Abstract
Due to the role of nitric oxide (NO) in regulating a variety of biological functions in humans, numerous studies on different NO releasing/generating materials have been published over the past two decades. Although NO has been demonstrated to be a strong antimicrobial and potent antithrombotic agent, NO-releasing (NOrel) polymers have not reached the clinical setting. While increasing the concentration of the NO donor in the polymer is a common method to prolong the NO-release, this should not be at the cost of mechanical strength or biocompatibility of the original material. In this work, it was shown that the incorporation of S-nitroso-penicillamine (SNAP), an NO donor molecule, into Elast-eon E2As (a copolymer of mixed soft segments of polydimethylsiloxane and poly(hexamethylene oxide)), does not adversely impact the physical and biological attributes of the base polymer. Incorporating 10 wt % of SNAP into E2As reduces the ultimate tensile strength by only 20%. The inclusion of SNAP did not significantly affect the surface chemistry or roughness of E2As polymer. Ultraviolet radiation, ethylene oxide, and hydrogen peroxide vapor sterilization techniques retained approximately 90% of the active SNAP content, where sterilization of these materials did not affect the NO-release profile over an 18 day period. Furthermore, these NOrel materials were shown to be biocompatible with the host tissues as observed through hemocompatibility and cytotoxicity analysis. In addition, the stability of SNAP in E2As was studied under a variety of storage conditions, as they pertain to translational potential of these materials. SNAP-incorporated E2As stored at room temperature for over 6 months retained 87% of its initial SNAP content. Stored and fresh films exhibited similar NO release kinetics over an 18 day period. Combined, the results from this study suggest that SNAP-doped E2As polymer is suitable for commercial biomedical applications due to the reported physical and biological characteristics that are important for commercial and clinical success.
Keywords: Nitric Oxide, SNAP, physical properties, cytotoxicity, biocompatibility
Introduction
Over the past 20 years, nitric oxide (NO) has emerged as a key player in regulating a number of biological functions, including angiogenesis, inflammation, vasodilation, thrombosis, smooth muscle cell proliferation and migration, wound healing, cardiovascular diseases, nervous system diseases, prevention of infection, cell growth and tumor formation in humans and other mammals.[1-7] It has been shown that NO is the primary regulator in inhibiting platelet activation and adhesion [8-11], and is naturally released from vasculature at an estimated rate of 0.5 – 4 × 10−10 mol min−1 cm−2.[12] In addition, NO has broad-spectrum antibacterial properties, and is effective against both gram-positive and gram-negative bacteria.[13-18] These research results show the potential of using NO-releasing materials for biomedical device applications with improved biocompatibility and reduced foreign body response. In order to translate the NO research from benchtop to bedside, there is an urgent need to understand the physical and biological characteristics of NO-releasing polymers.
Nitric oxide is a free radical, water soluble, ubiquitous gas with a very short half-life and hence acts in a localized manner.[19] The NO exposure to a biological system can have significant consequences in a dose dependent manner; therefore, concentration of NO must be controlled carefully to provide reproducible results. For instance, micromolar concentrations of NO are required for inhibiting the growth of tumor cells, while picomolar concentrations have an angiogenic effect.[20] Recently, the development of NO donor molecules has allowed for localized delivery of NO, drastically increasing the possible applications for NO-releasing (NOrel) materials. Among various NO donors that have been investigated, synthetic S-nitrosothiols (RSNOs) have been studied extensively.[10, 11, 18, 21-29] Release of NO from these materials can occur through thermal decomposition, catalysis (using metals ions such as Cu+) [30], or by exposure to light [29, 31, 32], resulting in disulfide species (RSSR) formation. S-nitroso-acetylpenicillamine (SNAP) is a synthetic RSNO which has shown to exhibit significant antimicrobial and antithrombotic effects in a various medical grade polymers.[11, 14, 23, 33] The delivery of NO from SNAP leads to a molecule of NO and the backbone of the donor, N-acetylpenicillamine (NAP). N-acetylpenicillamine has been used to treat cystinuria at dosages of 2-4 g/day, where only 1 transient episode of facial swelling due to hypersensitivity was seen out of 155 treatment days.[34] Among different polymers studied, Elast-eon E2As polymer (a copolymer of mixed soft segments of polydimethylsiloxane and poly(hexamethylene oxide)), possesses excellent intrinsic biocompatibility properties, exhibiting low levels of blood protein adhesion.[35, 36] In a previous study, diazeniumdiolates (NONOate) added to the E2As coating was able to retain 97 ± 10% of platelet counts in vivo in a 4 hour extracorporeal circuit (ECC) in a rabbit model.[10] These promising in vivo results have prompted the study of SNAP as the NO donor in E2As, where E2As-SNAP ECC loops preserved 100 ± 7% of the platelet count in a 4 hour ECC study in rabbits, while showing great stability of the NO donor after 2 months of storage.[23] Longer terms studies have been reported recently of SNAP-E2As catheters, and showed reduce bacterial adhesion and thrombus formation for 7 days in sheep.[11] As evident from the previous studies described above, SNAP-doped E2As possesses great potential for biomedical applications. While NO-releasing polymers have been shown to give excellent results in research settings, antibacterial activity and prevention of thrombus formation are not the only properties to consider when moving towards clinically relevant polymers. Due to the reactivity of NO donors with heat and light, sterilization and storage of these materials continue to limit use of these polymers in clinical devices. [23] Furthermore, the addition of NO donors into polymers should not have cytotoxic effects on cells or induce hemolysis, while maintaining the mechanical and surface properties of the base polymer. In the absence of fundamental research focusing on characterization of NO-releasing polymers in terms of mechanical strength, hemocompatibility, cytotoxicity, and ability to be stored and sterilized, nitric oxide releasing materials will never reach clinical use.
In this work, the effects of incorporating the NO donor SNAP into Elast-eon E2As were investigated in terms of its physical and biological properties. The effects of the addition of various levels of SNAP on the physical and mechanical properties (surface roughness, wettability, and maximum loading) are also shown through atomic force microscopy, static contact angle measurements, and tensile strength testing respectively. Standard methods were used to measure the hemolytic activity (ASTM F756 and ISO 10993-4) and cytotoxicity (ISO 10993-5) to determine biocompatibility of these NO-releasing materials. Furthermore, various levels of SNAP doped films (5 and 10 wt%) were stored under various conditions (ranging from −20°C to 37°C) to examine the shelf life of these materials for up to 6 months, as well as the effect of storage on the release kinetics of NO over a period of 18 days. The SNAP content of these materials was examined before and after a number of common sterilization techniques, including ultraviolet light exposure, autoclaving, hydrogen peroxide vapor, and ethylene oxide.
