Abstract
Foxp3+ regulatory T cells in peripheral tissues (pTregs) are instrumental in limiting inflammatory responses to non-self antigens. Within the intestine, pTregs are located primarily in the lamina propria, while intraepithelial CD4+ T cells (CD4IELs), which also exhibit anti-inflammatory properties and depend on similar environmental cues, reside in the epithelium. Using intravital microscopy, we show distinct cell dynamics of intestinal Tregs and CD4IELs. Upon migration to the epithelium, Tregs lose Foxp3 and convert to CD4IELs in a microbiota-dependent fashion, an effect attributed to the loss of the transcription factor ThPOK. Finally, we demonstrate that pTregs and CD4IELs perform complementary roles in the regulation of intestinal inflammation. These results reveal intra-tissue specialization of anti-inflammatory T cells shaped by discrete niches of the intestine.
The gut mucosa is exposed to large amounts of both harmless and potentially pathogenic stimuli on a daily basis, hence diverse immune regulatory mechanisms must operate to avoid inflammatory diseases (1). Peripheral Foxp3-expressing regulatory T cells (pTregs) mediate suppression of a variety of immune cells and actively prevent inflammatory bowel diseases and food allergies (2–7). Similar to pTregs, Foxp3−CD8αα+CD4+ intraepithelial lymphocytes (CD4IELs) depend on retinoic acid (RA) and transforming growth factor (TGF)-β signaling for their development and also have anti-inflammatory properties (4, 8–13). However, while CD4IELs accumulate in the intestinal epithelium, very few total Tregs (including pTregs or thymically-derived Tregs) can be found at this site (fig. S1A and B). We asked whether and how the intestinal environment segregates pTregs and CD4IELs, the transcriptional control involved in this regulation and how this balance affects gut inflammation.
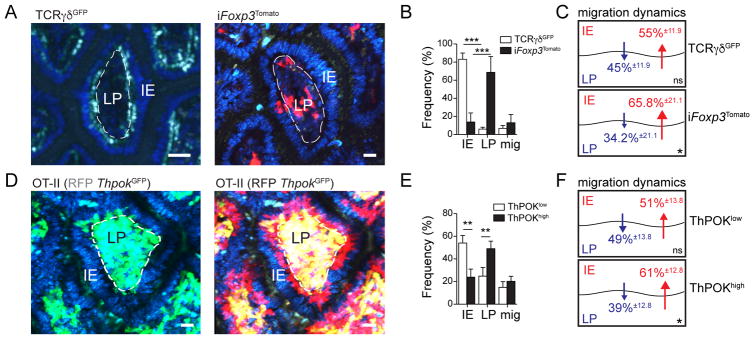
We used intravital multi-photon microscopy (IVM) to investigate whether Tregs are actively excluded from the gut epithelium. For tracking in vivo Treg dynamics, we utilized tamoxifen-inducible Foxp3CreER-eGFP:Rosa26lsl-tdTomato (iFoxp3Tomato) mice (14), which allow Treg fate mapping and the distinction between cells that currently express and cells that once expressed Foxp3. We compared Treg movement patterns in these mice shortly (24 hours) after tamoxifen administration, allowing the visualization of bona fide Tregs (Tomato+), to thymus-derived γδ IEL cell movement patterns (15). We found that more than 80% of TCRγδGFP cells preferentially remained in the epithelium, while 68% and 14% of Tomato+ cells were considered lamina propria and IE residents, respectively, throughout the duration of multiple independent image recordings (Fig. 1A, B and movies S1 and 2). Among the remaining (~18%) migrating Tomato+ cells, cell tracking indicated that cells moved into the epithelial layer from the lamina propria more frequently than vice-versa (Fig. 1B, C and movie S2). Because ex vivo analysis of the intestinal epithelium revealed a low frequency of Foxp3+ Tregs (fig. S1A), these IVM results suggested either preferential cell-death of Tregs or Treg conversion into another T cell subtype in this layer.
Fig. 1. ThPOK levels correlate with reciprocal Treg and CD4IEL localization and migration dynamics in the intestine.
