Abstract
Purpose of Review
To discuss and review the role for elective treatment of the neck in maxillary squamous cell carcinoma. Improvements in survival have been seen due to improved local therapies and control, therefore the treatment of the neck has become a topic of debate.
Recent findings
The risk of occult metastases in neck nodes is higher for T 3-4 tumors. The rate of nodal relapse in the N0 neck without elective treatment is 8-15%. With elective irradiation the nodal relapse rate decreases. However, most nodal relapses are accompanied by local failure or distant disease. Local failure remains the most common site of failure and cause of death in this patient population.
Summary
Treatment failure occurs overall in 62% of all patients, with local recurrence by far the most common site of treatment failure which is rarely amenable to salvage therapy. Therefore elective neck irradiation is not routinely indicated in the clinically N0 neck; those who recur only in the neck can be surgically salvaged more than 50% of the time.
Keywords: maxillary carcinoma, elective neck therapy
Introduction
Primary malignant tumors of the sinonasal tract are rare. They account for less than 10% of head and neck cancers with an annual incidence of 0.5-1.0 per 100,000 people in the United States1. The maxillary sinus is the second most common sub-site, preceded only by the nasal cavity for neoplasia of epithelial origin arising in this area. Squamous cell carcinoma (SCC) is by far the most prevalent malignant histology, accounting for 57-80% of all malignant tumors. This is followed by minor salivary gland tumors, sarcoma, esthesioneuroblastoma, lymphoma, sino nasal undifferentiated carcinoma (SNUC) and melanoma which occur in decreasing frequency. As in the remainder of the head and neck region, smoking predisposes one to squamous cell carcinoma. SCC can also arise from inverting papillomas in 10% of cases and rarely in the background of chronic sinusitis. More unique risk factors however are exposure to wood dust, chemicals such as chromium, nickel and formaldehyde, which are associated with other malignant tumors, such as adenocarcinoms and clear cell carcinoma2.
Presentation
Neoplasms of maxillary sinus or antrum of Highmore rarely present with symptoms at an early stage (Figure 1) as many lesions are asymptomatic until they become quite large1. Symptoms of obstruction or local invasion of adjacent tissue (soft tissue mass, loose teeth, anesthesia of V2, diplopia, proptosis, and trismus,) are often the first presenting sign. As such, 82% of patients present with advanced stage of disease (T3 or higher) (Table 1)1. However, even with advanced T stage, it is thought that the incidence of nodal metastasis is low, as the maxillary sinus does not have an extensive lymphatic drainage network3.
Figure 1.
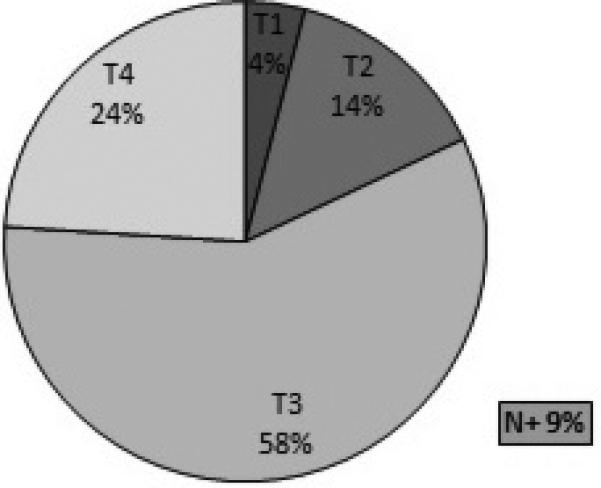
Distribution of the T categories of squamous cell carcinoma of the maxilla at the time of diagnosis1 (Data from MSKCC)
Table 1.
Tumor staging of maxillary sinus carcinoma1
| Tstage | Description |
|---|---|
| T1 | Tumor limited to maxillary sinus mucosa with no erosion or destruction of bone |
| T2 | Tumor causing bone erosion or destruction including extension into the hard palate and/or middle nasal meatus, except extension to posterior wall of maxillary sinus and pterygoid plates |
| T3 | Tumor invades any of the following: bone of the posterior wall of maxillary sinus, subcutaneous tissues, floor or medial wall of orbit, pterygoid fossa, or ethmoid sinuses |
| T4a | Moderately advanced local disease. Tumor invades anterior orbital contents, skin of cheek, pterygoid plates, infratemporal fossa, cribriform plate, or sphenoid or frontal sinuses |
| T4b | Very advanced local disease. Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V2), nasopharynx, or clivus |
In the 1930s Öhngren described an imaginary plane defined by a line joining the medial canthus of the eye to the angle of the mandible. This plane divides the region of the nasal cavity and maxillary sinus into half: the infrastructure (Antero inferior) and suprastructure (Postero superior) (Figure 2). This line was to delineate the easily resectable tumors of the infrastructure, from the more difficult, and harder to cure tumors of the suprastructure. Tumors of the infrastructure of the maxillary antrum may extend through the floor of the antrum into the oral cavity, through the medial wall into the nasal cavity and through the anterior wall to the soft tissues of the cheek or through the lateral wall into the masticator space. Lesions involving the suprastructure spread by extension through the posterior wall into the pterygomaxillary space, pterygopalatine fossa, infratemporal fossa and the middle cranial fossa; through the roof of the sinus into the orbit; or via the ethmoid cavities to the anterior cranial fossa (Figure 3). Tumors may also track along the infraorbital nerve to the gasserian ganglion4. Tumors of the infrastructure, generally produce symptoms early, and thus present at an earlier stage, and are more readily amenable to a satisfactory resection with an excellent chance for local control than lesions of the suprastructure.
