Abstract
Aim:
C-reactive protein (CRP) is a commonly used biomarker of sepsis, the leading cause of mortality in Intensive Care Units (ICUs). However, sufficient data are still lacking to strongly recommend it in clinical practice. The present study is aimed to find out its reliability in diagnosing sepsis.
Materials and Methods:
CRP was measured in ICU-admitted patients with systemic inflammatory response syndrome and compared using a cutoff of 50 mg/L with the gold standard for diagnosing sepsis, taken as isolation of organism from a suspected source of infection or the Centers for Disease Control criteria for clinical sepsis.
Results:
CRP had a sensitivity and specificity of 84.3% and 46.15%, respectively. Area under the receiver operating characteristics curve was calculated to be 0.683 (±0.153, P < 0.05). The cutoff value with the best diagnostic accuracy was found to be 61 mg/L.
Conclusion:
CRP is a sensitive marker of sepsis, but it is not specific.
Keywords: C-reactive protein, diagnosis, sepsis
Introduction
Sepsis is recognized as a systemic inflammatory response syndrome (SIRS) due to infection, and it is one of the leading causes of death in critically ill patients. Despite the advancement in medical technology, sepsis remains a major obstacle, with 18 million new cases every year and a mortality of up to 30%.[1]
Surviving sepsis campaign has repeatedly emphasized the significance of early diagnosis in the prognosis of the disease as routine screening of potentially infected patients allows earlier implementation of goal-directed therapy. However, diagnosing sepsis has been one of the fundamental challenges, as the manifestation of sepsis is clinically so protean, and laboratory confirmation of infection has been so inconsistent.
Many biomarkers have been assessed for diagnosing sepsis. C-reactive protein (CRP), an acute-phase reactant secreted by the liver during inflammation, is considered one of such markers and has been extensively studied with interest. Although it has wide applicability potentials, its characteristic response to infection is that it can rise very high (sometimes >1000 times),[2] unlike in any other inflammatory condition. Though a newer biomarker, procalcitonin, has been preferred over CRP as a marker of sepsis, it is not widely available and still not the ideal biomarker. The present study is aimed to find out whether CRP can help diagnose sepsis, especially in resource-limited places, where newer markers such as procalcitonin may not be available.
Materials and Methods
This study was conducted in a multi-disciplinary adult Intensive Care Unit (ICU) of a community-based tertiary care hospital in East Nepal from June 2012 to May 2013. Ethical clearance from the Institutional Ethical Board and informed consent from patients’ relatives were obtained. Consecutive patients admitted to the ICU with the fulfillment of criteria for SIRS were included in the study. SIRS was defined according to the ACCP/SCCM Consensus Conference Committee 1992. Two or more of the above conditions if met were considered as SIRS: (a) Fever (>38°C) or hypothermia (<36°C), (b) tachycardia (heart rate >90 beats/min), (c) tachypnea (respiratory rate 20/min) or PaCO2 <32 mmHg, and (d) leukocytosis (white blood cell count [WBC] >12,000/μL) or leukopenia (WBC < 4000/μL) or >10% of immature neutrophils (band cells).
CRP analyzed with a point-of-care device (NycoCard Reader II) within 48 h of diagnosis was compared with cultures sent from the suspected site of infection. The sources comprised endotracheal tube or sputum, blood, urine, body fluids and pus, and results were recorded after 24–72 h of incubation as per the standard microbiological technique followed in the hospital. There was an arbitrary cutoff value of 50 mg/L as it had the best cutoff value in a previous study.[3]
The results were compared to the gold standard for diagnosing sepsis taken as a positive culture and in those without a positive culture, the Centers for Disease Control (CDC) criteria for sepsis. According to the CDC, clinical sepsis can be diagnosed when the patient has fever (>38°C), hypotension (systolic pressure <90 mm), or oliguria (<20 cm3/h), and all the following: (1) Blood culture not done or no organisms or antigen detected in blood, (2) no apparent infection at another site, and (3) physician institutes appropriate antimicrobial therapy for sepsis.
Results
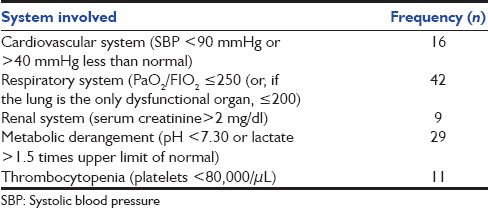
A total of 64 patients were studied in the 1-year period. There were 41 males and 23 females with age range from 15 to 85 years (mean 43 ± 19). The details of distribution of SIRS manifestations are as shown in Table 1, and those of organ dysfunction in Table 2.
