Abstract
The Food and Drug Administration (FDA), Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute for Drug Abuse (NIDA) report that no sound scientific studies support the medicinal use of cannabis. Despite this lack of scientific validation, many patients routinely use “medical marijuana,” and in many cases this use is for pain related to nerve injury. We conducted a double-blinded, placebo-controlled, crossover study evaluating the analgesic efficacy of smoking cannabis for neuropathic pain. Thirty-eight patients with central and peripheral neuropathic pain underwent a standardized procedure for smoking either high-dose (7%), low-dose (3.5%), or placebo cannabis. In addition to the primary outcome of pain intensity, secondary outcome measures included evoked pain using heat-pain threshold, sensitivity to light touch, psychoactive side effects, and neuropsychological performance. A mixed linear model demonstrated an analgesic response to smoking cannabis. No effect on evoked pain was seen. Psychoactive effects were minimal and well-tolerated, with some acute cognitive effects, particularly with memory, at higher doses.
Keywords: Neuropathic pain, analgesia, cannabis, clinical trial, neuropsychological testing
The case for the clinical utility of cannabis as an analgesic derives from experimental studies as well as anecdotal reports. Activation of the endocannabinoid system suppresses behavioral responses to acute and persistent noxious stimulation through both central71 and peripheral45 mechanisms. Cannabinoid receptors are localized in neuroanatomic regions intimately involved with transmission and modulation of pain signals: The periaqueductal gray (PAG), the rostral ventromedial medulla (RVM),40,66 and the dorsal horn of the spinal cord.66 Animal experimentation has clearly demonstrated that synthetic and endogenous cannabinoids not only produce analgesia but also interact in some manner to potentiate opioids,18,70 particularly in neuropathic pain.41
Surveys involving the use of medicinal marijuana reveal that pain, sleep, and mood improve with only modest side effects.72,73 In one human pain experiment, subjects had a significant dose-dependent antinociception (increased finger withdrawal latency) effect that was not reversed by opioid antagonism.31 In a somewhat contradictory manner, hyperalgesic activity and enhancement of the perception of pain on acute exposure in chronic users of marijuana was reported.20 Experience with cancer pain revealed that 120 mg codeine and 20 mg delta-9-tetrahydrocannabinol (9-THC) were similar to each other and significantly superior to placebo for the sum of the pain intensity differences and total pain relief.55,56 However, there was a clear dose-response relationship for sedation, mental clouding, and other central nervous system (CNS) related side effects from the 9-THC.
When taken alone, 9-THC or dronabinol does not fully replicate the effect of the total cannabis preparation, indicating that there might be other active cannabinoids needed for a full range of effects.77 As a result, combinations of cannabinoids are being sought for clinical implementation. Sativex is one of the first cannabis-based medicines to have been approved as a prescription medicine in Canada.5 It has been found to be effective in reducing pain and sleep disturbances in patients with multiple sclerosis who have central neuropathic pain, and it appears to be well-tolerated.62 The rationale for a combination is that the cannabidiol, normally present in insignificant concentrations in cannabis, purportedly antagonizes undesirable effects of 9-THC such as intoxication, sedation, and tachycardia while contributing analgesic, anti-emetic, and anti-carcinogenic properties.64 However, in one direct comparison between this combination and 9-THC alone, additional effectiveness was not evident.11 Therefore, evaluating herbal cannabis remains a worthwhile endeavor awaiting more definitive proof of a specific combination of cannabinoids that can enhance effectiveness.
Despite support from the basic and clinical sciences, the clinical utility of cannabis in the United States remains mired in controversy.13,48,57 Akin to the medical and social controversy surrounding the use of opioids in chronic pain,23 clinical trials will be a critical factor in the debate concerning medical marijuana. In defense of this position, the National Institutes of Health (NIH) Workshop on the Medical Utility of Marijuana1 concluded, “Inhaled marijuana merits testing in controlled, doubleblind, randomized trials …”. Furthermore, the NIH panel concluded that neuropathic pain is a condition in which currently available analgesics are, at best, marginally effective, suggesting that cannabis might hold promise as a treatment. To address this issue, we examined whether smoking cannabis produces dose-dependent analgesia on both spontaneous and evoked pain in patients with neuropathic pain. In addition, we studied the adverse effects of cannabis to better understand its potential detrimental effects on patients.
Materials and Methods
Patients
This study was approved by the Human Subjects Institutional Review Boards at the UC Davis Medical Center (UCDMC) and the Veterans Affairs of Northern California Health Care System (VANCHCS). At the state level, endorsement by the Research Advisory Panel of California was obtained to proceed with the investigation of a Schedule I controlled substance. The approval process also included national review by the Food and Drug Administration, the National Institute on Drug Abuse, and the Department of Health and Human Services.
Participants were recruited from the UCDMC and VANCHCS pain clinics through initial contact by providers intimately involved in the patient’s care as well as newspaper advertisements and postings in newsletters. All candidates were initially screened via a brief telephone interview. Qualified candidates with complex regional pain syndrome (CRPS type I), spinal cord injury, peripheral neuropathy, or nerve injury were interviewed and examined by the principal investigator who invited those meeting inclusion and exclusion criteria to enroll in the study. The diagnostic criteria for CRPS type I followed a decision rule compiled by a research consortium working with the International Association for the Study of Pain (IASP),14,27,33 which required at least 2 signs and 4 symptoms to be positive. The specific historic and physical findings included burning pain, skin sensitivity to light touching or cold, skin color changes, swelling, limited movement of the affected body part, motor neglect or abnormalities in skin temperature, hair growth, nail growth, and/or sweating.
To reduce the risk of adverse psychoactive effects in naive individuals, previous cannabis exposure was required of all participants. All participants were required to refrain from smoking cannabis or taking oral synthetic delta-9-THC medications (ie, Marinol; Solvay Pharmaceuticals, Inc., Marietta, GA) for 30 days before study sessions to reduce residual effects; each participant underwent urine toxicology screening to confirm this provision. To further reduce unsystematic variation, subjects were instructed to take all other concurrent medications as per their normal routine during the 3- to 4-week study period.
To ensure that potential subjects did not have depression profound enough to compromise their ability to tolerate the psychoactive effects of cannabis, the Beck Depression Inventory-II (BDI-II) was administered as a screening tool. Candidates with a BDI-II score of 17 or higher were then evaluated with the Composite International Diagnostic Interview, a structured interview used to assess mental disorders and to provide diagnoses according to the definitions and criteria of the ICD-10 (World Health Organization 1992, 1993) and DSM-IV (American Psychiatry Association, 1994). If the criteria for severe major depressive disorder were met, the candidate was excluded from participation. Because the effects of cannabis can exacerbate mental illness24,54 and have been linked to an increase in the risk of suicide,22 candidates with a history or diagnosis of schizophrenia or bipolar depression were also excluded. Medical illnesses were also evaluated, and exclusion criteria included uncontrolled hypertension, cardiovascular disease, chronic pulmonary disease (eg, asthma, chronic obstructive pulmonary disease), and active substance abuse. Routine laboratory analysis included a hematology screen, blood chemistry panel, and urinalysis. Urine drug toxicologies for opioids, benzoylecgonine (cocaine metabolite), benzodiazepines, cannabinoids, and amphetamines were also performed through the use of urine quick tests.
Design
The study used a randomized, double-blinded, placebocontrolled, crossover design, using high-dose cannabis (7% delta-9-THC), low-dose cannabis (3.5% delta-9-THC), and placebo cigarettes. Two doses of medication and a cumulative dosing scheme17,31 were used to determine dosing relationships for analgesia and psychoactive and cognitive effects.
The cannabis was harvested and machine-rolled into cigarettes at the University of Mississippi under the supervision of the National Institute on Drug Abuse (NIDA). NIDA routinely is able to provide cigarettes ($8 each) ranging in strength from 3% to 7% THC, subject to the availability of current crop potency. Placebo cigarettes are made from whole plant with extraction of cannabinoids. After overnight delivery, the cigarettes were stored in a freezer securely bolted to the floor of the Sacramento Veterans Administration Research Pharmacy. Further precautions against theft of the study drug included limited password access to the pharmacy, with a state-of-the-art entry detection system and a direct connection of the alarm system of the room housing the freezer to the Sacramento Veterans Administration Police Department. In addition to security precautions for storing the study drug, a background check of all members of the investigative team was performed by the Drug Enforcement Agency during the process of obtaining a Schedule I license.
Procedures
After informed consent was obtained, participants were scheduled for 3, 6-hour experimental sessions at the UC Davis/Sacramento VA Medical Center General Clinical Research Center (GCRC). The sessions were separated by at least 3 days to permit the metabolic breakdown of residual cannabis. The intervals between sessions ranged from 3 to 21 days, with a mean (SD) of 7.8 (3.4) days. Participants received either low-dose, high-dose, or placebo cannabis cigarettes at each visit in a crossover design using a Web-based random number–generating program, “Research Randomizer” (http://www.randomizer.org/). Each patient received each treatment once, in random order. The allocation schedule was kept in the pharmacy and concealed from other study personnel. Patients were assigned to treatment after they signed a consent form. Patients and assessors were blinded to group assignments.