Figure 2.
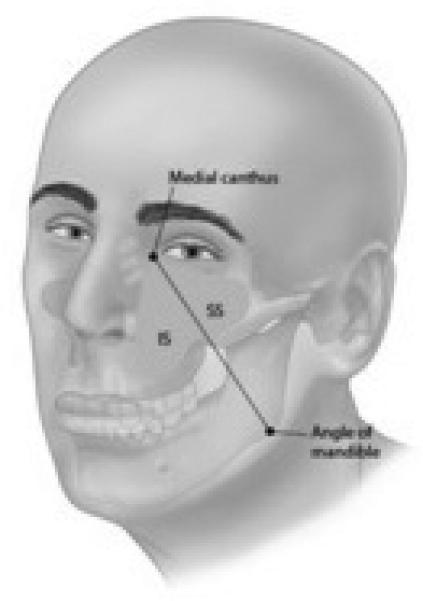
Öhngren's line - The anatomic region located anterior and inferior to this plane is called the infrastrucre and the region posterosuperior to this plane is called the suprastructure.1
Figure 3.
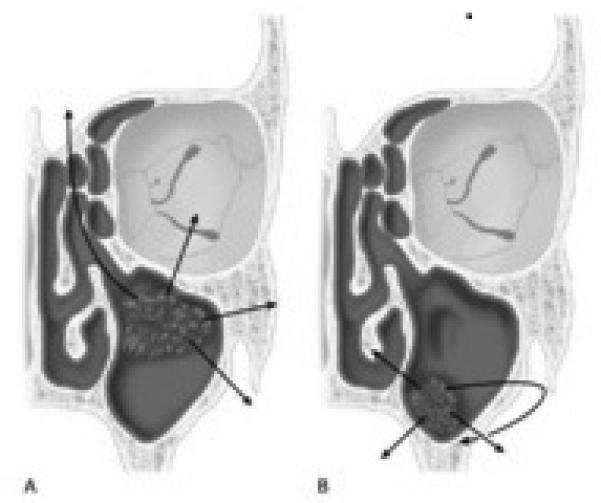
Routes of spread of tumors of the maxillary antrum. A- Suprastructure. B-Infrastructure1
Lymphatic Drainage of the Maxillary Sinus
The maxillary sinus is thought to be an area with paucity of lymphatic pathways with two main routes of lymphatic drainage: the ipsilateral jugulodigastric nodes and the retropharyngeal nodes. One drainage pathway runs from the maxillary gingiva to the submandibular nodes (level 1) through the buccal lymphatic vessels or buccinator nodes. The second pathway runs from the nasal floor to the upper jugular nodes through the retropharyngeal or parapharyngeal nodes5,6. Therefore the majority of observed nodal involvement occurs in level 1 and 23,7. A review of T4 maxillary sinus SCC patients by Homma et al8 (n=128) demonstrated that of those with cervical metastases at time of diagnosis, 96% had ipsilateral level 1b or 2 disease. However the retropharyngeal lymph nodes, level 3, level 4, and even contralateral neck can also be involved in a minority of patients, particularly those with advanced stage primary tumors, and those tumors which cross the midline (Figure 4). Yagi et al9, when looking at all stages and histology of tumors arising in the maxillary sinus, also noted that cervical metastatic disease was most commonly found in the ipsilateral upper jugular region, followed by the submandibular region and then the lower jugular region. Less frequently bilateral disease was seen. Also retropharyngeal node involvement has been reported in 7-16%6,8,10 of patients at time of diagnosis, as well in those developing regional failures11.
Figure 4.
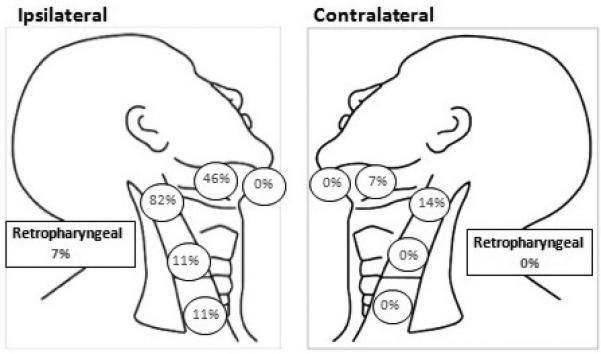
Lymph node metastases distribution at presentation for T4 maxillary sinus squamous cell carcinoma.
