Abstract
Background
Benzodiazepine (BZD) use disorders are a common clinical problem among methadone maintenance treatment patients and have adverse effects on clinical outcomes.
Objectives
To evaluate gabapentin for the outpatient treatment of BZD abuse or dependence in methadone maintenance patients.
Methods
Participants (n=19) using BZDs at least 4 days per week were enrolled into an eight-week randomized double-blind placebo-controlled outpatient pilot trial. All participants received a manual-guided supportive psychotherapy aimed to promote abstinence. Study medication was titrated over a two-week period to a maximum dose of gabapentin 1200 mg or placebo three times a day. BZD use was assessed using urine toxicology confirmed self-report. BZDs were not provided as part of study participation; participants were provided guidance to gradually reduce BZD intake.
Results
Sixteen participants had post-randomization data for analysis. Retention at week eight was 50%. The mean dose of gabapentin achieved by titration was 2666 mg/day (SD=±1446). There were no significant between group differences on BZD use outcomes (amount BZD per day (Mann-Whitney U = 27, p = .745), abstinent days per week (U = 28, p = .811)) and CIWA-BZD scale (U = 29.0, p = .913). One participant in the gabapentin group discontinued study medication because of peripheral edema. Two participants in the placebo group requested admission for inpatient detoxification treatment.
Conclusion
Gabapentin was not found to differ from placebo, although the small sample recruited for this trial may have limited the ability to detect a difference.
INTRODUCTION
Benzodiazepine (BZD) abuse and dependence is a serious clinical problem in the methadone maintenance treatment population. Estimates of the prevalence of BZD use disorders among methadone maintenance treatment (MMT) patients, range from 21% to 66% (1–7), with roughly half of patients starting BZD use after entering MMT (8). MMT patients with a BZD use disorder are more likely to be using other drugs, including heroin, engage in high risk behaviors, and have increased rates of depression, anxiety, and global psychopathology (1, 4, 9). Women in MMT who misuse BZDs during pregnancy have babies of significantly lower birth weight than those women who do not (10). MMT patients with BZD use disorders have an 8-fold likelihood of death compared to other MMT patients (11) and BZDs are also likely to contribute to deaths from methadone toxicity in patients receiving MMT (12, 13). In summary, BZD use disorders in MMT programs is widespread and is associated with a negative impact on treatment outcomes (14, 15). Despite the risk of BZD use problems on MMT patients, no clearly effective strategy for managing this clinical problem has been developed.
Gamma-aminobutyric acid (GABA) type A receptors are a family of ligand-gated ion channels responsible for inhibitory regulation of the central nervous system. BZDs bind directly to a specific modulatory site that is present on GABAA receptors (16, 17), which enhances the effect of the inhibitory neurotransmitter, but does not open the chloride channel in the absence of GABA. Chronic exposure to BZDs leads to alterations of GABAergic neurotransmission, which are manifested as the symptoms of tolerance, dependence and withdrawal. The mechanism of these changes is unclear, and may involve alterations in the expression of individual GABAA receptor subtypes (18), but are known to be linked to the dose and duration of BZD use (19). While the direct effects of BZDs are GABA-related, the withdrawal syndrome may be in part be mediated by calcium channel (20) and glutamatergic (21) mechanisms. In addition, neuroendocrine pathways, including regulation of the hypothalamo-pituitary-adrenocortical axis by corticotropin-releasing factor, may be involved in the mechanism of BZD dependence (22).
Research studying the management of discontinuation of therapeutic BZD treatment has generally supported the approach of a slow, gradual taper (23). For patients with BZD dependence, a consensus exists that gradual taper is preferred to abrupt discontinuation (24). Small clinical trials have tested propranolol (25, 26), buspirone (27), and progesterone (28) without any clear evidence of efficacy. Hydroxyzine can reduce anxiety symptoms associated with a BZD taper (29). Carbamazepine has shown promise as an adjunct to gradual taper of BZDs (30). Trazodone and valproate were associated with improved abstinence rates, but had no effect on withdrawal symptoms (31). Cyamemazine, an anxiolytic antipsychotic agent, has shown promise as a treatment for the BZD withdrawal syndrome (32). The use of a slow infusion of the BZD antagonist flumazenil has been shown to be feasible (33), but carries a high risk of seizures (34). No clearly effective pharmacotherapy for the treatment of BZD use disorder has been identified (24).
As for the specific circumstance of managing MMT patients with a BZD use disorder, limited research has been conducted. Potential treatment interventions that have been investigated, including BZD maintenance (6, 35) contingency reinforcement (36–38), and slow outpatient detoxification (39) have not been accepted into clinical practice. The optimal strategy for the treatment of BZD use disorders in MMT patients remains unknown.