Table 1.
Frequency of systemic inflammatory response syndrome manifestations
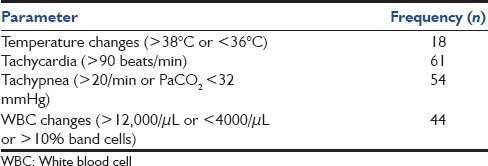
Table 2.
Frequency of organ dysfunctions
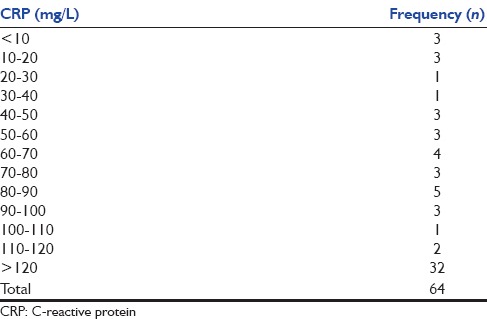
Fifty-one (80%) patients had CRP value above 50 mg/L. The values of CRP obtained are shown in Table 3. Even in the remaining patients, it was raised above the normal (10 mg/L) in all but three. The mean value of CRP could not be calculated as the point-of-care kit that was used could not measure values more than 120 mg/L and gave the reading as “>120 mg/L.”
Table 3.
Values of C-reactive protein obtained
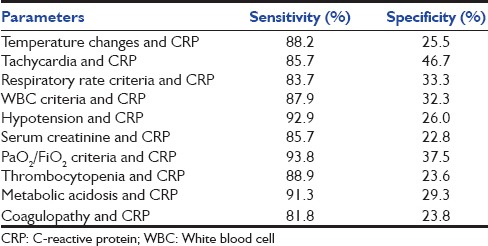
A total of 51 patients (80%) were diagnosed of having sepsis. Specimen from forty patients (62.5%) yielded bacterial growth and 11 (17%) met the CDC criteria for clinical sepsis. CRP had a sensitivity of 84.3%, specificity of 46.15%, positive predictive value of 84%, and negative predictive value of 42.8%. A comparison of CRP positivity with sepsis is presented as a contingency table in Table 4. The area under receiver operating characteristic (ROC) curve was 0.683 (0.529–0.836, P = 0.043) as shown in Figure 1 and Table 5, and the cutoff value with the best diagnostic accuracy was found to be 61 mg/L for this point-of-care testing. It gave a sensitivity of 84.3% and specificity of 53.8%. The diagnostic accuracy was also tested when CRP was combined with other parameters of sepsis. The results are shown in [Table 6]. Even after combining with CRP, the sensitivity and specificity could not increase above that provided by CRP alone.
Table 4.
2×2 contingency table for C-reactive protein and sepsis

Figure 1.
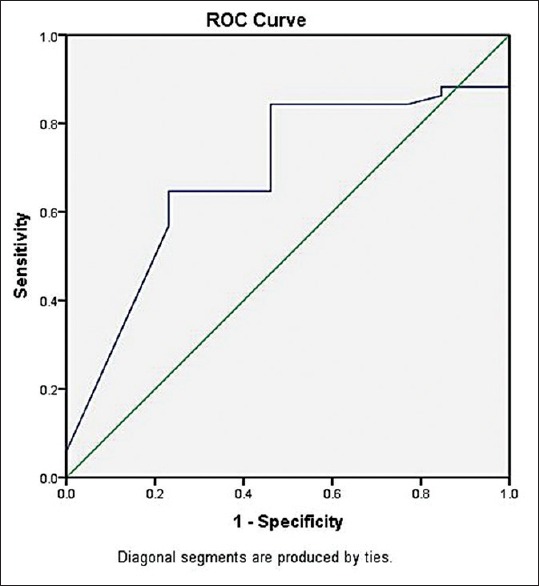
Receiver-operating characteristics curve for the performance of C-reactive protein as a diagnostic marker of sepsis
Table 5.
Area under the receiver-operating characteristics curve

Table 6.
Sensitivity and specificity of various parameters in combination with C-reactive protein
Discussion
From this study, using 50 mg/L as a cutoff value, quantitative assay of CRP was found to be sensitive for sepsis (84.3%), but not specific enough (46.1%), with area under curve of 0.683 (confidence interval, 0.529–0.836). Its diagnostic accuracy did not increase even when combined with other parameters of sepsis. Similarly, CRP had a positive predictive value of 84%, but the negative predictive value was only 42.8%. From the ROC curve that was extrapolated from the data, the best cutoff value was 61 mg/L in our point-of-care setting (84.3% sensitivity and 53.8% specificity), albeit the specificity still being low.
