Abstract
BACKGROUND
An accurate staging system is crucial for cancer management. Evaluations for continual suitability and improvement are needed as staging and treatment methods evolve.
METHODS
This was a retrospective study of 1609 patients with nasopharyngeal carcinoma investigated by magnetic resonance imaging, staged with the 7th edition of the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) staging system, and irradiated by intensity-modulated radiotherapy at 2 centers in Hong Kong and mainland China.
RESULTS
Among the patients without other T3/T4 involvement, there were no significant differences in overall survival (OS) between medial pterygoid muscle (MP)±lateral pterygoid muscle (LP), prevertebral muscle, and parapharyngeal space involvement. Patients with extensive soft tissue involvement beyond the aforementioned structures had poor OS similar to that of patients with intracranial extension and/or cranial nerve palsy. Only 2% of the patients had lymph nodes>6cm above the supraclavicular fossa (SCF), and their outcomes resembled the outcomes of those with low extension. Replacing SCF with the lower neck (extension below the caudal border of the cricoid cartilage) did not affect the hazard distinction between different N categories. With the proposed T and N categories, there were no significant differences in outcome between T4N0-2 and T1-4N3 disease.
CONCLUSIONS
After a review by AJCC/UICC preparatory committees, the changes recommended for the 8th edition include changing MP/LP involvement from T4 to T2, adding prevertebral muscle involvement as T2, replacing SCF with the lower neck and merging this with a maximum nodal diameter>6 cm as N3, and merging T4 and N3 as stage IVA criteria. These changes will lead not only to a better distinction of hazards between adjacent stages/categories but also to optimal balance in clinical practicability and global applicability.
Keywords: nasopharyngeal cancer, prognostication, TNM staging system
INTRODUCTION
An accurate staging system is crucial in cancer management for predicting the prognosis, guiding clinicians in treatment decisions for different risk groups, and evaluating the results of treatment between centers. The prognostic significance of a staging system changes with advances in investigation and treatment methods. Evaluations of staging systems to ensure continual suitability and exploration for further improvement are essential.
It is well recognized that the natural behavior of and therapeutic considerations for nasopharyngeal carcinoma (NPC) are different from those for other head and neck cancers. A major improvement in the TNM staging system by the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) was the adoption of a customized system for NPC in the 5th edition.1,2 With data from large retrospective series from Asia, where NPC is most prevalent, the staging criteria were developed through the merging of the strengths of the 4th edition of the AJCC/UICC system and Ho’s system.3,4 This was a milestone development that has gained global acceptance as studies from different countries (endemic and nonendemic) unanimously confirmed substantial improvements in comparison with prior systems.
No change was recommended in the 6th edition5,6 except for the addition of the term masticator space as a synonym for infratemporal fossa (one of the T4 criteria) because, although the intended extent was described in the staging handbook, the latter was not a clearly defined space with universal acceptance. In the current 7th edition,7,8 both terms are retained as T4 criteria; however the term masticator space now uses the boundaries described in a classic anatomy textbook instead of the demarcation used for infratemporal fossa. Additional changes included downstaging of tumors with extension to the nasal fossa/oropharynx without parapharyngeal extension (previously T2a) to T19,10 and a clear definition of retropharyngeal lymph node involvement (unilateral or bilateral) as N1.11
The management of NPC has undergone substantial evolution in the past 2 decades. More accurate imaging methods have allowed better delineation of the tumor extent and early detection of occult metastases. The transition from 2-dimensional conventional radiotherapy to 3-dimensional conformal and intensity-modulated radiotherapy (IMRT) has led to increasing conformity of tumor coverage and sparing of noninvolved structures. The use of combination chemotherapy has further improved tumor control and cure rates, especially for advanced locoregional disease. It is, therefore, important that the new staging system be based on data from patients managed with contemporary methods.12
The preparatory processes for the 8th edition of the AJCC/UICC staging system included an extensive literature review and validation of recommendations by contemporary series before consensus was attained by international multidisciplinary experts. Among the suggestions reported in the literature, 4 issues demand serious consideration: 1) the controversy about the significance of the masticator space,13-18 2) the uncertainty about the significance of prevertebral muscle invasion,19-21 3) the possibility of replacing the supraclavicular fossa (SCF)3 with anatomic nodal levels,22-27 and 4) the simplification of unnecessary subgroups by elimination.27,28
In this study, we evaluated patients who were staged with magnetic resonance imaging (MRI) and irradiated with IMRT at 2 hospitals (one in Hong Kong and the other in Fujian in mainland China) to address these issues and to develop consensus recommendations by AJCC and UICC for the coming 8th edition.
MATERIALS AND METHODS
Patients
A total of 1609 consecutive patients with nondisseminated NPC who were treated at Fujian Provincial Cancer Hospital and Pamela Youde Nethersole Eastern Hospital from June 2005 to December 2010 were analyzed (Table 1). All patients had histological confirmation: 99.2% had nonkeratinizing (differentiated/undifferentiated) carcinoma and 0.8% had keratinizing squamous cell carcinoma according to the World Health Organization classification. The median age was 47 years (range, 11-84 years); 75% were male, and 25% were female. The median follow-up for the whole cohort was 5 years (range, 0.2-9.3 years).
TABLE 1.
Patient Characteristics
| Age, median (range), y | 47 (11-84) |
| Sex, No. (%) | |
| Male | 1212 (75) |
| Female | 397 (25) |
| Performance status, No. (%) | |
| 0 | 1424 (88.5) |
| 1 | 172 (10.7) |
| 2 | 11 (0.7) |
| 3 | 2 (0.1) |
| Histology, No. (%) | |
| Keratinizing squamous cell | 13 (1) |
| Nonkeratinizing, differentiated | 68 (4) |
| Nonkeratinizing, undifferentiated | 1528 (95) |
| Radiotherapy | |
| Total dose, median (range), Gy | 69.75 (61.6-86.7) |
| Overall treatment time, median (range), d | 43 (36-96) |
| Chemotherapy (cisplatin-based) | |
| Total patients treated, No. (%) | 1359 (85) |
| Sequence, stage II/stage III-IVB,% | |
| Concurrent±induction/adjuvant | 36/56 |
| Adjuvant | 2/1 |
| Induction | 16/17 |
| Induction+adjuvant | 20/18 |
| Nil | 27/8 |
This retrospective study was approved by the respective local hospitals.
