Abstract
Purpose/Objective(s)
We examined practice patterns using the National Cancer Database (NCDB) to determine risk factors for prolonged diagnosis to treatment interval (DTI) and survival outcomes in patients receiving chemoradiation for oropharyngeal squamous cell carcinoma (OPSCC).
Methods and Materials
We identified 6,606 NCDB patients with Stage III-IV OPSCC receiving chemoradiation from 2003-2006. We determined risk factors for prolonged DTI (>30 days) using univariate and multivariable logistic regression models. We examined overall survival (OS) using Kaplan Meier and multivariable Cox proportional hazards models.
Results
3,586 (54.3%) patients had prolonged DTI. Race, IMRT, insurance status, and high volume facilities were significant risk factors for prolonged DTI. Patients with prolonged DTI had inferior OS compared to DTI ≤ 30 days (Hazard Ratio (HR)=1.12, 95% CI 1.04-1.20, p=0.005). For every week increase in DTI there was a 2.2% (95% CI 1.1%-3.3%, p<0.001) increase in risk of death. Patients receiving IMRT, treatment at academic, or high-volume facilities were more likely to experience prolonged DTI (High vs. Low volume: 61.5% vs. 51.8%, adjusted OR 1.38, 95% CI 1.21-1.58; Academic vs. Community: 59.5% vs. 50.6%, adjusted OR 1.26, 95% CI 1.13-1.42; non-IMRT vs. IMRT: 53.4% vs. 56.5%; adjusted OR 1.17, 95% CI 1.04-1.31).
Conclusions
Our results suggest that prolonged DTI has a significant impact on survival outcomes. We observed disparities in DTI by socioeconomic factors. However, facility level factors such as academic affiliation, high volume, and IMRT also increased risk of DTI. These findings should be considered in developing efficient pathways to mitigate adverse effects of prolonged DTI.
Keywords: oropharyngeal cancer, national cancer database, treatment delays
INTRODUCTION
Delays in cancer treatment not only adversely affect patient reported satisfaction but may also lead to disease progression and inferior oncologic outcomes.1–5 Treatment of head and neck squamous cell cancer (HNSCC) requires complex multidisciplinary care, involving providers from a wide range of clinical specialties, and timely care delivery may be particularly challenging for both providers and patients.
There is moderate evidence suggesting that a delay between surgery and post-operative radiotherapy for HNSCC has a detrimental effect on disease outcomes. A subset analysis from a large, multi-institutional randomized control trial conducted in the United States found a decrease in locoregional control for HNSCC patients associated with delay from the time of surgery to completion of post-operative radiation of >11 weeks.6 Additionally, a prior systematic review on the effect of delays in the treatment of HNSCC suggested that patients with a 6 week or greater interval between surgery and postoperative radiation had significantly worse local tumor control rates.1 However, prior analyses of treatment delays in patients receiving definitive chemoradiation have not shown a consistent correlation between treatment delay and local control, metastatic disease, or overall survival.7–9 Furthermore, it is unclear whether treatment delays have a detrimental effect for patients with OPSCC, the majority of whom have HPV-associated tumors.10 For example, it has been hypothesized that the significance of progression prior to treatment may decrease as the incidence of HPV positive tumors increases.9 A Dutch study including a significant number of OPSCC patients also calls into question the significance of delays to treatment initiation for these patients.11
Delays in the initiation of cancer therapy may result from a variety of patient and provider related factors. The treatment of head and neck cancer involves multifaceted care prior to treatment start – including surgical evaluation, dental procedures, nutritional assessments, evaluation for chemotherapy, and complex radiation treatment planning – all of which may prolong the time before the start of treatment. Initiation of treatment may also be delayed through patient non-compliance or barriers related to socioeconomic status.12,13 Potential causative factors at the health systems level may be modifiable with implementation of care pathways, facilitation of care coordination through electronic medical records, and appropriate management of the supply of healthcare resources.
Delays in cancer care, by their very nature, are not amenable to study through prospective randomized trials. Patient and provider level factors associated with treatment delays in this setting are not well understood. Furthermore, prior studies of such delays have examined the outcomes of relatively small numbers of patients treated at academic medical centers. We therefore undertook this study to better understand risk factors for prolonged diagnosis to treatment interval (DTI) at the national level and the associated impact on survival outcomes in patients diagnosed with locally advanced oropharyngeal squamous cell cancer (OPSCC) receiving definitive chemoradiation in a large national cancer registry.
METHODS AND MATERIALS
Data Source
We conducted a retrospective, observational cohort study using the National Cancer Data Base (NCDB). The NCDB is a large national oncology registry sponsored by the American College of Surgeons and the American Cancer Society encompassing over 1,500 Commission on Cancer (CoC) institutions. Approximately 70 percent of new nationwide cancer diagnoses are recorded in this database.14
Cohort
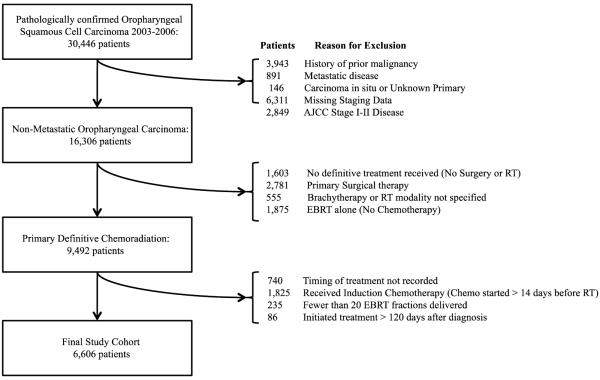
The definition of the study cohort is illustrated in Figure 1. We identified 6,606 patients with non-metastatic, Stage III or IV, biopsy-confirmed OPSCC receiving definitive chemoradiation from January 1, 2003 to December 31, 2006 in the NCDB. We selected patients receiving definitive chemoradiation as initial treatment because prior studies of treatment delays have more often focused on patients receiving primary surgery where delays may be largely influenced by post-operative complication events. Furthermore, the use of definitive chemoradiation for OPSCC has increased over time.15 The study period was selected to allow for risk adjusted survival analysis with inclusion of comorbidity data. Patients with OPSCC were eliminated from the cohort if they received primary surgical therapy (n=2,781), radiation alone without chemotherapy (n=1,875), chemotherapy more than 14 days before radiation (n= 1,825), fewer than 20 external beam radiation treatment (EBRT) fractions (n=235), no definitive treatment (n=1,603), or if treatment was initiated more than 120 days after diagnosis (n=86).