(A to F) Intravital microscopy (IVM) analysis of ileal villi. Mice were injected with Hoechst prior to imaging to visualize all nuclei (blue). Scale bar=10μm. (A) Time-stacked image of TCRγδGFP (left, green channel) mice and iFoxp3Tomato (right, red channel) mice, 24 hours after tamoxifen administration. Images are representative of 20–22 villi from at least 3 independent experiments. (B, C) Frequency of intraepithelial (IE), lamina propria (LP) or migratory TCRγδGFP and iFoxp3Tomato cells. (C) Percentages within migrating cells. (D to F) Sorted naïve CD4+ T cells from OT-II (RFP ThpokGFP) mice were transferred to Rag1−/− mice and recipient mice were fed an OVA-containing diet for 7 days before IVM analysis. (D) Time-stacked image of GFP+ (left, green channel and blue channel overlay) and GFP+ (yellow) and GFP− (red) cells (right, green, red and blue channel overlay). Time-stacked images are representative of at least 50 villi from 4 independent experiments. (E) Quantification of tracked GFP+ and GFP− cell dynamics from 4 different movies (total 6-paired villi) in 2 independent experiments. (F) Percentages within migrating cells. Cells were tracked using Imaris software (Bitplane UK). Data are expressed as mean ± SD from independent movies. ns: not significant, *P<0.05, **P<0.01, ***P<0.001 (Student’s t test).
While all Tregs express the CD4-lineage transcription factor ThPOK, or Zbtb7b, CD8αα+CD4+ and more than 50% of Foxp3−CD8α− CD4+ cells in the small intestine epithelium lack ThPOK expression (fig. S1C). We thus asked whether down-modulation of ThPOK during CD4IEL differentiation influences CD4+ T cell dynamics. We performed IVM using an ovalbumin (OVA)-specific T cell receptor (TCR) transgenic system (OT-II), in which oral OVA exposure leads to incremental ThPOK loss by intestinal CD4+ T cells (10, 16). To allow for the continuous visualization of T cells upon ThPOK loss, we crossed OT-II (Rag1−/−) with ThpokGFP knockin reporter and ubiquitous mRFP1 mice. Sorted naïve (RFP+GFP+) CD4+ T cells from OT-II (RFP ThpokGFP) mice were transferred to Rag1−/− mice, and recipient mice were kept on an OVA-containing diet for one week and then analyzed by IVM. Computational tracking revealed that roughly 60% of the transferred cells that downregulated ThPOK (RFP+GFP−), and only 20% of ThPOKhigh cells (RFP+GFP+) remained in the epithelial layer (Fig. 1D, E and movie S3). Migrating ThPOKhigh cells showed similar movement patterns to Treg cells, with preferential displacement from the lamina propria towards the epithelial layer, suggesting that part of these cells convert into ThPOKlow cells, or die, in that compartment (Fig. 1F). These observations indicate that loss of ThPOK corresponds to an IEL-like behavior in CD4+ T cells. Additionally, the discrepancy between the capacity of Tregs to visit the intestinal epithelium and their low frequency suggests that this environment may favour Treg plasticity.
To directly address Treg plasticity in the gut tissue, we performed Treg fate mapping using naïve adult Foxp3Cre-YFP:Rosa26lsl-DsRed (Foxp3DsRed) mice (17). Analysis of peripheral lymphoid tissues isolated from Foxp3DsRed mice revealed an almost complete concurrence between Foxp3 reporter (YFP) and DsRed expression, confirming previously the described stability of the Treg lineage (14, 18). However, over 40% of DsRed+ CD4+ T cells in the small intestine epithelium did not express Foxp3 (YFP−), indicating that a considerable number of Tregs, or cells that had once turned on the Foxp3 promoter, lost Foxp3 expression (fig. S2A). Because previous studies have demonstrated that the majority of “ex-Foxp3” cells in the steady state were derived from uncommitted precursors that transiently upregulated Foxp3 (18), we also performed fate mapping after pulse-labeling iFoxp3Tomato mice with tamoxifen (14), a strategy more likely to target bona fide Tregs (19). Nevertheless, while stable Foxp3 expression was again observed in several peripheral tissues examined, over 50% of Tomato+ CD4+ T cells that accumulated in the small intestine and almost 10% in the large intestine epithelium isolated from iFoxp3Tomato mice no longer expressed Foxp3 fives weeks post tamoxifen administration (fig. S2B and C). The contribution of Tomato+ cells to the CD8αα+ and CD8αβ+ CD4IEL pools was roughly 10% and 25%, respectively (fig. S2D). Consistent with a ThPOK-dependent process, ex-Tregs that underwent IEL differentiation showed low levels of ThPOK (fig. S2E). These results indicate that a substantial proportion of intestinal Tregs physiologically convert to CD4IELs.
