Abstract
The development of long-term human organotypic liver-on-a-chip models for successful prediction of toxic response is one of the most important and urgent goals of the NIH/DARPA’s initiative to replicate and replace chronic and acute drug testing in animals. For this purpose we developed a microfluidic chip that consists of two microfluidic chambers separated by a porous membrane. The aim of this communication is to demonstrate the recapitulation of a liver sinusoid-on-a-chip using human cells only for a period of 28 days. Using a step-by-step method for building a 3D microtissue on-a-chip, we demonstrate that an organotypic in vitro model that reassembles the liver sinusoid microarchitecture can be maintained successfully for a period of 28 days. In addition, higher albumin synthesis (synthetic), urea excretion (detoxification) was observed under flow compared to static cultures. This human liver-on-a-chip should be further evaluated in drug-related studies.
Keywords: liver-on-a-chip, microfludics, liver sinusoid, hepatocytes, 3D microtissue
The liver sinusoid is a functional repetitive microvascular unit connected to the portal vein and hepatic artery. The microanatomy of the sinusoid is primarily composed of highly polarized hepatocytes (parenchymal fraction) and liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs) and Kupffer cells (KCs) (non-parenchymal fraction) as shown in Figure 1e (Godoy et al. 2013). LSECs form the endothelium that lines the liver sinusoid and serve as a selective sieve between the blood and the hepatocytes. The role of the sieve in the liver has been demonstrated in various cell processes, such as in bidirectional macromolecular exchange, liposomal transport (Romero et al. 1999), regenerating liver (Popescu et al. 2000), nitric oxide synthesis (Yokomori et al. 2002) and in various diseases conditions (hyperlipoproteinemia, cirrhosis and cancer) (Braet and Wisse 2002). The endothelial cell barrier in the liver is an early target for several drugs and toxicants, as shown in studies with acetaminophen (McCuskey 2006). Like LSECs, HSCs are engaged in several important functions in the liver under both physiological and pathological conditions and reside in the space of Disse, which is situated between the hepatocytes and the LSECs (Sarem et al. 2006; Senoo 2004). Kupffer cells are macrophage cells that reside within the lumen of the liver sinusoids. Upon activation, KCs release various cytokines, prostanoides and nitric oxide that regulate the phenotype of KCs, as well as the neighboring cells (hepatocytes, stellate cells, endothelial cells). In addition, KCs are intimately involved in the liver's response to toxins (Bilzer et al. 2006).
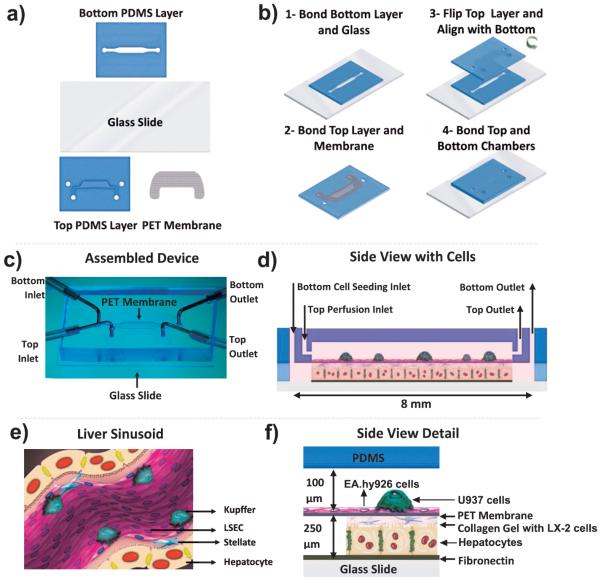
Figure 1.