Materials and Methods
N-Acetyl-D-penicillamine (NAP), sodium chloride, ethylenediaminetetraacetic acid (EDTA), potassium chloride, sodium phosphate dibasic, potassium phosphate monobasic, tetrahydrofuran (THF), sulfuric acid and N,N-dimethylacetamide (DMAc) were purchased from Sigma Aldrich (St. Louis, MO). Methanol, hydrochloric acid and sulfuric acid were obtained from Fisher Scientific (Pittsburgh, PA). Elast-eon E2As was obtained from AorTech International, plc (Scoresby, Victoria, Australia). Phosphate buffered saline (PBS), pH 7.4, containing 138 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate, and 100 mM EDTA was used for all in vitro experiments. Atomic force microscopy Tap 300-G tips were purchased from NanoandMore.
SNAP synthesis
A modified version of a previously reported method was used to synthesize SNAP [28]. Equimolar ratios of NAP and sodium nitrite were added to a 1:1 mixture of water and methanol containing 2 M HCl and 2 M H2SO4. After 30 min of stirring, the reaction vessel was cooled in an ice bath to precipitate the SNAP crystals. The crystals were collected by filtration, washed with water, and allowed to air dry. The reaction and crystals were protected from light at all the times. Each batch of SNAP was tested for purity using the Sievers Chemiluminescence Nitric Oxide Analyzer. All batches used recorded purity levels greater than 95%.
Preparation of SNAP-based polymer films
Polymer films containing 5, 10, and 15 wt% SNAP were prepared by solvent evaporation method. The casting solutions were prepared by dissolving 190, 180, or 170 mg Elast-eon E2As in 3 mL THF. SNAP (10, 20, 30 mg, respectively) was then added to the polymer solution and the mixture was stirred for 10 min to give 5, 10, and 15 wt% SNAP solutions. The film solution was casted in Teflon ring (d = 2.5 cm) and dried overnight under ambient conditions. Small disks (d = 0.7 cm) were cut from the parent films and were dip coated with a topcoat solution (200 mg polymer (no SNAP added) in 4 mL THF) and dried over-night under ambient conditions, followed by 48 h of drying under vacuum to remove any residual solvent. The weight of each small disk was recorded prior to top coating. All films and film solutions were protected from light throughout.
The use of solvent evaporation method for processing of the SNAP-E2As material provides highly uniform composites. This can be attributed to the casting of the materials using SNAP in a solvent at concentrations much lower than saturation. After evaporation of the solvent, the uniformity of the composite can be seen visually due to the characteristic green color from the addition of SNAP.
Tensile testing
Control and SNAP-loaded E2As films (5 wt%, 10 wt%, 15 wt%) were cast in dumbbell-shaped Teflon™ molds with an active area length of 4.5 cm and width of 1 cm using similar concentrations described in film preparation section. Tensile testing was performed by clamping samples in place on the jaws of the Instron tensile tester. The polymer was pulled with a constant cross-head speed of 20 mm/min and the force at break (N) was recorded. Tensile testing was conducted at room temperature (23 °C).
Surface characterization
Film preparation: Films were spin coated onto glass slides using a CHEMAT Technology KW-4A spin coater at a fixed E2As concentration of 50 mg/mL and 15 wt% of SNAP in THF, and spun for 60 seconds at 2000 rpm. A common approach for limiting NO release from SNAP loaded films is the addition of a top coat. E2As solution (50 mg/mL) was applied over the primary E2As layer to match the top coat thickness from dip coated top coats on SNAP-doped films.
Wettability
Static contact angle was measured using Kruss DA 100 drop shape analyzer. A 1 μL droplet of water was placed on E2As and E2As-SNAP films that were spin coated on glass slides, and the average of left and right contact angles were measured via the Kruss software.
Surface Roughness
Films were then analyzed using Bruker Multimode atomic force microscopy (AFM) to determine surface roughness over a 4 μm2 square region using tapping mode. Each film was analyzed in three random areas, with n=3 for each condition (E2As, SNAP-E2As). The average surface roughness value was taken as the Ra parameter, which is the centerline average between the highest and lowest point of the surface irregularities, and was calculated by built in software (Nanoscope, Digitial Instruments, CA, USA).[37]
Storage stability of SNAP/E2As films
SNAP/E2As films (consisting of 5 wt% or 10 wt% SNAP) were placed under the following conditions in vials with desiccant: 23°C, 37°C in dark, refrigerator (4°C), and in the freezer (−20°C). All samples were protected from the light during the storage tests. The amount of SNAP remaining in the films was measured after 1, 3, and 6 months of storage at each condition. Films were dissolved in DMAc and the UV-Vis spectra were recorded to determine the % SNAP remaining in the film, as compared to the initial SNAP films. The NO release kinetics of the films stored for 6 months at 23°C were compared to the freshly made films containing 5 and 10 wt% SNAP.
Nitric oxide release measurements
Nitric oxide released from the films was measured using a Sievers Chemiluminescence Nitric Oxide Analyzer (NOA) 280 (Boulder, CO). The Sievers chemiluminescence Nitric Oxide analyzer (model 280i) is considered to be a gold standard for detecting nitric oxide. It is widely used for measurement of nitric oxide released from materials due to the ability to limit interfering species, such as nitrates and nitrites, as they are not transferred from the sample vessel to the reaction cell.[38-40] Films were placed in the sample vessel immersed in PBS (pH 7.4) containing 100 mM EDTA. Nitric oxide was continuously purged from the buffer and swept from the headspace using nitrogen sweep gas and bubbler into the chemiluminescence detection chamber. Films were submerged in PBS with EDTA and stored in glass vials and kept at 37°C between NO-release measurements. Fresh PBS solution was used for each NO-release measurement, and films were kept in fresh PBS solution for storage after each measurement.
UV-Vis spectra
All UV-Vis spectra were recorded in the wavelength range of 200-700 nm using a UV-Vis spectrophotometer (Lambda 35, Perkin Elmer, MA) at room temperature. The presence of the S-NO group of SNAP provides characteristic absorbance maxima at 340 and 590 nm.[29, 41, 42] E2As films were dissolved in DMAc and absorbance values were measured at 340nm. Figure 1shows a typical UV-Vis spectra of SNAP dissolved in DMAc.