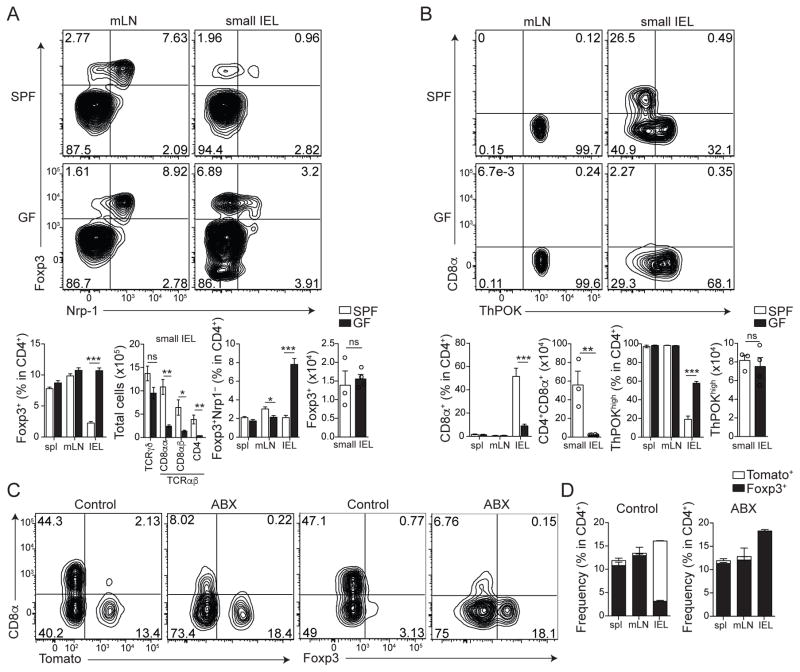
Commensal bacteria play a major role in the induction of lamina propria pTregs in the large intestine (3, 5–7, 20). In contrast, we observed an increased frequency of pTregs (Neuropilin-1− Foxp3+) in the small intestinal epithelium isolated from germ-free (GF) mice when compared to specific pathogen-free (SPF) controls (Fig. 2A). The total Treg number in the epithelial compartment was comparable between GF and SPF mice, even though GF mice showed an almost tenfold reduction in the number of intraepithelial CD4+ T cells (Fig. 2A). Consistent with a reciprocal ThPOK or Foxp3 expression and CD4IEL differentiation, we found an increased frequency of ThPOKhigh CD4+ T cells and significantly reduced CD4IELs in GF mice (Fig. 2B). We therefore reasoned that the instability of Tregs in the gut epithelium was influenced by the microbiota. To address this possibility, we treated iFoxp3Tomato mice with broad-spectrum antibiotics for five weeks, starting immediately after tamoxifen exposure. We observed that microbiota depletion completely prevented Foxp3 loss within the Tomato+ CD4+ T cell population, resulting in an accumulation of Tregs in the epithelial compartment (Fig. 2C, D). The direct contribution of microbial metabolites versus microbial or dietary antigens to the differentiation of CD4IELs or pTregs occupying the small intestinal epithelium (21) remains to be fully determined. Nevertheless, provision of a TCR ligand can overcome the strict microbiota requirement for CD4IEL differentiation, as demonstrated by the relatively normal CD4IEL differentiation in antibiotic-treated OT-II ThpokGFP Rag1−/− mice exposed to oral OVA (fig. S2F). Along with the above data, these observations substantiate a microbial-induced plasticity of Treg cells in the epithelium, corroborating intra-tissue specialization and conversion of gut CD4+ T cells.
Fig. 2. Microbiota-dependent plasticity of Tregs in the intestinal epithelium.