Schematic of the various steps during fabrication of a two-chambered microfluidic device and the 3D co-culture assembly within the device. (a) Schematic of the four parts that were used to assemble the device. (b-1) PDMS-glass bond bottom chamber assembled firmly by using an air plasma treatment, (b-2) PDMS-PET membrane top chamber prepared at 70 °C using a PDMS coating and (b-3,4) assembly of the bottom and upper chamber. (c) Assembled microfluidic two-chamber device. The metal tubing shown is only used for illustration purposes and are not used during the experiments. (d) Side view of the device with the four cell types. (e) Cross-sectional schematic of the liver sinusoidal microanatomy. (f) Detailed side view of the assembled device with four cells, mimicking the microanatomy of the liver sinusoid.
In this context, co-culture of hepatocytes with non-parenchymal cells has already been shown to maintain hepatocyte morphology and a variety of liver functions (synthetic, metabolic, and detoxification) that cannot be achieved in hepatocyte mono-cultures (Bale et al. 2014; Godoy et al. 2013). Thus, the ability of the non-parenchymal cell population to metabolically convert drugs and to regulate hepatocyte metabolism is of great importance for the overall hepatocyte function and plays a significant role in certain types of toxin-induced injuries (McCuskey 2006). Experimental approaches that can successfully maintain primary human hepatocyte function in a sinusoidal format using microfluidics over long periods of time (4 weeks) have not yet been reported. Microfluidic platforms have been shown to allow the creation of an artificially engineered, physiologically relevant cell culture microenvironment, which is not possible using standard plate cultures (Polini et al. 2014; Usta et al. 2015). Thus, the aim of this study was to mimic the liver sinusoidal microenvironment more accurately than standard culture techniques and re-create human liver physiology and functions on-a-chip. For this purpose, multiple cell types from human origins and microfluidics were combined in a 3D cell culture format. Our hypothesis was that by using constant flow in a microfluidic device, a co-culture of human primary hepatocytes with cell lines representing the non-parenchymal liver cell fraction can be maintained over a period of 28 days. Results for the main physiological liver parameters of albumin synthesis (synthetic), urea excretion (detoxification), and CYP3A4 activity (enzymatic) were accessed under flow and compared to static over a period of 28 days.
The microfluidic device is shown in (Figure 1a-d) and consists of two microfluidic chambers separated by a porous membrane. The device was fabricated at the BioMEMS Resource Center at the Massachusetts General Hospital and assembled in-house (McDonald et al. 2002). In these studies, primary cryopreserved human hepatocytes were used as the parenchymal fraction. Due to the limited availability of primary non-parenchymal human cells, for this early attempt to re-create a liver sinusoid on-a-chip, three cell lines of human origin EA.hy926, LX-2 and U937 that have been extensively characterized (Bouis et al. 2001; Castilho-Fernandes et al. 2011; Passmore et al. 2001) were used as substitutes for endothelial, stellate and KCs, respectively.
We used a step-by-step method to introduce the four cell types into the device. Briefly, the hepatocytes were introduced into the bottom chamber and allowed to attach as a monolayer of hepatocytes onto the glass slide for a period of 24 h. The LX-2 cells were mixed into a liquid collagen gel solution and added into the bottom chamber between the monolayer of attached hepatocytes and the porous membrane on the next day, and the collagen solution then gelled. Next, to allow formation of an endothelial monolayer, the EA.hy926 cells were introduced into the top chamber and seeded on the membrane as shown in (Figure 1f). The intervening porous membrane acts as a barrier that prevents the collagen gel from entering the top fluidic chamber, but also creates an empty microfluidic channel in which flow can be applied to the monolayer of endothelial cells, but not to the hepatocytes directly, as is found in the liver sinusoid. The U937 cells can be differentiated (Ferreira et al. 1991) and added in the devices to induce immune response reactions in experiments where a cytokine storm is required, such as in vitro experiments mimicking the acute liver response. In the current experimental set-up, the U937 cells were activated and added as a proof of concept at day 8 in small numbers (see supplementary data Figure Sa-c). The number of cells needed to cause a cytokine storm requires further evaluation. The assembled 3D model allows culture of human hepatocytes in small numbers (< 10 000) and in a low total volume of media of 24 μL /per day in flow conditions or 1 μL /per day in static condition for up to 28 days.