Clinical Staging and Treatment
All patients underwent a complete physical examination, fiber-optic nasopharyngoscopy, and MRI of the nasopharyngeal and cervical region. An additional metastatic evaluation was performed according to institutional polices. The 7th edition of the AJCC/UICC staging system7,8 was used for clinical staging at presentation.
All patients were treated with the IMRT technique with a median total dose of 69.8 Gy (range, 61.6-86.7 Gy). Details of IMRT planning and dose prescription have been described previously.29,30 Additional treatment with cisplatin-based chemotherapy (various schedules) was administered to 92% of patients with stage III disease and to 73% of patients with stage II disease (Table 1).
Statistical Analysis
The eligibility criteria set for this retrospective study included histologically confirmed NPC, no gross evidence of distant metastases, staging with MRI, and irradiation with radical intent with IMRT. The exclusion criteria were a history of previous treatment or prior malignancy. All consecutive eligible patients treated in the 2 participating centers from June 2005 to December 2010 were analyzed. The study period was chosen to ensure the most consistent staging and radiotherapy methods with a median follow-up of 5 years.
All events were measured from the date of histological diagnosis. The primary endpoint for this analysis was overall survival (OS; time to death due to any cause). Additional endpoints included distant failure-free survival (D-FFS; time to distant metastasis), local failure-free survival according to the T category (L-FFS; time to local persistence/recurrence), and nodal failure-free survival according to the N category (N-FFS; time to nodal persistence/recurrence). The unadjusted actuarial rates were calculated with the Kaplan-Meier method,31 and the differences were compared with a log-rank test.32 A Cox proportional hazards model33 was used to assess the hazard ratio with a 95% confidence level. Two-sided tests were used, and those with P values<.05 were considered statistically significant.
With the current sample size of 1609, the power was 88.5% for detecting a hazard ratio at 1.27 between adjacent stages with a 95% confidence level.34 The proportional hazard assumption was also tested. All the Cox models satisfied the proportional hazard assumption with P values>.05 for the proportional hazard test.35
The performances of the 7th edition of the AJCC/UICC staging system and the proposed 8th edition were also compared with the Akaike information criterion (AIC)36 and Harrell’s concordance index (c-index).37 Both the AIC and the c-index were calculated for the Cox proportional hazards regression model and were adjusted for age and sex. The AIC refers to the information loss of the selected model; a smaller AIC value suggests a better goodness of fit of the model. The c-index measures the ability to predict the outcomes; a higher c-index suggests a greater ability to discriminate the outcomes with the model (ie, better discriminatory power of the model). Internal validation for the AIC and the c-index was performed via bootstrapping with 1000 replications. All statistical analyses were conducted with SPSS 22 and R 3.1.3.
RESULTS
T Category
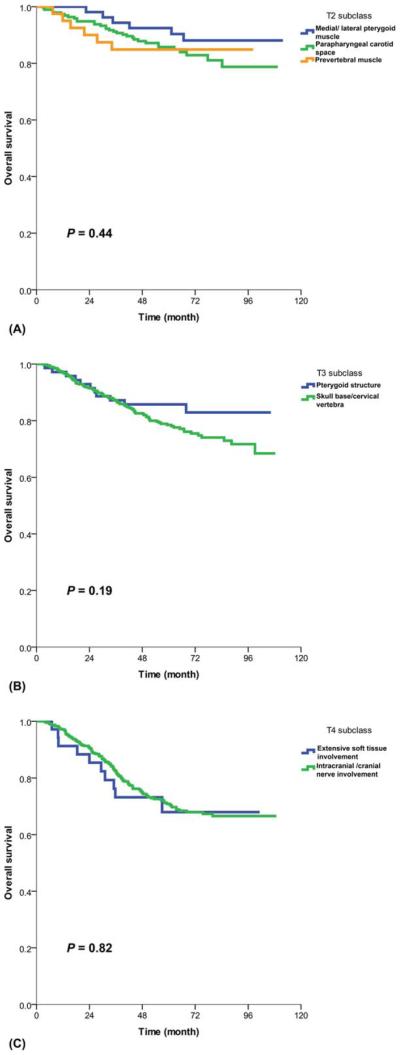
Among the patients categorized as T4 on the basis of the current definition of masticator space, those with medial pterygoid muscle (MP)±lateral pterygoid muscle (LP) involvement (n=590) had a significantly higher rate of association with other T3/T4 staging criteria in comparison with those without MP/LP involvement (n=1019; 91% vs 44%, P<.001). However, among the patients without other T3/T4 criteria, the subgroup of patients with MP/LP involvement (n=53) had much better OS than the patients with other T4 criteria (93% vs 71% at 5 years, P=.003); there were no significant differences in OS between those with MP/LP involvement, those with prevertebral muscle involvement, and those with parapharyngeal extension alone (Fig. 1A). On the other hand, the subgroup of patients with extensive soft tissue involvement (infiltration beyond the lateral surface of the LP, hypopharynx, orbital structures, and parotid gland) but no other T4 criteria had poor OS similar to that of the subgroup with intracranial extension and/or cranial nerve palsy (68% vs 73%, P=.816; Fig. 1C).
Figure 1.
Overall survival: (A) T2 subgroups (different adjacent soft tissue involvement), (B) T3 subgroups (pterygoid structures vs skull base erosion), and (C) T4 subgroups (extensive soft tissue involvement vs intracranial/cranial nerve involvement).