Gabapentin was initially synthesized as a structural analogue of the neurotransmitter GABA (40), and while it is not a GABA-mimetic agent (41), it has been shown to increase synaptic GABA levels (42, 43). Gabapentin binds to the α2δ-1 and α2δ-2 subunits of voltage-gated calcium channels (44–46) and inhibits calcium currents (47–49), which leads to attenuation of postsynaptic excitability (50, 51). Gabapentin is not appreciably metabolized in humans, does not bind to plasma proteins or induce hepatic enzymes, and is eliminated by renal excretion as an unchanged drug. It has limited abuse potential, few side effects, does not require blood monitoring, and does not affect the hepatic metabolism of other medications (52, 53). Even without titration, gabapentin is well tolerated (54, 55) at doses of 3,600 mg/day (56). Gabapentin has been shown to have generally mild effects on cognition (57–60). Gabapentin has anxiolytic (61, 62) and sedating properties (63), which may alleviate the symptoms associated with a reduction in BZD dose and make the achievement of reduction in use or abstinence more likely.
Gabapentin has been studied for the treatment of a variety of substance use disorders. Most promising, gabapentin has been for the treatment of alcohol use disorder (64–67) and cannabis use disorder (68). Given the promise that gabapentin has shown for alcohol use problems, and the similar pharmacodynamics properties of BZDs and alcohol, gabapentin could potentially have efficacy as a treatment for BZD.
We hypothesized that MMT patients with a BZD use disorder would have difficulty in reducing or discontinuing BZD use because of craving, anxiety, and sleep disturbance symptoms that accompany reduction in BZD use, and that gabapentin would help ameliorate those symptoms and promote achievement of abstinence from BZDs. The neuroinhibitory effects of gabapentin, its favorable pharmacokinetic and safety profile, make it a promising candidate medication to study for the treatment of BZD use disorder. Therefore, we conducted a pilot randomized controlled clinical trial comparing the efficacy of gabapentin to placebo in treating BZD abuse and dependence in MMT patients. The goal was to select a population of individuals in MMT with BZD use disorder who were appropriate for outpatient management, where study medication was provided, but BZD supply remained the participants responsibility.
METHODS
Study Design
The study took place at Narco Freedom: Bridge Plaza Treatment and Rehabilitation Clinic in Long Island City, NY February 1, 2007 through September 21, 2010. Bridge Plaza is an outpatient clinic that offers substance abuse and methadone maintenance treatment to approximately 440 patients.
Subjects
The Institutional Review Board of the New York State Psychiatric Institute approved this research protocol and all participants provided informed consent prior to study enrollment. Participation was confidential and voluntary and information was never shared with counselors or the remaining staff at the clinic. All of the participating individuals were receiving methadone treatment at Bridge Plaza at the time of the study. Ninety-five potential participants were screened. Participants were 19 men and non-pregnant women between the ages of 18–65 who met DSM-IV criteria for current benzodiazepine abuse or dependence, and opioid dependence, and werebeing treated for opioid dependence with methadone (see table 1.) Participants reported using benzodiazepines an average ≥ 4 days per week over the past 28 days and were seeking treatment for benzodiazepine abuse or dependence. Exclusion criteria included: 1) Any Axis I psychiatric disorder as defined by DSM-IV-TR that were unstable or would be disrupted by study medication or by an effort to discontinue benzodiazepines; 2) Acute physiological benzodiazepine withdrawal or a history of seizures during alcohol or sedative-hypnotic withdrawal; 3) Participants with cocaine dependence as their primary substance use disorder diagnosis; 4) Participants with unstable physical disorders or impaired kidney function; 5) Prescribed psychotropic medications, other than methadone or medications prescribed for pain syndromes that would be disrupted by study medication or by an effort to discontinue benzodiazepines; 6) Anticonvulsants prescribed for pain syndromes; 7) Known sensitivity to gabapentin; 8) Individuals who had exhibited suicidal or homicidal behavior within the past two years or had current active suicidal ideation; 9) Individuals physiologically dependent on any other drugs (excluding nicotine, caffeine, methadone); 10) Individuals currently prescribed gabapentin; 11) Individuals requiring pharmacological detoxification from BZDs in the past year and are unlikely to be able to tolerate taper off of benzodiazepines.
Table 1.