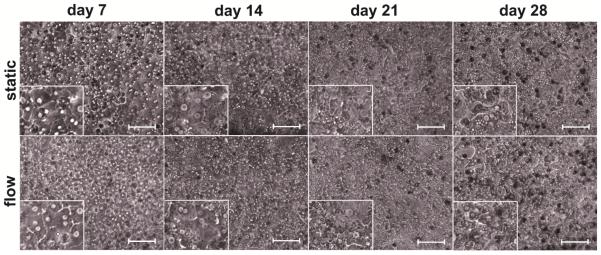
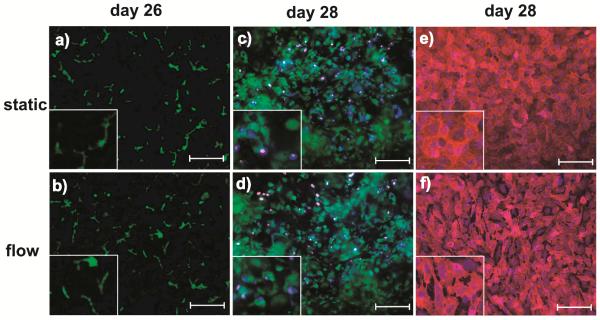
Hepatocyte morphology and monolayer integrity were assessed by phase contrast microscopy at days 7, 14, 21 and 28. Hepatocytes in both: static and flow cultures similarly showed the flattened cuboidal morphology that is typical for hepatocytes over a period of three weeks (Figure 2). Changes in cell morphology and cytoplasm, including lower nuclear clarity, were observed during the fourth week of culture, but the polarization of the hepatocytes remained present, as shown by the CMFDA bile canalicular network staining (Figure 3a). Nevertheless, the presence of bile canaliculi is one of the most important routes for drug elimination and we observed it at day 26. The viability of the LX-2 cells within the gel (bottom chamber) and the presence of the EA.hy926 cells on the PET membrane (upper chamber) were also observed with live/dead assay and CD31 staining respectively at day 28 (Figure 3b and 3c).
Figure 2.
Representative phase contrast images of primary human hepatocyte co-cultures maintained under static and flow conditions at days 7, 14, 21 and 28. The co-cultured cells showed consistent morphology and nuclear clarity during the first three weeks. Changes in the cell morphology and cytoplasm brining lower nuclear clarity were observed during the fourth week. Image scale bar: 20 μm.
Figure 3. Representative images of hepatic bile canaliculi, EA.hy926 and LX-2 cells in a microfluidic device.
(a,b) CMFDA staining showing the polarization of hepatocytes and formation of a bile canalicular network at day 26. Note that the bile canaliculi were visible in both static and flow conditions. (c,d) LIVE/DEAD staining showing LX-2 cells embedded in the collagen gel of the bottom chamber at day 28 of cell culture. Images were taken ~100 μm below the membrane. Green: live cells, red: dead cells, and blue: nucleus staining with DAPI. (e, f) CD31 staining specific for endothelial cells at day 28. Red: CD31 and blue: nucleus staining with DAPI. Image scale bar: 20 μm.
