Abstract
G protein–coupled receptors (GPCRs) relay diverse extracellular signals into cells by catalyzing nucleotide release from heterotrimeric G proteins, but the mechanism underlying this quintessential molecular signaling event has remained unclear. Here we use atomic-level simulations to elucidate the nucleotide-release mechanism. We find that the G protein α subunit Ras and helical domains—previously observed to separate widely upon receptor binding to expose the nucleotide-binding site—separate spontaneously and frequently even in the absence of a receptor. Domain separation is necessary but not sufficient for rapid nucleotide release. Rather, receptors catalyze nucleotide release by favoring an internal structural rearrangement of the Ras domain that weakens its nucleotide affinity. We use double electron-electron resonance spectroscopy and protein engineering to confirm predictions of our computationally determined mechanism.
G protein–coupled receptors (GPCRs), which represent the largest class of drug targets, trigger cellular responses to external stimuli primarily by activating heterotrimeric G proteins: an activated GPCR, upon binding an inactive, GDP-bound G protein, dramatically accelerates GDP release, thus allowing GTP to bind spontaneously to the vacated nucleotide-binding site (1-2). This nucleotide exchange initiates G protein–mediated intracellular signaling. Despite breakthroughs in GPCR structure determination (3-5), key aspects of the molecular mechanism by which GPCRs accelerate GDP release remain unresolved.
Heterotrimeric G proteins undergo a dramatic conformational change upon binding activated GPCRs (Fig. 1, A and B). Double electron-electron resonance (DEER) spectroscopy has demonstrated that the Ras and helical domains of the G protein α subunit (Gα), which tightly sandwich the nucleotide in all nucleotide-bound G protein crystal structures, separate by tens of angstroms upon GPCR binding and GDP release (6). A crystal structure of a GPCR–G protein complex (4), and accompanying deuterium exchange and electron microscopy data (7, 8), confirmed this dramatic domain separation.
Figure 1.
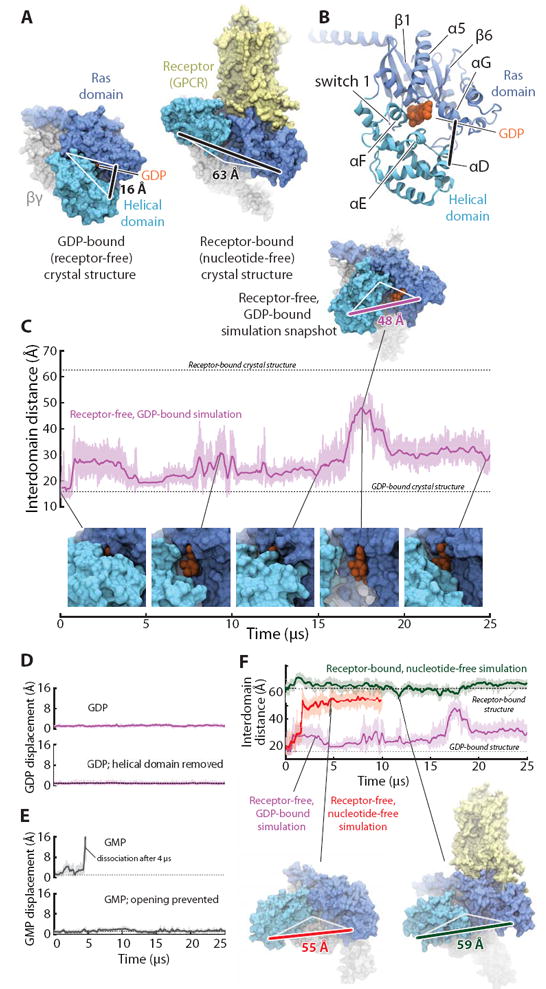
The Ras and helical domains of the G protein α subunit separate spontaneously and frequently when GDP is bound, even in the absence of a receptor. (A) The Ras and helical domains are tightly apposed in all nucleotide-bound G protein crystal structures, enveloping the nucleotide (left: GDP-bound Gt heterotrimer; PDB entry 1GOT), but are dramatically separated in the receptor-bound, nucleotide-free structure (right: β2-adrenergic receptor–Gs heterotrimer [β2AR–Gs] complex; PDB entry 3SN6). GDP is colored orange, the Ras domain blue, the helical domain cyan, Gβγ gray, and the receptor yellow. The degree of domain separation is represented by a thick black line connecting Ala134 and Glu272 in Gαt or the corresponding Ala161 and Glu299 in Gαs, with both ends connected by white lines to a pivot point near Thr166 (Gαt) or Ser193 (Gαs). (B) Key structural motifs of the α subunit, illustrated using the GDP-bound Gt structure. (C) Spontaneous domain separation provides an exit pathway for GDP. In simulations of receptor-free, GDP-bound Gt, the Ala134–Glu272 distance varies substantially as the domains fluctuate between apposed and separated conformations; raw (light purple) and smoothed (250-ns moving average; dark purple) data are shown. Representative molecular simulation snapshots (top: overview; bottom: nucleotide-binding site) display varying degrees of GDP exposure. Data are from simulation 2 (Table S1). (D) Domain separation is not sufficient for rapid nucleotide release. GDP remains tightly bound to receptor-free Gt (top), even with the helical domain removed (bottom; traces show displacement of the centroid of the