Participant Characteristics
| Gabapentin(n = 8) | Placebo(n = 11) | Statistic | |
|---|---|---|---|
| Age | 40 | 37 | t = −.631, df= 17, p=.53 |
| Male | 75% | 73% | Fisher’s exact = 1.0 |
| White | 50% | 64% | Fisher’s exact = .66 |
| Unmarried | 50% | 91% | Fisher’s exact = .11 |
| Employed | 0% | 20% | χ2 = .15 |
| Years education | 11.5 (±2.0) | 12.4 (±1.5) | t = 1.1; df= 16; p=.29 |
Treatment
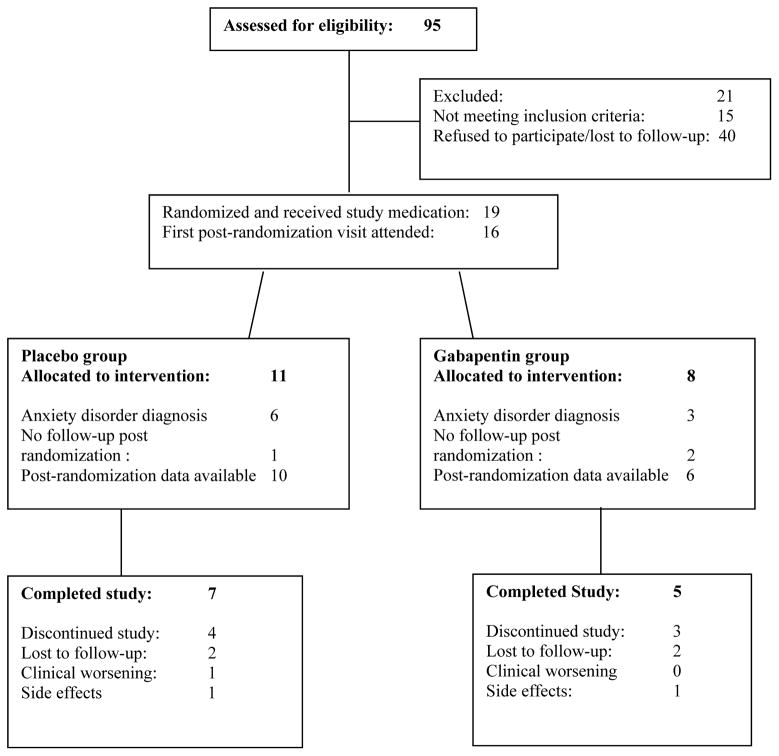
Ninety-five participants were screened for study participation and 19 were enrolled (see Figure 1). Participants were randomly allocated (1:1) to receive gabapentin or placebo stratified by the presence of comorbid anxiety disorders (panic disorder, social phobia, generalized anxiety disorder, obsessive-compulsive disorder, or post-traumatic stress disorder.) Recruitment was unbalanced (gabapentin n=8; placebo n=11) as an artifact of randomization. Gabapentin doses were gradually titrated from 400 mg three times daily to 1200 mg three times daily over a two week period. All capsules (placebo and gabapentin) were over-capsulated with riboflavin to assess compliance. The placebo group had a dosing schedule identical to the gabapentin group. This was a maximum tolerated dose study and dose reductions for tolerability problems were made by the research psychiatrist. All participants had to take a minimum of gabapentin/placebo 400 mg three times a day to remain in the study.
Figure 1.
Participants’ progress through the screening, entry, randomization and medication phases of the treatment trial
Pill counts were performed at each visit as a measure of compliance. Patients were compensated $10 per visit to return their pill bottle with any unused pills; compensation was not tied to ingesting the pills. A structured interview covering the time period since the last visit will account for every scheduled dose of medication.
All participants had a weekly individual psychotherapy session with the research psychiatrist using a structured compliance enhancement manual designed for pharmacotherapy trials in subjects with substance use disorders (69). The goal of compliance enhancement therapy was to provide abstinence-focused supportive treatment as well as consistency between treatment groups. Sessions were structured and focused on setting abstinence from benzodiazepine use as a goal, patient compliance, and current functioning. The plan to taper from BZDs was developed in the first session. The plan for tapering benzodiazepine use was individually determined in a collaborative effort between the research psychiatrist and the participant. The target rate of taper recommended to participants was 25% per week. No BZDs were provided by study staff; participants continued to obtain BZDs from their own sources, prescribed or non-prescribed. Weekly assessments for benzodiazepine withdrawal included vital signs, rating scales, and the clinical interview by the research psychiatrists. The rescue plan for all patients was referral for emergency inpatient detoxification. Study exit criteria included development of serious psychiatric symptomatology, clinically significant symptoms of benzodiazepine withdrawal, clinically significant worsening of benzodiazepine use, pregnancy. Vital signs (heart rate and blood pressure) and side effects were monitored at each visit. Urine toxicology was conducted on a weekly basis.