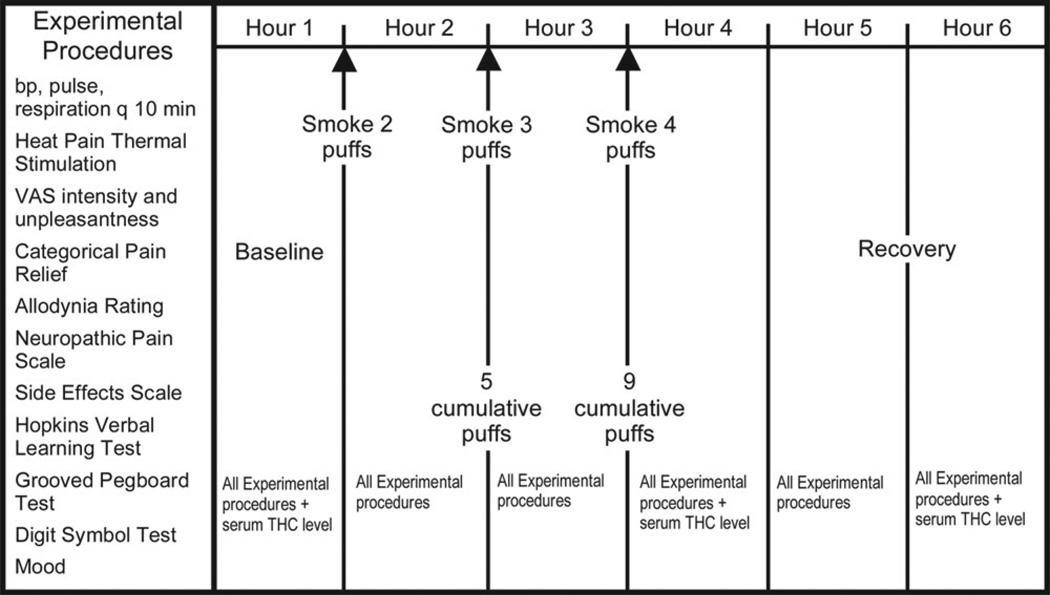
The cigarettes were stored in a freezer at −20°C until the day before use. At least 12 hours before each session, 2 marijuana cigarettes were thawed and humidified by placing them above a saturated NaCl solution in a closed humidifier at room temperature. The cigarettes were smoked under a standard laboratory fume hood with constant ventilation in ambient room temperature at 22°C and a humidity of 40% to 60%. A cued-puff procedure17 standardized the administration of the cannabis. Participants were verbally signaled to “light the cigarette” (30 seconds), “get ready” (5 seconds), “inhale” (5 seconds), “hold smoke in lungs” (10 seconds), “exhale,” and to wait before repeating the puff cycle (40 seconds). A nurse continuously supervised the participant during the smoking session via a closed-circuit monitor in an adjoining room. Participants were observed constantly and could signal that they wanted to stop smoking for whatever reason by raising their hand. Participants completed a standardized cued-puff procedure9,21 of 2 puffs after baseline measurements, 3 puffs an hour later, and 4 puffs an hour after that. The cumulative dose for each session was thus 9 puffs (Fig 1).
Figure 1.
Experimental procedures. THC, tetrahydrocannabinol; VAS, visual analog scale.
Hourly assessment periods were scheduled before and after each set of puffs and for 2 additional hours during the recovery period (Fig 1). Plasma levels for delta-9-THC, cannabidiol (CBD), cannabinol (CBN), 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (11-nor-9-carboxy THC), and 11-hydroxy-tetrahydrocannabinol (11-hydroxy-THC) were measured at baseline, 5 minutes after the first puff bout, and again at 3 hours after the last puff cycle. After each blood draw, plasma was separated by centrifugation and immediately frozen. Plasma samples were subsequently evaluated for enzyme-linked immunosorbent assay of delta-9-THC and metabolite content. Vital signs (aural temperature, blood pressure, respiratory rate, and heart rate) were recorded at baseline and at every hour.
Participants were allowed to engage in normal activities, such as reading or listening to music, between puff cycles and measurement periods. After each session, participants were accompanied home by a responsible adult. After completing all 3 study sessions, participants were debriefed and paid a modest stipend (prorated at $25 per hour) for their participation.
Outcome Measurements
Spontaneous pain relief, the primary outcome variable, was assessed by asking participants to indicate the intensity of their current pain on a 100-mm visual analog scale (VAS) between 0 (no pain) and 100 (worst possible pain). Pain unpleasantness, a measure of the emotional dimension of pain, was also measured by using a similar VAS. In addition, the degree of pain relief was monitored with a standard 7-point patient global impression of change scale.26
The Neuropathic Pain Scale,28 an 11-point box ordinal scale with several pain descriptors, was a secondary outcome. When present, allodynia (the sensation of unpleasantness, discomfort, or pain when the skin in a painful area of the patient’s body was stroked with a foam paint brush) was measured using a 100-mm VAS. Heatpain threshold was determined by applying mild-to-moderately painful heat to the most painful area of the subjects’ body32 with the commercially available Medoc TSA 2001 Peltier thermode (Medoc Ltd. Advanced Medical Systems, Ramat Yishai, Israel). This device was used to apply a constant 1°C per second increasing thermal stimulus until the patient pressed the response button to show that a temperature change was considered painful; the heat pain threshold (mean of 3 attempts) was recorded in degrees Centigrade. Subjective intensities for “any drug effect,” “good drug effect,” and “bad drug effect” were measured using a 100-mm VAS anchored by “no side effect” at 0 and “strongest side effect” at 100. In addition, psychoactive effects, including “high,” “drunk,” “impaired,” “stoned,” “like the drug effect,” “sedated,” “confused,” “nauseated,” “desire more of the drug,” “anxious,” “down,” and “hungry” were measured similarly. Mood was measured using 6, 100-mm VAS ratings for: Feeling sad versus happy; anxious versus relaxed; jittery versus calm; bad versus good; paranoid versus self-assured; and fearful versus unafraid. Subjects were prompted to provide their current rating for the foregoing items at each measurement of these subjective states.
Neurocognitive assessments focused on 3 domains: Attention and concentration, learning and memory, and fine motor speed. Subjects completed the Wechsler Adult Intelligence Scale (WAIS-III) Digit Symbol Test,75 a test of concentration, psychomotor speed, and graphomotor abilities. This pen and paper test involves having subjects substitute a series of symbols with numbers as quickly and accurately as possible during a 120-second period. The results are expressed as the number of correct substitutions. The Hopkins Verbal Learning Test Revised (HVLT)7 provided information on the ability to learn and immediately recall verbal information as well at the ability to retain, reproduce, and recognize this information after a delay. Alternate forms (A–F) were used to minimize practice effects.6,8 A list of 12 words (4 words from each of 3 semantic categories) were presented, and the subject was asked to recall as many words as possible in any order. After a 20-minute delay, the subject was asked to recall the words once again (ie, delayed recall). The Grooved Pegboard Test,47 a test of fine motor coordination and speed, was also administered. In this test, subjects were required to place 25 small metal pegs into holes on a 3 × 3-inch metal board as quickly as possible. All pegs are alike and have a ridge on one side, which corresponds to a randomly oriented notch in each hole on the metal board. First the dominant hand is tested and then repeated with the non-dominant hand, and the total time for each test is recorded. A 5-minute limit is used for those unable to complete the task.
Performance on neuropsychological tests often improves as a result of practice effects.39 This can be somewhat ameliorated by the use of alternate forms8 and, since the largest practice effects typically occur between the first and second testing,21 preexposure to the measures (ie, dual baselines) are recommended.6,69 For this study, we used 6 separate versions of the Hopkins Verbal Learning Test, and incorporated a practice testing session at the time of the screening interview to lessen early practice effects. Despite our attempts to limit practice effects (using alternate forms, conducting a prebaseline practice session), these effects cannot be completely eliminated when subjects are tested repeatedly over a brief period. However, this is likely to result in increased variance, thus attenuating the treatment effect. In addition, practice effects were also mitigated by the use of a placebo arm.
To estimate the level of functioning at baseline and to provide a common metric for interpreting treatment effects on cognition, the raw scores on each test were converted to demographically corrected T scores (adjusting for age, gender, highest educational level achieved, and ethnicity).37,38 In normal control groups, T scores have a mean of 50 with a standard deviation of 10. Based on previous research to determine the optimal cut-point that balances sensitivity and specificity in mild impairments,36 a T score below 40 was classified as an impaired performance. Neuropsychological test performance was also summarized using the global deficit score (GDS), a validated approach for detecting neuropsychological impairments across multiple measures.16 The GDS emphasizes both the number and the severity of deficits, giving less weight to average and above performances. T scores on the individual neuropsychological measures were converted using the following algorithm:
T score ≥ 40 = 0; no impairment
T score = 35 to 39 = 1; mild impairment
T score = 30 to 34 = 2; mild-to-moderate impairment
T score = 25 to 29 = 3; moderate impairment
T score = 20 to 24 = 4; moderate-to-severe impairment
T score < 20 = 5; severe impairment.
An arithmetic mean of the deficit scores was used to create the GDS.