nucleotide non-hydrogen atoms relative to the crystal structure). Data from simulations 2 and 33. (E) Domain separation is necessary for rapid nucleotide release. GMP dissociates spontaneously from receptor-free Gt (top) but remains bound when the interdomain distance is artificially restrained to prevent domain separation (bottom). Data from simulations 16 and 31. (F) Domain separation is greater in the absence of a nucleotide. In simulations initiated from the receptor-free, GDP-bound Gt crystal structure, but with the GDP removed, the Ras and helical domains exhibited extensive and prolonged separation (red trace; left-hand snapshot). In simulations of the β2AR–Gs complex, also nucleotide-free, the helical domain remained widely separated from the Ras domain, although it typically moved away from the membrane toward the beta propeller of Gβγ (green; right-hand snapshot). GDP-bound Gt simulation data from panel C are replicated for reference (purple). See SM for details on structural renderings. Data from simulations 2, 14, and 22.
These observations have raised several unresolved questions (4, 9). What is the role of domain separation in GDP release? Does a GPCR catalyze GDP release by forcing the domains to separate, or does the GPCR force out GDP, with the absence of GDP leading to subsequent domain separation? More generally, what is the structural mechanism by which a GPCR brings about GDP release?
To address these questions, we performed atomic-level molecular dynamics (MD) simulations of heterotrimeric G proteins with and without bound GPCRs. We initiated simulations from crystal structures of nucleotide-bound G protein heterotrimers (in particular, Gi (10) and a chimeric Gt (11)), including some in which we omitted the co-crystallized nucleotide, GDP (12). We also initiated simulations from the only crystal structure of a GPCR–G protein complex (β2-adrenergic receptor [β2AR]–Gs) (4), which is also the only structure of a nucleotide-free heterotrimeric G protein. All 66 simulations we performed, of length up to 50 μs each, are listed in Table S1.
In simulations of GDP-bound G protein heterotrimers, the Gα Ras and helical domains—which are tightly apposed in all nucleotide-bound crystal structures—unexpectedly and dramatically separated from one another (Fig. 1C, Figs. S1, S2). These domain-separated conformations recall the extreme open conformation of the nucleotide-free β2AR–Gs crystal structure (4): in both cases, the helical domain rotated as a rigid body (Fig. S3) from its nucleotide-bound crystallographic conformation about a loose hinge located on the distal (away from GDP) side of helix αF (Fig. S4). In GDP-bound simulations, the helical domain fluctuated between tightly apposed and separated positions. The maximal rotation observed, ~90°, was less extreme than the nearly 150° rotation of the β2AR–Gs structure. Nonetheless, the rotation observed in simulation, and the accompanying domain separation of up to ~30 Å (Fig. 1C), dramatically disrupted the interdomain nucleotide-binding site. Such domain separation is particularly remarkable because it occurred with GDP bound and in the absence of a receptor. Smaller interdomain motions have previously been observed in shorter MD simulations, including some with GDP bound (13-17).
Despite this substantial domain separation, GDP remained bound throughout our multi-microsecond simulations (Fig. 1D, Fig. S5), held in place by persistent, tight contacts with the Ras domain (Fig. S4); the few contacts with the helical domain appeared to be weaker, occasionally breaking and reforming. Indeed, GDP also remained bound to the Ras domain in a simulation with the entire helical domain deleted (Fig. 1D, Fig. S5), in accord with the experimental finding that the Ras domain alone is sufficient to bind nucleotides (18).