Measures
Benzodiazepine use was measured by urine confirmed self-report. Urine toxicology testing and a Timeline Followback calendar procedure was completed each week and urine toxicology screens were used to corroborate self-report. Benzodiazepine withdrawal symptoms were measured each week using the Clinical Institute Withdrawal Assessment-Benzodiazepines (CIWA-B) (70).
Data Analyses
Group differences with respect to BZD use outcomes and CIWA-BZD outcome were analyzed using independent-samples Mann-Whitney U tests. We compared baseline to last week as a paired sample in the gabapentin group using Wilcoxon signed ranks tests. Means and standard deviations were calculated and reported for BZD use outcome and CIWA-BZD outcome using the differences between baseline and last week in the gabapentin group.
RESULTS
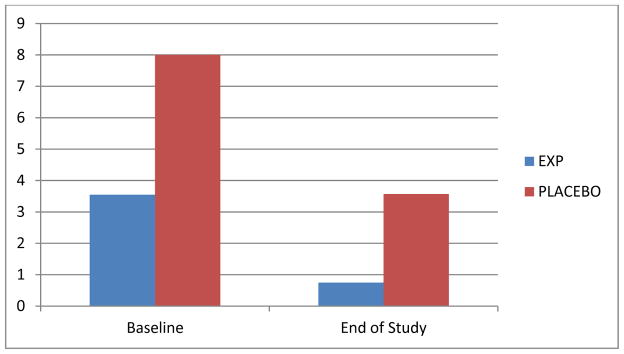
No significant between group differences on BZD use outcomes (amount BZD per day (Mann-Whitney U=27, p=.745), abstinent days per week (U=28, p=.811)) and CIWA-BZD scale (U=29.0, p=.913). Figure 1 displays the medians in each group at baseline and end of study; participants in both groups reduced their intake of BZD as compared to baseline. In the gabapentin group, the mean dose achieved was 2666 mg/day (SD=±1446). Overall retention at week eight was 50%. 10 participants (53%) met criteria for a substance-independent anxiety disorder. One gabapentin group participant discontinued study medication because of peripheral edema; two participants in the placebo group voluntarily sought admission for inpatient detoxification treatment. Over the study period, participants in the gabapentin group increased their abstinent days per week from 0.3 to 2.3 (SD=3.1; Z = −1.4, p=.16), significantly decreased their daily use of benzodiazepine by 4.0 mg per day (in lorazepam equivalents) (SD=4.0; Z = −2.2, p=.03), an 80.1% reduction (see Figure 2), and had mean reduction in CIWA-BZD scale scores of 5.7 (SD= 6.8; Z = −1.8, p=.08).
Figure 2.
Median Change in Benzodiazepine Use (Equivalent Lorazepam mg Per Day). Comparison of the experimental and placebo groups in the mean amount of benzodiazepine used per day calculated over the week prior to study entry (baseline) and the last week of study participation (end of study).
Adverse symptoms were reported by all six participants assigned to gabapentin, with fatigue (3 participants), somnolence (5 participants), and dizziness (4 participants) as the most common complaints. Five of the ten participants in the placebo group reported side effects, with fatigue (2 participants) and somnolence (2 participants) as the most common complaints.
DISCUSSION
There were no significant differences between gabapentin and placebo in changes in benzodiazepine use, although statistical power was limited due to the small sample size. Participants in both treatment groups reduced their use of BZDs as compared to baseline. The outpatient model of treatment for BZD use disorders tested here, in which study participation did not provide any BZDs, was found to be feasible. No patients removed from trial for BZD withdrawal related complications. MMT patients provided a convenient sample for recruitment, but may be a more difficult to treat population because of comorbidity and lack generalizability. The high rate of anxiety disorders (47%) suggests a possible association with risk for developing BZD use disorder. Gabapentin in divided doses of 3600 mg daily was well tolerated by methadone maintenance patients with benzodiazepine abuse or dependence. An outpatient clinical trial that does not provide BZDs is a feasible approach for studying potential pharmacotherapies for BZD use disorders. An adequately powered double-blind trial is required to further evaluate the utility of gabapentin in treating benzodiazepine use disorder. Recruitment from more than one program simultaneously is likely to be required to reach an adequate sample size.
The options for the clinical management of MMT patients with BZD use disorders are limited. MMT patients using BZDs are typically identified by urine toxicology and clinical observation (i.e., patient appears sedated or intoxicated). Patients who are identified to have BZD use disorders are either referred for inpatient detoxification or instructed to stop using BZDs. Often MMT programs will place patients with BZD use disorders on probation with the contingency of being discontinued from MMT for persistent BZD use. A confounding issue is the source of BZDs. BZDs can be obtained from licit or illicit sources or a combination of both. Not infrequently, MMT patients with BZD use disorders obtain prescriptions for BZDs, which then places the MMT program in the difficult position of second-guessing the prescription-writer as to the “legitimacy” of the indication for the use of BZDs. This is a particularly difficult management problem since a subset of BZD-using MMT patients are likely to have comorbid anxiety disorders that could potentially benefit from BZD therapy. Additionally, patients in MMT who use BZDs as prescribed for an appropriate psychiatric disorder can come under suspicion by the clinical staff and be inappropriately pressured to discontinue therapeutic BZD use.
