Abstract
The present issue of ‘Seminars in Immunology’ addresses the topic of macrophage biology, 100 years after the death of Elie Metchnikoff (May 1845–July 1916). As foreseen by Metchnikoff, macrophages function in the maintenance of homeostasis and immunity against pathogens and have become a broad and active area of investigation. This issue of ‘Seminars in Immunology’ highlights some of the recent progress in the biology of macrophages and their cousins, monocytes and dendritic cells. Over the past few years, a better understanding of the developmental biology of macrophages, monocytes, and dendritic cells has emerged, which we hope will lead to a molecular understanding of their functions within tissues in vivo, and guide comprehensive and efficient therapeutic interventions. In this issue, Gold and Brückner summarize their view of macrophage development and function in the fruit fly Drosophila melanogaster. McGrath and Palis, as well as Kierdorf, Prinz and Gomez-Perdiguero critically review the experimental work that recently transformed our view on the development of tissue-resident macrophages in mice, and Puhr et al. review the current understanding of dendritic cell differentiation. Ulland, Wang and Colonna review the functions of microglia in the brain with a focus on growth factors and activating receptors. Lauvau, Loke and Hohl give a detailed and comprehensive review of the mechanisms by which monocytes promote microbial clearance. Finally, Schultze and Schmidt review recent studies exploring transcriptional and epigenetic regulation in monocytes/macrophages under homeostatic and stress conditions. Overall, our improved understating of the myeloid system has led to a more complex view of the myeloid system that includes multiplicity of cell types with diverse developmental origins that do not fit into a textbook picture of a plastic and multifunctional macrophage. Therefore, We propose that further progress toward a quantitative and molecular understanding of myeloid cell biology in vivo and their roles in tissue homeostasis and remodeling will benefit from taking into account the developmental biology of macrophages in addition to their functional diversity. A tentative model to help in this pursuit and account for myeloid cells and macrophage diversity is discussed below.
Metchnikoff’s macrophage
Phagocytosis of microbes and foreign particles was observed and described by pathologists in the second half of the 19th century1. The unique contribution of Elie Metchnikoff, formulated in the 1880s, stemmed from his adhesion to the new theory of evolution proposed by Darwin. Metchnikoff hypothesized that phagocytosis was a physiological mechanism selected by evolution in metazoans for the purpose of removing unfit cells and microbes. This led him to study ‘intracellular digestion’ across the animal kingdom from unicellular organisms such as Amoebae to invertebrates and vertebrates during embryogenesis as well as in adults animals 2. Applying his comparative, experimental, systematic, and quantitative approach to vertebrates, Metchnikoff collected large amounts of data on phagocytosis by mesodermal cells during embryogenesis, tissue homeostasis, and the defense against infectious pathogens3,4. From these observations, he proposed a ‘Theory of cellular immunity’ that featured a central role for phagocytosis in the inflammatory response against pathogens. Against the long-held idea that inflammation was a local tissue degeneration, Metchnikoff wrote “Inflammation generally must be regarded as a phagocytic reaction on the part of the organism against irritants. This reaction is carried out by the mobile phagocytes, sometimes alone, sometimes with the aid of the vascular phagocytes (via diapedesis) or of the nervous system” 2.
After Metchnikoff, macrophages have been considered as the phagocyte ‘par excellence’, and numerous researchers have confirmed that macrophages are mesodermal cells, present as a distinct cell type across ontogeny, from embryo to adults, and across the animal kingdom from insects to vertebrates (see article by Gold et al., in this issue). Macrophages sense, scavenge, and whenever possible, digest dying and unfit cells, protein and lipid deposits, crystals, and microorganisms 5, 6, 7. Macrophages also produce a large spectrum of bioactive molecules such as cytokines and growth factors in response to signals from their environment. From an immunological perspective, their ability to present peptides from foreign protein to T-lymphocytes in many vertebrates is important for the mounting of an antigen specific immune memory 6, 7.