The Gα domain separation observed in simulations cleared an exit pathway for the bound nucleotide, eliminating steric barriers to its escape (Fig. S6). Indeed, GMP, which forms fewer contacts with the Ras domain and has a G protein binding affinity five to six orders of magnitude lower than that of GDP (7), consistently dissociated within microseconds in simulation (Fig. 1E, Fig. S5). GMP only dissociated when the domains had separated (Fig. S7), however, and when we prevented such separation by restraining the interdomain distance, GMP remained bound (Fig. 1E, Fig. S5). Loosening the restraint to permit partial domain separation of ~25 Å was sufficient to allow GMP dissociation (Fig. S5).
Lack of a bound nucleotide further promoted domain separation. In nucleotide-free simulations—still initiated from the tightly closed, GDP-bound conformation, in the absence of a receptor—domain separation was more dramatic and persistent (Fig. 1F, Fig. S1), approaching the level observed in the β2AR–Gs structure. This increased separation appeared to be due to the loss of nucleotide contacts with residues in and near the helical domain αF helix; αF generally remained in contact with the Ras domain α1 helix when GDP was bound, but readily separated from α1 in nucleotide-free simulations, adopting much the same position as in the β2AR–Gs structure (Fig. S8).
In simulations initiated from the β2AR–Gs structure, which also lacks a bound nucleotide, the domains consistently remained well-separated (Fig. 1F, Fig. S1). Indeed, the helical domain adopted conformations similar to those observed in receptor-free, nucleotide-free simulations.
Our nucleotide-bound G protein simulations indicate that a certain degree of Gα domain separation is necessary to clear an exit pathway for nucleotide release. Intriguingly, adequate separation occurs frequently and spontaneously even in the receptor-free, GDP-bound state, but separation alone is not sufficient for rapid GDP release. Rather, a weakening of nucleotide–Ras domain contacts also appears necessary. These observations suggest that an activated receptor could accelerate nucleotide release simply by favoring conformational changes in the Ras domain that weaken its interactions with GDP; the receptor need not promote further domain separation. Indeed, prior studies have indicated that binding of activated receptor promotes Ras domain conformational changes, particularly in the C-terminal α5 helix, but possibly also near the Gα N-terminus (4, 7, 19-22).
To investigate the nature of such conformational changes and how they might affect nucleotide affinity, we compared our simulations of receptor-free G proteins with and without bound GDP, focusing on those structural elements known to interact with receptors. Our guiding thesis was that a conformation that favors GDP release should itself be favored by the absence of GDP; that is, if affinity for GDP is weaker when the G protein adopts a particular conformation than when it does not, then removal of GDP will increase the equilibrium population of that conformation (Fig. S9).
Of the G protein structural elements that contact the receptor in the β2AR–Gs crystal structure, only the Ras domain α5 helix displayed clear conformational differences between simulations with and without bound GDP (Fig. S10). In the absence of GDP, α5 often moved away from the nucleotide-binding site (~5 Å translation along, and ~60° rotation around, the helical axis), adopting a conformation closely matching that observed in the β2AR–Gs structure (4) as well as in a rhodopsin–Gi model (19) (Fig. 2, A and B; Figs. S10, S11). The shift to this distal α5 conformation was facilitated by the increased mobility of the adjacent β6–α5 loop in the absence of a nucleotide; this loop directly contacts bound GDP and shifts position in its absence (Fig. S12). In receptor-free simulations, the distal α5 conformation was approximately 1,000 times more prevalent in the absence of GDP than in its presence (Fig. S10).
Figure 2.