Elective Management of the Neck in Maxillary Cancer
Due to the limited lymphatic drainage and rarity of regional disease at presentation, a topic of continued debate remains in regards to how best treat the N0 neck. Many papers have been published on this topic, including more recent reviews and a meta-analysis, underscoring the continued uncertainty and debate2,5,7,8,12-19. Current 2013 National Comprehensive Cancer Network (NCCN) guidelines recommend adjuvant radiation therapy to the N0 neck in the setting of T3 and T4a disease. However, for smaller primary lesions, (T1 and T2) no elective treatment of the neck is recommended. Most data on this topic consist of small series, with multiple histologies, multiple subsites, treated over a broad span of time with changing treatment patterns making interpretation difficult. It is accepted that different histologies have different propensities for nodal spread (Table 2) and as such, the risk of metastases changes based on tumor histology. Nodal metastases have been reported in as high as 28% of patients with squamous cell carcinoma, 25% for adenocarcinoma, 12% for undifferentiated carcinoma, and 10% for adenoid cystic carcinoma20. Another review has the incidence of lymph node involvement in nonsquamous cell carcinoma of the maxillary sinus at presentation even lower (in the range of 3-6%)21,22.
Table 2.
Survival of maxillary sinus carcinoma based on histology. Adapted from Bhattacharyya25
| Tumor Type | n | Percentage | Mean Survival (months) | Median Survival (months) | 5year survival (percentage) |
|---|---|---|---|---|---|
| Adenocarcinoma | 31 | 4.8 | 69 | 50 | 47.7 |
| Adenoid Cystic Carcinoma | 64 | 9.8 | 79 | 118 | 57.5 |
| Mucoepidermoid Carcinoma | 15 | 2.3 | 58 | 53 | 35.9 |
| Other | 70 | 10.8 | 53 | 27 | 38.7 |
| Sarcoma | 46 | 7.1 | 63 | 47 | 44.8 |
| Squamous Cell Carcinoma | 401 | 61.7 | 44 | 18 | 29.2 |
| Melanoma | 23 | 3.5 | 30 | 18 | 25.9 |
| Overall | 601 | 100 | 52 | 25 | 35 |
In this review we are focusing on squamous cell carcinoma as it is the most common histology and appears to be the most aggressive in regards to nodal metastasis. In the literature the rate of involvement of regional lymph nodes, for SCC varies widely. Le et al3 reports an overall incidence of lymph node involvement at diagnosis of 15.5% when only SCC pathology is taken into account. This is consistent with many reports5,7,9,12,13,23-25 and due to this low number most authors have advocated no elective neck treatment for patients with maxillary sinus carcinoma. However, in the 1990s Paulino et al7 and Jiang et al21 began to advocate elective ipsilateral neck irradiation in all patients with SCC due to high incidence of neck relapse associated with SCC histology (28% to 36% in their respective reviews) and the poor prognosis of those who relapsed in the neck7,21,26. A review of the literature however, shows regional failure in the N0 neck to be significantly lower at 4%24-17%14; especially when looking at patients who did not have synchronous local failure9,27(Table 3).
Table 3.
| Study | Overall % with nodes at presentation | SCC% with nodes at presentation | Treatment of N0 neck | Overall N0 patients with nodal failure | SCC N0 patients with nodal failure |
|---|---|---|---|---|---|
| Hinerman 20112! | -- | -- | None | -- | 2/22 (9%) |
| -- | -- | ENI unilat ENI bilat |
-- | 1/14 (4%)# 0/9 |
|
| Le 20003@ | 11/97 (11.3%) | 9/58 (15.5%) | None | 10/97 (10.3%) | 20% |
| -- | -- | ENI | 0/25 (0%) | -- | |
| Valentino 20105@ | 2/17 (11.7%) | 1/7 (14.3%) | none | -- | -- |
| Paulino 19977! | -- | 4/42 (9.5%) | None | -- | 11/38 (28.9%) |
| Homma 20148# | -- | 28/128 (21.9%) | none | -- | 8/83 (9.6%) |
| Yagi 20019@ | 9/118 (7.9%) | -- | None | 9/109 (8.3%) | -- |
| Cantu 200812@ | 33/399 (8.3%) | 16/156 (10.3%) | None | 51/399 (12.5) | -- |
| Kim 199913! | -- | 12/116 (10.3%) | None | 14/104 (13.5%) | 14/104 (13.5%) |
| Mirghani 201314$ | 8/155 (5.2%) | None | 16/133 (12.0%) | ||
| Jaing 199121@ | 6/73 (8.2%) | -- | none | 11/50 | -- |
| -- | -- | ENI | 0/17 | -- | |
| Dulguerov 200124@ | 3/103 (2.9%) | -- | none | (4%) | -- |
| SEER 1988-199825@ | 45/601 (7.5%) | -- | -- | -- | -- |
| Giri 199126@ | 3/41 (7.5%) | -- | -- | 3/35 (8%) | |
| Syners 200927$ | 18/168 (11%) | 10/55 (18%) | 11% | 11% |
ENI, elective neck irradiation
SCC of maxillary sinus
includes all malignant histologies originating in the maxillary sinus
T4 SCC lesions of maxillary sinus
includes all histologies and subsites in paranasal sinuses
In an attempt to address the rate of nodal failure, one paper in the literature focused on the use of elective neck dissection in the management of SCC of the maxillary sinus. In Brown15 et al's series, 13 patients with N0 necks underwent elective neck dissection. Even after neck dissection they had two neck recurrences in the ipsilateral dissected neck. This 15% (2/13) is consistent with their and our review of the literature of isolated neck recurrences in the non-treated neck (12%, range 5-36%)15. This is the only paper describing elective neck dissection in this patient population. One reason for this is that surgical clearance of the retropharyngeal nodal basin, that likely is at risk of occult nodal disease, is not possible. Therefore most other studies and the NCCN guidelines focus on elective irradiation to the neck in advanced tumors as irradiation to the ipsilateral upper jugular lymph nodes is not thought to bring about much additional morbidity and is well tolerated by the patient.