Among the patients with current T3 criteria, there was no statistically significant difference in OS between those with involvement of pterygoid structures alone (medial/lateral pterygoid plate, pterygoid body/process, pterygomaxillary fissure, and pterygopalatine fossa) and those with erosion of the skull base and/or cervical vertebra (86% vs 79%, P=.186; Fig. 1B).
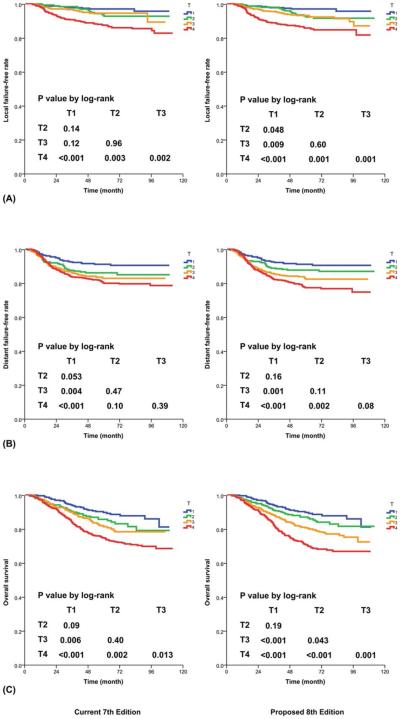
Hence, the changes recommended for the 8th edition include changing the criterion of MP/LP involvement without other T3/T4 criteria from T4 to T2 and adding prevertebral muscle involvement as a T2 criterion (Fig. 2A and Table 2). With the proposed changes, the differences in L-FFS between T1 and T2 (P=.048), in D-FFS between T2 and T4 (P=.002), and in OS between T2 and T3 (P=.043) now reached statistical significance (Fig. 3). In comparison with the 7th edition,7,8 the proposed 8th edition led to a lower AIC and a higher c-index for all endpoints (Table 3).
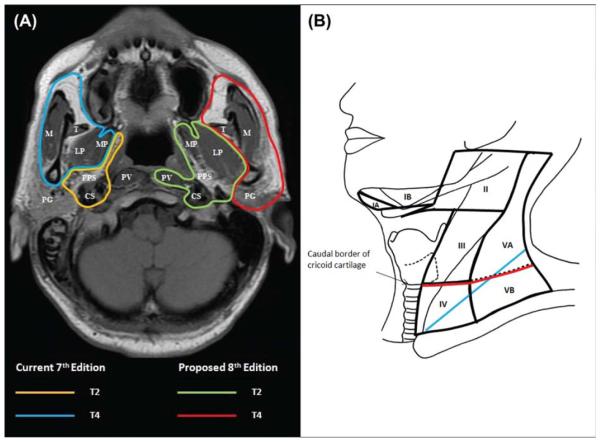
Figure 2.
Differences in defining criteria between the current 7th edition and the proposed 8th edition: (A) changing the extent of soft tissue involvement as T2 and T4 criteria and (B) replacing the supraclavicular fossa (blue) with the lower neck (ie, below the caudal border of cricoid cartilage; red) as N3 criteria. CS indicates carotid space; LP, lateral pterygoid muscle; M, masseter muscle; MP, medial pterygoid muscle; PG, parotid gland; PPS, parapharyngeal space; PV, prevertebral muscle; T, temporalis muscle.
TABLE 2.
Classification Criteria and Stage Grouping According to the 7th Edition and Proposed 8th Edition of the American Joint Committee on Cancer/International Union Against Cancer Staging System
| T Category | |
| Current 7th Edition | Proposed 8th Edition |
| T1. Nasopharynx, oropharynx, nasal fossa | T1. Nasopharynx, oropharynx, nasal fossa |
| T2. Parapharyngeal extension | T2. Parapharyngeal extension, adjacent soft tissue involvement (medial pterygoid, lateral pterygoid, prevertebral muscles) |
| T3. Bony structure, paranasal sinuses | T3. Bony structure (skull base, cervical vertebra), paranasal sinuses |
| T4. Intracranial extension, cranial nerve, hypopharynx, orbit, infratemporal fossa, masticator space |
T4. Intracranial extension, cranial nerve, hypopharynx, orbit, extensive soft tissue involvement (beyond the lateral surface of the lateral pterygoid muscle, parotid gland) |
| N Category | |
| Current 7th Edition | Proposed 8th Edition |
| N0. None | N0. None |
| N1. Retropharyngeal (regardless of laterality) Cervical: unilateral,≤6 cm, and above supraclavicular fossa |
N1. Retropharyngeal (regardless of laterality) Cervical: unilateral,≤6 cm, and above caudal border of cricoid cartilage |
| N2. Cervical: bilateral, ≤6 cm, and above supraclavicular fossa | N2. Cervical: bilateral, ≤6 cm, and above caudal border of cricoid cartilage |
| N3a.>6 cm | N3.>6 cm and/or below caudal border of cricoid cartilage (regardless of laterality) |
| N3b. In supraclavicular fossa | |
| Stage Group | |
| Current 7th Edition | Proposed 8th Edition |
| I. T1 N0 M0 | I. T1 N0 M0 |
| II. T1 N1 M0 T2 N0-1 M0 |
II. T1 N1 M0 T2 N0-N1 M0 |
| III. T1-2 N2 M0 T3 N0-2 M0 |
III. T1-2 N2 M0 T3 N0-2 M0 |
| IVA. T4 N0-2 M0 | IVA. T4 or N3 M0 |
| IVB. Any T N3 M0 | |
| IVC. Any T Any N M1 | IVB. Any T Any N M1 |
The nodal size was based on the maximum dimension in any direction.