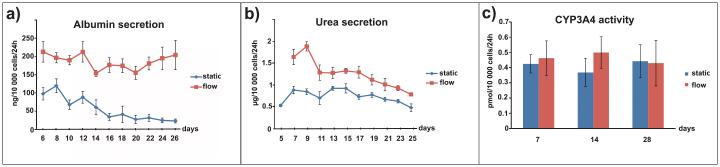
Hepatocyte cultures subjected to flow showed sustained and constant higher albumin and urea secretions for a period of 26 days compared with static cultures (Figure 4a and 4b), confirming previous studies (Hegde et al. 2014; McCarty et al. 2014). The difference in urea secretions under flow vs. static conditions was greater at the beginning of the culture period, followed by a decrease at day 12 before leveling off, but still remained higher under flow conditions at day 25. We expect that the higher albumin and urea production under flow conditions is a result of continuous delivery of nutrients (amino acids, carbohydrates, essential fatty acids) and continuous clearance of secreted factors (albumin, urea, toxic intermediates). The observed results are most likely driven by a homeostatic mechanism to maintain steady-state level of total (Pietrangelo et al. 1992) and/or specific proteins and are in line with basic chemical equilibrium (Le-Chatelier) principles. In contrast to albumin and urea secretions, there was no difference in CYP3A4 activity between the flow and static cultures (Figure 4c). CYP3A4 activity can be highly induced by a variety of drugs in humans and plays a crucial role in the clearance of numerous drugs in the liver (Zhou et al. 2009). Although there was no effect of flow on CYP3A4 activity, the results indicated that CYP3A4 can be sustained at stable levels over a relatively long time, which is important for drug-related studies. Inducing CYP3A4 activity with drugs in static and flow conditions would be interesting to examine in future experiments.
Figure 4. Main physiological liver parameters replicated in the microtissue over a period of 28 days.
(a) Hepatocyte co-cultures under continuous flow of media have higher and more stable albumin secretion. (b) Urea secretion was 2-fold higher in the first days of flow stimulation and remained higher during the next 25 days of culture. (c) CYP3A4 activity was maintained during the four weeks and was similar in both static and flow conditions. n=3. Data are presented as mean ± SEM, n = 6-9 devices derived from 3 different experiments. A significant effect of flow was found for albumin (P < 0.001) and urea (P < 0.001).
Scaling of flow parameters from the in vivo conditions to in vitro devices is an area of ongoing debate (Usta et al. 2015) where simple and straight forward translations would be to use linear flow velocities in the organ or to use residence times (Sin et al. 2004). Such translations, however, may not result in correct clearance behavior (Maguire et al. 2010) due to the significant difference in device dimensions (~ 100s of microns) as compared to liver sinusoid (~5-10 μm). Our choice of a relatively low flow rate (our linear velocity u is 2-3 orders of magnitude lower than in vivo values ) is based on approximately matching in vivo Peclet number (Pe = Hu/D where H is channel height, u is linear velocity and D is solute diffusion coefficient) in our device based on diffusion of albumin. This is to ensure approximately correct transport of solutes and to produce correct clearance values when drug studies are conducted. We also took into account our empirical observation that the 1 μL/h was an optimum in terms of stably maintaining our cultures for 4 weeks.
The 3-D aspect of our model is created by stacking 2D monolayers of cells (for hepatocytes, endothelial cell line, Ea.hy926 and Kupffer cell line, U937) along with the 3-D dispersal of stellate cell line, LX-2s, in a collagen gel. This is in contrast to other 3-D approaches such as spheroids or 3-D printing where cell aggregates are generated for high density cell packing (Godoy et al. 2013). In liver, the Space of Disse (thickness ~200-1000 nm)(Kuntz and Kuntz 2006) is defined as the semi-fluidic extracellular matrix space between endothelial cells lining the sinusoid and hepatocytes. Stellate cells mostly reside in this space and make contacts with both endothelial cells and hepatocytes (Wake 2006). In our model, the Space of Disse is approximately 250 μm collagen-gel thick layer between the hepatocytes and the endothelial cells (EA.hy 926) in which the stellate cells (LX-2 cell line) are dispersed. While this is an important improvement over traditional transwell model, a further reduction in the distance between these two cell types would be a significant improvement to better recapitulate cell-cell signaling events. To this end, we have developed an ultrathin collagen coating method for microfluidic devices in which the thickness of the collagen on top of the hepatocytes is only 150 nm (McCarty et al. 2014). Experiments using this novel method under flow conditions for long term culture are currently under investigation.