Given the public health consequences of BZD use disorders on MMT outcomes, more research is needed to determine the optimal management of BZD use disorders in MMT patients. With limited statistical power this study was unable to answer the question as to whether gabapentin is a potential treatment for individuals with BZD use disorder in MMT. However, the novel study design, an outpatient test of a medication to facilitate BZD taper, with no BZD provided by study participation, was found to be feasible.
Acknowledgments
Funding Source: Funding for this work was provided by NIDA.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00420771
References
- 1.DARKE S, SWIFT W, HALL W, ROSS M. Drug use, HIV risk-taking and psychosocial correlates of benzodiazepine use among methadone maintenance clients. Drug Alcohol Depend. 1993;34:67–70. doi: 10.1016/0376-8716(93)90047-t. [DOI] [PubMed] [Google Scholar]
- 2.IGUCHI MY, HANDELSMAN L, BICKEL WK, GRIFFITHS RR. Benzodiazepine and sedative use/abuse by methadone maintenance clients. Drug Alcohol Depend. 1993;32:257–266. doi: 10.1016/0376-8716(93)90090-d. [DOI] [PubMed] [Google Scholar]
- 3.KIDORF M, BROONER RK, KING VL, CHUTUAPE MA, STITZER ML. Concurrent validity of cocaine and sedative dependence diagnoses in opioid-dependent outpatients. Drug Alcohol Depend. 1996;42:117–123. doi: 10.1016/0376-8716(96)01268-9. [DOI] [PubMed] [Google Scholar]
- 4.BLEICH A, GELKOPF M, SCHMIDT V, HAYWARD R, BODNER G, ADELSON M. Correlates of benzodiazepine abuse in methadone maintenance treatment. A 1 year prospective study in an Israeli clinic. Addiction (Abingdon, England) 1999;94:1533–1540. doi: 10.1046/j.1360-0443.1999.941015339.x. [DOI] [PubMed] [Google Scholar]
- 5.GELKOPF M, BLEICH A, HAYWARD R, BODNER G, ADELSON M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1 year prospective study in an Israeli clinic. Drug Alcohol Depend. 1999;55:63–68. doi: 10.1016/s0376-8716(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 6.BLEICH A, GELKOPF M, WEIZMAN T, ADELSON M. Benzodiazepine abuse in a methadone maintenance treatment clinic in Israel: characteristics and a pharmacotherapeutic approach. Isr J Psychiatry Relat Sci. 2002;39:104–112. [PubMed] [Google Scholar]
- 7.SPECKA M, BONNET U, HEILMANN M, SCHIFANO F, SCHERBAUM N. Longitudinal patterns of benzodiazepine consumption in a German cohort of methadone maintenance treatment patients. Hum Psychopharmacol. 2011;26:404–411. doi: 10.1002/hup.1222. [DOI] [PubMed] [Google Scholar]
- 8.CHEN KW, BERGER CC, FORDE DP, D’ADAMO C, WEINTRAUB E, GANDHI D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry. 2011;11:90. doi: 10.1186/1471-244X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CONDELLI WS, DUNTEMAN GH. Exposure to methadone programs and heroin use. Am J Drug Alcohol Abuse. 1993;19:65–78. doi: 10.3109/00952999309002666. [DOI] [PubMed] [Google Scholar]
- 10.MCCARTHY JE, SINEY C, SHAW NJ, RUBEN SM. Outcome predictors in pregnant opiate and polydrug users. Eur J Pediatr. 1999;158:748–749. doi: 10.1007/s004310051193. [DOI] [PubMed] [Google Scholar]
- 11.CAPLEHORN JR, DALTON MS, HALDAR F, PETRENAS AM, NISBET JG. Methadone maintenance and addicts’ risk of fatal heroin overdose. Subst Use Misuse. 1996;31:177–196. doi: 10.3109/10826089609045806. [DOI] [PubMed] [Google Scholar]
- 12.ERNST E, BARTU A, POPESCU A, ILEUTT KF, HANSSON R, PLUMLEY N. Methadone-related deaths in Western Australia 1993–99. Aust N Z J Public Health. 2002;26:364–370. doi: 10.1111/j.1467-842x.2002.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 13.CAPLEHORN JR, DRUMMER OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26:358–362. doi: 10.1111/j.1467-842x.2002.tb00185.x. discussion 362–353. [DOI] [PubMed] [Google Scholar]
- 14.BRANDS B, BLAKE J, MARSH DC, SPROULE B, JEYAPALAN R, LI S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis. 2008;27:37–48. doi: 10.1080/10550880802122620. [DOI] [PubMed] [Google Scholar]