Figure 1.
Representative UV spectra of SNAP. Maxima occur at 340 and 590 nm, corresponding to the S-NO bond.
Cytotoxicity
In vitro Cytotoxicity Study Using the ISO Elution Method
To ascertain whether leachable extracted from the 10 wt% SNAP-E2As films would cause any toxic effects on mammalian cells, cytotoxicity testing using ISO 10993-5 Elution Method was used. The cytotoxicity testing was performed by NAMSA® following aseptic Standard Operating Procedures. A single preparation of the 10 wt% SNAP-E2As films was extracted in single strength Minimum Essential Medium (1X MEM) supplemented with 5% fetal bovine serum, 2% antibiotics (100 units/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL amphotericin B) and 1% (2 mM) L-glutamine (1X MEM) at 37°C for 24 hours. The negative control (high density polyethylene), reagent control (1X MEM), and positive control (Powder-Free Latex Gloves) were similarly prepared using MEM medium. The 10 wt% SNAP-E2As films were prepared and returned to the freezer until testing. The extracts were not centrifuged, filtered, or otherwise altered prior to dosing. However, the extracts were continuously agitated during extraction. The 1X MEM extraction was performed using serum to optimize extraction of both polar and non-polar components. Mouse fibroblast cells (L-929) were propagated and maintained in flasks containing 1X MEM at 37°C with 5% carbon dioxide (CO2). Thereafter, cells were seeded in 10 cm2 cell culture wells and incubated at 37°C in the presence of 5% CO2 to obtain subconfluent monolayers of cells. Triplicate cultures with subconfluent cell monolayer were then used for leachate exposure. The growth medium contained in the triplicate cultures was replaced with 2.0 mL of the test extract, reagent control, negative control, or positive control extract depending on the experiment. The wells were incubated at 37°C in 5% CO2 for 48 hours. The color of the test medium was observed to determine any change in pH. In general, a color shift toward yellow indicates an acidic pH range, and a color shift toward magenta to purple indicates an alkaline pH range.
Hemolysis
The potential of 10 wt% SNAP-E2As films to cause hemolysis was studied in vitro by NAMSA®. The films were tested using ASTM F756, Standard Practice for Assessment of Hemolytic Properties of Materials and ISO 10993-4. Whole blood samples collected from four New Zealand White rabbits into vacuum tubes containing EDTA as the anticoagulant were maintained at room temperature and used within four hours of collection. Anticoagulated whole rabbit blood was pooled, diluted, and added to tubes with the test sample in calcium and magnesium-free phosphate buffered saline (CMF-PBS). Negative controls, positive controls, and blanks were prepared in the same manner. Sterile water for injection (SWFI) and CMF-PBS were used as blanks and positive controls respectively while high density polyethylene (HDPE) was used as the negative control for this study. Clot-free blood samples were collected from each animal into 7 mL vacuum tubes containing 12 mg of EDTA on the same day the test was performed. The blood collected from each animal was pooled into a borosilicate screw cap tube and mixed gently to prevent mechanical hemolysis. The pooled blood was diluted with CMF-PBS to a total hemoglobin concentration of 10 ± 1.0 mg/mL. In a ratio of 1.0 mL diluted blood to 7.0 mL CMF-PBS, samples were prepared in triplicates both for direct contact as well as extraction. The samples were capped, inverted gently to mix the contents, and then maintained for at least 3 hours at 37°C with periodic inversions at approximately 30-minute intervals. Following incubation, the blood-CMF-PBS mixtures were transferred to separate disposable centrifuge tubes. These tubes were centrifuged for 15 minutes at 700-800 x g. The condition of the supernatant was recorded and a 1.0 mL aliquot of each test article, negative control, positive control, and blank supernatant was added to individual 1.0 mL portions of Drabkin's reagent (hemoglobin reagent) and allowed to stand for 15 minutes at room temperature. The condition of the test article supernatants were recorded a second time. The absorbance of each test article, negative control, positive control, and blank solution was measured at 540 nm using a spectrophotometer. The condition of the test article supernatants was then recorded a third, and the final time.
The hemoglobin concentration of each test article, negative control, positive control and blank solution was then calculated from the standard curve. The blank corrected percent hemolysis was calculated for each test article and the negative and positive controls as follows (where ABS = absorbance):
For the suitability of the system to be confirmed, the negative control must have had a blank corrected % hemolysis value <2% and the positive control must have had a blank corrected % hemolysis value of >5%. If either of these values were not within the acceptable range, the test was repeated with fresh blood.
The mean blank corrected % hemolysis (BCH) was calculated by averaging the blank corrected % hemolysis values of the triplicate test samples. In the event the BCH resulted in a value less than zero, the value was reported as 0.00. The standard deviation for the replicates was determined. An average hemolytic index of the triplicate test samples was also calculated as follows:
Sterilization methods of E2As-SNAP films
A number of sterilization techniques have become common practice in the fields of medical devices and bacteria/cell culture. Various commonly used sterilization techniques were compared to sterilize 10 wt% SNAP containing E2As films. Autoclaving, hydrogen peroxide vapor, ethylene-oxide (EO), as well as ultra-violet light exposure were investigated. The samples were tested for the wt% SNAP content before and after each sterilization method by UV-Vis analysis. Polymer samples were weighed and rapidly dissolved in DMAc for determination of SNAP by UV-Vis. Details of each sterilization method is described below.
Autoclave
The films were steam sterilized at 121°C for 30 min in a Steris 3033 steam sterilizer.
Hydrogen Peroxide vapor
SNAP-loaded polymer samples were sterilized using a Steris VHP MD140X for 28 minutes at the University Of Georgia College of Veterinary Medicine. The sterilization chamber is evacuated and hydrogen peroxide solution is injected from a cassette and is vaporized in the sterilization chamber to a concentration of 6 mg/L.
Ethylene Oxide Sterilization
SNAP-loaded polymers samples were sterilized by ethylene oxide at the University of Michigan Hospital Central Sterile Processing Department. The EO sterilization procedure is conducted at 54 °C and 40–80% humidity. Samples were conditioned for 1 h, exposed to EO gas for 2-3 h, and aerated for 14 h.