Figure 1.
Definition of the Study Cohort
Exposure
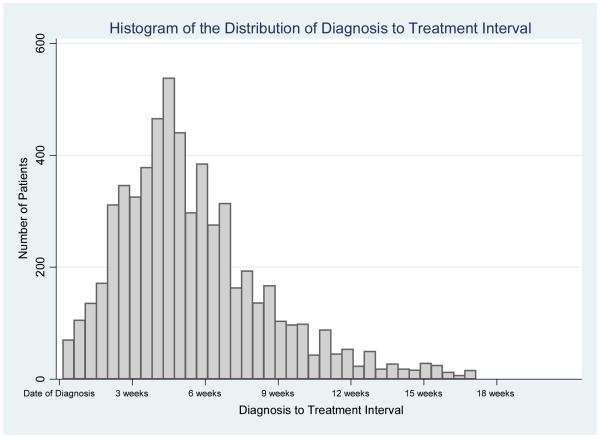
We defined a diagnosis to treatment interval (DTI) for each patient as the time in days between biopsy and initiation of treatment for each patient. In cases where patients did not start chemotherapy and radiation therapy on the same day, we defined the DTI based on whichever treatment was started first. For the purposes of categorical comparisons, we considered a DTI >30 days to be prolonged. A DTI of 30 days was deemed to be clinically relevant and approximated our median DTI within the patient cohort of 32 days (see Figure 2). In addition, the influence of delay on survival was also examined as both a continuous variable (measured in weeks) and in approximate tertiles of DTI ≤ 3 weeks, DTI 3-6 weeks, and DTI > 6 weeks to assess the incremental effects of treatment delay on survival outcomes.
Figure 2.
Histogram of the Distribution of Diagnosis to Treatment Interval
Primary Study Outcome
The primary study outcome of interest was the impact of prolonged DTI on patient overall survival (OS). Overall survival was defined as the time of biopsy until the date of death or last follow up.
Secondary Endpoints
In addition, we examined patient, clinical, and demographic factors associated with prolonged DTI in order to define risk factors for prolonged DTI.
Independent Variables
Patient characteristics included age at diagnosis, gender, and race. Clinical characteristics included primary tumor site, clinical T stage, clinical N stage, and comorbidity index (Charlson/Deyo score). We categorized radiation technique as intensity modulated radiation treatment (IMRT) or non-IMRT. Treatment facilities were classified as community versus academic and those with low versus high case-volume. Facility classification is based on CoC designation of community versus academic.16 Facility case-volume was divided by quartiles of study patients per facility, and facilities in the upper quartile were considered high volume. Demographic characteristics included year of diagnosis, geographic region, distance from treatment center, and insurance status.
Statistical Analysis
We investigated risk factors for prolonged DTI (>30 days) using univariate and multivariable logistic regression models. We examined the impact of prolonged DTI on patient overall survival (OS) using unadjusted Kaplan Meier (KM) analyses and log-rank statistics. In multivariable Cox proportional hazards models, we estimated the impact of DTI on patient overall survival. DTI was classified categorically as ≤ 30 days vs. > 30 days for the primary analysis. The multivariable analysis was then repeated with DTI included as a continuous variable (in weeks) and in approximate tertiles of DTI (≤ 3 weeks, DTI 3-6 weeks, and DTI > 6 weeks) in order to assess the incremental effect of delay on patient OS. Variables included in the final multivariable models were selected using a combination of purposeful and backwards stepwise selection with p-value cut off of 0.10. In unplanned subset analyses, we compared survival outcomes by radiation technique and facility treatment volume, to assess the impact of DTI in these patient subsets. We also conducted a sensitivity analysis to examine the potential influence of an unmeasured confounding variable such as HPV status on our observed results. HPV associated OPSCC has an improved prognosis compared to non-HPV associated OPSCC.17 African Americans diagnosed with OPSCC are less likely to have HPV associated tumors than Caucasian patients with OPSCC.18 Since non-Hispanic Black race was associated with increased risk of delay in our study, we repeated the multivariable Cox proportional hazards models in the subset of patients with non-Hispanic white race. All statistical analyses were completed using Stata, version 12.1 (StataCorp LP, College Station, TX). Associations were noted as statistically significant at p <0.05. All tests were two-tailed.
RESULTS
Characteristics of the Study Population
Characteristics of the study population are displayed in Table 1. The majority of patients were male (82.2%), of non-Hispanic White race (83.3%), and under the age of 65 (76.5%). Most patients received treatment at community treatment facilities (58.5%), and relatively few (28%) received intensity modulated radiation therapy (IMRT). The majority of patients had commercial health insurance at the time of treatment (53.1%), and overall 90.1% of patients were known to have had either health insurance or government provided health benefits.
Table 1.