An additional concern for our device as well as most other microfluidic culture devices that utilize PDMS for manufacturing is the hydrophobic nature of this rapid prototyping material. PDMS poses a challenge for drug screening experiments due to the nonspecific adsorption of hydrophobic drugs, proteins and other analytes (Toepke and Beebe 2006). Thus, in order to develop a fully drug testing compatible microfluidic tissue, it is important to use methods that can modify the hydrophobic character of the PDMS by using oxygen plasma treatment and appropriate storage medium (Kim et al. 2004), replace PDMS with inert plastic materials or use PDMS alternatives such as SEBS (Borysiak et al. 2013).
Although we used primary human hepatocytes in our model and the results showed that the function of the tissue constructs can be maintained over a period of 28 days, replacement of the EA.hy926, LX-2 and U937 cell lines with primary human NPCs may be important for the accurate recapitulation of liver physiology. NPCs are involved in both physiological and pathological conditions (e.g. hyperlipoproteinemia, cirrhosis, fibrosis, NASH, microsteatosis, inflammatory response and cancer), and the direct comparison of tissue constructs with different cell configurations in which LSECs, stellate cells, and Kupffer cells are added and compared to hepatocyte cultures will be of great interest for the creation of human disease models in which the effect of the non-parenchymal cells can be studied in more detail.
In conclusion, we have developed an organotypic in vitro model in which human hepatocytes grown in a co-culture 3D configuration that reassembles the liver sinusoid microarchitecture were maintained successfully for a period of 28 days. We observed that continuous flow of media at 1 μL/h can induce faster albumin and urea responses. Further, hepatocytes in the flow co-cultures showed a higher albumin and urea production as compared to static cultures over a period of 26 days. The volume of media per hepatocyte under flow in the device is relatively low (24 μL/day) when compared to current in vitro flow liver models, (Godoy et al. 2013) which is important for obtaining concentrated metabolite solution. In addition, due to the interspecies difference, the use of hepatocytes from animal origins lack specificity and cannot always predict metabolic formation. Thus, the development of simple organotypic in vitro models for culturing primary human hepatocytes may be of high interest for PK/PD modeling studies. However, the presented microfluidic human organotypic liver-on-a-chip model should be further evaluated in drug-related studies to confirm the importance of the non-parenchymal fraction and liver sinusoidal microarchitecture, as well as to fully exploit their importance in drug-related studies.
Materials and Methods
Fabrication of microfluidic device
Silicon wafer templates served as negative molds to generate the top chamber of the device in poly(dimethyl)siloxane (PDMS, Sylgard 184, Dow Corning) using standard soft lithography protocols. Two sets of inlet and outlet ports were punched into the top PDMS chamber using a 0.75 mm dermal punch (0.75 mm punch, Harris Unicore). The bottom chamber was made of two components: 1) ~250μm thick PDMS sheet (HT-6420 Transparent 0.10", Rogers Corporation) that was cut using a laser cutter and 2) a glass slide (Glass slides, 75 mm × 25 mm, Fischer Scientific) as on Figure 1a. The glass slide and the cut sheet were bonded by air plasma treatment to form an intact bottom chamber (Figure 1b). The dimensions of the top chamber were 10 mm2 × 0.1 mm (surface area × height) and 10 mm2 × 0.25 mm for the bottom chamber. The total volume in both chambers (whole device) was ~ 3.5 μL. A 1.0 μm pore-sized polyethylene terephthalate (PET) membrane (1.6 × 106 pores cm−2 and ~6 mm2 exposed surface area cm−2) obtained from a transwell membrane insert (Falcon™ Cell Culture Inserts, 6 well size, BD Biosciences) with a thickness of 10 μm was cut to the desired shape. The top PDMS chamber was first bonded to the membrane. Briefly, a thin layer of a PDMS pre-polymer was spin-coated onto a clean glass coverslip (Glass coverslip, 50 mmx 24 mm, Fisherfinest™ Premium Cover Glasses, Fischer Scientific) and a clean top chamber was placed onto it for the PDMS to spread on the surface around the channel. The laser-cut membrane was placed on the PDMS pre-polymer coated surface of the top chamber and bonded firmly after baking at 70 °C for 1-2 hours (Figure 1b). The membrane-bond top chamber and the bottom chamber were treated with air plasma for 30 sec. Next, the two chambers were aligned and brought in conformal contact prior to baking at 70 °C for 30 min.