UV Sterilization
SNAP-loaded polymer samples were sterilized using ultra-violet light administered from a 1300 Series A2 Thermo Scientific Biosafety cabinet for 1.5 hours.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Comparison of SNAP-doped E2As films to control was done using a two tailed Student’s t-test, where p < 0.05 was considered statistically significant for all tests.
Results and Discussions
Nitric oxide releasing materials have been shown to drastically increase the biocompatibility of materials in vivo in both short and long term applications, particularly the use of SNAP-E2As composites.[11, 23] One of the prime objectives in developing NOrel materials is to control as well as prolong the NO-release from these devices. Increasing the lifetime of these materials is generally done through increasing the amount of NO donor that is incorporated in the host material. However, the increase in NO donor concentration can also provide an initial burst of NO. Similarly, leaching of NO donors from their base material is a common metric to determine how effective the NO is being delivered. Leaching of SNAP from E2As has been studied previously, and shows how the application of top coats of pure E2As to SNAP-E2As devices will prevent the leaching of SNAP over a period of time.[23] This technique for preventing leaching of SNAP has also been described for other polymeric materials as well.[43] For this reason, the characterization of SNAP-E2As was solely done in combination with pure E2As top coats.
Tensile strength
The use of additives in polymers can have a detrimental effect on a number of the mechanical properties of the base material, such as the tensile strength. The addition of SNAP was seen to reduce the ultimate tensile strength (UTS) of E2As, and is shown as a percentage of the pure polymer UTS in Figure 2. As E2As have very low water uptake (<2%), the UTS is not expected to change significantly when implemented in physiological conditions.[23] Base polymer E2As was found to have an UTS of 55.8 ± 4.3 N. Films of E2As with 5 wt% SNAP had a ultimate tensile strength of 47.0 ± 10.4 N, and were shown to not be significantly different than the pure E2As films (p=0.13). The UTS of SNAP-E2As between 5 and10 wt% was not found to change significantly (p=0.31), where 10 wt% SNAP-E2As had an UTS of 45.0 ± 4.4 N. However, as SNAP concentrations are increased past 10 wt%, sharp decreases in material strength were observed, with an UTS of 35.1 ± 5.6 N for films with 15 wt% SNAP (64% of pure E2As). Therefore, concentrations between 5 and 10 wt% SNAP are optimal for retaining the properties of the base material. Optimization of these materials will need to be performed for each type of device, as NO-release characteristics and physical strength requirements can vary drastically from application to application. As the sharp decrease in UTS was seen for SNAP concentration at 15 wt%, only SNAP concentration of 5 wt% and 10 wt% were used in the subsequent studies. When stored at room temperature for 6 months, only 13% loss of SNAP was observed (10% to 8.7% by weight). Therefore, only small changes in UTS are expected for stored samples (discussed further in storage stability below).
Figure 2.
Ultimate tensile strength of E2As films with various concentrations of SNAP at break point, as measured by Instron tensile strength instrument.
Surface characterization
Surfaces properties of medical devices, blood contacting devices in particular, are some of the most important characteristics of the device in determining biocompatibility. It is well known that increasing roughness as well as hydrophobicity of surfaces can potentially lead to higher amounts of protein adsorption on the surface.[44-46] Therefore, increasing the wettability and decreasing the roughness of these materials is desirable, or any modifications to existing materials should not decrease wettability or increase roughness. While the overall biocompatibility of E2As has been shown to be drastically improved when used in combination with SNAP in vivo, the effects on the surface properties of the base material should not be adversely affected by the addition of SNAP.[11, 23] Results for the surface characteristics are shown in Table 1, and show the addition of SNAP has no noticeable effect on the surface roughness or wettability of the base polymer.
Table 1.
Effect of SNAP on surface characteristics of E2As films as measured using atomic force microscopy (AFM) and contact angle measurements.
| Film | Surface Roughness (nm) |
Contact Angle (Degrees) |
|---|---|---|
| Elast-eon E2As | 11.04 ± 1.97 | 111.7 ± 3.30 |
| 10 Wt % SNAP | 11.65 ± 1.08 | 110.7 ± 0.47 |
The high biocompatibility of the pure E2As polymer can partially be attributed to the higher affinity of albumin binding to the surface when compared to fibrinogen, which has been determined to be the primary protein to initiate the coagulation cascade and induce clot formation.[36, 47] Therefore, altering the surface chemistry of E2As can have large implication on the effectiveness of the material to resist clot formation. Contact angle was measured for pure E2As (111.7 °± 3.3) and 10 wt% SNAP-E2As films (110.7° ± 0.4) (p= 0.20). Therefore, it is predicted that interactions between blood proteins and the surface will remain unchanged by the addition of SNAP.
Increasing surface roughness has been shown to increase the adsorption of fibrinogen.[44] This stems from geometrical changes in the arrangement of the adsorbed fibrinogen, as it is a non-globular protein. Therefore, it is important to ensure that the addition of the NO donor will not have a detrimental effect on the average surface roughness. Surface roughness of E2As and SNAP-E2As films were measured using AFM over a 4 μm2 region. Pure E2As films were found to have an average roughness value of 11.04 ± 1.97 nm, while SNAP-E2As films had a roughness value of 11.65 ± 1.08 nm (p=0.38). As the addition of SNAP was insignificant in changing the wettability or surface morphology, it is expected the interactions of blood proteins with the E2As surface remain unchanged.