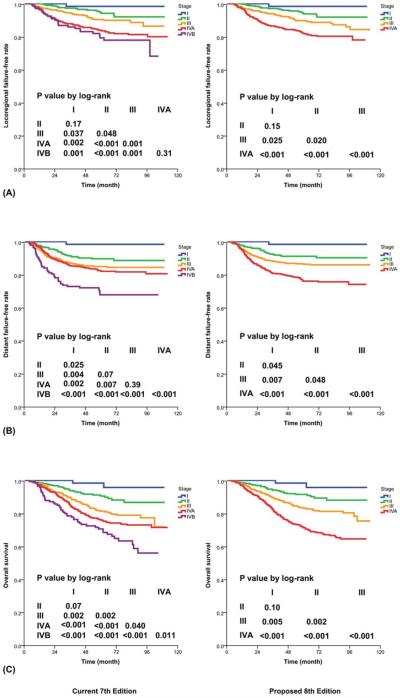
Figure 3.
Prognostication by T category with (Left) the current 7th edition and (Right) the proposed 8th edition: (A) local failure-free rate, (B) distant failure-free rate, and (C) overall survival.
TABLE 3.
Distribution and Prognostication (Failure-Free/Survival Rates at 5 Years) According to the 7th Edition and Proposed 8th Edition of the American Joint Committee on Cancer/International Union Against Cancer Staging System
| Current 7th Edition |
Proposed 8th Edition |
||||||||
| T Category | No. (%) | Local FFS, % | Distant FFS, % | Overall Survival, % | T Category | No. (%) | Local FFS, % | Distant FFS, % | Overall Survival, % |
|
| |||||||||
| T1 | 342 (21) | 97 | 91 | 90 | T1 | 342 (21) | 97 | 91 | 90 |
| T2 | 233 (15) | 93 | 83 | 86 | T2 | 286 (18) | 92 | 88 | 87 |
| T3 | 294 (18) | 94 | 83 | 81 | T3 | 485 (30) | 93 | 82 | 81 |
| T4 | 740 (46) | 87 | 80 | 75 | T4 | 496 (31) | 86 | 77 | 71 |
| Overall P | <.001 | .001 | <.001 | Overall P | <.001 | <.001 | <.001 | ||
| AICa | 1814.3 (1558.8-2057.8) | 3555.7 (3242.2-3906.3) | 4474.6 (4107.9-4840.2) | AICa | 1814.0 (1561.1-2054.1) | 3548.8 (3238.3-3900.4) | 4462.2 (4097.0-4828.6) | ||
| c-indexa | 0.688 (0.650-0.723) | 0.604 (0.575-0.634) | 0.690 (0.667-0.715) | c-indexa | 0.691 (0.652-0.728) | 0.611 (0.583-0.640) | 0.696 (0.671-0.721) | ||
|
| |||||||||
| Current 7th Edition |
Proposed 8th Edition |
||||||||
| N Category | No. (%) | Nodal FFS, % | Distant FFS, % | Overall Survival, % | N Category | No. (%) | Nodal FFS, % | Distant FFS, % | Overall Survival, % |
|
| |||||||||
| N0 | 194 (12) | 99 | 94 | 91 | N0 | 194 (12) | 99 | 94 | 91 |
| N1 | 727 (45) | 97 | 86 | 84 | N1 | 711 (44) | 97 | 87 | 85 |
| N2 | 547 (34) | 93 | 81 | 75 | N2 | 479 (30) | 94 | 81 | 75 |
| N3a | 37 (2) | 90 | 72 | 75 | N3 | 225 (14) | 89 | 72 | 70 |
| N3b | 104 (7) | 86 | 66.4 | 69 | |||||
| Overall P | <.001 | <.001 | <.001 | Overall P | <.001 | <.001 | <.001 | ||
| AICa | 1070.3 (876.7-1258.8) | 3534.7 (3223.4-3876.8) | 4470.2 (4106.3-4843.6) | AICa | 1073.0 (878.9-1264.0) | 3536.4 (3225.3-3886.7) | 4468.1 (4103.5-4835.8) | ||
| c-indexa | 0.683 (0.634-0.729) | 0.626 (0.597-0.655) | 0.691 (0.668-0.715) | c-indexa | 0.674 (0.627-0.723) | 0.624 (0.595-0.653) | 0.691 (0.668-0.716) | ||
|
| |||||||||
| Current 7th Edition |
Proposed 8th Edition |
||||||||
| Stage Group | No. (%) | Locoregional FFS, % | Distant FFS, % | Overall Survival, % | Stage Group | No. (%) | Locoregional FFS, % | Distant FFS, % | Overall Survival, % |
|
| |||||||||
| I | 63 (4) | 98 | 98 | 98 | I | 63 (4) | 98 | 98 | 98 |
| II | 309 (19) | 94 | 90 | 91 | II | 323 (20) | 94 | 91 | 92 |
| III | 434 (27) | 90 | 85 | 81 | III | 565 (35) | 90 | 86 | 83 |
| IVA | 662 (41) | 84 | 82 | 76 | IVA | 658 (41) | 82 | 76 | 71 |
| IVB | 141 (9) | 79 | 68 | 71 | |||||
| Overall P | <.001 | <.001 | <.001 | Overall P | <.001 | <.001 | <.001 | ||
| AICa | 2580.9 (2293.0-2871.5) | 3533.6 (3226.7-3879.0) | 4457.4 (4093.0-4825.9) | AICa | 2585.6 (2296.8-2874.7) | 3528.1 (3215.7-3877.9) | 4436.4 (4074.9-4801.0) | ||
| c-indexa | 0.668 (0.641-0.701) | 0.622 (0.593-0.651) | 0.700 (0.677-0.725) | c-indexa | 0.668 (0.637-0.701) | 0.636 (0.608-0.664) | 0.711 (0.688-0.735) | ||
Abbreviations: AIC, Akaike information criterion; c-index, Harrell’s concordance index; FFS, failure-free survival.
The data are presented as medians and 90% ranges (in parentheses) from 1000 bootstrap replications.