Patient Characteristics by Diagnosis to Treatment Interval (DTI): OPSCC Receiving Primary Chemoradiotherapy 2003-2006
| Patient Characteristic | Total n=6,606 |
DTI <= 30 Days n=3,020 |
DTI > 30 Days n=3,586 |
p-value |
|---|---|---|---|---|
| Age at Diagnosis (years) | ||||
|
| ||||
| < 65 | 76.5% | 76.8% | 76.3% | 0.64 |
| ≥ 65 | 23.5% | 23.2% | 23.7% | |
| Mean Age (SD) | 57.6 (9.9) | 57.5 (9.8) | 57.7 (9.9) | 0.41 |
|
| ||||
| Gender | ||||
|
| ||||
| Male | 82.2% | 82.2% | 82.3% | 0.93 |
| Female | 17.8% | 17.8% | 17.7% | |
|
| ||||
| Race | ||||
|
| ||||
| Non-Hispanic White | 83.3% | 85.9% | 81.0% | <0.001 |
| Non- Hispanic Black | 11.1% | 9.4% | 12.5% | |
| Hispanic | 3.4% | 2.4% | 4.3% | |
| Other/Unknown | 2.3% | 2.4% | 2.1% | |
|
| ||||
| Primary Tumor Site | ||||
|
| ||||
| Base of Tongue | 46.6% | 47.6% | 45.8% | 0.22 |
| Tonsil | 43.5% | 42.4% | 44.3% | |
| Pharyngeal Wall/Soft Palate |
3.9% | 3.6% | 4.1% | |
| Oropharynx NOS | 6.1% | 6.4% | 5.8% | |
|
| ||||
| Clinical T-Stage | ||||
|
| ||||
| T1 | 16.3% | 18.1% | 14.7% | 0.003 |
| T2 | 32.2% | 31.2% | 33.1% | |
| T3 | 25.0% | 24.8% | 25.2% | |
| T4 | 24.5% | 23.7% | 25.1% | |
| TX | 2.0% | 2.1% | 2.0% | |
|
| ||||
| Clinical N-Stage | ||||
|
| ||||
| N0 | 9.6% | 9.3% | 9.9% | 0.118 |
| N1 | 22.8% | 24.0% | 21.8% | |
| N2 | 57.9% | 56.8% | 58.8% | |
| N3 | 8.6% | 8.6% | 8.5% | |
| NX | 1.2% | 1.4% | 1.0% | |
|
| ||||
| AJCC Clinical Stage Group |
||||
|
| ||||
| III | 26.4% | 27.4% | 25.4% | 0.097 |
| IV | 73.6% | 72.7% | 74.5% | |
|
| ||||
| Comorbidity Index | ||||
|
| ||||
| None | 87.5% | 87.3% | 87.6% | 0.33 |
| Moderate (1) | 10.7% | 11.1% | 10.3% | |
| High (2) | 1.9% | 1.7% | 2.0% | |
|
| ||||
| Year of Diagnosis | ||||
|
| ||||
| 2003 | 20.4% | 20.7% | 20.1% | 0.53 |
| 2004 | 23.0% | 23.6% | 22.5% | |
| 2005 | 26.7% | 26.2% | 27.1% | |
| 2006 | 29.9% | 29.4% | 30.4% | |
|
| ||||
| Radiation Technique | ||||
|
| ||||
| IMRT | 28.0% | 26.7% | 29.2% | 0.02 |
| Non-IMRT | 72.0% | 73.3% | 70.8% | |
|
| ||||
| Treatment Facility | ||||
|
| ||||
| Academic | 41.6% | 36.8% | 45.5% | <0.001 |
| Community | 58.5% | 63.2% | 54.5% | |
|
| ||||
| Facility Case-Volume | ||||
|
| ||||
| Low Case-Volume | 74.2% | 78.3% | 70.8% | <0.001 |
| High Case-Volume | 25.8% | 21.7% | 29.2% | |
|
| ||||
| Geographic Region | ||||
|
| ||||
| Northeast | 20.6% | 19.4% | 21.7% | <0.001 |
| Southeast | 28.2% | 27.4% | 28.9% | |
| Midwest | 37.3% | 40.3% | 34.9% | |
| West | 13.8% | 12.9% | 14.6% | |
|
| ||||
| Patient Distance from Treatment Center (Miles) | ||||
|
| ||||
| <25 | 72.7% | 73.1% | 72.3% | 0.15 |
| 25-100 | 18.8% | 18.3% | 19.2% | |
| >100 | 3.9% | 3.5% | 4.2% | |
| Unknown | 4.7% | 5.1% | 4.3% | |
|
| ||||
| Insurance Status | ||||
|
| ||||
| Commercial | 53.1% | 57.5% | 49.4% | <0.001 |
| Insurance | ||||
| Medicare | 24.2% | 23.8% | 24.6% | |
| Medicaid | 10.6% | 8.7% | 12.2% | |
| Government | 2.2% | 1.9% | 2.4% | |
| Uninsured | 7.5% | 5.7% | 9.0% | |
| Unknown | 2.4% | 2.3% | 2.4% | |
Risk Factors for Prolonged DTI
Overall, 54.3% of patients (n=3,586) had a prolonged DTI >30 days. Patients of non-Hispanic Black race, or Hispanic ethnicity were significantly more likely to experience a prolonged DTI compared to non-Hispanic White patients [Non-Hispanic Black vs. Non-Hispanic White: 61.2% vs. 52.8%, adjusted odds ratio (OR) 1.24, 95% Confidence Interval (CI) 1.04-1.46; Hispanic vs. Non-Hispanic White: 69.5% vs. 52.8%, adjusted OR 1.74, 95% CI 1.29-2.34]. Patients with Medicaid coverage or uninsured status were significantly more likely to have a prolonged DTI when compared to patients with commercial insurance (Medicaid vs. Commercial: 62.5% vs. 50.5%, adjusted OR 1.58, 95% CI 1.32-1.88; Uninsured vs. Commercial: 65.1% vs. 50.5%, adjusted OR 1.90, 95% CI 1.55-2.33). In addition, patients receiving treatment at academic centers or high case volume facilities were more likely to have prolonged DTI compared to patients receiving treatment at community centers or low case volume facilities (High volume vs. Low volume: 61.5% vs. 51.8%, adjusted OR 1.38, 95% CI 1.21-1.58; Academic vs. Community: 59.5% vs. 50.6%, adjusted OR 1.26, 95% CI 1.13-1.42). Patients receiving treatment with IMRT were also more likely to experience delays in treatment (non-IMRT vs. IMRT: 53.4% vs. 56.5%; adjusted OR 1.17, 95% CI 1.04-1.31).