- 15.EIROA-OROSA FJ, HAASEN C, VERTHEIN U, DILG C, SCHAFER I, REIMER J. Benzodiazepine use among patients in heroin-assisted vs. methadone maintenance treatment: findings of the German randomized controlled trial. Drug Alcohol Depend. 2010;112:226–233. doi: 10.1016/j.drugalcdep.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 16.SCHOCH P, MOHLER H. Purified benzodiazepine receptor retains modulation by GABA. Eur J Pharmacol. 1983;95:323–324. doi: 10.1016/0014-2999(83)90656-8. [DOI] [PubMed] [Google Scholar]
- 17.SCHOFIELD PR, DARLISON MG, FUJITA N, BURT DR, STEPHENSON FA, RODRIGUEZ H, et al. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- 18.WAFFORD KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Current opinion in pharmacology. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 19.ALLISON C, PRATT JA. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacology & therapeutics. 2003;98:171–195. doi: 10.1016/s0163-7258(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 20.XIANG K, TIETZ EI. Benzodiazepine-induced hippocampal CA1 neuron alpha-amino-3-hydroxy-5-methylisoxasole-4-propionic acid (AMPA) receptor plasticity linked to severity of withdrawal anxiety: differential role of voltage-gated calcium channels and N-methyl-D-aspartic acid receptors. Behavioural pharmacology. 2007;18:447–460. doi: 10.1097/FBP.0b013e3282d28f2b. [DOI] [PubMed] [Google Scholar]
- 21.SONG J, SHEN G, GREENFIELD LJ, JR, TIETZ EI. Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in Hippocampal CA1 neurons. J Pharmacol Exp Ther. 2007;322:569–581. doi: 10.1124/jpet.107.121798. [DOI] [PubMed] [Google Scholar]
- 22.HEBERLEIN A, BLEICH S, KORNHUBER J, HILLEMACHER T. Neuroendocrine pathways in benzodiazepine dependence: new targets for research and therapy. Hum Psychopharmacol. 2008;23:171–181. doi: 10.1002/hup.911. [DOI] [PubMed] [Google Scholar]
- 23.NARDI AE, FREIRE RC, VALENCA AM, AMREIN R, DE CERQUEIRA AC, LOPES FL, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30:290–293. doi: 10.1097/JCP.0b013e3181dcb2f3. [DOI] [PubMed] [Google Scholar]
- 24.DENIS C, FATSEAS M, LAVIE E, AURIACOMBE M. Pharmacological interventions for benzodiazepine mono-dependence management in outpatient settings. Cochrane Database Syst Rev. 2006;3:CD005194. doi: 10.1002/14651858.CD005194.pub2. [DOI] [PubMed] [Google Scholar]
- 25.CANTOPHER T, OLIVIERI S, CLEAVE N, EDWARDS JG. Chronic benzodiazepine dependence. A comparative study of abrupt withdrawal under propranolol cover versus gradual withdrawal. Br J Psychiatry. 1990;156:406–411. doi: 10.1192/bjp.156.3.406. [DOI] [PubMed] [Google Scholar]
- 26.HALLSTROM C, CROUCH G, ROBSON M, SHINE P. The treatment of tranquilizer dependence by propranolol. Postgraduate medical journal. 1988;64(Suppl 2):40–44. [PubMed] [Google Scholar]
- 27.LADER M, OLAJIDE D. A comparison of buspirone and placebo in relieving benzodiazepine withdrawal symptoms. J Clin Psychopharmacol. 1987;7:11–15. [PubMed] [Google Scholar]
- 28.SCHWEIZER E, CASE WG, GARCIA-ESPANA F, GREENBLATT DJ, RICKELS K. Progesterone co-administration in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal severity and taper outcome. Psychopharmacology (Berl) 1995;117:424–429. doi: 10.1007/BF02246214. [DOI] [PubMed] [Google Scholar]
- 29.LEMOINE P, TOUCHON J, BILLARDON M. Comparison of 6 different methods for lorazepam withdrawal. A controlled study, hydroxyzine versus placebo. L’Encephale. 1997;23:290–299. [PubMed] [Google Scholar]
- 30.SCHWEIZER E, RICKELS K, CASE WG, GREENBLATT DJ. Carbamazepine treatment in patients discontinuing long-term benzodiazepine therapy. Effects on withdrawal severity and outcome. Arch Gen Psychiatry. 1991;48:448–452. doi: 10.1001/archpsyc.1991.01810290060012. [DOI] [PubMed] [Google Scholar]
- 31.RICKELS K, SCHWEIZER E, GARCIA ESPANA F, CASE G, DEMARTINIS N, GREENBLATT D. Trazodone and valproate in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal symptoms and taper outcome. Psychopharmacology (Berl) 1999;141:1–5. doi: 10.1007/s002130050798. [DOI] [PubMed] [Google Scholar]