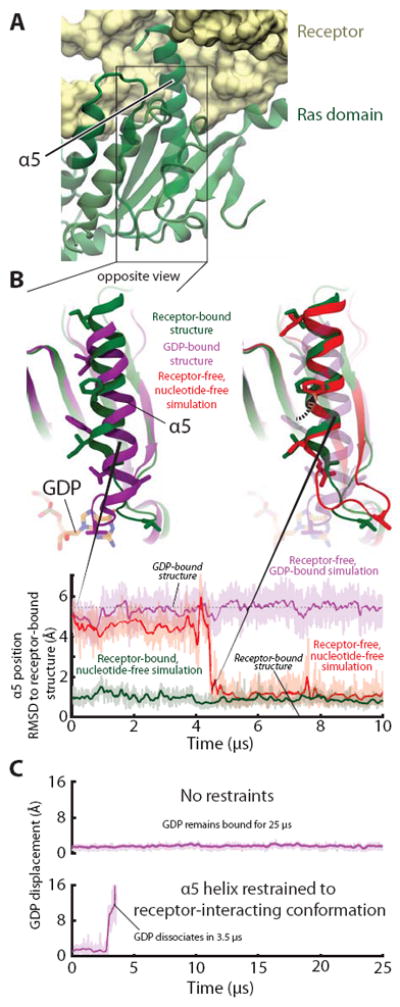
Receptor-induced displacement of the Gα C-terminal α5 helix disrupts key GDP contacts, thereby promoting nucleotide release. (A) In the receptor-bound, nucleotide-free crystal structure (PDB entry 3SN6) crystal structure, α5 docks into receptor. (B) (Top, left) Superimposition of receptor-free, GDP-bound (PDB entry 1GOT; purple) and receptor-bound, nucleotide-free (PDB entry 3SN6; green) crystal structures shows the displacement of α5, relative to the rest of the Ras domain, that occurs when a G protein binds to an activated receptor. (Top, right) In a simulation initialized from a receptor-free, GDP-bound Gt structure but with GDP removed (red), α5 spontaneously rotated 60° and translated 5 Å, adopting a position distal from the nucleotide-binding site that closely matched that of the β2AR–Gs complex (green). Several side chains in the α5 helix and α5–β6 loop are shown to facilitate comparison between structures. (Bottom) The position of α5 in this simulation (red) changed abruptly at ~4.5 μs to match that of the β2AR–Gs complex; the α5 position was stable in simulations of receptor-free, GDP-bound Gt (purple) and of the β2AR–Gs complex (green). Data are from simulations 5, 12, and 22 (Table S1). (C) Forcing α5 into the distal conformation accelerates nucleotide release in simulation. Temperature-accelerated MD simulations allow observation of GDP release on computationally accessible timescales, but only when α5 is restrained to the distal conformation (i.e., the conformation observed in the β2AR–Gs complex). Receptor-free, GDP-bound Gt was simulated without (top) or with (bottom) restraints on α5 (see SOM). GDP displacement is measured as in Fig. 1. Data are from simulations 55 and 56.
Our simulations thus indicate that a repositioning of α5 reduces the affinity of bound GDP. This α5 motion shifts the β6–α5 loop away from the guanine ring of GDP, thereby weakening its contacts with the Ras domain. Previous computational and experimental work has shown that the distal α5 conformation is favored by the activated receptor (19); indeed, the β2AR–Gs crystal structure shows that only when α5 is distally positioned can it dock fully into the receptor (4) (Fig. S13). The distal α5 conformation, which is adopted only rarely in our simulations of a receptor-free, GDP-bound G protein (Fig. S10), apparently becomes the dominant conformation once the G protein binds an activated receptor (19), thus facilitating GDP dissociation.
Indeed, mimicking the effect of the receptor by restraining the α5 helix to the distal conformation substantially accelerated GDP release in temperature-accelerated MD simulations (Fig. 2C; Fig. S14). Release of GDP led to increased domain separation, but the receptor-mimicking restraints were not observed to increase domain separation prior to GDP release, suggesting that a receptor accelerates nucleotide release primarily by weakening the Ras domain’s nucleotide affinity rather than by favoring domain separation.
Our simulations thus suggest the following nucleotide exchange mechanism. The Ras and helical domains of GDP-bound Gα separate spontaneously even in the absence of a receptor (Fig. 3). Such separation is necessary but not sufficient for rapid GDP release. Binding of an activated receptor favors conformational changes within the Ras domain—rotation and translation of the α5 helix away from the nucleotide-binding site, leading to rearrangement of the adjacent β6–α5 loop—that weaken its interactions with GDP, thereby enabling GDP to unbind when the helical and Ras domains spontaneously separate. Because GDP helps stabilize closed domain conformations, nucleotide dissociation shifts the equilibrium toward conformations with the two domains widely separated.
Figure 3.