Storage stability of SNAP-doped E2As
While a number of NO donors have been studied, all share a similar sensitivity to heat. The ability for these products to be stored will play a critical role in the feasibility of these materials to be clinically applicable. Films with 10 wt% SNAP were stored at a variety of temperatures (−20°C to 37°C) to mimic possible storage conditions. Films for measurement of NO release were fabricated as stated in the methods section. Little variation in NO release was seen between the sections of the parent film, confirming the uniform distribution of SNAP within the film. All samples were protected from the light during the storage tests. Even after 6 months at the elevated temperature (37°C), 81.2 ± 4.3 % of SNAP remained in the E2As films. This amount increased as storage temperature decreased. Room temperature conditions being most realistic for commercial/clinical applications, retained 88.4 ± 2.7 % SNAP after 1 month, and 87.1 ± 2.8 % SNAP after 6 months. Optimum storage conditions were found to be at −20 °C, where 100% ± 3.1 SNAP was remaining after 3 months, and 95 ± 2.1% remained after 6 months. A complete description of the storage data for all temperatures can be found in Figure 3. Films containing 5 wt% SNAP were also stored at these conditions, with 57.4 ± 2.8 % (37°C), 61.1 ± 3.5 % (25°C), 90.8 ± 0.9 % (4°C), and 94.7 ± 6.5 % (−20°C) remaining after 6 months. This increased stability of SNAP at higher concentrations was recently discovered to be due to the solubility of SNAP (ca. 3.4 – 4.0 wt%) when preparing polymer films through solvent evaporation [43]. While SNAP concentration at 5 wt% have less effect on the mechanical properties, but do not contain enough crystalline SNAP to maintain NO during storage. Higher concentrations (e.g., 10 wt%) allow for increased crystal formation within the polymer matrix through intermolecular hydrogen bonding, resulting in the enhanced storage stability. This crystallization processes also plays an important role in determining the release kinetics of NO from the polymer, as discussed below. While stability of SNAP in the polymer films is important, maintaining the release profile from the films is equally critical for controlled release. NO-release from 5 and 10 wt% SNAP-E2As films were measured directly after film preparation, as well as after 6 months storage at room temperature. While the initial 24 hour release rates of NO from both 5 and 10 wt% stored films after 6 month storage are significantly lower, the release rates for both compositions after 24 hours maintain similar profiles over the remaining lifetime of the fresh films. While the release rates for stored films are lower on day 0, they remain in the physiological range of NO released from the natural endothelium (0.5 – 4 × 10−10 mol min−1 cm−2).[12] The higher levels of NO-release from films on Day 0 can be attributed to the SNAP soluble in the polymer, which is unstable and rapidly releases its NO payload. During storage, this soluble SNAP decomposes, while the crystalline SNAP is stabilized by intermolecular hydrogen bonding. The SNAP in crystalline form provides the long-term NO-release and is minimally affected by the shelf storage. This increased crystallinity not only is important for the storage of the material, but also plays a pivotal role in the control of the release. This lower NO-flux for the 6 month stored films can be attributed to the loss of SNAP that is observed in the UV-vis results. Release measurements of films stored for 6 months as compared to control films are shown in Figure 4.
Figure 3.
Storage stability of SNAP in E2As. Films were stored under various conditions ( - −20°C,
- −20°C,  - 4°C,
- 4°C,  - 23°C,
- 23°C,  -37°C) and evaluated for SNAP content by dissolving in DMAc and measuring absorbance at 340 nm via UV-Vis.
-37°C) and evaluated for SNAP content by dissolving in DMAc and measuring absorbance at 340 nm via UV-Vis.
Figure 4.
Nitric oxide release profiles of:  -fresh 5% SNAP-E2As,
-fresh 5% SNAP-E2As,  - 5% SNAP-E2As after 6 month storage,
- 5% SNAP-E2As after 6 month storage,  - fresh 10% SNAP-E2As,
- fresh 10% SNAP-E2As,  -10% SNAP-E2As after 6 month storage as measured by chemiluminescence in PBS buffer at 37°C. The dotted line represents lower limit to physiological levels.
-10% SNAP-E2As after 6 month storage as measured by chemiluminescence in PBS buffer at 37°C. The dotted line represents lower limit to physiological levels.
Cytotoxicity
Treating cells with a chemical agent can result in a variety of cell fates like altered metabolism, decrease in cell viability, necrosis death and change in the genetic program of cells ultimately resulting in apoptosis (programed cell death). After incubation with the SNAP-E2As, L-929 mouse fibroblast cells were examined microscopically (100X) following the cytotoxicity testing using ISO 10993-5 elution method to evaluate cellular characteristics (abnormal morphology and cellular degeneration) and percent lysis. For the test to be valid, the reagent control and the negative control must have had a reactivity of none (grade 0) and the positive control must have moderate (grade 3) or severe (grade 4) reactivity. In the event of 100% cell lysis, percent rounding and percent cells without intracytoplasmic granules were not evaluated. The reagent control, negative control, and the positive control performed as anticipated (grade 0, grade 0, and grade 4 respectively). The 10 wt% SNAP-E2As films showed no evidence of causing cell lysis or toxicity when tested for 48 hours on L-929 mouse fibroblast cells (grade 0), where the positive control showed 100% cell death.
Hemolysis
Hemolysis testing is considered to be the most common method to evaluate the hemolytic properties of any blood contacting biomaterial or device. The test is based on erythrocyte (red blood cells) lysis induced by contact, toxins, metal ions, leachables, and surface charge or any other cause of red blood cells lysis. The hemolytic potential of 10 wt% SNAP-E2As films was tested in vitro for 3 hours at 37°C on blood samples sourced from rabbit using ISO 10993-4 protocol. In the event the hemolytic index resulted in a value less than zero, the value was reported as 0.0. The hemolytic index for 10 wt% SNAP-E2As films in direct contact with blood was 0.05 ± 0.1% when compared to negative and positive controls (0%, 102 ± 1.7% respectively). Extract from the films were also tested, where and the hemolytic index for SNAP-E2As was 0.05 ± 0.1%, with negative and positive controls of 0.23 ± 0.1% and 99.77 ± 2.3%. Thus, 10 wt% SNAP-E2As films in direct contact with blood and the sample extract were found to be non-hemolytic in a 3 h study.
Sterilization of SNAP-doped E2As
Infection continues to be a serious problem in clinical settings, where 1.7 million healthcare associated infections result in 99,000 deaths per year in the United States alone.[48] Providing a sterile environment is necessary for surgical procedures to be successful. For device related infections, bacteria congregate at the device site, or have adhered to the device itself prior to implantation. While various sterilization techniques have greatly reduced the risk of infection, the threat of infection has yet to be eliminated. Active antibacterial materials have been developed to help reduce the attachment of bacteria throughout the lifetime of the device. However, sterilization of these materials must be plausible for their implementation into clinical use. As mentioned above, SNAP is both heat and light sensitive, and is a limiting factor in the sterilization of these materials. A number of common sterilization techniques were evaluated to determine the best sterilization method for materials incorporating SNAP.
Autoclave
Autoclaving is a widely used technique to sterilize any equipment/material using a high pressure saturated steam at 121°C for around 30 minutes.[49] Sterilization autoclaves are commonly used in microbiology, veterinary science, mycology, prosthetics fabrication, and medicine. Only 7.2 ± 0.1 wt% of the initial SNAP concentration was retained after autoclaving and hence is not recommended to sterilize SNAP-doped polymers.