- 32.LEMOINE P, KERMADI I, GARCIA-ACOSTA S, GARAY RP, DIB M. Double-blind, comparative study of cyamemazine vs. bromazepam in the benzodiazepine withdrawal syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:131–137. doi: 10.1016/j.pnpbp.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 33.HOOD S, O’NEIL G, HULSE G. The role of flumazenil in the treatment of benzodiazepine dependence: physiological and psychological profiles. J Psychopharmacol. 2009;23:401–409. doi: 10.1177/0269881108100322. [DOI] [PubMed] [Google Scholar]
- 34.LUGOBONI F, FACCINI M, QUAGLIO GL, ALBIERO A, CASARI R, PAJUSCO B. Intravenous flumazenil infusion to treat benzodiazepine dependence should be performed in the inpatient clinical setting for high risk of seizure. J Psychopharmacol. 2011;25:848–849. doi: 10.1177/0269881110393050. [DOI] [PubMed] [Google Scholar]
- 35.WEIZMAN T, GELKOPF M, MELAMED Y, ADELSON M, BLEICH A. Treatment of benzodiazepine dependence in methadone maintenance treatment patients: a comparison of two therapeutic modalities and the role of psychiatric comorbidity. Aust N Z J Psychiatry. 2003;37:458–463. doi: 10.1046/j.1440-1614.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 36.STITZER M, BIGELOW G, LIEBSON I. Contingent reinforcement of benzodiazepine-free urines from methadone maintenance patients. NIDA Res Monogr. 1982;41:282–287. [PubMed] [Google Scholar]
- 37.STITZER ML, IGUCHI MY, FELCH LJ. Contingent take-home incentive: effects on drug use of methadone maintenance patients. J Consult Clin Psychol. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- 38.CHUTUAPE MA, SILVERMAN K, STITZER M. Contingent reinforcement sustains post-detoxification abstinence from multiple drugs: a preliminary study with methadone patients. Drug Alcohol Depend. 1999;54:69–81. doi: 10.1016/s0376-8716(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 39.MCDUFF DR, SCHWARTZ RP, TOMMASELLO A, TIEGEL S, DONOVAN T, JOHNSON JL. Outpatient benzodiazepine detoxification procedure for methadone patients. J Subst Abuse Treat. 1993;10:297–302. doi: 10.1016/0740-5472(93)90078-g. [DOI] [PubMed] [Google Scholar]
- 40.TAYLOR CP, GEE NS, SU TZ, KOCSIS JD, WELTY DF, BROWN JP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 41.MANEUF YP, GONZALEZ MI, SUTTON KS, CHUNG FZ, PINNOCK RD, LEE K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;60:742–750. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ERRANTE LD, WILLIAMSON A, SPENCER DD, PETROFF OA. Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Res. 2002;49:203–210. doi: 10.1016/s0920-1211(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 43.PETROFF OA, ROTHMAN DL, BEHAR KL, LAMOUREUX D, MATTSON RH. The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Annals of neurology. 1996;39:95–99. doi: 10.1002/ana.410390114. [DOI] [PubMed] [Google Scholar]
- 44.GEE NS, BROWN JP, DISSANAYAKE VU, OFFORD J, THURLOW R, WOODRUFF GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. The Journal of biological chemistry. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 45.WANG M, OFFORD J, OXENDER DL, SU TZ. Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. The Biochemical journal. 1999;342(Pt 2):313–320. [PMC free article] [PubMed] [Google Scholar]
- 46.MARAIS E, KLUGBAUER N, HOFMANN F. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- 47.STEFANI A, SPADONI F, BERNARDI G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 48.ALDEN KJ, GARCIA J. Differential effect of gabapentin on neuronal and muscle calcium currents. J Pharmacol Exp Ther. 2001;297:727–735. [PubMed] [Google Scholar]
- 49.SUTTON KG, MARTIN DJ, PINNOCK RD, LEE K, SCOTT RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SILLS GJ. The mechanisms of action of gabapentin and pregabalin. Current opinion in pharmacology. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 51.CHENG JK, CHIOU LC. Mechanisms of the antinociceptive action of gabapentin. Journal of pharmacological sciences. 2006;100:471–486. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- 52.BEYDOUN A, UTHMAN BM, SACKELLARES JC. Gabapentin: pharmacokinetics, efficacy, and safety. Clin Neuropharmacol. 1995;18:469–481. [PubMed] [Google Scholar]