Survival Analysis
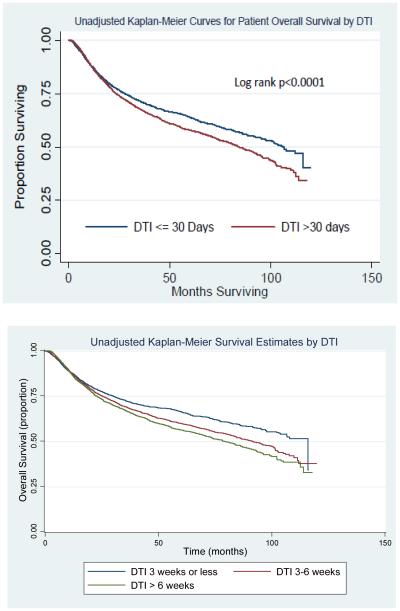
Unadjusted Kaplan Meier survival curves for patients classified by DTI are shown in Figure 3. The results of our multivariable Cox proportional hazards model are displayed in Table 3. In the multivariable model, a prolonged DTI > 30 days was associated with significantly worse overall survival (HR 1.12, 95% CI 1.03-1.20, p=0.005). In a separate model that measured DTI continuously in weeks, there was a 2.2% (95% CI 1.1%-3.3%, p<0.001) increase in the risk of death due to any cause for every 1 week increase in the DTI. When DTI was classified into tertiles, there was a progressive rise in the risk of death within each cohort of increasing delays (DTI 3-6 weeks: HR 1.15, 95% CI 1.05-1.27, p=0.003); DTI > 6 weeks: HR 1.22, 95% CI 1.10-1.35, p<0.001). Additional factors significantly associated with patient survival included treatment at high case volume facilities and receipt of IMRT (High Volume vs. Low Volume: HR 0.84, 95% CI 0.76-0.93, p=0.001; IMRT vs. Non-IMRT: HR 0.75, 95% CI 0.68-0.82, p<0.0001; respectively).
Figure 3.
Unadjusted Kaplan Meier Curves for Patient Overall Survival
Table 3.
Cox Proportional Hazards Model for Patient Overall Survival
| Patient Characteristic | Adjusted HR (95% CI) |
p value |
|---|---|---|
|
| ||
| Age at Diagnosis Race |
1.023 (1.02-1.03) | <0.001 |
|
| ||
| Non-Hispanic White | - | - |
| Non-Hispanic Black | 1.55(1.39-1.72) | <0.001 |
| Hispanic | 0.87 (0.70-1.09) | 0.23 |
| Other | 0.88 (0.66-1.17) | 0.37 |
|
| ||
| Primary Tumor Site | ||
|
| ||
| Base of Tongue | - | - |
| Tonsil | 1.00 (0.93-1.09) | 0.92 |
| Pharyngeal Wall/ Soft Palate | 1.70 (1.44-2.01) | <0.001 |
| Oropharynx NOS | 1.48 (1.29-1.70) | <0.001 |
|
| ||
| Clinical Stage | ||
|
| ||
| III | - | - |
| IV | 1.37 (1.26-1.50) | <0.001 |
|
| ||
| Comorbidity Index | ||
|
| ||
| None | - | - |
| Moderate (1) | 1.49 (1.34-1.66) | <0.001 |
| High (2) | 1.47 (1.17-1.84) | 0.001 |
|
| ||
| Year of Diagnosis | ||
|
| ||
| 2003 | - | - |
| 2004 | 0.96 (0.86-1.07) | 0.41 |
| 2005 | 1.04 (0.93-1.15) | 0.51 |
| 2006 | 1.01 (0.90-1.13) | 0.89 |
|
| ||
| Radiation Technique | ||
|
| ||
| Non-IMRT | - | - |
| IMRT | 0.75 (0.68-0.82) | <0.001 |
|
| ||
| Treatment Facility | ||
|
| ||
| Community | - | - |
| Academic | 1.01 (0.93-1.10) | 0.82 |
|
| ||
| Facility Case-Volume | ||
|
| ||
| Low Case-Volume | - | - |
| High Case-Volume | 0.84 (0.76-0.93) | 0.001 |
|
| ||
| Geographic Region | ||
|
| ||
| Northeast | - | - |
| Southeast | 0.99 (0.89-1.11) | 0.91 |
| Midwest | 1.00 (0.90-1.11) | 0.99 |
| West | 0.87 (0.76-0.99) | 0.04 |
|
| ||
| Insurance Status | ||
|
| ||
| Commercial Insurance | - | - |
| Medicare | 1.74 (1.56-1.94) | <0.001 |
| Medicaid | 2.10 (1.86-2.37) | <0.001 |
| Uninsured | 2.05 (1.79-2.36) | <0.001 |
| Government | 1.69 (1.32-2.16) | <0.001 |
| Unknown | 1.80 (1.42-2.27) | <0.001 |
|
| ||
| Diagnosis to Treatment Interval | ||
|
| ||
| DTI ≤ 30 days | - | - |
| DTI > 30 days | 1.12 (1.03-1.20) | 0.005 |
| DTI (measured in weeks) | 1.02 (1.01-1.03) | <0.001 |
| DTI in Tertiles | ||
| DTI ≤ 3 weeks | - | - |
| DTI 3-6 weeks | 1.15 (1.05-1.27) | 0.003 |
| DTI > 6 weeks | 1.22 (1.10-1.35) | <0.001 |
Subset Analyses
In the subset of patients receiving IMRT treatment (n=1,850), delay in initiation of treatment (measured continuously in weeks) significantly increased the risk of death due to any cause (HR 1.032, 95% CI 1.01-1.06, p=0.009). This indicates a 3.2% increase in the risk of death due to any cause for every 1 week increase in DTI.