It is suggested that lesions which invade the hard palate, and present in the oral cavity, have a higher rate of nodal failure. This is due to the rich lymphatic pathways of the palate, and therefore they act more as an oral cavity primary rather than maxillary sinus tumor5,8,13,15,28,29. In Cantu's12 series of 156 patients with SCC of the maxillary sinus, 16 patients (10%) had regional recurrence; 11 of which (68.7%) were T2 N0 at presentation. In addition, T4 lesions with extension to the hard palate have been reported to have a significantly decreased local control rate, as compared to all T4 maxillary sinus tumors30,31. A recent review of maxillary SCC originating in the oral cavity noted 38%16 of patients with cervical metastases at diagnosis, of which 33% (17/52) had N2c disease. These lesions originated in the hard palate, and thus were not truly maxillary sinus cancers, and support the existing theory that there is increased drainage and lymphatic spread from the oral cavity as compared to the maxillary sinus16.
While the true rate of nodal metastases at presentation and subsequent nodal failure in the untreated neck has been the recent topic of discussion, it is noted in these studies that local recurrence is still the most common cause of treatment failure, with a very morbid course, and low salvage rates. Giri et al26 in 1991 noted that 49% (18 of 37) of their patients had a treatment failure with local recurrence as the most common site of failure in 16 out of the 18 cases. In contrast only three patients (8%) who were originally N0 and received no elective treatment of the neck developed cervical metastases. Two of the three of these recurrences also had recurrence at the primary site. Due to the frequency of local failure, routine neck irradiation was not recommended as the added morbidity of radiation did not overweight the small risk of having isolated neck failure. Kim et al13 also reported a local failure rate of 68.1% as compared to a regional failure rate of 19% during follow-up (median, 6 years) of their patients.
Our review of the literature (Table 4) and MSKCC data (Figure 5) shows an overall recurrence rate of 44-68%. Local recurrence in each study was most common, accounting for 43-75% of all recurrences. Nodal failure was less often, occurring in 8-33% of all failures. Isolated nodal failure was observed in 4.8%24 – 7.3%17 of all patients when the primary site was controlled. Isolated neck failure accounted for 38-82% of all the cervical failures. The rate of isolated neck failure is similar to the rate of patients with distant failures24. In the largest series published, Cantu et al, reported 31 isolated neck failures out of 230 recurrences in 399 maxillary sinus cancer. Of these 30 (97%) were able to be surgically salvaged12 and only 2 died as a result of isolated neck metastasis12. Similarly Dirix33 and Porceddu34 reported the salvageability of isolated neck failures. These isolated failures occurred in the ipsilateral level 1b and 2 and as such allowed for identification and appropriate intervention. The occurrence of retropharyngeal nodes and overall medical status were the limiting factors keeping isolated nodal recurrences from being surgically salvaged. While neck disease is able to be salvaged > 50% of the time, recurrent local disease is extremely morbid and only 89,32 - 20%9 of isolated local failures are salvaged. Local failure is the most common cause of death in these patients32.
Table 4.
| Study | Overall Recurrence |
Local Recurrence |
Isolated Local Failure |
Nodal Recurrence |
Isolated Regional Failure |
Distant Disease |
Isolated Distant Failure |
|---|---|---|---|---|---|---|---|
| Le 20003@ | 65/97(67%) | 46/97 (47%) | 45/46 (98%) | 8/97 (8%) | 7/8 (86%) | 12/97 (12%) | -- |
| Valentino 20105@ | 1/7(14%) | 1/7 (14%) | 1/1 (100%) | 0 | -- | 0 | -- |
| Paulino 19977! | 28/42 (67%) | 19/42 (45%) | 13/19 (68%) | 14/42 (33%) | 7/14 (50%) | 5/42 (12%) | 0/5 (0%) |
| Yagi 20019@ | -- | 35/106 (33%) | -- | 9/ 106 (8.3%) | 7/9 (78%) | -- | -- |
| Guan11# | 26/59 (44%) | 18/26 (69%) | 12/18 (67%) | 7/26 (27%) | 3/7 (43%) | 7/26 (27%) | 3/9 (33%) |
| Kim 199913! | 79/116 (68%) | 79/116 (68%) | 57/79 (72%) | 22/116 (19%) | 0/22 (0%) | -- | -- |
| Mirghani 201314$ | 68/133 (51%) | 51/68 (75%) | 40/51 (78%) | 16/68 (24%) | 6/16 (38%) | 16/68 (24%) | 9/16 (56%) |
| Brown15! | -- | 9/18 (50%) | -- | 2/18 (11%) | 0/2 (0%) | -- | -- |
| Jaing 199121@ | 33/73 (45%) | 14/73 (19%) | 10/14 (71%) | 11/73 (15%) | 9/11 (82%) | 12/73 (16%) | 8/12 (67%) |
| Dulguerov 200124@ | 114/220 (52%) | 86/103 | 73/86 (85%) | 14/103 (14%) | 8/14 (57%) | 18/103 (17%) | 9/18 (50%) |
| Giri 199126@ | 18/37 (49%) | 16/37 (43%) | NR | 5/37 (14%) | 2/5 (40%) | 1/18 (6%) | -- |
| Syners 200927$ | |||||||
| Kreeft 201132! | -- | 20/69 (29%) | -- | 16/69 (23%) | -- | 2/69 (3%) | -- |
SCC of maxillary sinus
All histologies present in maxillary sinus carcinoma
SCC of paranasal sinuses
Includes all histologies and subsites in paranasal sinuses
Figure 5.