- 53.WANG PW, KETTER TA. Pharmacokinetics of mood stabilizers and new anticonvulsants. Psychopharmacology Bulletin. 2002;36:44–66. [PubMed] [Google Scholar]
- 54.BEYDOUN A, FAKHOURY T, NASREDDINE W, ABOU-KHALIL B. Conversion to high dose gabapentin monotherapy in patients with medically refractory partial epilepsy. Epilepsia. 1998;39:188–193. doi: 10.1111/j.1528-1157.1998.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 55.MCLEAN MJ, MORRELL MJ, WILLMORE LJ, PRIVITERA MD, FAUGHT RE, HOLMES GL, et al. Safety and tolerability of gabapentin as adjunctive therapy in a large, multicenter study. Epilepsia. 1999;40:965–972. doi: 10.1111/j.1528-1157.1999.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 56.BERGEY GK, MORRIS HH, ROSENFELD W, BLUME WT, PENOVICH PE, MORRELL MJ, et al. Gabapentin monotherapy: I. An 8-day, double-blind, dose-controlled, multicenter study in hospitalized patients with refractory complex partial or secondarily generalized seizures. The US Gabapentin Study Group 88/89. Neurology. 1997;49:739–745. doi: 10.1212/wnl.49.3.739. [DOI] [PubMed] [Google Scholar]
- 57.LEACH JP, GIRVAN J, PAUL A, BRODIE MJ. Gabapentin and cognition: a double blind, dose ranging, placebo controlled study in refractory epilepsy. J Neurol Neurosurg Psychiatry. 1997;62:372–376. doi: 10.1136/jnnp.62.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DODRILL CB, ARNETT JL, HAYES AG, GAROFALO EA, GREELEY CA, GREINER MJ, et al. Cognitive abilities and adjustment with gabapentin: results of a multisite study. Epilepsy Res. 1999;35:109–121. doi: 10.1016/s0920-1211(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 59.MEADOR KJ, LORING DW, RAY PG, MURRO AM, KING DW, NICHOLS ME, et al. Differential cognitive effects of carbamazepine and gabapentin. Epilepsia. 1999;40:1279–1285. doi: 10.1111/j.1528-1157.1999.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 60.SALINSKY MC, BINDER LM, OKEN BS, STORZBACH D, ARON CR, DODRILL CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002;43:482–490. doi: 10.1046/j.1528-1157.2002.22501.x. [DOI] [PubMed] [Google Scholar]
- 61.PANDE AC, DAVIDSON JR, JEFFERSON JW, JANNEY CA, KATZELNICK DJ, WEISLER RH, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19:341–348. doi: 10.1097/00004714-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 62.PANDE AC, POLLACK MH, CROCKATT J, GREINER M, CHOUINARD G, LYDIARD RB, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol. 2000;20:467–471. doi: 10.1097/00004714-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 63.KARAM-HAGE M, BROWER KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry. 2000;157:151. doi: 10.1176/ajp.157.1.151. [DOI] [PubMed] [Google Scholar]
- 64.BROWER KJ, MYRA KIM H, STROBBE S, KARAM-HAGE MA, CONSENS F, ZUCKER RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32:1429–1438. doi: 10.1111/j.1530-0277.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ANTON RF, MYRICK H, WRIGHT TM, LATHAM PK, BAROS AM, WAID LR, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168:709–717. doi: 10.1176/appi.ajp.2011.10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MASON BJ, QUELLO S, GOODELL V, SHADAN F, KYLE M, BEGOVIC A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA internal medicine. 2014;174:70–77. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FURIERI FA, NAKAMURA-PALACIOS EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691–1700. doi: 10.4088/jcp.v68n1108. [DOI] [PubMed] [Google Scholar]
- 68.MASON BJ, CREAN R, GOODELL V, LIGHT JM, QUELLO S, SHADAN F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.CARROLL KMOMS, NURO K. Yale University Psychotherapy Development Center Training Series No 4. West Haven, CT: 1999. Compliance Enhancement: A Manual for the Psychopharmacology of Drug Abuse and Dependence. [Google Scholar]
- 70.BUSTO UE, SYKORA K, SELLERS EM. A clinical scale to assess benzodiazepine withdrawal. Journal of Clinical Psychopharmacology. 1989;9:412–416. [PubMed] [Google Scholar]