Cell culture
Cryopreserved primary human hepatocytes were thawed according to the manufacturer protocol (Lot number 4025, Triangle Research Lab) before the start of every experiment. The viability after thawing the cells was in the range of 80% to 85%. The EA.hy926 (CRL-292™, ATCC) and LX-2 (kindly provided by Dr. Raymond T Chung, Department of Medicine, Massachusetts General Hospital, MA, USA) cells were maintained in Dulbecco’s modified Eagle medium, 10% FBS and 1% penicillin/streptomycin (all from Invitrogen Life Technologies) and passaged with trypsin regularly when cells reached 75% to 90% confluence. U937 cells (CRL- 1593.2™, ATCC) were maintained with the same media as EA.hy926 and LX-2, but in suspension culture. All 4 cell types were cultured at 37 °C in a humidified atmosphere of 5% CO2.
Cell seeding in the device
The devices were UV-sterilized for 15 min and the glass slide in the bottom chamber was first coated with 50 μg mL−1 fibronectin (fibronectin from bovine plasma, Sigma-Aldrich) in 1 × PBS and maintained at 37 °C for 1 h. A second collagen coating (1 parts 1.25 mg mL−1 type I rat tail collagen mixed with 19 parts 1 × PBS) was introduced on top of the fibronectin coating for 45 min at 37 °C. Primary human hepatocytes were thawed according to the manufacturer protocol (Lot number 4025, Triangle Research Lab), and a cell suspension was prepared in plating media (human hepatocyte plating medium, Triangle Research Lab) at a final concentration of 6.5 × 106 (M) mL−1. The cell suspension was introduced into the bottom chamber using a pipette tip. Four hours after the cell seeding, the plating media was replaced with maintenance media (human hepatocyte maintenance medium, Triangle Research Lab). The following day, collagen/LX-2 cell suspension was prepared by mixing one part of collagen gel solution (9 parts 1.25 mg mL−1 type I rat tail collagen mixed with 1 part 10 × DMEM) with one part of LX-2 cells 0.5 × 106 (M) mL−1 LX-2 cells. The devices were flipped upside down and placed in the cell culture incubator (at 37 °C) to promote LX-2 cells distribution along the depth of bottom chamber as the collagen gel polymerized. After 4 h devices were flipped back. Further, to allow formation of endothelial barrier, EA.hy926 cells were added on top of the membrane in the top chamber with a density of 10 × 106 (M) mL−1 on day 2. At day 7, the U937 cell line was differentiated by exposing the cells (5 × 105 cells/ml) to 50 ng/ml of PMA (phorbol 12-myristate 13-acetate, Sigma-Aldrich) for 48 h in a 6 well plate. After the differentiation the U937 were trypsinized and introduced in the top chamber with a cell density of 0.25 × 106 (M) mL−1. The devices were maintained in maintenance media (Triangle Research Lab) at 37 °C for 28 days. Media in the top chamber was replaced and collected for albumin and urea analyses every 24 h in the devices maintained in static condition. The devices maintained under flow were constantly perfused trough the top chamber with fresh media at 1 μL/h. Tygon tubing (0.01"ID × 0.03"OD, Cole Parmer) was used for all fluidic connections and media perfusion.
Statistical analysis
Quantitative data were plotted as the mean ± standard error of the mean (SEM) from 6-9 devices, derived from 3 separate experiments (n=3). The effects of flow on albumin and urea secretions were assessed using 2-way ANOVA. Tukey post-hoc tests were used to determine differences between groups where significant (P < 0.05) effects were found.