Statistical Methodology
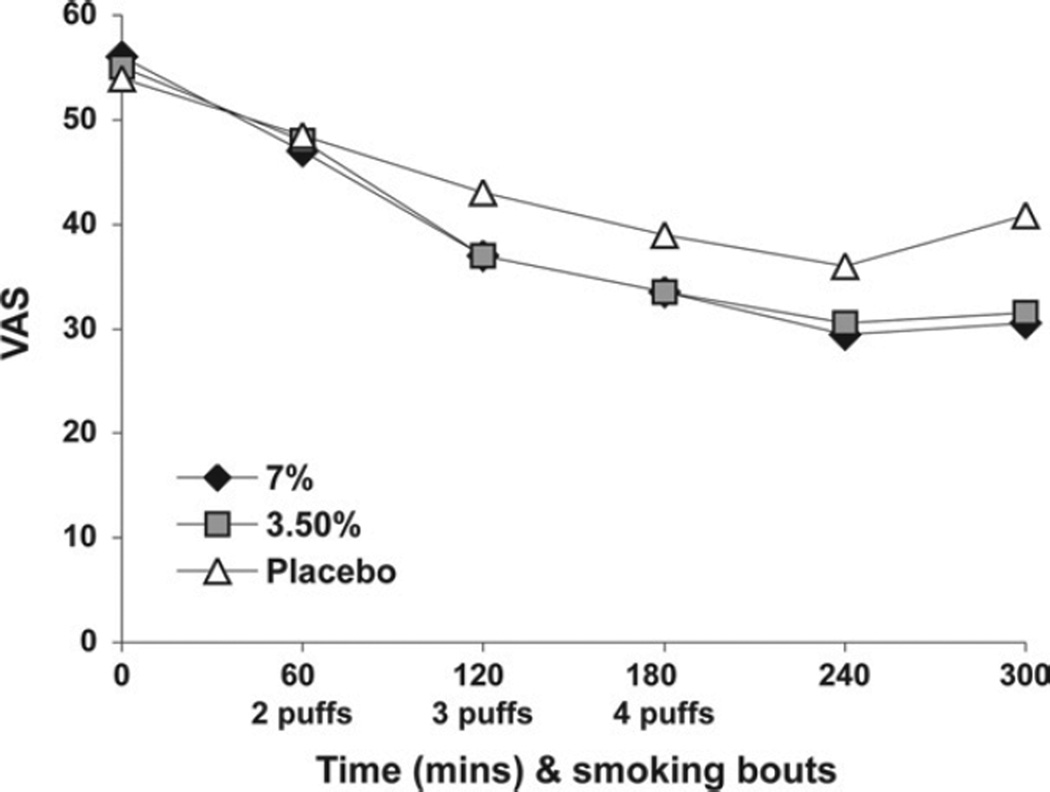
A linear mixed model with a random intercept was used to model pain intensity (the primary response measure) and secondary outcomes (pain unpleasantness, global impression of change, neuropathic pain scale, allodynia, quantitative sensory testing score, mood, subjective, and psychoactive effects, and neuropsychological tests). The random intercept term is used to model the subject-specific component of the response that is shared by measurement performed on the same subject but differs between subjects. Time is modeled as a continuous variable. To reproduce the U-shaped character of the response (recovery phase) noted toward the end of observation period on the subjects (eg, Fig 2, which represents the primary outcome measure VAS pain intensity), a quadratic term in time was introduced. Treatment (high dose, low dose, and placebo) is modeled as a categorical variable with a simple contrast. The main effects of time (linear and quadratic terms) in this analysis model the response pattern over time from the baseline values.
Figure 2.
Visual analog scale (VAS) pain intensity.
The main effect of treatment as well as treatment by time interaction effects were considered in the model. The main effect of treatment models treatment differences in mean response at any time point, including the baseline measurement at hour 1 (Fig 1). If subjects do not show any difference at this time, before the treatment is administered, this term would not be significant with all of the possible treatment effect expressed as an interaction. This is the situation shown in Fig 2, which indicates that response curves start at the same point at the beginning of hour 1. Overall treatment difference modification over time as well as treatment differences at specific time points over the course of treatment are modeled and tested using treatment by time interaction terms. All available patient data, including information from patients who did not complete all experimental sessions, was included in this model. α was set at 0.05, and all tests were 2-tailed. No adjustment for multiple statistical comparisons was performed. Models were fitted using maximum likelihood methods to enable Wald and likelihood ratio tests of statistical hypotheses. R statistics software was used for all analyses.
Results
Recruitment and Withdrawals
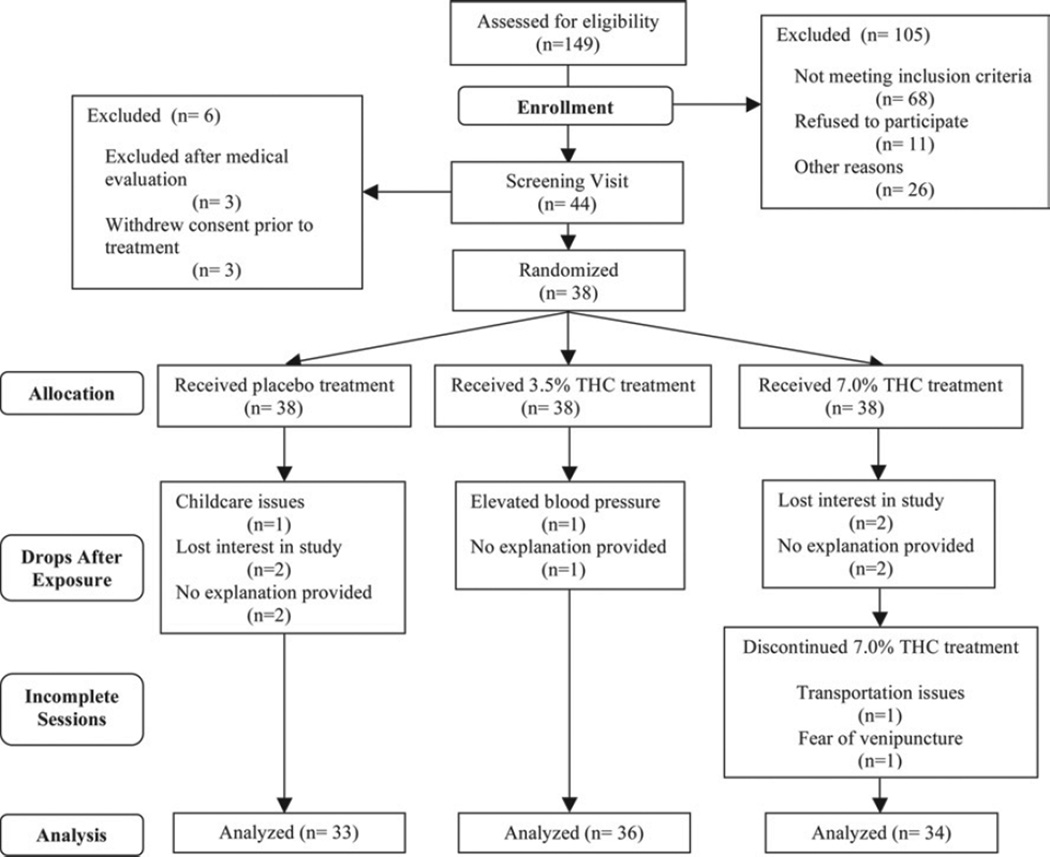
Of 44 patients recruited between June 2004 and February 2006, 23 were men and 21 were women. The mean age (range) was 46 years (21–71 years). Six subjects were excluded and did not receive study medication, 3 because they withdrew consent before commencing the study and 3 because they were excluded after medical evaluation. Of the remaining 38 patients (Table 1), 32 completed all 3 study sessions, 1 completed 2 sessions, and 5 completed only 1 session; a total of 103 study sessions took place. One participant was removed from the study because of high blood pressure that manifested itself before their third session. Five participants did not complete 10 other sessions because of personal reasons not related to the study. Two subjects did not complete all 6 hours of their high-dose sessions; neither dropped because of medical issues. One had to leave early and the other left before the final hour of the study to avoid a repeat blood draw (Fig 3, consort flowchart). There were no adverse cardiovascular side effects, and no participant dropped out because of an adverse event related to an experimental intervention.
Table 1.
Demographics and Characteristics of Patients (N = 38)
| Sex (No.) | |
| Male | 20 |
| Female | 18 |
| Age (y) | |
| Median | 46 |
| Range | 21–71 |
| Education level (y) | |
| Median | 14 |
| Range | 12–21 |
| Race | |
| Caucasian | 33 |
| African American | 1 |
| Hispanic | 1 |
| Asian American | 1 |
| American Indian | 1 |
| Other | 1 |
| Cause of pain (No.) | |
| CRPS type I | 22 |
| Spinal cord injury | 6 |
| Multiple sclerosis | 4 |
| Diabetic neuropathy | 3 |
| Ilioinguinal neuralgia | 2 |
| Lumbosacral plexopathy | 1 |
| Intensity of pain at baseline | 5.6 ± 2.10 |
| Duration of pain | |
| Mean (y) | 6 |
| Range (mo) | 10–290 |
| Concomitant medications (No.) | |
| Opioids | 31 |
| Antidepressants | 19 |
| NSAIDS | 9 |
| Anticonvulsants | 22 |
Abbreviations: CRPS, complex regional pain syndrome; NSAIDS, nonsteroidal anti-inflammatory drugs.
Figure 3.
Consort flow chart. THC, tetrahydrocannabinol.
The average (SD) pain intensity at baseline was 55 (21) on a 0- to 100-mm VAS. The minimally acceptable VAS at baseline was 30/100. Subjects were studied if they did not meet the 30-mm minimal VAS score if they had completed at least the first session. None of the 34 patients were below this minimal score during the 7% visits, 4 of 36 patients were below this score at the time of the 3.5% visits, and 2 of 33 subjects were below 30/100 at the placebo visit.
Of the 38 patients who completed the study, 22 met the IASP diagnostic criteria for CRPS type I,14,27,33 10 had central neuropathic pain related to spinal cord injury or multiple sclerosis, and 6 had peripheral neuropathic pain related to diabetic neuropathy or focal nerve injury. Mean (range) time from the diagnosis of neuropathic pain to study enrollment was 6 years (10 months to 24 years). All patients had used cannabis before, as required by the protocol. The median (range) time from previous exposure, disclosed by historic account during the screening interview, was 1.7 years (31 days to 30 years), with a median (range) exposure duration of 2 years (1 day to 22 years). As required by the inclusion criteria, urine toxicology screening for cannabis was negative in all patients before study entry.