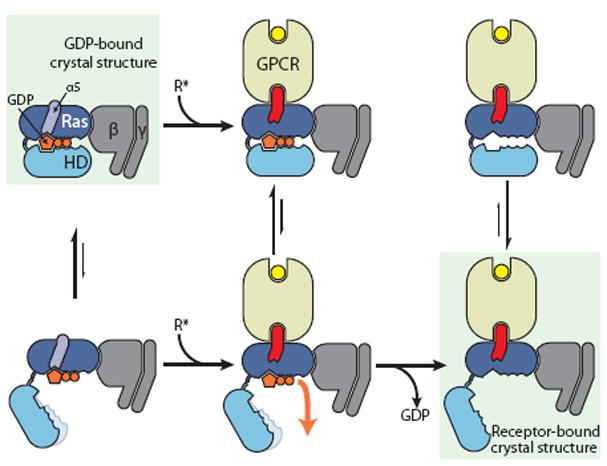
Proposed mechanism of receptor-catalyzed nucleotide release. (Left) The Ras and helical domains (Ras and HD) separate frequently, even in the absence of a receptor, but such separation does not usually lead to GDP release. This rapid (relative to overall GDP release) equilibrium favors the closed state (top). (Middle) Binding of an activated receptor induces a Ras domain conformational change—displacement of α5 away from GDP—that weakens interactions between GDP and the Ras domain, allowing GDP to escape when the Gα domains happen to spontaneously separate (bottom). (Right) Loss of GDP shifts the equilibrium toward Gα conformations with widely separated domains (bottom).
Our computationally determined mechanism predicts that the Ras and helical domains separate spontaneously and frequently, even with GDP bound and in the absence of a receptor. Although no crystal structure of a nucleotide-bound G protein has captured a domain-separated conformation—perhaps because such conformations are less populated and less amenable to crystallization than one with the domains in tight contact—the DEER spectroscopy study that originally demonstrated domain separation upon receptor binding also noted a small peak at large distances in GDP-bound Gi interdomain distance distributions (cf. Fig. 1 of ref. 6).
To better characterize this peak, we performed improved DEER experiments by using a Gi construct with no inserted purification tags (to avoid altering protein dynamics), substantially longer dipolar evolution times (to increase confidence in the measured distance distribution at large distances), and an experimental protocol that delivers an improved signal-to-noise ratio (12). We also performed similar experiments on Gs. In both GDP-bound Gi and GDP-bound Gs, we found clear evidence for a minority population exhibiting substantial domain separation (Fig. 4A; Fig. S15). The results of a recently published DEER study on GDP-bound Gαi1 in the absence of the βγ subunit also suggest a minority population with separated domains (23).
Figure 4.
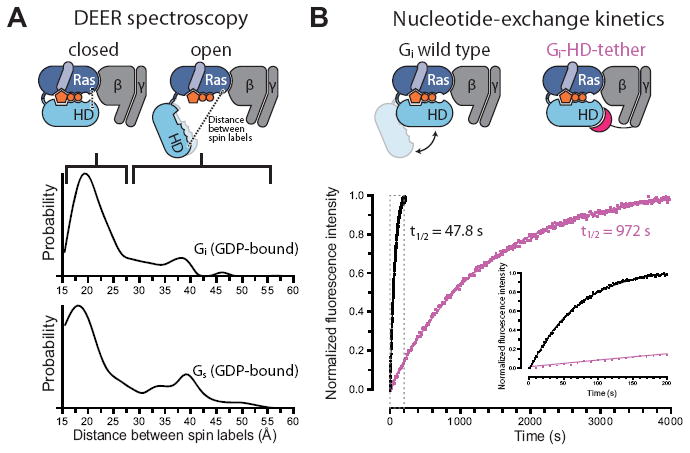
Experimental validation of spontaneous Gα domain separation in GDP-bound heterotrimeric G proteins and its role in nucleotide exchange. (A) DEER distance distributions measured between spin labels attached to the Ras and helical domains of Gi (Glu238 and Arg90) and Gs (Asn261 and Asn112) show multiple distance peaks, consistent with an equilibrium between closed and open conformations of the α subunit in the presence of GDP, despite the absence of an activated receptor. These distance distributions extend to much larger values than would be expected if the G proteins maintained their crystallographic nucleotide-bound conformations (Fig. S15). (B) Domain separation impacts the basal GDP release rate. The Gi-HD-tether construct (Fig. S16), designed to restrict domain separation, exchanges nucleotides 20-fold more slowly than Gi wild type, under conditions where GDP release is rate-limiting. GDP release was monitored by BODIPY-GTPγS binding kinetics, shown for Gi wild type (black) and Gi-HD-tether (purple). The inset corresponds to the gray dashed box.