Supplementary Material
Supplementary Figure S1. Phase contrast images of the U937 cells located on top of the membrane seeded with endothelial cells. (a) Before adding U937. (b) U937 cells 24 h after being added into the device. (c) U937 cells 24 h after induction of flow.
Acknowledgments
This work was supported by NIH grants, including a Microphysiological Systems Consortium grant from the NCATS (UH2TR000503), a Ruth L. Kirschstein NRSA Postdoctoral Fellowship from the NIDDK (F32DK098905 for WJM), and a K99 – Path to Independence award from the NIDDK (K99DK095984 for AB). We acknowledge support from the MGH BioMEMS Resource Center (NIH 2P41EB002503) in fabricating microdevices.
Footnotes
Supporting Information
Additional supporting information for methods can be found in the online version of this article at the publisher’s web-site.
References
- Bale SS, Vernetti L, Senutovitch N, Jindal R, Hegde M, Gough A, McCarty WJ, Bakan A, Bhushan A, Shun TY. In vitro platforms for evaluating liver toxicity. Experimental biology and medicine. 2014;239(9):1180–91. doi: 10.1177/1535370214531872. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver international : official journal of the International Association for the Study of the Liver. 2006;26(10):1175–86. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- Borysiak MD, Bielawski KS, Sniadecki NJ, Jenkel CF, Vogt BD, Posner JD. Simple replica micromolding of biocompatible styrenic elastomers. Lab on a chip. 2013;13(14):2773–84. doi: 10.1039/c3lc50426c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouis D, Hospers GA, Meijer C, Molema G, Mulder NH. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 2001;4(2):91–102. doi: 10.1023/a:1012259529167. [DOI] [PubMed] [Google Scholar]
- Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comparative hepatology. 2002;1(1):1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho-Fernandes A, de Almeida DC, Fontes AM, Melo FU, Picanco-Castro V, Freitas MC, Orellana MD, Palma PV, Hackett PB, Friedman SL. Human hepatic stellate cell line (LX-2) exhibits characteristics of bone marrow-derived mesenchymal stem cells. Experimental and molecular pathology. 2011;91(3):664–72. doi: 10.1016/j.yexmp.2011.09.002. and others. [DOI] [PubMed] [Google Scholar]
- Ferreira OC, Jr., Valinsky JE, Sheridan K, Wayner EA, Bianco C, Garcia-Pardo A. Phorbol ester-induced differentiation of U937 cells enhances attachment to fibronectin and distinctly modulates the alpha 5 beta 1 and alpha 4 beta 1 fibronectin receptors. Experimental cell research. 1991;193(1):20–6. doi: 10.1016/0014-4827(91)90533-z. [DOI] [PubMed] [Google Scholar]
- Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Bottger J. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Archives of toxicology. 2013;87(8):1315–530. doi: 10.1007/s00204-013-1078-5. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M, Jindal R, Bhushan A, Bale SS, McCarty WJ, Golberg I, Usta OB, Yarmush ML. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab on a chip. 2014;14(12):2033–9. doi: 10.1039/c4lc00071d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Peterson ETK, Papautsky I. Long-term stability of plasma oxidized PDMS surfaces. 2004 Sept.2004:5013–5016. doi: 10.1109/IEMBS.2004.1404385. [DOI] [PubMed] [Google Scholar]
- Kuntz E, Kuntz H-D. Hepatology, Principles and practice: history, morphology, biochemistry, diagnostics, clinic, therapy. Springer Science & Business Media; 2006. [Google Scholar]
- Maguire T, Usta OB, Yarmush ML. Computational Fluid Dynamic Analysis of a Cell-Based Microfluidic Drug Screening Platform. Nano LIFE. 2010;01:185–194. [Google Scholar]