Primary Efficacy Measurement: Pain Intensity
The primary analysis compared patients’ mean VAS pain intensity before and after smoking marijuana (Table 2). Predictably, no treatment differences were found at baseline before the treatment administration started (3.5% vs 7% at time 0: P = .93; placebo vs 7% at time 0: P = .35). A “ceiling effect” was noted with cumulative dosing as the 3.5% and 7% cigarettes produced equal antinociception at every time point with no difference between the 3.5% and 7% doses over time (treatment by time interaction: P = .95, Table 2). Significant analgesia expressed as a 0.0035 reduction in VAS pain intensity per minute was noted from both 3.5% and 7% cannabis compared with placebo (Fig 2; combined 3.5% and 7% treatment group vs placebo difference per minute: −0.0035, 95% CI: [−0.0063,−0.0007], P = .016). Analysis by specific time points was done using a categorical effect of time. Although a trend for separation of the active agents from placebo is visible by time 120 minutes (Fig 2), significant separation for a specific time point occurred only after a cumulative dose of 9 puffs at time 240 minutes (time = 60, 2 puffs, P = .13; time = 120, 3 puffs, P = .11; time = 180, 4 puffs, P = .11, time = 240, recovery hour 1, P = .02).
Table 2.
Visual Analog Scale (VAS) Pain Intensity Primary Efficacy Analysis
| Effect | Mean Difference |
Standard Error |
Confidence Interval | P Value | ||
|---|---|---|---|---|---|---|
| Differences at baseline |
7% vs 3.5% | −0.02 | 0.23 | −0.47 | 0.43 | .93 |
| 7% vs placebo | −0.21 | 0.23 | −0.66 | 0.24 | .35 | |
| 3.5% vs placebo | −0.19 | 0.23 | −0.64 | 0.26 | .40 | |
| Dose effect on top of basic pattern, treatment by time interaction |
7% vs 3.5% | −0.0001 | 0.0017 | −0.0034 | 0.0032 | .95 |
| 7% vs placebo | −0.0035 | 0.0017 | −0.0068 | −0.0002 | .04 | |
| 3.5% vs placebo | −0.0036 | 0.0017 | −0.0069 | 0.0003 | .03 | |
| 7%+ 3.5% vs placebo | −0.0035 | 0.0014 | −0.0063 | −0.0007 | .02 | |
| Basic analgesia pattern, placebo, combined 3.5% + 7% fit |
Pain intensity reduction per minute, time linear term |
−0.0050 | 0.0012 | −0.0073 | −0.0026 | < .01 |
| Pain intensity reduction over time, quadratic term |
0.00003 | 0.0001 | 0.00002 | 0.00005 | < .01 | |
| Absolute effects by time, Pain intensity reduction per minute |
Placebo | −0.0040 | 0.0010 | −0.0060 | −0.0021 | < .01 |
| 3% dose | −0.0085 | 0.0010 | −0.010 | −0.0066 | < .01 | |
| 7% dose | −0.0085 | 0.0010 | −0.010 | −0.0065 | < .01 | |
NOTE. Significant results (P< .05) are bolded. Point estimates of differences at baseline represent mean difference in pain intensity at time before treatment Dose effect point estimate represents a difference in VAS pain intensity change per minute (slope) between 2 dose levels. A zero dose effect point estimate and zero difference at baseline would produce identical mean VAS curves over time in the 2 groups.
The linear main effect of time coefficient was negative (the downward sloping lines on the left in Fig 2), signifying a basic pattern of increasing analgesia; mean reduction VAS pain intensity per minute in the placebo group [−0.0050, 95% CI: (−0.0073, −0.0026)]. The quadratic time coefficient for recovery was positive (ie, represented by the U-shaped pattern seen on the right-hand side of Fig 2), signifying a change in direction toward baseline; with the quadratic term being 3.3 × 10−5, 95% CI: (0.00002, 0.00005). Both of these time effects were highly significant (P < .0001), suggesting that cannabis produced an analgesic response with cumulative dosing that began to reverse within 1 to 2 hours after the last dose.
Using the model, we considered whether there is any evidence that the results might differ by the type of pain condition. No significant differences were found; a test of no effect of pain type showed a P value of .39. Pairwise tests did not show any significant differences, either. It should be noted, however, that the sample size in the above analysis was small, and a type II error may have been present.
Order of treatment administration (placebo, 3.5% or 7%) in this crossover study was not a significant factor (P = .37) in analyzing the primary outcome variable. However, the study may not have enough power to detect order or carryover effects. Generous spacing of patient visits was designed to alleviate this concern.
Secondary Outcomes
A sample of the results of model fit to secondary pain end points is shown in Table 3.
Table 3.
Secondary Pain Measures Analysis
| Pain Measure | Effect | Mean Difference |
Standard Error |
Confidence Interval | P Value | ||
|---|---|---|---|---|---|---|---|
| Unpleasantness | Basic analgesia pattern, placebo | Time, linear term | 23.67 | 8.42 | 7.16 | 40.18 | < .01 |
| Time, quadratic | 0.14 | 0.050 | 0.044 | 0.23 | < .01 | ||
| Treatment effect, interaction by time |
3.5% vs placebo | −0.21 | 0.06 | −0.33 | −0.09 | < .01 | |
| 7% vs Placebo | −0.21 | 0.06 | −0.33 | −0.09 | < .01 | ||
| Global impression of change |
Basic analgesia pattern, placebo | Time, linear term | −22.62 | 4.04 | −30.53 | −14.70 | < .01 |
| Time, quadratic | −0.13 | 0.023 | −0.18 | −0.08435 | < .01 | ||
| Treatment effect, interaction by time |
3.5% vs placebo | 0.12 | 0.029 | 0.064 | 0.18 | < .01 | |
| 7% vs Placebo | 0.12 | 0.029 | 0.065 | 0.18 | < .01 | ||
| Allodynia | Basic pattern | Time, linear term | 3.66 | 3.66 | −3.50203 | 10.83 | .32 |
| Time, quadratic | 0.022 | 0.021 | −0.01938 | 0.063 | .30 | ||
| Treatment effect, interaction by time |
3.5% vs placebo | 0.00007 | 0.034 | −0.066 | 0.066 | .99 | |
| 7% vs placebo | −0.009 | 0.034 | −0.076 | 0.058 | .79 | ||
| Heat stimuli | Basic pattern | Time, linear term | −2.64 | 6.52 | −15.42 | 10.14 | .69 |
| Time, quadratic | −0.015 | 0.038 | −0.089 | 0.059 | .69 | ||
| Treatment effect, interaction by time |
3.5% vs placebo | 0.11 | 0.06 | −0.0046 | 0.23 | .06 | |
| 7% vs placebo | 0.085 | 0.060 | −0.034 | 0.20392 | .16 | ||
NOTE. Significant results (P < .05) are bolded.
Pain Unpleasantness
Pain unpleasantness, a measure of the emotional response to pain, was also measured by using a similar 100-mm VAS bordered by “not at all” at 0 and “extremely unpleasant” at 100. A trend for the treatment difference increase over time is found to be the same in 3.5% and 7% dose groups (mean difference change per minute = −0.21, 95% CI: (−0.33, −0.09), P < .01), indicating that pain was more tolerable at higher cumulative doses of cannabis that it was with placebo.
Global Impression of Change
In addition to VAS ratings for pain intensity and unpleasantness, the degree of relief was monitored by a 7-point scale of patient global impression of change. As with the VAS ratings, cannabis provided a greater degree of relief than placebo (3.5% or 7% placebo = 0.12, 95% CI: (0.064, 0.18), P < .01). Once again, the low- and high-dose groups showed virtually identical results and did not differ significantly (P = .76).
Neuropathic Pain Scale
Measurements from the Neuropathic Pain Scale (NPS) indicate that smoking cannabis positively affected several of the multidimensional pain descriptors associated with neuropathic pain. Modeling of sharp (P < .001), burning (P < .001), aching (P < .001), sensitive (P = .03), superficial (P< .01) and deep pain (P< .001) showed that cannabis improved pain scores more than placebo. The higher dose provided no additional benefit on these dimensions, except that the high dose lowered superficial pain more than the low dose (P = .04). Cannabis improved neither cold nor itching over placebo (P > .05 for both dimensions).
Allodynia
The mean values of allodynia were relatively low throughout all sessions, with the average VAS for the 6 hourly measurements ranging between 20 and 35 on a 100 millimeter scale. These low scores are related to the fact that 15 of the 38 participants (39%) did not have allodynia. No effect of treatment with different concentrations of cannabis (P = .40) or cumulative dose (P = .29) was observed.
Quantitative Sensory Testing
Mild to moderately painful heat stimuli delivered to the most painful area of the participant’s body produced no significant change in response to treatment over time (P> .2) as well as no indication of any trend in treatment differences (P > .1).
Subjective and Psychoactive Effects
The linear mixed effects modeling explored side effects data using several variables. A continuous linear effect of time, a categorical main effect of treatment (low dose vs high dose vs placebo), and an interaction of time with treatment was used.
Subjective Effects
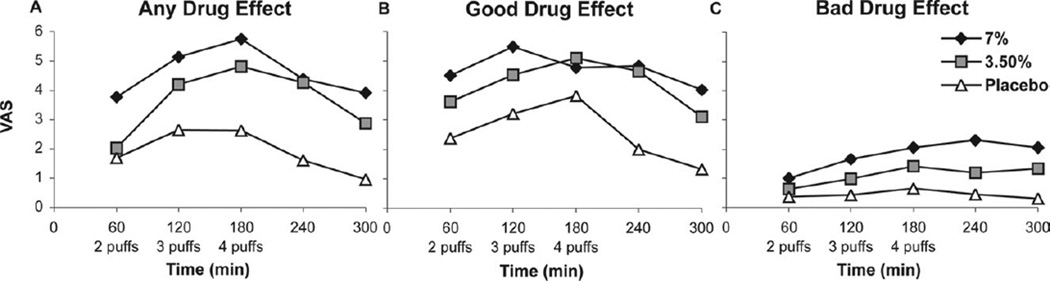
The “any drug effect” approached a VAS of 60/100 in the high-dose group after the maximum cumulative dose, but the effect receded rapidly thereafter (Fig 4A). The analysis of this end point showed significant main effect of treatment (Fig 4A), with the low-dose and placebo values being lower than the corresponding responses for the high-dose values (P = .002, P < .001, respectively). Time did not modify this treatment effect (P > .17).