Among the subset of patients receiving treatment at high case-volume facilities (n=1,704), delay in initiation of treatment (measured continuously in weeks) significantly increased the risk of death due to any cause (HR of 1.046, 95% CI 1.02-1.07; p<0.0001). This indicates a 4.6% increase in the risk of death due to any cause for every 1 week increase in DTI.
We also conducted a subset analysis in the non-Hispanic white population to assess the consistency of observed results. HPV positive tumors are known to have better overall survival outcomes than HPV negative tumors.17 Data regarding HPV status was not available in the data source for these patients and so HPV is a potential unmeasured confounding variable. Recent data from the CDC indicates that patients of black race are less likely to have HPV positive OPSCC (50.7% of black patients vs. 73.6% of white patients).18 We conducted the subset analysis among non-Hispanic white patients with the rationale that HPV status should be less variable within this subgroup. In this subset analysis we continued to find a significant increase in the risk of death due to prolonged DTI in the non-Hispanic white population alone (DTI > 30 days: HR 1.15, 95% CI 1.05-1.25, p=0.002; DTI measured continuously in weeks: HR 1.028, 95% CI 1.02-1.04, p< 0.001).
DISCUSSION
The objective of our study was to identify population level risk factors and national trends for prolonged delays in receipt of treatment among head and neck cancer patients, and to examine the effects of treatment delay on patient long-term survival outcomes. We observed significantly worse overall survival among patients experiencing a delay in the initiation of treatment greater than 30 days. For every week in treatment delay, there was a 2.2% increase in the risk of death within our study cohort. Furthermore, receipt of IMRT as well as treatment at academic or high volume medical centers was associated with a significantly increased risk of prolonged DTI. This finding suggests that the requisites of care coordination, particularly at high volume and academic centers with more specialized cancer care, in the setting of treatment with novel and complex radiation technologies such as IMRT, may inadvertently be associated with delays in care. We also observed significant disparities in DTI by race and insurance status, consistent with prior studies demonstrating healthcare disparities in these patient groups.12,13
Our results are consistent with and extend the findings of a number of other studies regarding the impact of treatment delays in patients with HNSCC. The importance of “package time” in the treatment of HNSCC has been suggested in prior studies. A subset analysis of a multi-institutional randomized controlled trial reported that an interval between surgery and completion of postoperative radiation therapy of more than 11 weeks had a negative effect on both local control and survival. Five year overall survival for patients completing treatment in < 11 weeks was 48% versus 27% for 11-13 weeks, and 27% for package times > 13 weeks (p = 0.03). completion6 A number of single institution retrospective series have also established the importance of reducing delays in treatment for patients with HNSCC.4,5,19 A series from the University of Pennsylvania demonstrated that a total treatment package time >100 days from surgery to RT completion was associated with both decreased local control and overall survival in patients with locally advanced HNSCC (p = 0.013 and p = 0.021, respectively).5 A reduction of overall radiation treatment time to less than 6 weeks has also been shown to provide an overall survival benefit in oral cavity patients, from 50% for RT > 8 weeks in duration to 74% when reduced to < 6 weeks.19
Delays in time to radiation treatment have been of particular interest in publicly funded health care systems where wait times for RT have been a significant concern.20,21 Feasible models have been implemented in Denmark to reduce waiting time from diagnosis of HNSCC to treatment with “package solutions” comprised of pre-booked appointments for diagnostic procedures. With this fast track pathway, they have demonstrated a decrease in time from referral to diagnosis from 24 to 7-10 days.22
A number of driving factors have been studied to address the causal pathway between delays in treatment and worse survival outcomes. Waaijer and colleagues demonstrated that for patients with OPSCC in the Netherlands there was a mean increase in tumor volume of 70% between the time of diagnostic CT and simulation CT while patients waited for RT initiation (mean waiting time 56 days).23 Another study found that delays in treatment resulted in larger radiation treatment volumes by radiologic response evaluation criteria, and even increased the risk of regional lymph node disease.24 A prior systematic review of the literature on the effect of waiting time for RT found a relative risk of local recurrence of 1.15 per month (95% CI 1.02-1.29) for patients receiving definitive radiation in the treatment of head and neck cancer.2 Huang et al. similarly found a local control detriment for head and neck cancer patients on their review of the literature with receipt of postoperative radiation more than 6 weeks after surgery (OR 2.98; CI 1.70-5.21).1 In addition to disease progression during delays, socioeconomic status and access to healthcare may also contribute to clinical outcomes. In a study of Medicaid patients treated for head and neck cancer, even while controlling for tumor stage and site, black patients were less likely to be treated with surgery and had worse overall survival.13 In our study, we find that both race and Medicaid or uninsured status were significantly related to prolonged DTI. These findings suggest that in addition to disease progression that may occur during delays to definitive treatment, patients with increased DTI are more likely to have poor access to healthcare that may lead to worse treatment tolerance and overall clinical outcomes.