(A, B) Flow cytometry analysis of lymphocytes from spleen (spl), mesenteric lymph nodes (mLN) and small intestine epithelium (IEL) of wild-type C57BL/6 mice maintained under specific pathogen-free (SPF) or germ-free (GF) conditions. (A) Surface neuropilin-1 (Nrp-1) and intracellular Foxp3 expression by TCRβ+CD4+CD8β− cells. Bar graphs represent frequency and total number of Foxp3+ (left) or Foxp3+Nrp-1− (pTregs) (right) among TCRβ+CD4+CD8β− cells. Total cell number for T cell populations isolated from the sIELs is also shown. (B) Surface CD8α and intracellular ThPOK expression by TCRβ+CD4+CD8β− cells. Bar graphs represent frequency and total number of CD8α+ (left) or ThPOKhigh (right) among TCRβ+CD4+CD8β− cells. Data expressed as mean ± SD of individual mice (n=6), representative of 3 independent experiments. (C, D) Flow cytometry analysis of lymphocytes from spl, mLN and small intestine IEL of Foxp3CreER-eGFP:Rosa26lsl-tdTomato (iFoxp3Tomato) mice treated with tamoxifen and maintained with broad spectrum antibiotics for 5 weeks (ABX) or sucralose (CTRL). (C) Surface CD8α and Tomato expression or intracellular Foxp3 among TCRβ+CD4+ cells. (D) Frequency of Foxp3+ (black) and Tomato+ (white) among TCRβ+ CD4+ cells in each tissue. Data expressed as mean ± SD of individual mice (n=3), representative of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 (Student’s t test).
Next, we interrogated whether the reciprocal properties of pTregs and CD4IELs were influenced by lineage-defining transcription factors. We first determined the role of ThPOK by crossing mice in which exons 2 and 3 of Thpok are flanked by loxP sites (Thpokfl/fl)(22) with Cd4CreER mice (fig. S3A)(23). In vivo administration of tamoxifen to iCd4(ΔThpok) mice (16) one week prior to analysis resulted in a 32% to 48% reduction in the frequency of Foxp3+ Tregs in lymphoid and intestinal tissues, respectively, whereas an increase in CD4IELs was observed only in intestinal tissues (Fig. 3A, B). To examine the Treg cell-intrinsic nature of these findings, we next crossed Thpokfl/fl with Foxp3CreER-eGFP (fig. S3B)(16). Ex vivo analysis of T cells from iFoxp3(ΔThpok) mice one week after tamoxifen treatment did not show a significant decrease in Foxp3 levels in the tissues examined, suggesting that ThPOK was not required for short term stability of the lineage. However, analysis of T cells from iFoxp3(ΔThpok) mice five weeks post-tamoxifen pulse revealed a significant reduction in the frequency and levels of Foxp3 in CD4+ T cells located in most compartments analyzed, with the exception of the large intestine (Fig. 3C, D). Reciprocally, we found an accumulation of CD8αα+ CD4+ T cells in the small intestine (Fig. 3C, D). These data provides further support for a ThPOK role in the regulation of the Treg de novo conversion and stability.
Fig. 3. ThPOK expression by intestinal epithelial CD4+ T cells plays a key role in the reciprocal development of Tregs and CD4IELs.
(A–D) Flow cytometry analysis of lymphocytes from spleen (spl), mesenteric lymph nodes (mLN), small and large intestine epithelium (IEL) and lamina propria (LPL) of inducible conditional Thpok-deficient mice. (A, B) Cd4CreER:Thpokfl/fl (iCd4(ΔThpok)) and (Cre−) Thpokf/f littermate control mice 7 days post-tamoxifen administration. (A) Representative contour plot for surface CD8α and intracellular Foxp3 among TCRβ+CD4+CD8β− cells. (B) Frequency of CD8α+ (upper) and Foxp3+ (lower) among TCRβ+CD4+CD8β− or among total CD45+ cells in the indicated tissues. Data expressed as means ± SD of individual mice (n=3–6), representative of 6 independent experiments. (C, D) Foxp3CreER:Thpokfl/fl (iFoxp3(ΔThpok)) and (Cre+)Thpok+/+ littermate control mice 7 days (shown in D) or 35 days post-tamoxifen administration. (C) Representative contour plot for surface CD8α and intracellular Foxp3 among TCRβ+CD4+CD8β− cells. (D) Frequency of CD8α+ (upper) and Foxp3+ (lower) among TCRβ+CD4+CD8β− or among total CD45+ cells in the indicated tissues. Data expressed as means ± SD of individual mice (n=3–8), representative of 3 independent experiments. (E) Frequency and total number of CD8α+ and Foxp3+ among TCRβ+CD4+CD8β− cells from sIEL of Ox40(ΔTbx21), Cd4(ΔRunx3), E8I(ΔTbx21), and wild-type (WT) mice. Data expressed as mean ± SD of individual mice (n=3–9), representative of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 (Student’s t test (B), one-way ANOVA with Tukey post-test (D, E)).