N Category
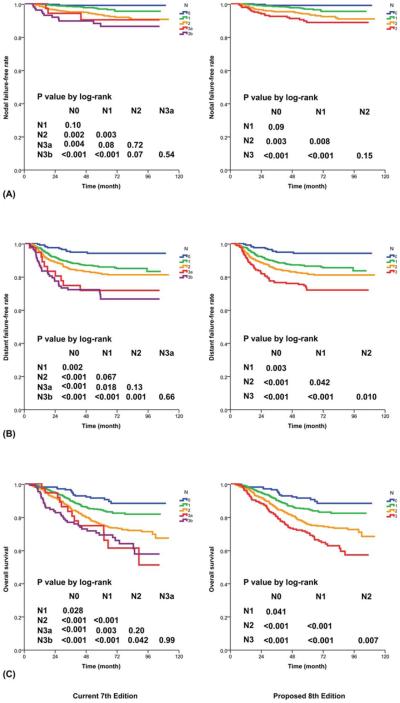
Only 37 patients (2%) had a lymph node larger than 6 cm without extension into the SCF; the differences between N3a and N2 and between N3a and N3b were statistically insignificant for all endpoints. The overall trends were closer to N3b, particularly in terms of D-FFS and long-term OS (Fig. 4). Grouping with N3b without further subclassification is hence suggested.
Figure 4.
Prognostication by N category with (Left) the current 7th edition and (Right) the proposed 8th edition: (A) nodal failure-free rate, (B) distant failure-free rate, and (C) overall survival.
Replacing the N3b criterion of the SCF with the lower neck (defined as an extension below the caudal border of the cricoid cartilage with the criteria of levels IV and Vb of Som et al22 did not affect the N category in 1505 patients but led to upstaging from N2 to N3 in 100 patients and downstaging from N3 to N2 in 4 patients. The 5-year OS for those with extension to the lower neck (70%) was similar to the 5-year OS for those with extension to the SCF (69%). Only 11 patients had a lymph node larger than 6 cm without extension to the lower neck.
Hence, the changes recommended for the 8th edition include changing the criterion of the SCF to the lower neck (defined as extension below the caudal border of the cricoid cartilage) and merging this with a size>6 cm as N3 criteria (Fig. 2B and Table 2). With the proposed changes, the differences between N2 and N3 in terms of D-FFS (P=.010) and OS (P=.007) and the difference in D-FFS between N1 and N2 (P=.042) now reached statistical significance (Fig. 4). In comparison with the 7th edition,7,8 although the proposed 8th edition did not lead to an improvement in the AIC or the c-index for N-FFS and D-FFS, it did lead to a lower AIC and maintained the same c-index for OS (Table 3).
Stage Group
Only 63 patients (4%) presented with T1N0 disease. There was no statistically significant difference between stages I and II in terms of locoregional failure-free survival (FFS; P=.15) and only a trend toward significance in OS (98% vs 92%, P=.098). However, the survival curves were clearly separated, and there was a significant difference in D-FFS (98% vs 91%, P=.045; Fig. 5). With adjustments for age and sex, the hazard of deaths (from all causes) increased from 1 for stage I to 3.5 for stage II, to 6.1 for stage III, and to 11.0 for stage IVA (Table 4).
Figure 5.
Prognostication by stage group with (Left) the current 7th edition and (Right) the proposed 8th edition: (A) locoregional failure-free rate, (B) distant failure-free rate, and (C) overall survival.
TABLE 4.
HRs According to the 7th Edition and Proposed 8th Edition of the American Joint Committee on Cancer/International Union Against Cancer Staging System
| Current 7th Edition |
Proposed 8th Edition |
||||||
| T Category | Local FFS | Distant FFS | Overall Survival | T Category | Local FFS | Distant FFS | Overall Survival |
|
| |||||||
| T1 | Reference | Reference | Reference | T1 | Reference | Reference | Reference |
| T2 | 2.28 (1.19-4.38) .013 | 1.58 (0.97-2.58) .067 | 1.38 (0.88-2.17) .161 | T2 | 2.38 (1.28-4.43) .006 | 1.40 (0.87-2.27) .167 | 1.35 (0.87-2.08) .178 |
| T3 | 2.03 (1.07-3.88) .031 | 1.98 (1.26-3.10) .003 | 1.86 (1.23-2.83) .003 | T3 | 2.47 (1.39-4.41) .002 | 1.99 (1.32-3.01) .001 | 1.94 (1.34-2.82) <.001 |
| T4 | 4.16 (2.43-7.11) <.001 | 2.24 (1.52-3.30) <.001 | 2.56 (1.81-3.62) <.001 | T4 | 4.82 (2.79-8.32) <.001 | 2.52 (1.68-3.76) <.001 | 2.96 (2.07-4.23) <.001 |
| Overall P | <.001 | <.001 | <.001 | Overall P | <.001 | <.001 | <.001 |
|
| |||||||
| Current 7th Edition |
Proposed 8th Edition |
||||||
| N Category | Nodal FFS | Distant FFS | Overall Survival | N Category | Nodal FFS | Distant FFS | Overall Survival |
|
| |||||||
| N0 | Reference | Reference | Reference | N0 | Reference | Reference | Reference |
| N1 | 1.32 (0.72-2.43) .365 | 2.52 (1.38-4.59) .003 | 2.12 (1.30-3.44) .003 | N1 | 1.35 (0.74-2.48) .331 | 2.44 (1.34-4.45) .004 | 2.04 (1.25-3.33) .004 |
| N2 | 2.84 (1.59-5.08) <.001 | 3.23 (1.77-5.88) <.001 | 3.17 (1.96-5.11) <.001 | N2 | 2.90 (1.62-5.21) <.001 | 3.27 (1.79-5.98) <.001 | 3.06 (1.88-4.96) <.001 |
| N3a | 3.29 (1.31-8.26) .011 | 4.92 (2.12-11.39) <.001 | 3.74 (1.84-7.58) <.001 | N3 | 2.82 (1.50-5.32) .001 | 4.90 (2.63-9.12) <.001 | 4.24 (2.57-7.00) <.001 |
| N3b | 3.40 (1.68-6.89) .001 | 6.17 (3.18-11.99) <.001 | 4.43 (2.54-7.73) <.001 | ||||
| Overall P | <.001 | <.001 | <.001 | Overall P | <.001 | <.001 | <.001 |
|
| |||||||
| Current 7th Edition |
Proposed 8th Edition |
||||||
| Stage Group | Locoregional FFS | Distant FFS | Overall Survival | Stage Group | Locoregional FFS | Distant FFS | Overall Survival |
|
| |||||||
| I | Reference | Reference | Reference | I | Reference | Reference | Reference |
| II | 3.45 (0.49-24.07) .212 | 7.17 (0.99-51.88) .051 | 3.61 (0.90-14.51) .071 | II | 3.68 (0.53-25.53) .187 | 6.21 (0.86-45.00) .071 | 3.35 (0.83-13.53) .090 |
| III | 6.13 (0.91-41.25) .062 | 10.64 (1.49-75.79) .018 | 6.80 (1.74-26.61) .006 | III | 6.82 (1.02-45.49) .047 | 9.64 (1.36-68.43) .023 | 6.18 (1.58-24.17) .009 |