Ethylene Oxide
Ethylene oxide gas has been used since the 1950s for heat-sensitive and moisture-sensitive medical devices.[49] Films exposed to EO sterilization retained 89.4 ± 2.0 % of SNAP. The ability for SNAP to withstand EO sterilization is very important as it is a standard method used in many healthcare facilities for heat sensitive products. The ability for these products to be sterilized without the need for specialized equipment will make SNAP-E2As an easily implemented material.
Hydrogen peroxide vapor
Hydrogen peroxide sterilization works by producing destructive hydroxyl free radicals that can attack the membrane lipids, DNA, and other essential cell components, and is effective against bacteria, yeasts, fungi, viruses, and spores.[49] Hydrogen peroxide sterilization was able to retain 89.4 ± 6.7 wt%. This can be a promising alternative for facilities lacking EO sterilization.
UV Exposure
Ultra-violet radiation has been become a common technique for sterilization, providing a bactericidal effect between 210 and 328 nm, with the maximum bactericidal effect using light between 240 and 280 nm.[50] Numerous health studies have shown that ultraviolet (UV) light is very effective against bacteria, spores, mold, fungi, mildew and viruses by breaking down their RNA or DNA. Ultra-violet radiation has been implanted in the disinfection of drinking water[51], air[50], titanium implants[52], and contact lenses[53].UV sterilization for 1.5 hours was found to retain the highest amount of SNAP at 99 ± 0.003 wt%. Effects of UV exposure on the retention of SNAP in the polymer will have an increasingly detrimental effect as you approach 340 nm (corresponding to the S-NO bond within SNAP).[29]
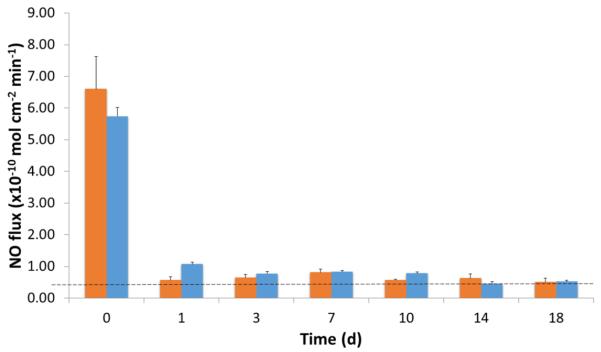
A summary of the various sterilization techniques on the retention of SNAP is found in Figure 5. Autoclaving the SNAP-doped E2As films was the most detrimental to the NO release properties of the films due to the prolonged exposure to high temperatures. While UV sterilization may be not be applicable for all applications, such as ECC tubing or catheter-type applications, it remains a viable option for devices requiring surface coatings. For devices requiring more intensive sterilization, EO and H2O2 methods are viable approaches. Similarly to storage, the sterilization of these materials should not alter the NO-release profile. As EO sterilization is the most clinically accepted method for sterilization of heat sensitive products, NO-release from sterilized and nonsterilized 10 wt% SNAP-E2As films were measured over 18 day period. The sterilization of the SNAP-E2As films had minimal effects on the NO-release profile, as shown in Figure 6.
Figure 5.
Effect of various sterilization methods on retention of SNAP in E2As films. Films were sterilized by the various methods, and then dissolved in DMAc for rapid determination of the SNAP content via UV-Vis.
Figure 6.
Effect of EO sterilization ( - no sterilization,
- no sterilization,  - EO sterilized) on NO-release profile from 10 wt% SNAP-E2As films measured by chemiluminescence in PBS buffer at 37°C.The dotted line represents lower limit to physiological levels.
- EO sterilized) on NO-release profile from 10 wt% SNAP-E2As films measured by chemiluminescence in PBS buffer at 37°C.The dotted line represents lower limit to physiological levels.
Conclusions
As researchers look to extend the lifetime of NOrel devices, the physical properties and biomcompatibility of these materials hold precedent on the clinical feasibility of medical devices utilizing these polymers. In this study, different aspects of a SNAP doped polymer as they pertain to commercialization and clinical application were investigated, including the effects of SNAP on mechanical and physical properties, hemocompatibility, and cytotoxicity. In addition, stability of SNAP during shelf storage and exposure to various sterilization techniques was evaluated. Ultimate tensile strength of SNAP-doped E2As films was 80% of the pure polymer when used at a concentration from 5-10 wt%, declining rapidly at SNAP concentrations of 15 wt% (~63% of pure E2As). Further mechanical testing of SNAP incorporated polymers is recommended for medical devices that undergo high or cyclical loading, such as roller pump tubing for rotary blood pumps. The addition of SNAP did not alter the surface roughness or surface chemistry, as measured using AFM and contact angle. Concentrations of SNAP at and below 10 wt% have been shown in previous works to adequately prevent platelet adhesion and activation, as well as antimicrobial properties in vivo. These biological attributes are complemented by our current work, demonstrating the non-cytotoxic and hemocompatible properties of SNAP-doped E2As polymer. Furthermore, the present study demonstrated NO releasing E2As (10 wt% SNAP) can be stored for 6 months at room temperature while retaining 87.1 ± 2.8 % of initial SNAP content, and retained 81.2 ± 4.3 % at harsh conditions (37°C). Films containing 5 wt% SNAP were found to be less stable (61.1 ± 3.5 % after 6 months at room temperature), and are consistent with previous findings on SNAP-doped polymers. Furthermore, these stored materials maintain similar release kinetics over an 18 day period as compared to freshly prepared SNAP-doped E2As. Various sterilization methods were tested, and showed H2O2, EO, and UV sterilization methods all to be promising methods for sterilization (>90% SNAP retained). To confirm the sterilization of the SNAP-E2As films had minimal effects on the NO-release profile, NO-release from EO sterilized films were compared to fresh films over an 18 day period.
While adding increasing amounts of SNAP may seem to be an attractive approach for increasing and prolonging the NO-release from polymeric materials, this can have detrimental effects on the mechanical properties of the device. Contrarily, reducing the amount of SNAP below 5 wt % will have detrimental effects in terms of stability and shelf-life. It is observed that polymers with low water uptake containing SNAP in the range of 5-10 wt% provide high stability, long durations of NO release, with minimal effects on strength. To conclude, the successful demonstration of NO-releasing E2As as a stable, and physically and biologically compatible material, makes it a potential material for biomedical device and implant applications.