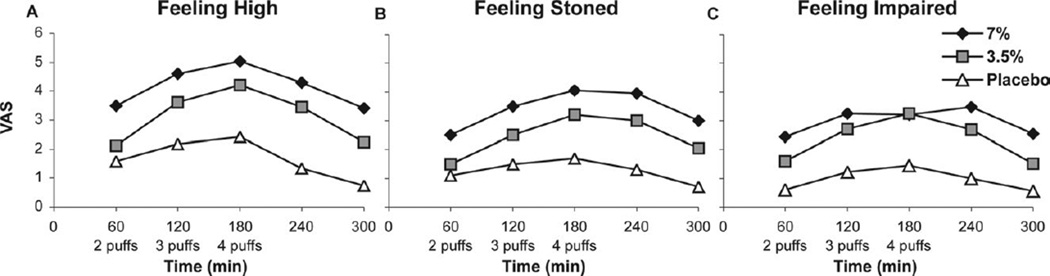
Figure 4.
Subjective side effects.
The low-dose and high-dose groups had more of a “good drug effect” (Fig 4B) than placebo (P < .001). The maximum “good drug effect” was between 30/100 and 50/100 for the 2 doses (Fig 4B) and was greater in magnitude than the 25/100 recorded for a “bad drug effect” (Fig 4C). A “bad drug effect” (Fig 4C) was not evident for the low-dose group when compared with placebo (P > .2), and initially the high dose-group did not differ significantly from placebo either. Eventually, however, this effect built up with time for the high dose (effect change per unit time = 0.275, P = .03).
Psychoactive Effects
“Feeling high” (Fig 5A) scored greatest for the high-dose group (P < .001), and both dose groups differed from placebo (P< .05). Recovery was gradual after smoking cessation; no interaction with time occurred (P > .2), implying that the differences between active and placebo cigarettes remained similar at all time points despite cumulative dosing. “Feeling stoned” (Fig 5B) was also scored greater for the high-dose group (P = .001); again, both dose groups differed from placebo (P < .05). The treatment groups differed from placebo on “Feeling drunk” (P = .054), but this was of questionable clinical significance as the VAS was only 10/100 for both groups over time.
Figure 5.
Psychoactive side effects.
Somewhat more clinically relevant was the sensation of being “impaired” (Fig 5C), which rose just above 30 on a 100 millimeter VAS for both dose groups and differed from placebo (P = .003), and then declined with time. There was no change in “desire more of the drug” with time in either of the 2 treatment groups (P = .72). In the placebo group, however, the “desire more of the drug” decreased (probably because smoking cigarettes was unpleasant), and this decrease resulted in a significant difference between the treatment groups and placebo over time (P = .03). There was no difference between the 2 dose groups (P = .99) as to “desire more of the drug.”
Sedation occurred in both dose groups compared with placebo (P< .01), but there was no interaction with time (P = .82). Cannabis produced significantly more confusion than placebo (P = .03). Hunger increased over time in both treatment groups compared with placebo (P < .001), and the difference between the dose groups was not significant (P = .61). Anxiety was not a prominent effect of marijuana in this study. The only significant difference was between the high-dose and placebo groups (P < .02), but the maximum VAS value was less than 20/100. Similarly, feeling down was not a major factor; all the VAS values were just above 10/100 and did not differ significantly between groups (P > .05).
Mood
Mood was measured using VAS for feeling happy versus feeling sad, feeling relaxed versus feeling anxious, feeling calm versus feeling jittery, feeling good versus feeling bad, feeling self-assured versus feeling paranoid, feeling unafraid versus feeling fearful. There was no clear indication that mood changes accompanied marijuana use. Calmness was more noticeable over time with the 3.5% and placebo cigarettes (P < .03) but not with 7% cigarettes (P = .6). However, the effect size was approximately 1 and thus probably not an important consideration. The other measurements were similarly of little clinical significance.
Neuropsychological Testing
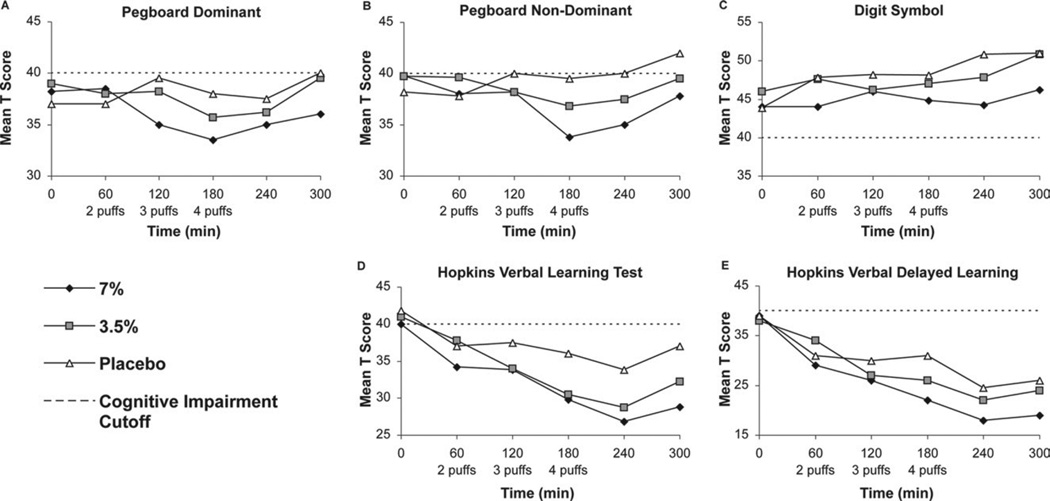
The linear mixed effects modeling for this data includes the main effect of time (continuous), a categorical main effect of treatment (7% vs 3.5% vs placebo cannabis), an interaction of time with treatment, and a categorical effect associated with the last time point (recovery effect). The recovery effect involving measurement of the last time point was performed as this point estimate showed departure from the linear pattern (Fig 6, A–E) of earlier measurements in other models. For this reason, we used this special model term to address reversal of the neuropsychological decline. Detailed results for the normalized data are presented in Table 4.
Figure 6.
Neuropsychological test scores.
Table 4.
Neuropsychological T Scores
| Score | Effect | Mean Difference |
Standard Error |
Confidence Interval |
P Value | ||
|---|---|---|---|---|---|---|---|
| Pegboard dominant hand |
Initial impairment | 7% vs 3.5% | 0.07 | 1.6 | −3.07 | 3.21 | .97 |
| 7% vs placebo | −1.77 | 1.63 | −4.96 | 1.42 | .28 | ||
| Change per minute in mean difference |
7% vs 3.5% | 0.46 | 0.41 | −0.34 | 1.26 | .27 | |
| 7% vs placebo | 1.14 | 0.42 | 0.32 | 1.96 | .007 | ||
| 3.5% vs placebo | 0.68 | 0.41 | −0.12 | 1.48 | .1 | ||
| Recovery effect | Last point vs linear trend | 3.22 | 1.03 | 1.20 | 5.24 | .002 | |
| Pegboard nondominant hand |
Initial impairment | 7% vs 3.5% | 0.29 | 1.51 | −2.67 | 3.25 | .85 |
| 7% vs placebo | −2.52 | 1.53 | −5.52 | 0.48 | .1 | ||
| Change per minute in mean difference |
7% vs 3.5% | 0.33 | 0.39 | −0.43 | 1.09 | .39 | |
| 7% vs placebo | 1.34 | 0.39 | 0.58 | 2.10 | < .001 | ||
| 3.5% vs placebo | 1.01 | 0.39 | 0.25 | 1.77 | < .01 | ||
| Recovery effect | Last point vs linear trend | 3.19 | 0.96 | 1.31 | 5.07 | < .01 | |
| Digit symbol test | Initial impairment | 7% vs 3.5% | 2.09 | 1.82 | −1.48 | 5.66 | .25 |
| 7% vs placebo | 0.18 | 1.85 | −3.45 | 3.81 | .92 | ||
| Change per minute in mean difference |
7% vs 3.5% | 0.43 | 0.47 | −0.49 | 1.35 | .36 | |
| 7% vs placebo | 0.93 | 0.48 | −0.01 | 1.87 | .051 | ||
| 3.5% vs placebo | 0.50 | 0.47 | −0.42 | 1.42 | .28 | ||
| Recovery effect | Last point vs linear trend | 1.30 | 1.16 | −0.97 | 3.57 | .001 | |
| HVLT–Learning | Initial impairment | 7% vs 3.5% | 1.59 | 2.22 | −2.76 | 5.94 | .47 |
| 7% vs placebo | 0.15 | 2.26 | −4.28 | 4.58 | .95 | ||
| Change per minute in mean difference |
7% vs 3.5% | 0.28 | 0.57 | −0.84 | 1.40 | .62 | |
| 7% vs placebo | 1.31 | 0.58 | 0.17 | 2.45 | .02 | ||
| 3.5% vs placebo | 1.03 | 0.57 | −0.09 | 2.15 | .07 | ||
| Recovery effect | Last point vs linear trend | 6.19 | 1.42 | 3.41 | 8.97 | < .001 | |
| HVLT–Recall | Initial impairment | 7% vs 3.5% | 1.5 | 2.25 | −2.91 | 5.91 | .5 |
| 7% vs placebo | −0.45 | 2.30 | −4.96 | 4.06 | .84 | ||
| Change per minute in mean difference |
7% vs 3.5% | 0.48 | 0.58 | −0.66 | 1.62 | .41 | |
| 7% vs placebo | 1.30 | 0.59 | 0.14 | 2.46 | .03 | ||
| 3.5% vs placebo | 0.82 | 0.58 | −0.32 | 1.96 | .16 | ||
| Recovery effort | Last point vs linear trend | 6.16 | 1.44 | 3.34 | 8.98 | < .000 | |
Significant results (P < .05) are bolded. HVLT, Hopkins Verbal Learning Test Revised.