| IVA | 11.02 (1.66-72.98) .013 | 12.33 (1.74-87.21) .012 | 9.14 (2.36-35.42) .001 | IVA | 12.18 (1.84-80.54) .010 | 16.50 (2.34-116.39) .005 | 11.41 (2.94-44.33) <.001 |
| IVB | 13.61 (2.00-92.75) .008 | 23.15 (3.22-166.22) .002 | 12.38 (3.13-48.97) <.001 | ||||
| Overall P | <.001 | <.001 | <.001 | Overall P | <.001 | <.001 | <.001 |
Abbreviations: CI, confidence interval; FFS, failure-free survival; HR, hazard ratio.
The data are presented as HRs and 95% CIs (in parentheses); the corresponding P values are shown beneath them. Cox regression models with adjustments for age and sex were used to calculate HRs.
There were no significant differences between the subgroup with T4N0-2 disease and the subgroup with T1-4N3 disease for all endpoints, including D-FFS (78% vs 72%, P=.080) and OS (72% vs 70%, P=.114). Hence, the change recommended for the 8th edition is to merge T4 and N3 as the criteria for advanced locoregional disease (stage IVA) without further subclassification (Table 2). With the proposed system, the difference in D-FFS between stages II and III now reached statistical significance (P=.048; Fig. 5). In comparison with the 7th edition,7,8 the proposed 8th edition led to a lower AIC and a higher c-index for D-FFS and OS, and it maintained the same c-index for locoregional FFS (Table 3).
DISCUSSION
Since the milestone change in the 5th edition of the AJCC/UICC staging system,1,2 with the development of a customized staging system for NPC based on a combination of the strengths of Ho’s system3,4 and the AJCC/UICC system, substantial support has been attained in both endemic and nonendemic regions. As staging and treatment methods evolve, evaluations for continual suitability and improvement are important. A specific Literature Watch program has been implemented by the UICC to capture the studies on staging systems reported in the literature, and feedback has been obtained from an international expert panel regarding whether changes should be considered for subsequent editions. However, the reported studies on the evaluation of the current 7th edition of the AJCC/UICC staging system7,8 and suggestions for further improvement are based largely on series managed with past methods. Validation by more contemporary series is needed.
The current study was initiated to address the concerns outlined in the introduction and to explore potential optimal changes for the coming 8th edition of the AJCC/UICC staging system. This series of 1609 patients with a median follow-up of 5 years, all staged with MRI and irradiated with IMRT at 2 major centers in Hong Kong and mainland China, provides useful data for this validation. Furthermore, extensive discussions by the AJCC and UICC preparatory committee were conducted to review the results and attain a consensus among international multidisciplinary experts to ensure not only prognostic accuracy but also optimal balance in clinical practicability and global applicability. The main weakness of the current study is that this is a retrospective study; further validation by prospective data will be useful.
It would be ideal if the current study could be further validated by data from nonendemic regions; however, most centers in nonendemic regions have only small series of patients, and even major centers rarely capture details of tumor extent as in the current study. Nevertheless, we are confident that the current conclusions should be applicable across countries as evidenced by the global support for the 5th edition of the AJCC/UICC staging system, a landmark development when we used data from Hong Kong to combine the strengths of the 4th edition of the AJCC/UICC staging system and Ho’s staging system to design a customized system for NPC.
As for the T categories, the main area for improvement is defining the extent of soft tissue involvement as a T4 criterion. According to the definition stated in the 7th edition of the AJCC cancer staging handbook, the masticator space primarily consists of the muscles of mastication encompassed within the superficial layer of the deep cervical fascia and extends from the medial and lateral pterygoid (MP/LP) muscles to the masseter and temporalis muscles (Fig. 2A). The current study concurs with the study by Sze et al17: the involvement of the MP/LP per se did not lead to poor survival similar to that of patients with extensive infiltration beyond the lateral surface of the LP. The claim that patients with MP/LP have a poor prognosis is likely due to the intrinsic association with other sinister criteria. Our data showed that for patients without other T3/T4 criteria, there were no significant differences in OS among those with infiltration of adjacent soft tissue, including the MP/LP, prevertebral muscles, and parapharyngeal space alone (Fig. 1A). Involvement of pterygoid structures should remain as T3 because the prognosis was similar to that with other skull base bony erosions (Fig. 1B). Our data also confirmed that patients with extensive soft tissue infiltration beyond these structures did have a poor prognosis similar to that of patients with intracranial extension/cranial nerve palsy (Fig. 1C). Therefore, tumors involving these structures should continue to be classified as T4.