Acknowledgements
The authors declare this work is supported by the National Institutes of Health, Grants K25HL111213 and R44HL114148.
References
- [1].Marín J, Rodríguez-Martínez MA. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacology & Therapeutics. 1997;75:111–34. doi: 10.1016/s0163-7258(97)00051-x. [DOI] [PubMed] [Google Scholar]
- [2].Vural K, Bayazit M. Nitric oxide: implications for vascular and endovascular surgery. European Journal of Vascular and Endovascular Surgery. 2001;22:285–93. doi: 10.1053/ejvs.2001.1448. [DOI] [PubMed] [Google Scholar]
- [3].Radomski MW, Moncada S. Regulation of vascular homeostasis by nitric oxide. Thrombosis and Haemostasis. 1993;70:36–41. [PubMed] [Google Scholar]
- [4].Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacologica Sinica. 2005;26:259–64. doi: 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- [5].Feldman PL. The surprising life of nitric oxide. Chem Eng News. 1993;71:26–38. [Google Scholar]
- [6].Kuo PC, Schroeder RA. The emerging multifaceted roles of nitric oxide. Annals of Surgery. 1995;221:220. doi: 10.1097/00000658-199503000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cai TB, Wang PG, Holder AA. NO and NO donors for pharmaceutical and biological applications. Wiley VCH; Weinheim: 2005. [Google Scholar]
- [8].Radomski MW, Palmer RM, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochemical and biophysical research communications. 1987;148:1482–9. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- [9].Radomski MW, Moncada S. Mechanisms of Platelet Activation and Control. Springer; 1993. The biological and pharmacological role of nitric oxide in platelet function; pp. 251–64. [DOI] [PubMed] [Google Scholar]
- [10].Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH. Hemocompatibility comparison of biomedical grade polymers using rabbit thrombogenicity model for preparing nonthrombogenic nitric oxide releasing surfaces. Journal of Materials Chemistry B. 2014;2:1059–67. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brisbois EJ, Davis RP, Jones AM, Major TC, Bartlett RH, Meyerhoff ME, et al. Reduction in thrombosis and bacterial adhesion with 7 day implantation of S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As catheters in sheep. Journal of Materials Chemistry B. 2015 doi: 10.1039/C4TB01839G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reactionrates in tissue by use of a mathematical model. American Journal of Physiology-Heart and Circulatory Physiology. 1998;274:H2163–H76. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- [13].Regev-Shoshani G, Ko M, Miller C, Av-Gay Y. Slow release of nitric oxide from charged catheters and its effect on biofilm formation by Escherichia coli. Antimicrobial Agents and Chemotherapy. 2010;54:273–9. doi: 10.1128/AAC.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ren H, Colletta A, Koley D, Wu J, Xi C, Major TC, et al. Thromboresistant/anti-biofilm catheters via electrochemically modulated nitric oxide release. Bioelectrochemistry. 2014 doi: 10.1016/j.bioelechem.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nablo BJ, Chen T-Y, Schoenfisch MH. Sol-gel derived nitric-oxide releasing materials that reduce bacterial adhesion. Journal of the American Chemical Society. 2001;123:9712–3. doi: 10.1021/ja0165077. [DOI] [PubMed] [Google Scholar]
- [16].Nablo BJ, Schoenfisch MH. Antibacterial properties of nitric oxide–releasing sol-gels. Journal of Biomedical Materials Research Part A. 2003;67:1276–83. doi: 10.1002/jbm.a.20030. [DOI] [PubMed] [Google Scholar]
- [17].Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. Journal of Investigative Dermatology. 2009;129:2463–9. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- [18].Pegalajar-Jurado A, Wold KA, Joslin JM, Neufeld BH, Arabea KA, Suazo LA, et al. Nitric oxide-releasing polysaccharide derivative exhibits 8-log reduction against Escherichia coli, Acinetobacter baumannii and Staphylococcus aureus. Journal of Controlled Release. 2015;217:228–34. doi: 10.1016/j.jconrel.2015.09.015. [DOI] [PubMed] [Google Scholar]
- [19].Ignarro LJ. Nitric oxide: biology and pathobiology. Academic press; 2000. [Google Scholar]
- [20].Mocellin S, Bronte V, Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Medicinal research reviews. 2007;27:317–52. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- [21].Shin JH, Schoenfisch MH. Improving the biocompatibility of in vivo sensors via nitric oxide release. Analyst. 2006;131:609–15. doi: 10.1039/b600129g. [DOI] [PubMed] [Google Scholar]
- [22].Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chemical Society Reviews. 2006;35:780–9. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- [23].Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials. 2013;34:6957–66. doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Frost M, Meyerhoff ME. In vivo chemical sensors: tackling biocompatibility. Analytical Chemistry. 2006;78:7370–7. doi: 10.1021/ac069475k. [DOI] [PubMed] [Google Scholar]
- [25].Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26:1685–93. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- [26].Frost MC, Meyerhoff ME. Synthesis, characterization, and controlled nitric oxide release from S-nitrosothiol-derivatized fumed silica polymer filler particles. Journal of Biomedical Materials Research Part A. 2005;72:409–19. doi: 10.1002/jbm.a.30275. [DOI] [PubMed] [Google Scholar]
- [27].Cha W, Meyerhoff ME. Catalytic generation of nitric oxide from S-nitrosothiols using immobilized organoselenium species. Biomaterials. 2007;28:19–27. doi: 10.1016/j.biomaterials.2006.08.019. [DOI] [PubMed] [Google Scholar]
- [28].Chipinda I, Simoyi RH. Formation and stability of a nitric oxide donor: S-nitroso-N-acetylpenicillamine. The Journal of Physical Chemistry B. 2006;110:5052–61. doi: 10.1021/jp0531107. [DOI] [PubMed] [Google Scholar]