In addition to improved outcomes with time from diagnosis to treatment less than 30 days, we also found that treatment at high case-volume centers and receipt of IMRT predicted for improved overall survival outcomes. This survival benefit seen in our study with the use of IMRT and treatment at high volume facilities is consistent with previously published data.25–27 A recent study of RT technique for HNSCC using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database demonstrated an increase in cause-specific survival with the use of IMRT (84.1%) versus non-IMRT technique (66%, p <0.001).25 A study of patients with laryngeal cancer using the NCDB found lowest HR for death at 90 days, 1 year, and 4 years for patients treated at high volume facilities.27 Another recent analysis of patients treated on Radiation Therapy Oncology Group protocol 0129 found that treatment at historically low-accruing centers was associated with worse overall survival compared to historically high-accruing centers (OS at 5 years: 51.0% vs. 59.1%, p=0.002; HR 1.91, 95% CI, 1.37-2.65).28 However, subset analyses in our study among patients receiving IMRT or treatment at high volume facilities continued to show a significant association of delays in treatment with inferior survival outcomes. Thus, the benefits of IMRT or provider experience may be attenuated by treatment delays in this setting since receipt of IMRT or treatment at high volume facilities were significant risk factors for treatment delay. Our results suggest that high-tech treatment and associated complex requirements of care coordination, especially at high volume or academic centers, may exacerbate delays in time from diagnosis to treatment.
Limitations of this study include its retrospective nature, and the inherent potential for biases associated with such studies. However, we used univariate and multivariable logistic regression models as well as multivariable Cox proportional hazards models to adjust for differences between the comparison groups. Furthermore, we were limited in the scope of our study by the data available in the NCDB. For instance, we were unable to further examine causes of delayed treatment, such as dental procedures, enteric feeding tube placement, performance status, patient compliance, or hospitalization events. Additionally, the treatment years for our study, 2003-2006, encompassed a period of early IMRT adoption, which may have led to increased delay to initiation of treatment due to more complex treatment planning. The NCDB did not capture HPV status during the time period associated with this study, and so there is potential for unmeasured confounding. To address this, we conducted a subset analysis among non-Hispanic white patients, since HPV may be differentially prevalent among racial groups, and found that the associations of delay on survival remained consistent. However, smoking prevalence is generally higher among socially disadvantaged groups, and thus patients with delayed treatment driven by socioeconomic factors may have had higher rates of tobacco use and/or higher rates of HPV negative disease. Although our multivariable analysis adjusts for many other surrogates of socioeconimc status, there is potential for residual unmeasured confounding. Finally, while our study provides a snapshot of practice patterns across the nation with 70% of cancer diagnoses represented, only data from CoC accredited institutions is collected for this database, which may have more cancer-centered care available to patients than non-COC institutions.29
In conclusion, our study identifies risk factors for treatment delays in a large cohort of patients receiving chemoradiation for OPSCC in the United States. In addition to factors commonly associated with poor access to the healthcare such as minority race and insurance status, treatment delays were more common in patients receiving IMRT and treatment at high case-volume facilities. We found that increases in DTI beyond 30 days were associated with significantly worse patient overall survival. Patients receiving IMRT or treatment at high case-volume facilities had improvements in survival outcomes. However, these benefits were attenuated by prolonged DTI within these patient subsets suggesting that the adoption of more complex radiation technologies and treatment at specialized centers may increase DTI due to associated requisites of care coordination. Additionally, the issue of package time is particularly important to address in OPSCC due to the growing incidence of this cancer in the HPV era coupled with increased use of IMRT for primary treatment. Future research should evaluate implementation of more efficient care pathways to potentially mitigate the adverse effects of prolonged DTI in this setting while taking into account the increasing requisites of care coordination associated with the adoption of complex treatment technologies.