- McCarty WJ, Usta OB, Luitje M, Bale SS, Bhushan A, Hegde M, Golberg I, Jindal R, Yarmush ML. A novel ultrathin collagen nanolayer assembly for 3-D microtissue engineering: Layer-by-layer collagen deposition for long-term stable microfluidic hepatocyte culture. Technology. 2014;2(1):67–74. doi: 10.1142/S2339547814500083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuskey RS. Sinusoidal endothelial cells as an early target for hepatic toxicants. Clinical hemorheology and microcirculation. 2006;34(1-2):5–10. [PubMed] [Google Scholar]
- McDonald JC, Chabinyc ML, Metallo SJ, Anderson JR, Stroock AD, Whitesides GM. Prototyping of microfluidic devices in poly(dimethylsiloxane) using solid-object printing. Analytical chemistry. 2002;74(7):1537–45. doi: 10.1021/ac010938q. [DOI] [PubMed] [Google Scholar]
- Passmore JS, Lukey PT, Ress SR. The human macrophage cell line U937 as an in vitro model for selective evaluation of mycobacterial antigen-specific cytotoxic T-cell function. Immunology. 2001;102(2):146–56. doi: 10.1046/j.1365-2567.2001.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangelo A, Panduro A, Chowdhury JR, Shafritz DA. Albumin gene expression is down-regulated by albumin or macromolecule infusion in the rat. J Clin Invest. 1992;89(6):1755–60. doi: 10.1172/JCI115778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polini A, Prodanov L, Bhise NS, Manoharan V, Dokmeci MR, Khademhosseini A. Organs-on-a-chip: a new tool for drug discovery. Expert opinion on drug discovery. 2014;9(4):335–52. doi: 10.1517/17460441.2014.886562. [DOI] [PubMed] [Google Scholar]
- Popescu D, Movileanu L, Ion S, Flonta ML. Hydrodynamic effects on the solute transport across endothelial pores and hepatocyte membranes. Physics in medicine and biology. 2000;45(11):N157–65. doi: 10.1088/0031-9155/45/11/404. [DOI] [PubMed] [Google Scholar]
- Romero EL, Morilla MJ, Regts J, Koning GA, Scherphof GL. On the mechanism of hepatic transendothelial passage of large liposomes. FEBS letters. 1999;448(1):193–6. doi: 10.1016/s0014-5793(99)00364-6. [DOI] [PubMed] [Google Scholar]
- Sarem M, Znaidak R, Macias M, Rey R. [Hepatic stellate cells: it's role in normal and pathological conditions] Gastroenterologia y hepatologia. 2006;29(2):93–101. doi: 10.1157/13083906. [DOI] [PubMed] [Google Scholar]
- Senoo H. Structure and function of hepatic stellate cells. Medical electron microscopy : official journal of the Clinical Electron Microscopy Society of Japan. 2004;37(1):3–15. doi: 10.1007/s00795-003-0230-3. [DOI] [PubMed] [Google Scholar]
- Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol Prog. 2004;20(1):338–45. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]
- Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab on a chip. 2006;6(12):1484–6. doi: 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- Usta OB, McCarty WJ, Bale S, Hegde M, Jindal R, Bhushan A, Golberg I, Yarmush ML. Microengineered cell and tissue systems for drug screening and toxicology applications: Evolution of in-vitro liver technologies. Technology. 2015;03(01):1–26. doi: 10.1142/S2339547815300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake K. Hepatic stellate cells: Three-dimensional structure, localization, heterogeneity and development. Proc Jpn Acad Ser B Phys Biol Sci. 2006;82(4):155–64. doi: 10.2183/pjab.82.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori H, Oda M, Ogi M, Sakai K, Ishii H. Enhanced expression of endothelial nitric oxide synthase and caveolin-1 in human cirrhosis. Liver. 2002;22(2):150–8. doi: 10.1034/j.1600-0676.2002.01588.x. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug metabolism reviews. 2009;41(2):89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Phase contrast images of the U937 cells located on top of the membrane seeded with endothelial cells. (a) Before adding U937. (b) U937 cells 24 h after being added into the device. (c) U937 cells 24 h after induction of flow.