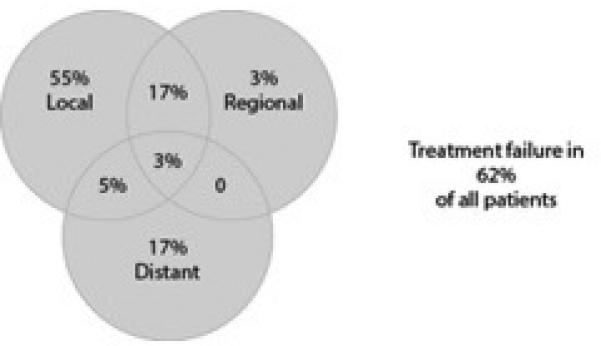
MSKCC data on site of failure of maxillary sinus SCC.1
Le3 and Guan11 had patients, originally N0, who underwent elective neck irradiation (ENI) to the retropharyngeal and cervical nodes. Though both were small series (n= 25 and 11 respectively) none of the patients who received ENI developed nodal metastasis. However 10%3 and 18%11 of those who did not receive ENI suffered neck failures. Therefore they support the use of elective irradiation to the ipsilateral level 1b and 2a during primary treatment, as there are few critical adjacent structures so late complications related to ENI of these echelons would be rare. Jiang21 looked at the survival for those who underwent ENI vs those who recurred in the neck that were able to be salvaged. The 10y disease specific survival (DSS) was 34% in those with no ENI and 58% in those who received ENI. Based on this MD Anderson changed their treatment practice to irradiate the neck in T2-4 SCC or undifferentiated maxillary sinus carcinoma.
Survival
Over the past decades, better imaging assessments of the primary tumor have allowed adequate surgery and adjuvant therapies leading to improved local control24,25,35. Thus attention is now focused to metastatic disease and survival. In Takes18 et al's review, median survival of patients who remained N0 was 80 months while those with any cervical involvement was 25 months (p=0.05). This is similar to Wu's36 and Kim's12 reviews that noted how node involvement at presentation affected survival. The 5 year survival rate with negative nodes was 31.3%12 - 55%36 while for those with positive nodes it was 16.7%12 – 17%36 (p=0.03). Lee et al3 also reported that patients with nodal relapse had a significantly higher risk of distant metastasis based on univariate (p=0.02) and multivariate analysis (HR=4.5, p=0.006)2; with the 5year actuarial risk of distant relapse being 29% for patients with neck control as compared to 81% for patients with neck failure3. However, the most powerful prognostic factor for overall survival remained T stage25,29(p=0.02635) followed by nodal involvement (p=0.03635). Therefore, while the presence of nodal relapse has been associated with decreased survival it is rarely the cause of death. The presence of nodal disease is a sign of an aggressive and advanced phenotype; one associated not only with neck disease but local failure and distant metastatic disease. A meta-analysis, published this year, found that elective neck irradiation can significantly reduce the rate of nodal recurrences19 however it is unclear whether this improved regional control impacted on survival.
Conclusion
A majority of patients with malignant tumors of the maxillary sinus present at an advanced T stage which directly affects survival (Figure 6 and 7). Treatment failure occurs in 62% of all patients, with local recurrence by far the most common site of treatment failure which is rarely amenable to salvage therapy24,37-39. Regional failure is less common and occurs in only 3-20% of all treated patients. Most cervical relapse is accompanied with uncontrollable primary or distant relapse, explaining the poor survival of those with cervical relapse. Patients with cervical relapse alone can be salvaged in 50-70% of cases29. Local failure is the primary cause of failure in any stage29 and as such, aggressive therapy to achieve maximum local control of the primary tumor is considered to be more important than elective neck treatment13. Thus, based on our own experience, elective treatment of the neck can only be considered justifiable in patients with advanced stage primary carcinomas (T3-4) of the maxillary sinus. If the neck on the ipsilateral side is entered for example, to accomplish vascular anastomosis of a free flap, then elective neck dissection is performed at that time. If the neck is not entered, then elective irradiation of the neck, in conjunction with postoperative RT to the primary site, is recommended for T 3-4 tumors. Elective treatment of the neck in early stage tumors (T 1-2), is not recommended.
Figure 6.
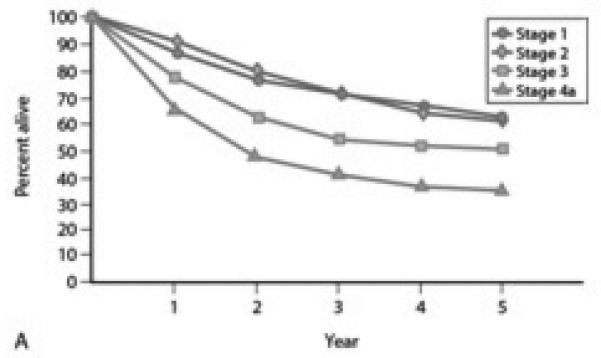
Overall 5y survival of nasal and paranasal disease with all histology by stage1
Figure 7.