Our simulations suggest that the minority domain-separated population in GDP-bound G proteins arises due to rapid fluctuations between closed and open conformations, and that this spontaneous opening plays an essential role in nucleotide exchange. This implies that constraining domain opening would substantially slow basal nucleotide exchange, and, in particular, GDP release. To test this prediction, we engineered a Gi variant to restrict domain opening. In this construct, the N-terminus of the γ subunit was fused to a peptide fragment designed to bind the helical domain without impinging on either the nucleotide-binding site or the Ras domain (Fig. S16). Binding kinetics measured by fluorescence quenching showed that this “helical domain tether” slowed basal nucleotide exchange 20-fold, under conditions where GDP release is rate limiting (Fig. 4B).
Our nucleotide-release mechanism is consistent with earlier mutagenesis studies. Point mutations to the Ras domain β6–α5 loop (24) accelerate nucleotide exchange in the absence of a receptor substantially more than mutations that weaken contacts between the Ras and helical domains (25), suggesting that weakening interactions between β6–α5 and the GDP guanine ring facilitates nucleotide release to a greater extent than does increasing domain separation. Mutations to α5 that energetically favor the distal conformation increase both receptor-catalyzed and basal nucleotide exchange rates, whereas those disfavoring that conformation decrease nucleotide exchange rates (21) (Fig. S10D).
Several caveats are in order. First, because we did not simulate the complete process of receptor–G protein association, we have not determined the sequence of steps by which a receptor couples to a G protein, nor addressed the question of whether a G protein might associate with a receptor prior to receptor activation (26-28). Second, although our simulations are orders of magnitude longer than previous atomistic G protein simulations, they still lack sufficient length, and perhaps sufficient accuracy, to reliably determine equilibrium populations of the various conformations; they do, however, strongly imply the existence of certain conformations and of dynamical interchange among them. We cannot rule out the possibility that additional conformational changes to the G protein would manifest themselves on longer timescales; the GPCR might thus also induce GDP release in part through other mechanisms, such as displacement of the β1 strand of Gα (7). Third, because crystal structures of nucleotide-bound and receptor-bound heterotrimers are not available for the same G protein, our analysis combines data from different G proteins, under the common assumption that their high level of structural homology implies similar functional mechanisms (1-2).
Why might heterotrimeric G proteins have evolved to fluctuate spontaneously between open and closed conformations? Tight apposition of the Ras and helical domains appears to be essential for efficient hydrolysis of GTP to GDP (29). In the closed conformation, the helical domain may substitute for the GTPase-activating proteins (GAPs) required by small G proteins—which contain only a Ras domain—for efficient catalysis (18). Conversely, our results suggest that rapid GDP release requires an open conformation. Spontaneous fluctuation of the helical domain position thus provides an elegant solution to the conflicting needs of catalysis and nucleotide release.
Supplementary Material
Acknowledgments
We thank Albert Pan for assistance with TAMD, Kim Palmo for help with force field parameterization, James Valcourt and Hillary Green for advice on figures, and Mollie Kirk for editorial assistance. Portions of this work were funded by NIH grant R01GM083118 to Brian Kobilka. D.H. was supported by the German Academic Exchange Service (DAAD).