The main effect of time models the cognitive impairment associated with the cumulative dose of cannabis. The pretreatment scores (intercept terms) were equal because participants did not have residual effects from previous treatments and had been instructed not to use marijuana for 30 days before study entry or during the intervals between study sessions. Cannabis produced a general cognitive decline, as indicated by the difference in the slopes of scores over time between treatment groups. The high-dose and placebo groups differed significantly on all dimensions, except the Digit Symbol Test, where the difference bordered on significant, at P = .051. The low- and high-dose groups did not differ significantly; however, point estimates (Table 4) indicate that the high-dose group had greater cognitive impairment. More notably, many of the neurocognitive results in the low-dose group did not differ significantly from those in the placebo group. The deviation of the last point from the general time pattern is modeled by the categorical recovery effect constructed using an indicator dummy variable representing the last time point. Recovery at the last observed time point after discontinuation of cannabis was significant for all scores, with the average score showing a P value of < .01.
The analysis comparing the effect of smoking cannabis in the low- and high-dose and placebo sessions using mean values could minimize group differences on neuropsychological testing since above average performers may offset poor performance by others. To obviate this potential bias, deficit scores were used to reduce the influence of the high-functioning individuals.16 Using this approach, both low- and high-dose cannabis induced moderate to severe impairment for verbal learning and recall (Table 5). Of note, subjects on placebo also declined in learning and recall, most likely due to proactive interference resulting from exposure to multiple learning lists in a short time frame. Nonetheless, despite this pattern, performance on these measures still differed by cannabis levels, and one would expect that any potential confounding might likely lessen any discernible treatment effects. Since cannabis may affect individuals differently and the impact on cognitive performance may be obscured if one just analyzes individual tests, the global deficit score was used to determine whether treatment with low-and high-dose cannabis affected overall cognitive performance. As can be seen in Table 5, there were significant group differences on global cognitive functioning at each treatment level. Participants using low-dose cannabis had poorer cognitive function than placebo and performed least well when on the high dose.
Table 5.
Global Deficit Scores (GDS)
| Treatment | 0 Min | 60 Min 2 Puffs |
120 Min 3 Puffs |
180 Min 4 Puffs |
240 Min | 300 Min | |
|---|---|---|---|---|---|---|---|
| Pegs Dom | 7% | 1.3 | 1.5 | 2.0 | 2.0 | 2.0 | 1.7 |
| 3.5% | 1.3 | 1.5 | 1.4 | 1.8 | 1.7 | 1.3 | |
| Placebo | 1.8 | 1.4 | 1.2 | 1.4 | 1.6 | 1.3 | |
| Pegs | 7% | 1.2 | 1.4 | 1.5 | 2.0 | 1.9 | 1.7 |
| Nondom | 3.5% | 1.1 | 1.3 | 1.4 | 1.5 | 1.5 | 1.3 |
| Placebo | 1.4 | 1.4 | 1.2 | 1.4 | 1.3 | 1.1 | |
| Digit Symbol | 7% | 0.9 | 0.9 | 0.9 | 1.1 | 1.3 | 0.8 |
| 3.5% | 0.6 | 0.8 | 0.9 | 0.7 | 0.7 | 0.5 | |
| Placebo | 0.8 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | |
| HVLT–Learn | 7% | 1.2 | 2.2 | 2.4 | 2.8 | 3.3 | 2.9 |
| 3.5% | 1.1 | 1.9 | 2.2 | 2.7 | 2.9 | 2.7 | |
| Placebo | 1.2 | 1.6 | 1.3 | 1.6 | 2.1 | 1.7 | |
| HVLT–Delay | 7% | 1.5 | 2.8 | 3.3 | 3.7 | 4.2 | 3.8 |
| 3.5% | 1.6 | 2.3 | 3.1 | 3.4 | 3.9 | 3.5 | |
| Placebo | 1.5 | 2.5 | 2.6 | 2.5 | 3.5 | 3.3 | |
| Average GDS |
7% | 1.2 | 1.8 | 2.0 | 2.3 | 2.5 | 2.2 |
| 3.5% | 1.1 | 1.5 | 1.8 | 2.0 | 2.2 | 1.8 | |
| Placebo | 1.3 | 1.5 | 1.4 | 1.5 | 1.8 | 1.6 |
Abbreviation: HVLT, Hopkins Verbal Learning Test Revised.
NOTE. Significant results (P< .05) are bolded.
Categorization of each raw deficit score was performed using clinically relevant cutoff points.
T ≥ 40 (deficit score (DS = 0; no impairment), 35 ≤ T ≤ 39 (DS = 1; mild impairment), 30 ≤ T ≤ 34 (DS = 2; mild-to-moderate impairment), 25 ≤ T ≤ 29 (DS = 3; moderate impairment), 20 ≤ T ≤ 24 (DS = 4; moderate-to-severe impairment), T < 20 (DS = 5; severe impairment).
In summary, the 7% cannabis demonstrated evidence of neurocognitive impairment in attention, learning and memory, and psychomotor speed, whereas, the 3.5% cannabis resulted in a decline in learning and memory only. When looking across all measures, subjects on 7% cannabis had greater impairment than those on 3.5%, who in turn had greater impairment than subjects on placebo. Of note, a significant proportion of subjects had cognitive impairment at baseline: Grooved Pegboard Dominant (71%) and non-Dominant (68%), Digit Symbol (61%), and HVLT Learning (76%) and Recall (76%). Twenty-nine of the 38 subjects (76%) had a global deficit score in the impaired range (>.50) at baseline before smoking cannabis.
Cannabinoid Levels
The mean (range) consumption of cigarettes was 550 mg (200–830 mg) during the low-dose sessions and 490 mg (270–870 mg) for the high-dose sessions. These amounts represent smoking slightly more than about one-half of a cigarette. The amount of delta-9-THC consumed was estimated to be 19 mg during the low-dose sessions and 34 mg during the high-dose sessions. Serum levels of the primary active cannabinoid D9-THC, secondary active cannabinoids, cannabidiol (CBD), cannabinol (CBN), the primary active metabolite, 11-hydroxy THC, and the primary inactive metabolite 1-NOR THC were evaluated using linear mixed modeling, with results presented in Table 6. There was no correlation of these serum levels with analgesia. As expected, several psychomimetic effects correlated with levels of delta-9-THC, CBD, and the active metabolite 1-NOR THC. However, neuropsychological testing did not show a relationship with serum values with the exception of delta-9-THC levels and performance on the Digit Symbol test (P = .034).
Table 6.
Linear Mixed Model Fit of Psychometric Responses Regressed on a Panel of Blood Levels of 5 Cannabinoids
| Blood Level | Psychomimetic Response | Regression Coefficient |
Standard Error |
Confidence Interval | P Value | |
|---|---|---|---|---|---|---|
| Cannabidiol | High | 0.73 | 0.30 | 0.13 | 1.00 | .019 |
| Impaired | 0.59 | 0.27 | 0.07 | 1.00 | .029 | |
| Stoned | 0.79 | 0.24 | 0.31 | 1.00 | .002 | |
| VAS Intensity | −0.24 | 0.23 | −0.69 | 0.22 | .315 | |
| Delta-9-THC | High | 0.01 | 0.004 | 0.00 | 0.02 | .007 |
| Impaired | 0.007 | 0.004 | 0.00 | 0.01 | .061 | |
| Stoned | 0.007 | 0.003 | 0.00 | 0.01 | .031 | |
| VAS Intensity | −0.001 | 0.003 | −0.01 | 0.00 | .662 | |
| Cannabinol | High | −0.003 | 0.10 | −0.20 | 0.20 | .971 |
| Impaired | −0.01 | 0.09 | −0.19 | 0.17 | .894 | |
| Stoned | −0.11 | 0.08 | −0.27 | 0.05 | .194 | |
| VAS Intensity | −0.08 | 0.08 | −0.23 | 0.08 | .334 | |
| 11-Hydroxy THC | High | −0.05 | 0.13 | −0.29 | 0.20 | .706 |
| Impaired | −0.03 | 0.11 | −0.25 | 0.19 | .783 | |
| Stoned | 0.05 | 0.10 | −0.14 | 0.25 | .593 | |
| VAS Intensity | −0.02 | 0.003 | −0.02 | −0.01 | .662 | |
| 1-NOR THC | High | −0.01 | 0.006 | −0.03 | 0.00 | .036 |
| Impaired | −0.003 | 0.006 | −0.01 | 0.01 | .574 | |
| Stoned | −0.005 | 0.006 | −0.02 | 0.01 | .424 | |
| VAS Intensity | 0.004 | 0.005 | −0.01 | 0.01 | .734 | |
Abbreviations: THC, tetrahydrocannabinol; VAS, visual analog scale.
NOTE. Regression coefficients represent mean change of response as a result of unit change in the respective blood level. Significant results (P< .05) are bolded.