Hence, the changes recommended for the 8th edition include changing the criterion of MP/LP involvement from T4 to T2, adding prevertebral muscle involvement as a T2 criterion (Fig. 2), and replacing the ambiguous terms masticator space and infratemporal fossa with a specific description of soft tissue involvement (Table 2). This change could help to refine the decision on the addition of chemotherapy. Although aggressive chemotherapy with a concurrent±adjuvant/induction sequence is indicated for patients with stage IV, the benefit for patients with stage II is less certain (especially for patients irradiated with the optimal intensity-modulated technique), and even if chemotherapy is used, the concurrent-alone sequence is the one generally recommended. Hence, with more accurate prognostication by downstaging pterygoid muscle involvement to T2, these patients might be spared unnecessary chemotherapy if there are no other unfavorable prognostic factors.
Although the differences in D-FFS and OS between T1 and T2 remained insignificant, the difference in L-FFS now reached statistical significance (P=.048). In addition, although the differences in L-FFS and D-FFS between T2 and T3 remained insignificant, the difference in OS now reached statistical significance (P=.043). Therefore, continuing to classify them as a discrete T category is recommended (Fig. 3).
As for the N categories, the current 7th edition of the AJCC/UICC staging system7,8 subdivides N3 into N3a and N3b with a nodal diameter>6 cm (maximum dimension) and extension into the SCF3 as respective criteria. The significance of the size criterion was difficult to evaluate because few patients had a large lymph node without reaching the SCF3 (n=35) or the lower neck (n=11). Because the outcome pattern of N3a was similar to that of N3b, particularly for long-term D-FFS and OS (Fig. 4), continuing to use this as an N3 criterion was supported. However, in agreement with the study by Lee et al,28 further subclassification could be discontinued as the number of patients was too few for meaningful impact.
The feasibility of replacing the SCF3 with the lower neck (defined as extension below the caudal border of the cricoid cartilage22; ie, levels IV and Vb; Fig. 2B) without affecting the prognostic significance was first reported by Ng et al23 in 2007. This was adopted as the criterion for N3 in the Chinese system (2008 version).24-27 There is little controversy that this leads to easier and more reproducible demarcation by imaging instead of clinical palpation. Although the current data showed that this did not lead to an improvement in the AIC or the c-index for N-FFS and D-FFS, the c-index for OS was maintained (Table 3). Together with merging with nodes>6 cm (maximum dimension), differences in D-FFS and OS were significant between all adjacent N categories in the proposed 8th edition.
As for the stage grouping scheme, the current article concurs with the study by Lee et al28: subclassification into IVA and IVB was unnecessary because the OS of patients with T4N0-2 disease was similar to the OS of those with T1-4N3 disease (72% vs 70% at 5 years, P=.114). Hence, all T4 and N3 can be merged into 1 substage (IVA) for advanced locoregional disease. How-ever, further simplification by the merging of stages I and II was not recommended. Even though only 4% of patients presented with T1N0 disease and there was no significant difference between stages I and II in locoregional FFS (P=.15) or OS (98% vs 92%, P=.098), the difference in D-FFS was significant (98% vs 91%, P=.045). Because of the concern that stage II patients might benefit from the addition of chemotherapy, keeping stages I and II separate is recommended.
NPC has a highly skewed global distribution, with 80% of the global burden in Asian countries. According to the statistics from GLOBOCAN in 2012,38 the total number of new cases of NPC in the world was 86,691, and 33,198 of these cases (38%) were registered in China. It is understandable that centers from China take great interest in developing the best possible staging system for this cancer. Although most countries adopt the AJCC/UICC system, China is the only country that still commonly uses a different system. A comparison of the 2 systems by Pan et al25 showed that the prognostic value of the T category in the 7th edition of the AJCC/UICC staging system7,8 was superior, whereas the prognostic value of the N category in the 2008 Chinese edition was superior; for the prediction of OS by stage group, the 2 systems were comparable. The study by OuYang et al26 also supported the superiority of the N category in the Chinese 2008 edition in the IMRT era. Our current analyses support merging the strengths of the 2 systems.
In summary, the proposed 8th edition adopts the easily reproducible definition of the lower neck as an N3 criterion, clarifies the appropriate T2 criteria, avoids ambiguous terms, and discontinues subclassifications that have little impact. These changes will lead to a better distinction of hazards between adjacent stages/categories (Figs. 2–4) and improvements in both the AIC and the c-index by the proposed T category for all endpoints and by the proposed stage group for D-FFS and OS. This represents a concerted effort by endemic centers together with multidisciplinary international experts to develop the optimal staging system and work toward global unity.
After the optimization of the fundamental TNM staging system, further refinement of prognostication by additional independent factors will be useful for guiding treatment and cost-effective use of health care resources. There are increasing data showing that tumor volume is one of the most promising factors, but this is not incorporated into the current TNM system because the measurement of this criterion is not globally available and consensus on the cutoff value has yet to be attained. Further studies to develop a nomogram with the incorporation of additional prognostic factors are now ongoing in an attempt to work toward a personalized treatment strategy tailored to an individual’s risk pattern.
Footnotes
FUNDING SUPPORT
No specific funding was disclosed.
CONFLICT OF INTEREST DISCLOSURES
Quynh Thu Le reports a grant from Amgen to Stanford University to conduct a long-term follow-up study of patients enrolled in past trials.