References
- 1.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 2.Sprang SR. G protein mechanisms: Insights from structural analysis. Ann Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen SGF, et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 6.Van Eps N, et al. Interaction of a G protein with an activated receptor opens the interdomain interface in the alpha subunit. Proc Natl Acad Sci USA. 2011;108:9420–9424. doi: 10.1073/pnas.1105810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KY, et al. Conformational changes in the G protein Gs induced by the β2-adrenergic receptor. Nature. 2011;477:611–615. doi: 10.1038/nature10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westfield GH, et al. Structural flexibility of the Gαs α-helical domain in the β2-adrenoceptor Gs complex. Proc Natl Acad Sci USA. 2011;108:16086–16091. doi: 10.1073/pnas.1113645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohlman HG, Jones JC. Signal activation and inactivation by the Gα helical domain: A long-neglected partner in G protein signaling. Sci Signal. 2012;5:re2. doi: 10.1126/scisignal.2003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall MA, et al. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 11.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 12.Materials and methods are available as supplementary materials on Science Online
- 13.Mello LV, van Aalten DM, Findlay JB. Dynamic properties of the guanine nucleotide binding protein alpha subunit and comparison of its guanosine triphosphate hydrolase domain with that of ras p21. Biochemistry. 1998;37:3137–3142. doi: 10.1021/bi971402v. [DOI] [PubMed] [Google Scholar]
- 14.Ceruso MA, Periole X, Weinstein H. Molecular dynamics simulations of transducin: Interdomain and front to back communication in activation and nucleotide exchange. J Mol Biol. 2004;338:469–481. doi: 10.1016/j.jmb.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Khafizov K, Lattanzi G, Carloni P. G protein inactive and active forms investigated by simulation methods. Proteins. 2009;75:919–930. doi: 10.1002/prot.22303. [DOI] [PubMed] [Google Scholar]
- 16.Louet M, Perahia D, Martinez J, Floquet N. A concerted mechanism for opening the GDP binding pocket and release of the nucleotide in hetero-trimeric G-proteins. J Mol Biol. 2011;411:298–312. doi: 10.1016/j.jmb.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Jones JC, Jones AM, Temple BR, Dohlman HG. Differences in intradomain and interdomain motion confer distinct activation properties to structurally similar Gα proteins. Proc Natl Acad Sci USA. 2012;109:7275–7279. doi: 10.1073/pnas.1202943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markby DW, Onrust R, Bourne HR. Separate GTP binding and GTPase activating domains of a G alpha subunit. Science. 1993;262:1895–1901. doi: 10.1126/science.8266082. [DOI] [PubMed] [Google Scholar]
- 19.Alexander NS, et al. Energetic analysis of the rhodopsin G-protein complex links the α5 helix to GDP release. Nat Struct Mol Biol. 2014;21:56–63. doi: 10.1038/nsmb.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onrust R, et al. Receptor and βγ binding sites in the α subunit of the retinal G protein transducin. Science. 1997;275:381–384. doi: 10.1126/science.275.5298.381. [DOI] [PubMed] [Google Scholar]
- 21.Marin EP, Krishna AG, Sakmar TP. Rapid activation of transducin by mutations distant from the nucleotide-binding site: Evidence for a mechanistic model of receptor-catalyzed nucleotide exchange by G proteins. J Biol Chem. 2001;276:27400–27405. doi: 10.1074/jbc.C100198200. [DOI] [PubMed] [Google Scholar]
- 22.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol. 2006;13:772–777. doi: 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- 23.Van Eps N, Thomas CJ, Hubbell WL, Sprang SR. The guanine nucleotide exchange factor Ric-8A induces domain separation and Ras domain plasticity in Gαi1. Proc Natl Acad Sci USA. 2015;112:1404–1409. doi: 10.1073/pnas.1423878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iiri T, Herzmark P, Nakamoto JM, van Dop C, Bourne HR. Rapid GDP release from Gsa in patients with gain and loss of endocrine function. Nature. 1994;371:164–168. doi: 10.1038/371164a0. [DOI] [PubMed] [Google Scholar]
- 25.Marin EP, Krishna AG, Archambault V, Simuni E, Fu WY, Sakmar TP. The function of interdomain interactions in controlling nucleotide exchange rates in transducin. J Biol Chem. 2001;276:23873–23880. doi: 10.1074/jbc.M101197200. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann R, Heck M, Henklein P, Hofmann KP, Ernst OP. Signal transfer from GPCRs to G proteins: Role of the Gα N-terminal region in rhodopsin-transducin coupling. J Biol Chem. 2006;281:30234–30241. doi: 10.1074/jbc.M600797200. [DOI] [PubMed] [Google Scholar]
- 27.Scheerer P, et al. Structural and kinetic modeling of an activating helix switch in the rhodopsin-transducin interface. Proc Natl Acad Sci USA. 2009;106:10660–10665. doi: 10.1073/pnas.0900072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elgeti M, et al. Precision vs flexibility in GPCR signaling. J Am Chem Soc. 2013;135:12305–12312. doi: 10.1021/ja405133k. [DOI] [PubMed] [Google Scholar]
- 29.Shnerb T, Lin N, Shurki A. What is the role of the helical domain of GsR in the GTPase reaction. Biochemistry. 2007;46:10875–10885. doi: 10.1021/bi700585w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