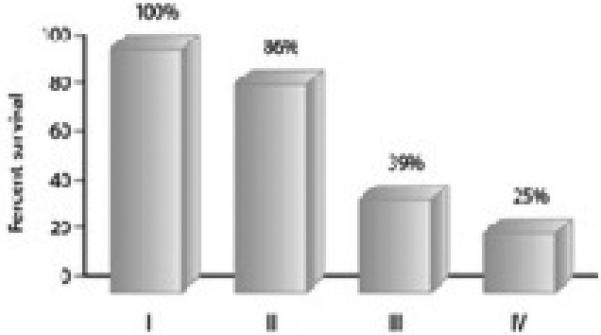
Overall 5y survival of maxillary sinus SCC by stage1
Key Points.
Treatment failure occurs in 62% of all patients, with local recurrence by far the most common site of treatment failure in any stage; local failure is rarely amenable to salvage therapy
Most cervical relapse is accompanied with uncontrollable primary or distant relapse, explaining the poor survival of those with cervical relapse.
Patients with cervical relapse alone can be salvaged in 50-70% of cases.
Aggressive therapy to achieve maximum local control of the primary tumor is considered to be more important than elective neck treatment.
Elective treatment of the neck can only be considered justifiable in patients with advanced stage primary carcinomas (T3-4) of the maxillary sinus.
Acknowledgements
We would like to thank Raia Mohammed and Benjamin Hegel for their assistance with this article and continued support of our program's endeavors.
Footnotes
No funding or conflicts of interest to disclose
Financial support and sponsorship: None
Conflicts of interest: None
References
- 1.Shah JP, Patel SG, Singh B. Jatin Shah's Head and Neck Surgery and Oncology. 4th ed Elsevier Mosby; Philidelphia PA: 2012. [Google Scholar]
- 2.Hinerman RW, Indelicato DJ, Morris CG, Kirwan JM, Werning JW, Vaysberg M, Mendenhall WM. Radiotherapy with or without surgery for maxillary sinus squamous cell carcinoma: should the clinical N0 neck be treated? Am J Clin Oncol. 2011 Oct;34(5):483–7. doi: 10.1097/COC.0b013e3181f942c7. [DOI] [PubMed] [Google Scholar]
- 3.Le QT, Fu KK, Kaplan MJ, et al. Lymph node metastasis in maxillary sinus carcinoma. Int J Radiat Oncol Biol Phys. 2000;2013:541–9. doi: 10.1016/s0360-3016(99)00453-8. [DOI] [PubMed] [Google Scholar]
- 4.McMahon JD, Wong LS, Crowther J, Taylor WM, McManners J, Devine JC, Wales C, Maciver C. Patterns of local recurrence after primary resection of cancers that arise in the sinonasal region and the maxillary alveolus. Br J Oral Maxillofac Surg. 2013 Jul;51(5):389–93. doi: 10.1016/j.bjoms.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Valentini V, Terenzi V, Battisti A, Cassoni A, Anelli A, Priore P, Petrinca P. Management of clinically negative neck in maxillary carcinoma. J Craniofac Surg. 2010 May;21(3):759–62. doi: 10.1097/SCS.0b013e3181d878d1. [DOI] [PubMed] [Google Scholar]
- 6.Umeda M, Minamikawa T, Komatsubara H, Ojima Y, Shibuya Y, Yokoo S. En bloc resection of the primary tumor and cervical lmph nodes through the parapharyngeal space in patients with squamous cell carcinoma of the maxilla: a preliminary study. Br J Oral Maxillofac Surg. 2005 Feb;43(1):17–22. doi: 10.1016/j.bjoms.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Paulino AC, Fisher SG, Marks JE. Is prophylactic neck irradiation indicated in patients with squamous cell carcinoma of the maxillary sinus? Int J Radiat Oncol Biol Phys. 1997;39:283–289. doi: 10.1016/s0360-3016(97)00293-9. [DOI] [PubMed] [Google Scholar]
- 8*.Homma A, Hayashi R, Matsuura K, Kato K, Kawabata K, Monden N, Hasegawa Y, Onitsuka T, Fujimoto Y, Iwae S, Okami K, Matsuzuka T, Yoshino K, Nibu K, Kato T, Nishino H, Asakage T, Ota I, Kitamura M, Kubota A, Ueda T, Ikebuchi K, Watanabe A, Fujii M. Lymph node metastasis in T4 maxillary sinus squamous cell carcinoma: incidence and treatment outcome. Recent article discussing management of the neck in maxillary sinus cancer. Ann Surg Oncol. 2014 May;21(5):1706–10. doi: 10.1245/s10434-014-3544-6. [DOI] [PubMed] [Google Scholar]
- 9.Yagi K, Kukuda S, Furuta Y, et al. A clinical study on the cervical lymph node metastasis of maxillary sinus carcinoma. Auris Nasus Larynx. 2001;28(Suppl):S77–81. doi: 10.1016/s0385-8146(01)00080-3. [DOI] [PubMed] [Google Scholar]
- 10.Watarai J, Seino Y, Kobayahi M, Shindo M, Kato T. CT of retropharyngeal lymph node metastasis from maxillary carcinoma. Acta Radiol. 1993;34:492–5. [PubMed] [Google Scholar]