The ‘umbrella’ macrophage
Altogether, a century of work on macrophages has established the idea that this evolutionary conserved cell type plays important roles in phagocytosis and production of bioactive molecules, immune and non-immune functions in embryogenesis, tissue homeostasis, protection against infectious diseases, and more recently in tumor growth 8, 9, 10. Yet, through the role of macrophages in disease remains complex and not well understood. This is primarily due to our limited knowledge of the developmental and molecular mechanisms that underlie macrophage diversity across tissues and the poorly defined nature of macrophages 11, 12. For comparison, the study of lymphoid cells in diseases has benefited tremendously from being rooted into a detailed knowledge of thymic and bone marrow lymphopoiesis. Metchnikoff discussed in evolutionary terms the heterogeneity of phagocytes, from tissue phagocytes already present in invertebrates to blood leukocytes that appear with the circulatory system and extravasate into inflamed tissues 13. This work was carried forward into the 20th century and resulted in distinction between dendritic cells and macrophages by Steinman and Cohn 14. However, the term macrophage is still an ‘umbrella’ for very different phagocytic cells types. The ‘mononuclear phagocyte system’ model (MPS), initially proposed by Van Furth and Cohn 15, 16, held that monocytes were the precursors of all tissue macrophages. It followed that resident phagocytes such as Kupffer cells in the liver, brain microglia, or alveolar macrophages, as well as blood inflammatory monocytes that enter tissues via diapedesis during inflammation, and myeloid cells that populate the lamina propria of the gut are all called macrophages and are frequently defined by a set of common markers, despite their developmental, molecular, and functional heterogeneity.
The in vitro models that have been developed to study macrophages have accordingly relied on the cultivation of blood monocytes or bone marrow progenitors, without always trying to recapitulate the biology and diversity of macrophages resulting from their different developmental origins. It is also the case that we frequently lack unique marker(s) to distinguish monocytes from macrophages in a given tissue, even more so when the tissue is the site of an inflammatory response, e.g. an atherosclerotic plaque. Likewise, current transgenic mouse models in use to target ‘macrophages’ and study their biology in vivo do not reliably distinguish distinct types of myeloid cells, in contrast to equivalent models useful for example to the study of B- and T-lymphocytes. To account for macrophage diversity, the MPS model was augmented by a differentiation/activation process of monocytes called ‘polarization’ and defined in vitro 17, where external cues such as Interferon gamma or interleukin-4 direct monocyte differentiation towards classical M1 and alternative M2 macrophages respectively 17, 18, 19. This paradigm was proposed to account for the diversity of macrophage phenotypes and functions in a given healthy or diseased tissue, and logically suggests that ‘re- or de- polarizing’ tissue macrophages may help restore homeostasis 17. However, this paradigm does not reflect the developmental origin of macrophages and how it may contribute to the observed phenotypic and functional diversity.
A stratified myeloid system
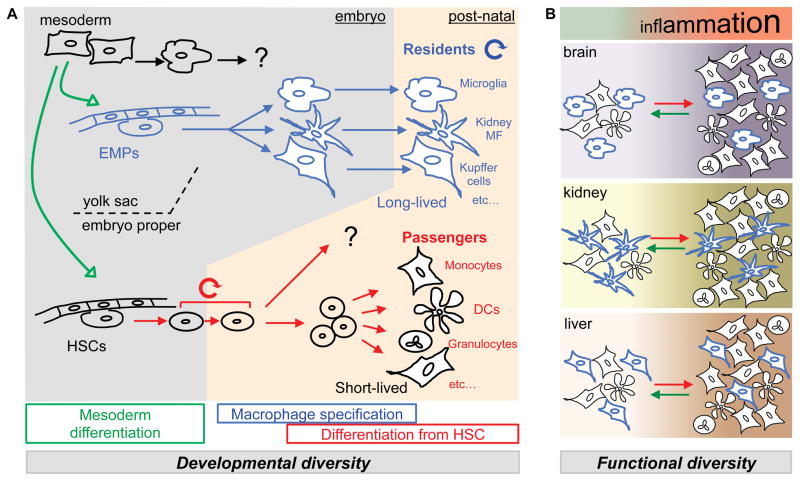
Although many investigators from the basic sciences, clinical sciences, and pharmaceutical industry ‘see’ macrophages through the MPS framework, numerous observations and experimental results from different laboratories do not fit into the aforementioned model, and strategies to target the ‘right’ macrophage represent a challenge. As it will be discussed below, it has become apparent that plasticity of monocytes do not solely account for macrophage diversity. Several layers of ‘hard wired’ macrophage diversity result from developmental processes that take place in the developing embryo and the post natal bone marrow to govern myeloid cell function in vivo (Figure 1A). This would be better referred to as a ‘stratified myeloid system’ (SMS) than a ‘mononuclear’ phagocyte system.
Figure 1. A stratified myeloid system.