REFERENCES
- 1.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC Cancer Staging Manual. 5th Lippincott-Raven; Philadelphia, PA: 1997. [Google Scholar]
- 2.Sobin LH, Wittekind CH, editors. TNM Classification of Malignant Tumours. 5th Wiley-Liss; New York, NY: 1997. [Google Scholar]
- 3.Ho JHC. Stage classification of nasopharyngeal carcinoma: a review. In: de The G, Ito Y, editors. Nasopharyngeal Carcinoma: Etiology and Control. International Agency for Research on Cancer; Lyon, France: 1978. pp. 99–113. [Google Scholar]
- 4.Lee AW, Foo W, Law SC, et al. Staging of nasopharyngeal carcinoma: from Ho’s to the new UICC system. Int J Cancer. 1999;84:179–187. doi: 10.1002/(sici)1097-0215(19990420)84:2<179::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Greene F, Page D, Fleming I, editors. AJCC Cancer Staging Manual. 6th Springer-Verlag; New York, NY: 2002. [Google Scholar]
- 6.Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumours. 6th Wiley-Liss; New York, NY: 2002. [Google Scholar]
- 7.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th Wiley-Blackwell; Oxford, United Kingdom: 2009. [Google Scholar]
- 8.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 7th Springer; New York, NY: 2010. [Google Scholar]
- 9.Lee AW, Au JS, Teo PM, et al. Staging of nasopharyngeal carcinoma: suggestions for improving the current UICC/AJCC staging system. Clin Oncol (R Coll Radiol) 2004;16:269–276. doi: 10.1016/j.clon.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Low JS, Heng DM, Wee JT. The question of T2a and N3a in the UICC/AJCC (1997) staging system for nasopharyngeal carcinoma. Clin Oncol (R Coll Radiol) 2004;16:581–583. doi: 10.1016/j.clon.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Li L, Mao Y, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma detected by magnetic resonance imaging: prognostic value and staging categories. Cancer. 2008;113:347–354. doi: 10.1002/cncr.23555. [DOI] [PubMed] [Google Scholar]
- 12.Zong J, Lin S, Lin J, et al. Impact of intensity-modulated radiotherapy on nasopharyngeal carcinoma: validation of the 7th edition AJCC staging system. Oral Oncol. 2015;51:254–259. doi: 10.1016/j.oraloncology.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Tang LL, Li WF, Chen L, et al. Prognostic value and staging categories of anatomic masticator space involvement in nasopharyngeal carcinoma: a study of 924 cases with MR imaging. Radiology. 2010;257:151–157. doi: 10.1148/radiol.10100033. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Liu LZ, Chen M, et al. Prognostic value of subclassification using MRI in the T4 classification nasopharyngeal carcinoma intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys. 2012;84:196–202. doi: 10.1016/j.ijrobp.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Luo DH, Yang J, Qiu HZ, et al. A new T classification based on masticator space involvement in nasopharyngeal carcinoma: a study of 742 cases with magnetic resonance imaging. BMC Cancer. 2014;14:653. doi: 10.1186/1471-2407-14-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang GY, Huang Y, Cai XY, et al. Prognostic value of grading masticator space involvement in nasopharyngeal carcinoma according to MR imaging findings. Radiology. 2014;273:136–143. doi: 10.1148/radiol.14132745. [DOI] [PubMed] [Google Scholar]
- 17.Sze H, Chan LL, Ng WT, et al. Should all nasopharyngeal carcinoma with masticator space involvement be staged as T4? Oral Oncol. 2014;50:1188–1195. doi: 10.1016/j.oraloncology.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y, Pan J, Chen Y, et al. The prognosis of nasopharyngeal carcinoma involving masticatory muscles: a retrospective analysis for revising T subclassifications. Medicine (Baltimore) 2015;94:e420. doi: 10.1097/MD.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng AC, Wu MC, Tsai SY, et al. Prevertebral muscle involvement in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:1026–1035. doi: 10.1016/j.ijrobp.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Lee CC, Chu ST, Chou P, et al. The prognostic influence of prevertebral space involvement in nasopharyngeal carcinoma. Clin Otolaryngol. 2008;33:442–449. doi: 10.1111/j.1749-4486.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou GQ, Mao YP, Chen L, et al. Prognostic value of prevertebral space involvement in nasopharyngeal carcinoma based on intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1090–1097. doi: 10.1016/j.ijrobp.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 22.Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000;174:837–844. doi: 10.2214/ajr.174.3.1740837. [DOI] [PubMed] [Google Scholar]
- 23.Ng WT, Lee AW, Kan WK, et al. N-staging by magnetic resonance imaging for patients with nasopharyngeal carcinoma: pattern of nodal involvement by radiological levels. Radiother Oncol. 2007;82:70–75. doi: 10.1016/j.radonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Li WF, Sun Y, Mao YP, et al. Proposed lymph node staging system using the international consensus guidelines for lymph node levels is predictive for nasopharyngeal carcinoma patients from endemic areas treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:249–256. doi: 10.1016/j.ijrobp.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Pan J, Xu Y, Qiu S, et al. A comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal carcinoma. Am J Clin Oncol. 2015;38:189–196. doi: 10.1097/COC.0b013e31828f5c96. [DOI] [PubMed] [Google Scholar]
- 26.OuYang PY, Su Z, Ma XH, Mao YP, Liu MZ, Xie FY. Comparison of TNM staging systems for nasopharyngeal carcinoma, and proposal of a new staging system. Br J Cancer. 2013;109:2987–2997. doi: 10.1038/bjc.2013.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue D, Xu YF, Zhang F, et al. Is replacement of the supraclavicular fossa with the lower level classification based on magnetic resonance imaging beneficial in nasopharyngeal carcinoma? Radiother Oncol. 2014;113:108–114. doi: 10.1016/j.radonc.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Lee AW, Ng WT, Chan LK, et al. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol. 2012;48:1007–1013. doi: 10.1016/j.oraloncology.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79:420–428. doi: 10.1016/j.ijrobp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Lin S, Pan J, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys. 2009;75:1071–1078. doi: 10.1016/j.ijrobp.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 32.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A Stat Soc. 1972;135:185–207. [Google Scholar]
- 33.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. 1972;34:187–220. [Google Scholar]
- 34.Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials. 2000;21:552–560. doi: 10.1016/s0197-2456(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 35.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503. [PubMed] [Google Scholar]
- 36.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 37.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 38.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. International Agency for Research on Cancer; Lyon, France: 2013. [Google Scholar]