Discussion
In the present study, standardized doses of smoked Cannabis sativa were administered using a uniform puff and breath-hold procedure.46 The analgesic, subjective, and neuropsychological effects of cannabis were then measured. A linear analgesic dose response for both 3.5% delta-9-THC and 7% delta-9-THC cannabis substantiated previous empirical reports of pain relief. Identical levels of analgesia were produced at each cumulative dose level by both concentrations of active agent (Fig 2). The plateau or “ceiling effect” indicates that within the range of the doses used, the top of the dose-response curve was reached. In addition to pain intensity, participants completed a rating scale to measure pain unpleasantness. This instrument has been validated in pain states amplified by emotional turmoil59 and provides insight into a drug’s relative effectiveness on alleviating the affective component of the pain experience as opposed to the more familiar sensory experience.60,61 In the present experiment, cannabis reduced pain intensity and unpleasantness equally. Thus, as with opioids,61 cannabis does not rely on a relaxing or tranquilizing effect (eg, anxiolysis) but rather reduces both the core component of nociception and the emotional aspect of the pain experience to an equal degree.
Separate appraisals using the patient global score and the multidimensional NPS revealed that both active agents alleviated pain compared with placebo. Interestingly, evoked pain brought about by lightly touching skin using a foam paintbrush or through testing heat pain threshold with the commercially available Medoc TSA 2001 Peltier thermode (Medoc, Ramat Yishai, Israel) did not confirm an analgesic effect of cannabis. These results are similar to those in a recent study demonstrating the efficacy of smoked cannabis in patients with human immunodeficiency virus (HIV)-associated sensory neuropathy.2 As in the present investigation, there was little effect on the painfulness of noxious heat stimulation. However, relief to experimentally induced hyperalgesia to both brush and von Frey hair stimuli was evident in the HIV-associated sensory neuropathy study.2 The lack of response to allodynia in the present study may reflect the challenge of alleviating this condition as it is notable for resistance to treatment.63 The lack of an increase in heat pain threshold in both the HIV study and the present analysis, however, has no apparent explanation. A simultaneous effect on heat pain induced experimentally and clinical pain has been documented with opioids60 and theoretically should have been evident in the present study provided the effect size was large enough to be discernable.
Undesirable consequences of smoking cannabis were clearly identifiable. However, consistent with the notion that these side effects are acceptable to patients with chronic pain,65,72 no participant withdrew because of tolerability issues. Subjects receiving active agent endorsed a “good drug effect” (Fig 4B) more than a “bad drug effect” (Fig 4C), and the latter was at issue only for the higher dose of cannabis. Similarly, feeling “high,” “stoned,” or “impaired” were less problematic for the lower strength cigarettes (Fig 5A – C). In general, side effects and changes in mood were relatively inconsequential. These findings are consistent with the observation that many patients find treatment with cannabis to be a satisfactory experience.65,72 A reasonable explanation would be that a patient self-titrates cannabis, balancing analgesia against side effects. However, beyond the benign psychoactive effects, administration of cannabis may be deleterious in that it impairs cognition. Previous investigations have reviewed processing speed, attention, memory, reaction time, and psychomotor abilities after smoking cannabis.25,53,58 Results have varied and depend on several variables including cigarette potency, smoking technique, individual variation in bioavailability, and previous exposure to the drug. As in the present study, although analgesia appears to be consistent across the low and high dosages, cognitive changes were more problematic with a high dose of delta-9-THC.4,35 This suggests that a therapeutic window may exist that might be exploited for clinical purposes. However, there is an additional problem with using cannabis in the chronic pain population. Severe pain coupled with psychological distress is associated with below average scores on cognitive performance tests.43,44,76 As in these previous reports, the patients in our study were either below or nearly below the cutoff for impairment before receiving study medication. Our study indicates that modest declines in cognitive performance occur with cannabis, particularly in learning and recall, and especially at higher doses. In combination with the deficits in baseline neurocognitive performance, however, cannabis compounds this problem. This finding necessitates caution in the prescribing of medical marijuana for neuropathic pain, especially in instances in which learning and memory are integral to a patient’s work and life-style.
Further vigilance is warranted in young patients because cannabis use in adolescence increases the risk of later schizophrenia-like psychoses, especially in genetically susceptible individuals.24 There is an increased risk of a psychosis in those who have ever used cannabis (pooled adjusted odds ratio = 1.41; 95% CI, 1.20–1.65) and a dose-response effect, with greater risk in subgroups consuming cannabis very frequently (pooled adjusted odds ratio = 2.09; 95% CI, 1.54–2.84).54 These effects of cannabis may be consequent on its impact on the dopamine system.54 There is less evidence of cannabis playing a role in other mental disorders (ie, depression and anxiety). Further research is needed to understand the biological mechanisms underlying the effects of cannabis on psychiatric conditions, but the health risks of cannabis in patients with any propensity for psychosis mandate caution in this population. Consistent with this risk, a history of schizophrenia and bipolar depression were exclusionary criteria in the present study.
It is tempting to speculate that a lower strength of cannabis might avoid or at least reduce the adverse neurocognitive profile noted above. As the 7% and 3.5% cannabis were equianalgesic, it would be certainly be appropriate to test a lower concentration to see if the analgesic profile is maintained while cognitive decline is reduced or even obviated. Even if the pain-relieving properties are less than robust, a case could be made for using the lowest possible strength despite attenuation in analgesic potency. Moreover, as polypharmacy is common in the management of chronic pain,12 the addition of the lowest effective dose of cannabis to another analgesic drug (ie, anticonvulsant, opioid, etc) might lead to an effective treatment of a neuropathic pain condition otherwise treatment resistant.18,19 Additionally, the diversion potential could be reduced as cannabis with a very low THC content is less desirable for recreational use.34 In addition to evaluating the efficacy of a lower concentration, the potential of use a cognitive enhancer with cannabis to reverse or at least mitigate cognitive impairment might be considered in the future. Such an agent (eg, modafinil) has been used with psychotropic medications to reduce sedation and cognitive impairment.9,10,49,67,68,74 New cognitive enhancers are on the horizon as researchers test cholinergic agents, biogenic amines, and neuropeptides to treat learning and memory deficits associated with neurodegenerative states. Psychostimulants, excitatory amino acids, and a heterogeneous group of compounds of diverse chemical composition that allegedly facilitate learning and memory or overcome natural or induced cognitive impairments50 might someday be useful in combination with cannabis or cannabinoids currently thought to be undesirable because of the depressant effects on the central nervous system that limit their use.15
In addition to the issues discussed above, the noxious pyrolytic byproducts released through combustion remain a public health deterrent to the use of smoked cannabis.42,51 However, a method has been devised to provide a safer and more efficient delivery system. Cannabis vaporization is a technique that avoids the production of irritating respiratory toxins by heating the herbal medicine to a temperature at which active cannabinoid vapors form but below the point of combustion where toxins are released.29,52 Gas chromatograph/mass spectrometer analysis reveals that the gas phase of the vapor consists overwhelmingly of cannabinoids. In contrast, more than 111 compounds are identifiable in samples of smoked cannabis, including several potentially harmful polynuclear aromatic hydrocarbons.30 In a pilot study involving healthy volunteers,3 vaporization delivered therapeutic doses of cannabinoids with a drastic reduction in pyrolytic smoke compounds. It is reasonable to assume that future clinical trials will utilize this alternative delivery method.
Perspective.
This study adds to a growing body of evidence that cannabis may be effective at ameliorating neuropathic pain, and may be an alternative for patients who do not respond to, or cannot tolerate, other drugs. However, the use of marijuana as medicine may be limited by its method of administration (smoking) and modest acute cognitive effects, particularly at higher doses.
Acknowledgments
We acknowledge the University of California Center for Medicinal Cannabis Research which provided critical support and guidance. They derived direct financial support from the California legislature as well as logistic and scientific support from several national stakeholders (ie, Food and Drug Administration, Department of Health and Human Services, National Institute on Drug Abuse, and the Drug Enforcement Agency), whose assistance was invaluable. This publication was also made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. We also thank Tom Lang for his editorial assistance in the preparation of the manuscript.
Supported by grant C01-DA-114 from the University of California Center for Medicinal Cannabis Research.