Supplementary Material
Table 2.
Risk of Prolonged Diagnosis to Treatment Interval (DTI): Patients with OPSCC Receiving Primary Chemoradiotherapy 2003-2006
| Patient Characteristic |
% DTI > 30
Days n=6,606 |
Unadjusted OR
(95% CI) |
Adjusted OR
(95% CI) |
|---|---|---|---|
|
| |||
| Age at Diagnosis (years) | |||
|
| |||
| < 65 | 54.1% | - | - |
| ≥ 65 | 54.8% | 1.03 (0.92-1.15) | 0.99 (0.84-1.18) |
|
| |||
| Gender | |||
|
| |||
| Male | 54.3% | - | - |
| Female | 54.2% | 0.99 (0.88-1.13) | 0.97(0.85-1.11) |
|
| |||
| Race | |||
|
| |||
| Non-Hispanic White | 52.8% | - | - |
| Non- Hispanic Black | 61.2% | 1.41(1.20-1.65)** | 1.24 (1.04-1.46)* |
| Hispanic | 69.5% | 2.03 (1.52-2.72)** | 1.74 (1.29-2.34)** |
| Other/Unknown | 51.0% | 0.93 (0.67-1.29) | 0.84 (0.60-1.17) |
|
| |||
| Primary Tumor Site | |||
|
| |||
| Base of Tongue | 53.4% | - | - |
| Tonsil | 55.4% | 1.08 (0.98-1.20) | 1.07 (0.97-1.19) |
| Pharyngeal Wall/Soft Palate |
57.2% | 1.17 (0.90-1.51) | 1.15 (0.88-1.51) |
| Oropharynx NOS | 51.6% | 0.93 (0.75-1.14) | 0.88 (0.71-1.09) |
|
| |||
| Clinical T-Stage | |||
|
| |||
| T1 | 49.0% | - | - |
| T2 | 55.7% | 1.31 (1.13-1.52)* | 1.24 (1.07-1.44)* |
| T3 | 54.7% | 1.26 (1.08-1.47)* | 1.12 (0.95-1.32) |
| T4 | 55.7% | 1.31 (1.12-1.53)* | 1.13 (0.95-1.33) |
| Tx | 51.9% | 1.12 (0.78-1.60) | 1.19 (0.80-1.77) |
|
| |||
| Clinical N-Stage | |||
|
| |||
| N0 | 56.0% | - | - |
| N1 | 51.9% | 0.85 (0.70-1.02) | 0.88 (0.72-1.07) |
| N2 | 55.1% | 0.97 (0.82-1.14) | 0.94 (0.78-1.13) |
| N3 | 54.2% | 0.93 (0.74-1.17) | 0.84 (0.66-1.07) |
| NX | 46.1% | 0.67 (0.42-1.08) | 0.59 (0.35-0.99)* |
|
| |||
| Comorbidity Index | |||
|
| |||
| None | 54.4% | - | - |
| Moderate (1) | 52.5% | 0.93 (0.79-1.08) | 0.94 (0.80-1.10) |
| High (2) | 59.3% | 1.22 (0.85-1.76) | 1.19 (0.82-1.72) |
|
| |||
| Year of Diagnosis | |||
|
| |||
| 2003 | 53.6% | - | - |
| 2004 | 53.0% | 0.98 (0.84-1.13) | 0.94 (0.81-1.09) |
| 2005 | 55.1% | 1.06 (0.92-1.22) | 1.03 (0.89-1.19) |
| 2006 | 55.1% | 1.06 (0.92-1.22) | 1.02 (0.88-1.19) |
|
| |||
| Radiation Technique | |||
|
| |||
| Non-IMRT | 53.4% | - | - |
| IMRT | 56.5% | 1.13 (1.02-1.26)** | 1.17 (1.04-1.31)** |
|
| |||
| Treatment Facility | |||
|
| |||
| Community | 50.6% | - | - |
| Academic | 59.5% | 1.43 (1.30-1.58)** | 1.26 (1.13-1.42)** |
|
| |||
| Facility Case-Volume | |||
|
| |||
| Low Case-Volume | 51.8% | - | - |
| High Case-Volume | 61.5% | 1.49 (1.33-1.67)** | 1.38 (1.21-1.58)** |
|
| |||
| Geographic Region | |||
|
| |||
| Northeast | 57.0% | - | - |
| Southeast | 55.6% | 0.94 (0.82-1.09) | 0.87 (0.75-1.01) |
| Midwest | 50.7% | 0.77 (0.68-0.89)** | 0.73 (0.64-0.84)** |
| West | 57.3% | 1.01 (0.85-1.20) | 1.06 (0.88-1.26) |
|
| |||
| Patient Distance from Treatment Center (Miles) | |||
|
| |||
| <25 | 54.0% | - | - |
| 25-100 | 55.4% | 1.06 (0.93-1.20) | 1.00 (0.88-1.14) |
| >100 | 59.0% | 1.22 (0.95-1.58) | 1.08 (0.83-1.41) |
| Unknown | 50.0% | 0.85 (0.68-1.07) | 0.82 (0.65-1.04) |
|
| |||
| Insurance Status | |||
|
| |||
| Commercial Insurance | 50.5% | - | - |
| Medicare | 55.1% | 1.20 (1.07-1.35)* | 1.27 (1.07-1.51)* |
| Medicaid | 62.5% | 1.64 (1.39-1.93)** | 1.58 (1.32-1.88)** |
| Uninsured | 65.1% | 1.83 (1.50-2.22)** | 1.90 (1.55-2.33)** |
| Government | 59.4% | 1.44 (1.02-2.01)* | 1.38 (0.97-1.95) |
| Unknown | 55.1% | 1.20 (0.87-1.66) | 1.25 (0.90-1.75) |
ACKNOWLEDGEMENT
Funding: This work was supported in part by a project grant from the University of, Pennsylvania Leonard Davis Institute of Health Economics.
Footnotes
Prior Presentation:
This research was presented in part at the American Society for Radiation Oncology (ASTRO) Annual Meeting 2014, San Francisco, CA
Author Contributions:
Conception and design: All authors
Collection and assembly of data: Swisher-McClure, Sharma
Manuscript Writing: All authors
Data analysis and interpretation: All authors
Financial Support: Swisher-McClure
Provision of study materials or patients: Swisher-McClure
Final approval of manuscript: All authors
Financial Disclosures: None
Disclaimer: The data used in this study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic and statistical methodology used or the conclusions drawn from these data by the investigator. The interpretation and reporting of these data are the sole responsibility of the authors.