CRP when used with its traditional cutoff of 2 standard deviations as suggested by the International Sepsis Definition Conference in 2001 would be too nonspecific and will probably include many noninfectious inflammatory conditions as well. Matson et al.[4] had stated in their study that “normal” value of CRP level in critically ill patients rarely lies in the normal range for a healthy population.
Diagnostic accuracy of CRP even when using such high cutoff values has been variable and debatable. Our results almost match those found by Cheval et al.,[5] who despite using a cutoff value double of ours, found CRP to have a 93% sensitivity and 40% specificity. A study by Póvoa et al.[6] probably has the best diagnostic accuracy for CRP, with values of 98.5% sensitivity and 75% specificity. Most other studies[7,8,9] have found a sensitivity of 70–75% and a specificity of 66–78%. The reasons for such differences in observation may be due attributed to the accuracy of the diagnostic kits used, causes of infections, and patient-related factors. Further, individual responses to sepsis as well as CRP levels are known to be influenced by genetic variation,[10] and this study may be the first of its kind to be conducted in an adult population of Nepal.
Diagnosing sepsis has been a great challenge ever since the term was coined by the ancient Greeks. An international consensus of the definition was first achieved using SIRS in 1991 and further modified in 2001. Currently, the SIRS criteria have been criticized for being poorly specific for sepsis, and the Third International Consensus Definitions for Sepsis and Septic Shock (sepsis-3) has recommended the use of sequential organ failure assessment to replace SIRS. However, sepsis-3 has also received a widespread criticism with the main concern being that this definition de-emphasizes intervention at earlier stages when the disease is most amenable to treatment.[11] Furthermore, sepsis-3 definition still does not add any information in truly identifying those with infection as the culprit.
Conclusion
Despite having a small size, our study has shown the usefulness of CRP in identifying patients with sepsis in those who present with the manifestation of SIRS. Furthermore, CRP could be very useful in resource-limited places, where newer biomarkers such as procalcitonin or interleukins are not available, and where there is no guidance of an intensivist or a trained sepsis expert. Such situations are more prevalent in most of the hospitals in Nepal. CRP due to its high sensitivity has a lesser risk on missing those who are at a higher risk of mortality, and treatment or referral to a higher center could begin early. However, further research on a larger scale is required to define an accurate cutoff value, which may prove to be invaluable in the diagnosis of sepsis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–40. [PubMed] [Google Scholar]
- 2.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid a protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 3.Póvoa P. C-reactive protein: A valuable marker of sepsis. Intensive Care Med. 2002;28:235–43. doi: 10.1007/s00134-002-1209-6. [DOI] [PubMed] [Google Scholar]
- 4.Matson A, Soni N, Sheldon J. C-reactive protein as a diagnostic test of sepsis in the critically ill. Anaesth Intensive Care. 1991;19:182–6. doi: 10.1177/0310057X9101900204. [DOI] [PubMed] [Google Scholar]
- 5.Cheval C, Timsit JF, Garrouste-Orgeas M, Assicot M, De Jonghe B, Misset B, et al. Procalcitonin (PCT) is useful in predicting the bacterial origin of an acute circulatory failure in critically ill patients. Intensive Care Med. 2000;26(Suppl 2):S153–8. doi: 10.1007/BF02900729. [DOI] [PubMed] [Google Scholar]
- 6.Póvoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragão A, et al. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998;24:1052–6. doi: 10.1007/s001340050715. [DOI] [PubMed] [Google Scholar]
- 7.Ugarte H, Silva E, Mercan D, De Mendonça A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27:498–504. doi: 10.1097/00003246-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Suprin E, Camus C, Gacouin A, Le Tulzo Y, Lavoue S, Feuillu A, et al. Procalcitonin: A valuable indicator of infection in a medical ICU? Intensive Care Med. 2000;26:1232–8. doi: 10.1007/s001340000580. [DOI] [PubMed] [Google Scholar]
- 9.Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–83. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Nelson GE, Mave V, Gupta A. Biomarkers for sepsis: A review with special attention to India. Biomed Res Int 2014. 2014 doi: 10.1155/2014/264351. 264351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson SQ. New sepsis criteria: A change we should not make. Chest. 2016;149:1117–8. doi: 10.1016/j.chest.2016.02.653. [DOI] [PubMed] [Google Scholar]


