especially for vascular procedures, was a marker of more severe comorbidity, in this case severe atherosclerosis, known to be associated with vascular dementia and MCI.42, 43 Nonetheless, these results do not exclude the possibility that exposures to anesthesia and surgery or procedures at a more advanced age may be associated with incident MCI.
Strengths and limitations
Important strengths of this study include utilizing a well-described, population-based cohort receiving multiple rigorous longitudinal assessments of cognitive function that allow for precise ascertainment of MCI. Through the REP,19 a wealth of details regarding medical comorbidity and anesthesia exposure history is also available. However, this and other observational studies have limitations. Primary among these is inability to distinguish between potential effects of the procedural experience and the underlying conditions which make the procedures necessary, such that procedures may simply serve as a marker for underlying factors that contribute to cognitive decline. In addition we cannot distinguish between the effects of anesthesia from the stress of the surgical procedure. Furthermore, our primary analysis examines exposures to surgical anesthesia over a very long period (since age 40), and it is likely that same types of surgeries over decades differ by the level of stress, postoperative morbidities, length of hospitalization (all risks for dementia): controlling for these variables in our retrospective study was not possible. Nonparticipation bias is also potentially problematic in several ways. We chose to study incident MCI, such that all subjects had normal cognition at baseline because we wanted to be certain that the onset of cognitive impairment did not precede the exposure to surgery and anesthesia. Although this approach has advantages in terms of analysis, it may also select for those individuals who are less vulnerable, as presumably the most vulnerable may have already developed cognitive deficits at the time of recruitment and may have been less likely to enroll. In addition, patients who undewernt surgery and had died prior to age 70 were not eligible for enrolment. Thus, it is important to reiterate that our findings are only relevant for persons who were healthy enough to reach up 70-89 years of age on October 1, 2004 and were cognitively normal. It is also possible that if procedures occurred shortly prior to a longitudinal assessment, any transient cognitive dysfunction may have contributed to a diagnosis of MCI. However, the lack of association between exposure within 5 years and MCI argues against this possibility. The racial and ethnic composition of Olmsted County is considerably less diverse than the overall US population, so our results may not apply to other populations. Finally, the analysis may not have been sufficiently powered to detect small effects.
In conclusion, in study participant who were cognitively normal at enrollment, cumulative exposure to surgical anesthesia after age 40 was not significantly associated with incident MCI. However, these data do not exclude the possibility that anesthetic exposures occurring later in life may be associated an increase in the rate of incident MCI, especially in patients undergoing vascular surgery
Summary statement.
In this population-based Mayo Clinic Study of Aging there was no association between exposure to general anesthesia for surgery and procedures after the age of 40 and mild cognitive impairment. A concern remains that cumulative anesthetic exposures which occur later in life (e.g., after age 60) may increase the risk for MCI.
Acknowledgement
Mrs. Shonie Buenvenida, RN, for study coordination, and to Mr Jeremiah Aakre, REP statistician for the data handling.
Role of the funding source: This study was supported by the NIH grants P50 AG016574, U01 AG006786, K01 MH068351, and K01 AG028573, by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's Disease Research Program, the Rochester Epidemiology Project (R01 AG034676, Principal Investigators: Walter A. Rocca, MD, and Barbara P. Yawn, MD, MSc) and the Mayo Clinic Center for Translational Sciences Activities (CTSA), grant number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Financial support for statistical analyses was provided by the Department of Anesthesiology Mayo Clinic.
Alphabetical List of Abbreviations
- AD
Alzheimer's dementia
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- POCD
postoperative cognitive dysfunction
- REP
Rochester Epidemiology Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: We the Authors declare that we have no competing interests.
Note: Juraj Sprung and Darrell Schroeder and Rosebud Roberts have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. They also have final responsibility for the decision to submit for publication.
Disclosures: D. Knopman serves as Deputy Editor for journal Neurology; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the Dominantly Inherited Alzheimer's Disease Treatment Unit. He has served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; served as a consultant to TauRX, was an investigator in clinical trials sponsored by Baxter and Elan Pharmaceuticals in the past 2 years; and receives research support from the NIH; R. Petersen is the Chair of Data Monitoring Committees for Pfizer and Janssen Alzheimer Immunotherapy, and has served as a consultant for Roche, Merck, and Genentech. He receives royalties from the publication of Mild Cognitive Impairment by Oxford University Press; R. Roberts receives research support from the NIH, the Driskill Foundation, and previously received research support from AbbVie Health Economics and Outcomes Research; J Sprung and T. Weingarten, D. Olive, J. Gappa, V. Sifuentes, T. Behren, J. Farmer, A. Hanson, D. Schroeder, and D. Warner have nothing to disclose.