REFERENCES
- 1.Huang J. Does Delay in Starting Treatment Affect the Outcomes of Radiotherapy? A Systematic Review. J Clin Oncol. 2003;21(3):555–563. doi: 10.1200/JCO.2003.04.171. doi:10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87(1):3–16. doi: 10.1016/j.radonc.2007.11.016. doi:10.1016/j.radonc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Astit LAB, Lot EMB, Ebourdeau PHD, et al. Influence of the delay of adjuvant postoperative radiation therapy on relapse and survival in oropharyngeal and hypopharyngeal head and neck cancers. Int J Radiat Oncol Biol Phys. 2001;49(1):139–146. doi: 10.1016/s0360-3016(00)01376-6. [DOI] [PubMed] [Google Scholar]
- 4.Fortin A, Bairati I, Albert M, Moore L, Allard J, Couture C. Effect of Treatment Delay on Outcome of Patients with Early-Stage Head-and-Neck Carcinoma Receiving Radical Radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929–936. doi: 10.1016/s0360-3016(01)02606-2. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal DI, Liu L, Lee JH, et al. Importance of the Treatment Package Time in Surgery and Postoperative Radiation Therapy for Squamous Carcinoma of the Head and Neck. 2002 Feb;:115–126. doi: 10.1002/hed.10038. doi:10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Trotti A, Brown B, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 7.Barton MB, Morgan G, Smee R, Tiver KW, Hamilton C, Gebski V. Does waiting time affect the outcome of larynx cancer treated by radiotherapy. Radiother Oncol. 1997;44(2):137–141. doi: 10.1016/s0167-8140(97)00093-5. [DOI] [PubMed] [Google Scholar]
- 8.Brouha XDR, Tromp DM, de Leeuw JRJ, Hordijk G-J, Winnubst J a. M. Laryngeal cancer patients: analysis of patient delay at different tumor stages. Head Neck. 2005;27(4):289–295. doi: 10.1002/hed.20146. doi:10.1002/hed.20146. [DOI] [PubMed] [Google Scholar]
- 9.Caudell JJ, Locher JL, Bonner JA. Diagnosis-to-Treatment Interval and Control of Locoregionally Advanced Head and Neck Cancer. Arch Otolaryngol Head Neck Surg. 2011;137(3):282–285. doi: 10.1001/archoto.2011.20. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi AK, Engels E a, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. doi:10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Harten MC, de Ridder M, Hamming-Vrieze O, Smeele LE, Balm AJM, van den Brekel MWM. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) patients in a Dutch comprehensive cancer center. Oral Oncol. 2014;50(4):282–290. doi: 10.1016/j.oraloncology.2013.12.018. doi:10.1016/j.oraloncology.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Munck K, Ali MJ, Murr AH, Goldberg AN. Impact of Socioeconomic Status on the Diagnosis to Treatment Interval in Waldeyer ’ s Ring Carcinoma. 2005 Jul;:1283–1287. doi: 10.1097/01.MLG.0000165382.83891.92. doi:10.1097/01.MLG.0000165382.83891.92. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian S, Chen A. Treatment patterns and survival among low-income medicaid patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139(5):489–495. doi: 10.1001/jamaoto.2013.2549. doi:10.1001/jamaoto.2013.2549. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Data Base Am Coll Surg. 2015 https://www.facs.org/quality programs/cancer/ncdb.
- 15.Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16–21. doi: 10.1097/01.mlg.0000240182.61922.31. doi:10.1097/01.mlg.0000240182.61922.31. [DOI] [PubMed] [Google Scholar]
- 16.CoC Accreditation Categories Am Coll Surg. 2015 https://www.facs.org/quality-programs/cancer/accredited/about/categories.
- 17.Ang KK, Weber R, Rosenthal DI, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Human Papillomavirus–Associated Cancers — United States, 2004–2008. Centers Dis Control Prev Morb Mortal Wkly Rep. 2012:258–261. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6115a2.htm. [PubMed]
- 19.Langendijk J, de Jong M, Leemans C, et al. Postoperative radiotherapy in squamous cell carcinoma of the oral cavity: The importance of the overall treatment time. Int J Radiat Oncol. 2003;57(3):693–700. doi: 10.1016/s0360-3016(03)00624-2. doi:10.1016/S0360-3016(03)00624-2. [DOI] [PubMed] [Google Scholar]
- 20.Mackillop WJ, Zhou Y, Quirt CF. A Comparison of Delays in the Treatment of Cancer with Radiation in Canada and the United States. Int J Radiat Oncol Biol Phys. 1995;32(2):531–539. doi: 10.1016/0360-3016(94)00662-5. [DOI] [PubMed] [Google Scholar]
- 21.Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84(1):1–4. doi: 10.1016/j.radonc.2007.05.006. doi:10.1016/j.radonc.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen JR, Johansen J, Gano L, et al. A “package solution” fast track program can reduce the diagnostic waiting time in head and neck cancer. Eur Arch Otorhinolaryngol. 2013 Jun; doi: 10.1007/s00405-013-2584-z. doi:10.1007/s00405-013-2584-z. [DOI] [PubMed] [Google Scholar]
- 23.Waaijer A, Terhaard CHJ, Dehnad H, et al. Waiting times for radiotherapy: consequences of volume increase for the TCP in oropharyngeal carcinoma. Radiother Oncol. 2003;66(3):271–276. doi: 10.1016/s0167-8140(03)00036-7. doi:10.1016/S0167-8140(03)00036-7. [DOI] [PubMed] [Google Scholar]
- 24.Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84(1):5–10. doi: 10.1016/j.radonc.2007.04.001. doi:10.1016/j.radonc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Beadle BM, Liao K-P, Elting LS, et al. Improved survival using intensity-modulated radiation therapy in head and neck cancers: a SEER-Medicare analysis. Cancer. 2014;120(5):702–710. doi: 10.1002/cncr.28372. doi:10.1002/cncr.28372. [DOI] [PubMed] [Google Scholar]
- 26.Yu JB, Soulos PR, Sharma R, et al. Patterns of care and outcomes associated with intensity-modulated radiation therapy versus conventional radiation therapy for older patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):e101–e107. doi: 10.1016/j.ijrobp.2011.11.067. doi:10.1016/j.ijrobp.2011.11.067. [DOI] [PubMed] [Google Scholar]
- 27.Chen AY, Fedewa S, Pavluck A, Ward EM. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116(20):4744–4752. doi: 10.1002/cncr.25364. doi:10.1002/cncr.25364. [DOI] [PubMed] [Google Scholar]
- 28.Wuthrick EJ, Zhang Q, Machtay M, et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol. 2015;33(2):156–164. doi: 10.1200/JCO.2014.56.5218. doi:10.1200/JCO.2014.56.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27(25):4177–4181. doi: 10.1200/JCO.2008.21.7018. doi:10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.