- 11.Guan X, Wang X, Liu Y, Hu C, Zhu G. Lymph node metastasis in sinonasal squamous cell carcinoma treated with IMRT/3D-CRT. Oral Oncol. 2013 Jan;49(1):60–5. doi: 10.1016/j.oraloncology.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Cantu G, Bimbi G, Miceli R, et al. Lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg. 2008;134:170–177. doi: 10.1001/archoto.2007.30. [DOI] [PubMed] [Google Scholar]
- 13.Kim GE, Chung EJ, Lim JJ, et al. Clinical significance of neck node metastasis in SCC of maxillary antrum. Am J Otolaryngol. 1999;20:383–390. doi: 10.1016/s0196-0709(99)90078-9. [DOI] [PubMed] [Google Scholar]
- 14.Mirghani H, Hartl D, Mortuaire G, et al. Nodal recurrence of sinonasal cancer: does the risk of cervical relapse justify a prophylactic neck treatment? Oral Oncol. 2013;49:374–380. doi: 10.1016/j.oraloncology.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 15*.Brown JS, Bekiroglu F, Shaw RJ, Woolgar JA, Triantafyllou A, Rogers SN. First report of elective selective neck dissection in the management of squamous cell carcinoma of the maxillary sinus. Br J Oral Maxillofac Surg. 2013 Mar;51(2):103–7. doi: 10.1016/j.bjoms.2012.04.004. [Recent article discussing management of the neck in maxillary sinus cancer.] [DOI] [PubMed] [Google Scholar]
- 16*.Sagheb K, Saheb Ka, Taylor KJ, Al-Nawas B, Walter C. Cervical metastases of squamous cell carcinoma of the maxilla: a retrospective study of 25 years. Clin Oral Invest. 2014;18:1221–1227. doi: 10.1007/s00784-013-1070-8. [Recent articles discussing management of the neck in maxillary sinus cancer.] [DOI] [PubMed] [Google Scholar]
- 17*.Mirghani H, Mortuaire G, Armas GL, Hartl D, Aupérin A, El Bedoui S, Chevalier D, Lefebvre JL. Sinonasal cancer: Analysis of oncological failures in 156 consecutive cases. Head Neck. 2014 May;36(5):667–74. doi: 10.1002/hed.23356. [Recent article discussing management of the neck in maxillary sinus cancer.] [DOI] [PubMed] [Google Scholar]
- 18*.Takes RP, Ferlito A, Silver CE, Rinaldo A, Medina JE, Robbins KT, Rodrigo JP, Hamoir M, Suárez C, Zbären P, Mondin V, Shaha AR, Mendenhall WM, Strojan P. The controversy in the management of the N0 neck for squamous cell carcinoma of the maxillary sinus. Eur Arch Otorhinolaryngol. 2014 May;271(5):899–904. doi: 10.1007/s00405-013-2591-0. [Recent article discussing management of the neck in maxillary sinus cancer.] [DOI] [PubMed] [Google Scholar]
- 19*.Abu-Ghanem S, Horowitz G, Abergel A, Yehuda M, Gutfeld O, Carmel NN, Fliss DM. Elective neck irradiation versus observation in squamous cell carcinoma of the maxillary sinus with N0 neck: A meta-analysis and review of the literature. Head Neck. 2014 Jun 10; doi: 10.1002/hed.23791. [First meta-analysis analyzing the rate of nodal recurrence in the irradiated and observed neck. Based on 129 patients in 4 studies they determined that elective neck irradiation can significantly reduce the rate of nodal recurrence in the N0 neck of patients with SCC of the maxillary sinus (OR 0.16, CI 0.04-0.67).] [DOI] [PubMed] [Google Scholar]
- 20.Le QT, 1, Fu KK, Kaplan M, Terris DJ, Fee WE, Goffinet DR. Treatment of maxillary sinus carcinoma: a comparison of the 1997 and 1977 American Joint Committee on cancer staging systems. Cancer. 1999 Nov 1;86(9):1700–11. [PubMed] [Google Scholar]
- 21.Jiang GL, Ang KK, Peters LJ, et al. Maxillary sinus carcinomas: Natural history and results of postoperative radiotherapy. Radiother Oncol. 1991;21:193–200. doi: 10.1016/0167-8140(91)90037-h. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi SS, Chaukar DA, Talole SD, et al. Clinical characteristics and outcomes of non-squamous cell malignancies of the maxillary sinus. J Surg Oncol. 2006;93:362–367. doi: 10.1002/jso.20500. [DOI] [PubMed] [Google Scholar]
- 23.Rho HJ, Kim SJ, Nam HY, Kim BS, Kim IJ, Kim YK, Park K. Detection and prediction of local recurrence of maxillary sinus cancer using F-18 FDG PET/CT. Eur J Surg Oncol. 2010 Feb;36(2):214–20. doi: 10.1016/j.ejso.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92:3012–3029. doi: 10.1002/1097-0142(20011215)92:12<3012::aid-cncr10131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya N. Factors Affecting Survival in Maxillary Sinus Cancer. J Oral Maxillofac Surg. 2003;61:1016–1021. doi: 10.1016/s0278-2391(03)00313-6. [DOI] [PubMed] [Google Scholar]