(A) Mesoderm-derived primitive hematopoiesis starts from embryonic day (E)7.5 and gives rise to primitive red blood cells (in vivo), as well as primitive macrophages (in vitro). Intra- and extraembryonic hemogenic endothelium also develops from the yolk sac mesoderm and gives rise to two developmentally distinct layers of immune cells. Erythro-Myeloid Progenitors (EMPs) emerge in the yolk sac around E8.5 and represent the first wave of definitive hematopoiesis, migrate to the fetal liver, and give rise to fetal macrophages, the precursors of post-natal resident macrophages in vivo (For review see 12 and McGrath and Palis, as well as Kierdorf, Prinz and Gomez-Perdiguero in the present issue). Within the embryo proper, the hemogenic endothelium of large arteries gives rise to Hematopoietic Stem Cells (HSCs) at E10.5 as the second wave of the definitive hematopoiesis. EMP-derived macrophages develop in the absence of Myb and persists in post-natal tissues as resident macrophages 20, 23. The contribution of primitive hematopoiesis to resident macrophages is debated (see McGrath and Palis in the present issue). HSCs migrate first to the fetal liver, and at E17.5, to the bone marrow, where they persist and self renew. HSCs continuously give rise to adult-type red blood cells, lymphoid cells and myeloid cells such as granulocyte, monocyte, and dendritic cell subsets (‘Passengers’). (B) Overall myeloid content of resting and inflamed tissues is determined by developmental lineages. Each tissue is characterized in a steady state by its myeloid composition, made of tissue-specific ‘Residents’ and ‘Passenger’ subsets. In inflammatory states numbers of cells in general increase and proportions of the myeloid subsets change. This process is dynamic, regulated in part by recruitment of short-lived cells. If signals that lead to recruitment are discontinued, cell numbers (passengers) will decrease and the tissue may go back to a steady state.
It is interesting to note in that regard that phagocyte diversity in Drosophila may also result from a layered developmental process (See Gold et al).
The first layer of diversity is due to the two distinct developmental lineages that contribute to myeloid cells in an adult mouse. On one hand, there are the hematopoietic stem cell (HSC) derived myeloid cells and on the other there are Yolk Sac (YS) derived adult resident tissue macrophages. These cells arise from distinct hematopoietic lineages and likely exert different functions (see McGrath and Palis, as well as Kierdorf, Prinz and Gomez-Perdiguero, in the present issue).
A second level of diversity is observed within each of these two compartments and can be largely attributed to specification of the cells within each compartment. Bone marrow HSC differentiate in a steady state along several lineages to generate a number of distinct cells types, including conventional dendritic cells, plasmacytoid dendritic cells, several monocyte subsets, and cells such as gut lamina propria ‘macrophages/DCs’ (see Puhr et al.). A common feature of HSC-derived cells is a short lifespan and constant renewal from the bone marrow HSC. YS derived resident tissue macrophages also undergo specification into different cell types, such as microglia and Kupffer cells, but this process is likely to take place during embryogenesis as tissue resident macrophages self-maintain after birth (Figure 1A).
Diversity of resident macrophages
It is now widely accepted that, in contrast to the central hypothesis of the MPS, most tissue resident macrophages are maintained in adult tissue via self-renewal independently of HSCs 20, 21, 22. Several groups have pursued this line of research to identify the precursors of tissue resident macrophages 12, 23, 24, 25 and the mechanisms responsible for their maintenance 11, 26, 27. Although the relative contribution of distinct embryonic and fetal precursors is still debated, and much work remains to be done to test these hypotheses, it is proposed that resident macrophages originate in their majority from yolk sac embryonic progenitors that are genetically and developmentally distinct from HSCs. In particular, Erythro-Myeloid Progenitors (EMPs) are responsible for the first transient wave of definitive hematopoiesis 23, 28 and give rise in a Myb-independent manner to self-renewing tissue-resident macrophages, such as Kupffer cells in the liver, Langerhans cells in the epidermis, alveolar macrophages, and microglia in the brain. Therefore, it ensues that monocytes are not, in general, the progenitors of tissue macrophages, thus generation of resident macrophage diversity in tissues from adult mouse cannot be obtained solely via polarization of the monocytes.
As outlined above, further hard-wired diversification occurs within the ‘resident’ compartment. Tissue resident macrophages are extremely diverse from one tissue to another, as it is easy to realize when comparing alveolar macrophages to microglia or to peritoneal macrophages26, 27. It is also possible to speculate that further functionally significant heterogeneity exists in some tissues, such as in the brain, where ‘microglia’ may be another umbrella that encompasses many different phagocyte types, depending on the area of the brain they reside in. Whether the diversity among resident macrophages that populate distinct tissues and anatomical microenvironments is relevant to the pathophysiology of diseases remains to be investigated.