- [29].Frost MC, Meyerhoff ME. Controlled photoinitiated release of nitric oxide from polymer films containing S-nitroso-N-acetyl-DL-penicillamine derivatized fumed silica filler. Journal of the American Chemical Society. 2004;126:1348–9. doi: 10.1021/ja039466i. [DOI] [PubMed] [Google Scholar]
- [30].Dicks A, Swift H, Williams D, Butler A, AlSadoni H, Cox B. Identification of Cu+ as the effective reagent in nitric oxide formation from S-nitrosothiols (RSNO) Journal of the Chemical Society, Perkin Transactions 2. 1996:481–7. [Google Scholar]
- [31].Sexton DJ, Muruganandam A, McKenney DJ, Mutus B. Visible light photochemical release of nitric oxide from S-nitrosoglutathione: Potential photochemotherapeutic applications. Photochemistry and Photobiology. 1994;59:463–7. doi: 10.1111/j.1751-1097.1994.tb05065.x. [DOI] [PubMed] [Google Scholar]
- [32].Wood PD, Mutus B, Redmond RW. The mechanism of photochemical release of nitric oxide from S-nitrosoglutathione. Photochemistry and Photobiology. 1996;64:518–24. doi: 10.1111/j.1751-1097.1994.tb05065.x. [DOI] [PubMed] [Google Scholar]
- [33].Salas E, Moro M, Askew S, Hodson H, Butler A, Radomski M, et al. Comparative pharmacology of analogues of S-nitroso-N-acetyl-dl-penicillamine on human platelets. British Journal of Pharmacology. 1994;112:1071–6. doi: 10.1111/j.1476-5381.1994.tb13192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stokes G, Potts J, Lotz M, Bartter F. New Agent in the Treatment of Cystinuria: N-acetyl-D-penicillamine. Br Med J. 1968;1:284–8. doi: 10.1136/bmj.1.5587.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Simmons A, Padsalgikar AD, Ferris LM, Poole-Warren LA. Biostability and biological performance of a PDMS-based polyurethane for controlled drug release. Biomaterials. 2008;29:2987–95. doi: 10.1016/j.biomaterials.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [36].Cozzens D, Luk A, Ojha U, Ruths M, Faust R. Surface characterization and protein interactions of segmented polyisobutylene-based thermoplastic polyurethanes. Langmuir. 2011;27:14160–8. doi: 10.1021/la202586j. [DOI] [PubMed] [Google Scholar]
- [37].Chung T-W, Liu D-Z, Wang S-Y, Wang S-S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials. 2003;24:4655–61. doi: 10.1016/s0142-9612(03)00361-2. [DOI] [PubMed] [Google Scholar]
- [38].Hetrick EM, Schoenfisch MH. Analytical chemistry of nitric oxide. Annual review of analytical chemistry (Palo Alto, Calif) 2009;2:409. doi: 10.1146/annurev-anchem-060908-155146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Coneski PN, Schoenfisch MH. Nitric oxide release: part III. Measurement and reporting. Chemical Society Reviews. 2012;41:3753–8. doi: 10.1039/c2cs15271a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Clough P, Thrush BA. Mechanism of chemiluminescent reaction between nitric oxide and ozone. Transactions of the Faraday Society. 1967;63:915–25. [Google Scholar]
- [41].Shishido SlM, Seabra AB, Loh W, de Oliveira MG. Thermal and photochemical nitric oxide release from S-nitrosothiols incorporated in Pluronic F127 gel: potential uses for local and controlled nitric oxide release. Biomaterials. 2003;24:3543–53. doi: 10.1016/s0142-9612(03)00153-4. [DOI] [PubMed] [Google Scholar]
- [42].Li Y, Lee PI. Controlled nitric oxide delivery platform based on S-nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Molecular Pharmaceutics. 2010;7:254–66. doi: 10.1021/mp900237f. [DOI] [PubMed] [Google Scholar]
- [43].Wo Y, Li Z, Brisbois EJ, Colletta A, Wu J, Major TC, et al. Origin of Long-Term Storage Stability and Nitric Oxide Release Behavior of CarboSil Polymer Doped with S-Nitroso-N-acetyl-D-penicillamine. ACS applied materials & interfaces. 2015 doi: 10.1021/acsami.5b07501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rechendorff K, Hovgaard MB, Foss M, Zhdanov V, Besenbacher F. Enhancement of protein adsorption induced by surface roughness. Langmuir. 2006;22:10885–8. doi: 10.1021/la0621923. [DOI] [PubMed] [Google Scholar]
- [45].Elam J-H, Nygren H. Adsorption of coagulation proteins from whole blood on to polymer materials: relation to platelet activation. Biomaterials. 1992;13:3–8. doi: 10.1016/0142-9612(92)90086-4. [DOI] [PubMed] [Google Scholar]
- [46].Lyman D, Brash J, Chaikin S, Klein K, Carini M. The effect of chemical structure and surface properties of synthetic polymers on the coagulation of blood. II. Protein and platelet interaction with polymer surface. ASAIO Journal. 1968;14:250–5. [PubMed] [Google Scholar]
- [47].Sivaraman B, Latour RA. Delineating the roles of the GPIIb/IIIa and GP-Ib-IX-V platelet receptors in mediating platelet adhesion to adsorbed fibrinogen and albumin. Biomaterials. 2011;32:5365–70. doi: 10.1016/j.biomaterials.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vertes A, Hitchins V, Phillips KS. Analytical challenges of microbial biofilms on medical devices. Analytical Chemistry. 2012;84:3858–66. doi: 10.1021/ac2029997. [DOI] [PubMed] [Google Scholar]
- [49].Rutala WA, Weber DJ, Control CfD. Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control (US); 2008. 2008. [Google Scholar]
- [50].Fraise AP, Lambert PA, Maillard J-Y. Russell, Hugo & Ayliffe's Principles and Practice of Disinfection, Preservation & Sterilization. John Wiley & Sons; 2008. [Google Scholar]
- [51].Hall KK, Giannetta ET, Getchell-White SI, Durbin LJ, Farr BM. Ultraviolet light disinfection of hospital water for preventing nosocomial legionella infection: A 13-year follow-up. Infection Control. 2003;24:580–3. doi: 10.1086/502257. [DOI] [PubMed] [Google Scholar]
- [52].Singh S, Schaaf NG. Dynamic sterilization of titanium implants with ultraviolet light. Internat J Oral Maxillofac Implants. 1989;4:139–46. [PubMed] [Google Scholar]
- [53].Dolman PJ, Dobrogowski MJ. Contact lens disinfection by ultraviolet light. American journal of ophthalmology. 1989;108:665–9. doi: 10.1016/0002-9394(89)90858-1. [DOI] [PubMed] [Google Scholar]