References
- 1.Ad Hoc Group of Experts. Report to the Director, National Institutes of Health: Workshop on the Medical Utility of Marijuana. 1997. Feb 19–20, [Google Scholar]
- 2.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 3.Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: A pilot study. Clin Pharmacol Ther. 2007;82:572–578. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- 4.Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: Effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–122. [PubMed] [Google Scholar]
- 5.Barnes MP. Sativex: Clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Exp Opin Pharmacother. 2006;7:607–615. doi: 10.1517/14656566.7.5.607. [DOI] [PubMed] [Google Scholar]
- 6.Beglinger LJ, Gaydos B, Tangphao-Daniels O, Duff K, Kareken DA, Crawford J, Fastenau PS, Siemers ER. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol. 2005;20:517–529. doi: 10.1016/j.acn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 8.Benedict RH, Zgaljardic DJ. Practice effects during repeated administrations of memory tests with and without alternate forms. J Clin Exp Neuropsychol. 1998;20:339–352. doi: 10.1076/jcen.20.3.339.822. [DOI] [PubMed] [Google Scholar]
- 9.Berigan TR. Modafinil treatment of excessive sedation associated with divalproex sodium. Can J Psychiatry. 2004;49:72–73. doi: 10.1177/070674370404900118. [DOI] [PubMed] [Google Scholar]
- 10.Berigan TR. Modafinil treatment of excessive daytime sedation and fatigue associated with topiramate. Prim Care Companion J Clin Psychiatry. 2002;4:249–250. doi: 10.4088/pcc.v04n0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: Results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Black DR, Sang CN. Advances and limitations in the evaluation of analgesic combination therapy. Neurology. 2005;65:S3–S6. doi: 10.1212/wnl.65.12_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 13.Bostrom BA. Gonzales v. Raich In the Supreme Court of the United States. Issues Law Med. 2005;21:47–56. [PubMed] [Google Scholar]
- 14.Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stanton-Hicks M. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria: International Association for the Study of Pain. Pain. 1999;81:147–154. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 15.Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001;323:13–16. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 17.Chait LD, Corwin RL, Johanson CE. A cumulative dosing procedure for administering marijuana smoke to humans. Pharmacol Biochem Behav. 1988;29:553–557. doi: 10.1016/0091-3057(88)90019-6. [DOI] [PubMed] [Google Scholar]
- 18.Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Cichewicz DL, McCarthy EA. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- 20.Clark WC, Janal MN, Zeidenberg P, Nahas GG. Effects of moderate and high doses of marihuana on thermal pain: A sensory decision theory analysis. J Clin Pharmacol. 1981;21:299S–310S. doi: 10.1002/j.1552-4604.1981.tb02608.x. [DOI] [PubMed] [Google Scholar]
- 21.Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9:419–428. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- 22.Day R, Wodak A, Chesher G. Cannabis and suicide. Med J Aust. 1994;160:731. [PubMed] [Google Scholar]
- 23.Dellemijn P. Are opioids effective in relieving neuropathic pain? Pain. 1999;80:453–462. doi: 10.1016/S0304-3959(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 24.Di Forti M, Morrison PD, Butt A, Murray RM. Cannabis use and psychiatric and cognitive disorders: The chicken or the egg? Curr Opin Psychiatry. 2007;20:228–234. doi: 10.1097/YCO.0b013e3280fa838e. [DOI] [PubMed] [Google Scholar]
- 25.Dornbush RL, Kokkevi A. Acute effects of cannabis on cognitive, perceptual, and motor performance in chronic hashish users. Ann N Y Acad Sci. 1976;282:313–322. doi: 10.1111/j.1749-6632.1976.tb49906.x. [DOI] [PubMed] [Google Scholar]
- 26.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 27.Galer BS, Bruehl S, Harden RN. IASP diagnostic criteria for complex regional pain syndrome: A preliminary empirical validation study: International Association for the Study of Pain. Clin J Pain. 1998;14:48–54. doi: 10.1097/00002508-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology. 1997;48:332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 29.Gieringer D. Marijuana research: Waterpipe study. MAPS (Multidisciplinary Association for Psychedelic Studies) Bull. 1996;6:59–66. [Google Scholar]
- 30.Gieringer D, St. Laurent J, Goodrich S. Cannabis vaporizer combines efficient delivery of THC with effective suppression of pyrolytic compounds. J Cannabis Ther. 2004;4 [Google Scholar]
- 31.Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 32.Hansson P, Lindblom U. Hyperalgesia assessed with quantitative sensory testing in patients with neurogenic pain. In: Willis WDJ, editor. Hyperalgesia and Allodynia. New York: Raven Press; 1992. pp. 335–343. [Google Scholar]
- 33.Harden RN, Bruehl S, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stanton-Hicks M. Complex regional pain syndrome: Are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211–219. doi: 10.1016/s0304-3959(99)00104-9. [DOI] [PubMed] [Google Scholar]
- 34.Harder S, Rietbrock S. Concentration-effect relationship of delta-9-tetrahydrocannabiol and prediction of psychotropic effects after smoking marijuana. Int J Clin Pharmacol Ther. 1997;35:155–159. [PubMed] [Google Scholar]
- 35.Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- 36.Heaton R, Grant I, Matthews C. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- 37.Heaton R, Miller S, Taylor M, Grant I. In: Revised Comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz F, editor. Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- 38.Heaton R, Taylor M, Manly J, editors. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2004. [Google Scholar]
- 39.Heaton RK, Marcotte TD. Clinical Neuropsychological Tests and Assessment Techniques. In: Boller F, Grafman J, Rizzolatti G, editors. Handbook of Neuropsychology. Amsterdam: Elsevier Science BV; 2000. [Google Scholar]
- 40.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- 42.Hiller FC, Wilson FJ, Jr, Mazumder MK, Wilson JD, Bone RC. Concentration and particle size distribution in smoke from marijuana cigarettes with different delta 9-tetrahy-drocannabinol content. Fundam Appl Toxicol. 1984;4:451–454. doi: 10.1016/0272-0590(84)90202-1. [DOI] [PubMed] [Google Scholar]
- 43.Iezzi T, Archibald Y, Barnett P, Klinck A, Duckworth M. Neurocognitive performance and emotional status in chronic pain patients. J Behav Med. 1999;22:205–216. doi: 10.1023/a:1018791622441. [DOI] [PubMed] [Google Scholar]
- 44.Iezzi T, Duckworth MP, Vuong LN, Archibald YM, Klinck A. Predictors of neurocognitive performance in chronic pain patients. Int J Behav Med. 2004;11:56–61. doi: 10.1207/s15327558ijbm1101_7. [DOI] [PubMed] [Google Scholar]
- 45.Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain. 2004;109:432–442. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Kelly TH, Foltin RW, Fischman MW. Effects of smoked marijuana on heart rate, drug ratings and task performance by humans. Behav Pharmacol. 1993;4:167–178. [PubMed] [Google Scholar]
- 47.Klove H. Clinical neuropsychology. In: Forster FE, editor. The Medical Clinics of North America. New York, NY: Saunders; 1963. [PubMed] [Google Scholar]
- 48.Lorber L. Gonzales v Raich: the US Supreme Court’s consideration captured the public policy debate about the medical use of marijuana. Gend Med. 2005;2:124–130. doi: 10.1016/s1550-8579(05)80040-3. [DOI] [PubMed] [Google Scholar]
- 49.Makela EH, Miller K, Cutlip WD2nd. Three case reports of modafinil use in treating sedation induced by antipsychotic medications. J Clin Psychiatry. 2003;64:485–486. doi: 10.4088/jcp.v64n0420h. [DOI] [PubMed] [Google Scholar]
- 50.Malik R, Sangwan A, Saihgal R, Jindal DP, Piplani P. Towards better brain management: Nootropics. Curr Med Chem. 2007;14:123–131. doi: 10.2174/092986707779313408. [DOI] [PubMed] [Google Scholar]
- 51.Matthias P, Tashkin DP, Marques-Magallanes JA, Wilkins JN, Simmons MS. Effects of varying marijuana potency on deposition of tar and delta9-THC in the lung during smoking. Pharmacol Biochem Behav. 1997;58:1145–1150. doi: 10.1016/s0091-3057(97)00328-6. [DOI] [PubMed] [Google Scholar]
- 52.McPartland JM, Pruitt PL. Medical marijuana and its use by the immunocompromised. Altern Ther Health Med. 1997;3:39–45. [PubMed] [Google Scholar]
- 53.Miller LL, McFarland D, Cornett TL, Brightwell D. Marijuana and memory impairment: Effect on free recall and recognition memory. Pharmacol Biochem Behav. 1977;7:99–103. doi: 10.1016/0091-3057(77)90191-5. [DOI] [PubMed] [Google Scholar]
- 54.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 55.Noyes R, Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975;18:84–89. doi: 10.1002/cpt197518184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noyes R, Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975;15:139–143. doi: 10.1002/j.1552-4604.1975.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 57.Okie S. Medical marijuana and the Supreme Court. N Engl J Med. 2005;353:648. doi: 10.1056/NEJMp058165. [DOI] [PubMed] [Google Scholar]
- 58.Pope HG, Jr, Gruber AJ, Yurgelun-Todd D. Residual neuropsychologic effects of cannabis. Curr Psychiatry Rep. 2001;3:507–512. doi: 10.1007/s11920-001-0045-7. [DOI] [PubMed] [Google Scholar]
- 59.Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. [DOI] [PubMed] [Google Scholar]
- 60.Price DD, Harkins SW, Rafii A, Price C. A simultaneous comparison of fentanyl’s analgesic effects on experimental and clinical pain. Pain. 1986;24:197–203. doi: 10.1016/0304-3959(86)90042-4. [DOI] [PubMed] [Google Scholar]
- 61.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 62.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 63.Rowbotham MC. Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65:S66–S73. doi: 10.1212/wnl.65.12_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 64.Russo E, Guy GW. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 65.Schnelle M, Grotenhermen F, Reif M, Gorter RW. Results of a standardized survey on the medical use of cannabis products in the German-speaking area. Forsch Komplementarmed. 1999;6((Suppl) 3):28–36. doi: 10.1159/000057154. [DOI] [PubMed] [Google Scholar]
- 66.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 67.Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004;55:1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Rob-bins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004;29:1363–1373. doi: 10.1038/sj.npp.1300457. [DOI] [PubMed] [Google Scholar]
- 69.Van Gorp WG, Lamb DG, Schmitt FA. Methodologic issues in neuropsychological research with HIV-spectrum disease. Arch Clin Neuropsychol. 1993;8:17–33. doi: 10.1016/0887-6177(93)90040-8. [DOI] [PubMed] [Google Scholar]
- 70.Vaughan CW, Christie MJ. An analgesic role for cannabinoids. Med J Aust. 2000;173:270–272. doi: 10.5694/j.1326-5377.2000.tb125638.x. [DOI] [PubMed] [Google Scholar]
- 71.Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- 72.Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain. 2003;102:211–216. doi: 10.1016/s0304-3959(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 73.Ware MA, Gamsa A, Persson J, Fitzcharles MA. Cannabis for chronic pain: Case series and implications for clinicians. Pain Res Manag. 2002;7:95–99. doi: 10.1155/2002/380509. [DOI] [PubMed] [Google Scholar]
- 74.Webster L, Andrews M, Stoddard G. Modafinil treatment of opioid-induced sedation. Pain Med. 2003;4:135–140. doi: 10.1046/j.1526-4637.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- 75.Wechsler D. Administration and Scoring Manual. Third. San Antonio, TX: The Psychological Corporation, Harcourt Brace & Co; 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 76.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 77.Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–1314. doi: 10.2165/00003495-200060060-00005. [DOI] [PubMed] [Google Scholar]