Diversity of passenger myeloid cells
A key distinction between resident macrophages subsets and the monocytes, gut lamina propria myeloid cells and dendritic cells is that the latter are short-lived and develop and constantly renew from HSC. A number of transcriptional regulators, growth factors, and chemotactic factors involved in this process have been identified (see the review by Puhr et al.).
In contrast to resident cells, the numbers of these ‘passengers’ within tissues is largely regulated by inflammatory signals. These signals regulate extravasation mediated by diapedesis from the blood into the tissue and myeloid cell production in the bone marrow, although the complex dynamics of hematopoietic progenitor differentiation under inflammatory conditions remain incompletely understood.
New tools and new concepts to study a stratified and dynamic myeloid system
Thus, in addition to the activation of resident macrophages, it is clear that the overall transcriptional changes observed in tissue myeloid cells during inflammation must correspond, at least in part, to the increased or decreased numbers of HSC-derived ‘passenger’ cells (Figure 1). It is thus important to distinguish residents from passengers to account for the behavior of the phagocyte pool present in a given tissue at a given time9. The study of phagocyte functions therefore requires the validation of new ‘markers’ to distinguish myeloid cell subsets and new genetic tools that will label or target selectively either HSC-derived cells or resident macrophages in vivo.
In a simplistic model, resident macrophages may be responsible for the ‘basic’ homeostatic functions of phagocytes, while passenger subsets would mostly mediate the inflammatory response 9. Differential targeting of resident and passengers would then be useful to consider for therapeutic strategies. Furthermore, in addition to the ‘high-level’ genetic and developmental distinction that can be drawn between long-lived, YS-derived, resident macrophages, and short-lived, HSC-derived, ‘passenger’ phagocytes that coexist in a given tissue, resident macrophages themselves are diverse, as outlined above, and harbor distinct transcriptional and chromatin landscapes 26, 27. They frequently require subset-specific key transcriptional regulators for their development, as in the case of Langerhans cells, large peritoneal macrophages, spleen marginal zone macrophages, and spleen red pulp macrophages 10, 29, 30,31, 32. Therefore, it is possible and may be prudent to selectively impair the development of tissue specific subsets by conditional inactivation of these transcriptional regulators in the resident lineage, in order to study the contribution of these subsets to tissue homeostasis and inflammation.
Acknowledgments
We are grateful to Lucile Crozet and Nehemiah Cox for their critical reading of the manuscript. Supported by grants from National Cancer Institute of the US National Institutes of Health (P30CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambrose CT. The Osler slide, a demonstration of phagocytosis from 1876 Reports of phagocytosis before Metchnikoff’s 1880 paper. Cellular immunology. 2006;240:1–4. doi: 10.1016/j.cellimm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Metchnikoff E. Lectures on the comparative pathology of inflammation; delivered at the Pasteur Institute in 1891. Dover Publications; New York: 1968. [Google Scholar]
- 3.Metchnikoff E. Researches on the Intracellular Digestion of Invertebrates. Quarterly Journal of Microscopical Science. 1884:89–111. [Google Scholar]
- 4.Metchnikoff E. Lecture on Phagocytosis and Immunity. British medical journal. 1891;1:213–217. doi: 10.1136/bmj.1.1570.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Current opinion in immunology. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual review of immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 9.Gomez Perdiguero E, Geissmann F. Myb-Independent Macrophages: A Family of Cells That Develops with Their Tissue of Residence and Is Involved in Its Homeostasis. Cold Spring Harbor symposia on quantitative biology. 2013 doi: 10.1101/sqb.2013.78.020032. [DOI] [PubMed] [Google Scholar]
- 10.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nature immunology. 2015;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 11.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nature immunology. 2015;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metchnikoff E. The Ancestral History of the Inflammatory Process. Quarterly Journal of Microscopical Science. 1884:112–117. [Google Scholar]
- 14.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. The Journal of experimental medicine. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. The Journal of experimental medicine. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Furth R, et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bulletin of the World Health Organization. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 17.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 19.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng J, Ruedl C, Karjalainen K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43:382–393. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Hoeffel G, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosselin D, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGrath KE, et al. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell reports. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohyama M, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosas M, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NAG, et al. The nuclear receptor LXRalpha controls the functional specialization of splenic macrophages. Nature immunology. 2013;14:831–839. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacker C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nature immunology. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]