References
- 1.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 2.Avidan MS, Evers AS. Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. J Alzheimers Dis. 2011;24(2):201–216. doi: 10.3233/JAD-2011-101680. [DOI] [PubMed] [Google Scholar]
- 3.Vanderweyde T, Bednar MM, Forman SA, Wolozin B. Iatrogenic risk factors for Alzheimer's disease: surgery and anesthesia. J Alzheimers Dis. 2010;22(Suppl):391–104. doi: 10.3233/JAD-2010-100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Z, Dong Y, Maeda U, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61(12):1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 5.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106(3):436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Crosby C, Culley DJ, Baxter MG, Yukhananov R, Crosby G. Spatial memory performance 2 weeks after general anesthesia in adult rats. Anesth Analg. 2005;101(5):1389–1392. doi: 10.1213/01.ANE.0000180835.72669.AD. [DOI] [PubMed] [Google Scholar]
- 7.Seitz DP, Shah PS, Herrmann N, Beyene J, Siddiqui N. Exposure to general anesthesia and risk of alzheimer's disease: a systematic review and meta-analysis. BMC Geriatr. 2011:1183. doi: 10.1186/1471-2318-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprung J, Jankowski CJ, Roberts RO, et al. Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clin Proc. 2013;88(6):552–561. doi: 10.1016/j.mayocp.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkstra JB, Van Boxtel MP, Houx PJ, Jolles J. An operation under general anesthesia as a risk factor for age-related cognitive decline: results from a large cross-sectional population study. J Am Geriatr Soc. 1998;46(10):1258–1265. doi: 10.1111/j.1532-5415.1998.tb04542.x. [DOI] [PubMed] [Google Scholar]
- 11.Eckenhoff RG, Laudansky KF. Anesthesia, surgery, illness and Alzheimer's disease. Prog Neuropsychopharmacol Bol Psychiatry. 2013:47162–166. doi: 10.1016/j.pnpbp.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankratz VS, Roberts RO, Mielke MM, et al. Predicting the risk of mild cognitive impairment in the Mayo Clinic Study of Aging. Neurology. 2015;84(14):1433–1442. doi: 10.1212/WNL.0000000000001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts RO, Cha RH, Knopman DS, Petersen RC, Rocca WA. Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis Assoc Disord. 2006;20(3):141–146. doi: 10.1097/00002093-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Roberts RO, Petersen RC. Subjective complaints in mild cognitive impairment make a difference. Acta Psychiatr Scand. 2010;121(4):241–242. doi: 10.1111/j.1600-0447.2009.01509.x. [DOI] [PubMed] [Google Scholar]
- 22.Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer's disease: a reanalysis of data from Rochester, Minnesota, 1975-1984. Am J Epidemiol. 1998;148(1):51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- 23.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48(7):725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 25.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 26.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7(5):486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 27.Fahn S, Elton RL, Committee UD. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Vol. 2. MacMillan Healthcare Information; Florham Park: 1987. pp. 153–163. [Google Scholar]
- 28.Wechsler DA. Wechsler Memory Scale-Revised. Psychological Corporation; New York: 1987. [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 31.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- 34.Bilotta F, Doronzio A, Stazi E, et al. Postoperative cognitive dysfunction: toward the Alzheimer's disease pathomechanism hypothesis. J Alzheimers Dis. 2010;22(Suppl):381–89. doi: 10.3233/JAD-2010-100825. [DOI] [PubMed] [Google Scholar]
- 35.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106(3):572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 37.Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer's disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7(4):319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- 38.Selnes OA, Royall RM, Grega MA, Borowicz LM, Jr., Quaskey S, McKhann GM. Cognitive changes 5 years after coronary artery bypass grafting: is there evidence of late decline? Arch Neurol. 2001;58(4):598–604. doi: 10.1001/archneur.58.4.598. [DOI] [PubMed] [Google Scholar]
- 39.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 40.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 41.Ghoneim MM, Block RI. Clinical, methodological and theoretical issues in the assessment of cognition after anaesthesia and surgery: a review. Eur J Anaesthesiol. 2012 doi: 10.1097/EJA.0b013e328356bd6e. [DOI] [PubMed] [Google Scholar]
- 42.Zanetti M, Ballabio C, Abbate C, Cutaia C, Vergani C, Bergamaschini L. Mild cognitive impairment subtypes and vascular dementia in community-dwelling elderly people: a 3-year follow-up study. J Am Geriatr Soc. 2006;54(4):580–586. doi: 10.1111/j.1532-5415.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 43.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363(9415):1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]