- 26.Giri SP, Reddy EK, Gemer LS, Krishnan L, Smalley SR, Evans RG. Management of advanced squamous cell carcinomas of the maxillary sinus. Cancer. 1992 Feb 1;69(3):657–61. doi: 10.1002/1097-0142(19920201)69:3<657::aid-cncr2820690310>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Snyers A, Janssen GO, Twickler MB, et al. Malignant tumors of the nasal cavity and paranasal sinuses: long-term outcome and morbidity with emphasis on hypothalamiz=pituitary deficiency. Int J Radiat Oncol Biol Phys. 2009;73:1343–1351. doi: 10.1016/j.ijrobp.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 28.Morris LG, Patel SG, Shah JP, Ganly I. High rates of regional failure in squamous cell carcinoma of the hard palate and maxillary alveolus. Head and Neck. 2011;33:824–30. doi: 10.1002/hed.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo M, Ogawa K, Inuyama Y, Yamashita S, Tominaga S, Shigematsu N, Nishiguchi I, Hashimoto S. Prognostic factors influencing relapse of squamous cell carcinoma of the maxillary sinus. Cancer. 1985 Jan 1;55(1):190–6. doi: 10.1002/1097-0142(19850101)55:1<190::aid-cncr2820550130>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Kano S, Hayashi R, Homma A, Matsuura K, Kato K, Kawabata K, Monden N, Hasegawa Y, Onitsuka T, Fujimoto Y, Iwae S, Okami K, Matsuzuka T, Yoshino K, Fujii M. Effect of local extension sites on survival in locally advanced maxillary sinus cancer. Head Neck. 2013 Aug 30; doi: 10.1002/hed.23483. [DOI] [PubMed] [Google Scholar]
- 31.Sakashita T, Homma A, Hatakeyama H, Kano S, Mizumachi T, Furusawa J, Yoshida D, Fujima N, Onimaru R, Tsuchiya K, Yasuda K, Shirato H, Fukuda S. The incidence of late neck recurrence in N0 maxillary sinus squamous cell carcinomas after superselective intra-arterial chemoradiotherapy without prophylactic neck irradiation. Eur Arch Otorhinolaryngol. 2013 Nov 9; doi: 10.1007/s00405-013-2806-4. [DOI] [PubMed] [Google Scholar]
- 32.Kreeft AM, Smeele LE, Rasch CR, Hauptmann M, Rietveld DH, Leemans CR, Balm AJ. Preoperative imaging and surgical margins in maxillectomy patients. Head Neck. 2012 Nov;34(11):1652–6. doi: 10.1002/hed.21987. [DOI] [PubMed] [Google Scholar]
- 33.Dirix P, Nuyts S, Geussens Y, et al. Malignancies of the nasal cavity and paranasal sinuses: long- term outcome with conventional or three-dimensional conformal radiotherapy. Int J Radiati Oncol Bio Phys. 2007;69:1042–1050. doi: 10.1016/j.ijrobp.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Porceddu S, Martin J, Shanker G, et al. Paranasal sinus tumors: Peter MacCallum Cacner Institute Experience. Head Neck. 2004;26:322–330. doi: 10.1002/hed.10388. [DOI] [PubMed] [Google Scholar]
- 35.Wu TH, Huang JS, Wang HM, Chang JW, Song GG, Wang CH, Yeh KY. Long-term survival after surgery for stage III-IV maxillary sinus carcinoma. B-ENT. 2010;6(1):35–41. [PubMed] [Google Scholar]
- 36.Hayashi T, Nonaka S, Bandoh N, et al. Treatment outcome of maxillary sinus squamous cell carcinoma. Cancer. 2001;92:1495–1503. doi: 10.1002/1097-0142(20010915)92:6<1495::aid-cncr1474>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 37*.Ansa B, Goodman M, Ward K, Kono SA, Owonikoko TK, Higgins K, Beitler JJ, Grist W, Wadsworth T, El-Deiry M, Chen AY, Khuri FR, Shin DM, Saba NF. Paranasal sinus squamous cell carcinoma incidence and survival based on Surveillance, Epidemiology, and End Results data, 1973 to 2009. Cancer. 2013 Jul 15;119(14):2602–10. doi: 10.1002/cncr.28108. [Recent SEER based review focusing on SCC of the paranasal sinuses. Demonstrated a small non-significant increase in 5y survival since the combination of radiation and surgery have become the standard of care in the 1980s.] [DOI] [PubMed] [Google Scholar]
- 38.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012 Jun;34(6):877–85. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 39.Fried DV, Zanation AM, Huang B, Hayes N, Weissler M, Hackman T, Shores C, Rosenman J, Morris DE, Funkhouser W, Varia M, Chera BS. Patterns of local failure for sinonasal malignancies. Pract Radiat Oncol. 2013 Jul-Sep;3(3):e113–20. doi: 10.1016/j.prro.2012.07.001. [DOI] [PubMed] [Google Scholar]