To target the CD4IEL lineage defining transcription factors, we generated mice with conditional deletion of Runx3 or Tbx21 (encoding T-bet), which mediate downregulation of ThPOK in developing CD4IELs (9, 10). We analyzed the epithelial compartment of Cd4(ΔRunx3) mice, and found a reduction in the frequency and in the number of CD4IELs and, conversely, enrichment in Tregs (Fig. 3E). Next, we analyzed mice in which Tbx21 was excised early (driven by Ox40Cre (24)), or late (driven by E8ICre (25)) during the CD4IEL differentiation (16). Ox40(ΔTbx21) mice also showed reduced CD4IEL and roughly a two-fold increase in Tregs in the epithelium, while E8I(ΔTbx21) mice showed reduced CD4IELs, but normal numbers of Tregs in the epithelium when compared to Cre− control mice (Fig. 3E). Collectively, the above data provide a possible mechanism for the reduced number of Tregs in the gut epithelium, where collaboration between Runx3 and T-bet results in downmodulation of ThPOK, and Foxp3, in CD4+ T cells (9, 10).
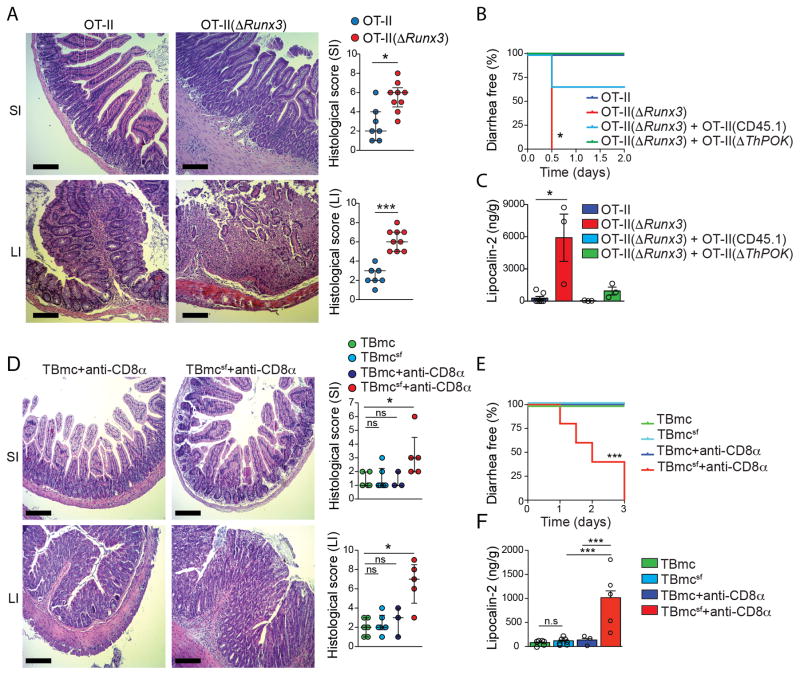
Oral exposure to TCR ligands results in both pTreg and CD4IEL differentiation in a TGF-β-dependent manner (4, 8, 10, 11, 13, 26). We asked whether the intra-tissue adaptation of pTregs and CD4IELs influences the outcome of T cell responses to dietary antigens by employing a transcription factor-based targeting of these lineages in OVA-specific TCR transgenic mice on Rag1−/− background (16). Conditionally targeting Runx3 in the OT-II model (OT-II(ΔRunx3)) prevented ThPOK loss and CD4IEL differentiation, and also impacted pTreg differentiation in the large intestine while no differences in cytokine production were found when compared to control OT-II mice (fig. S4A–D). Whereas OVA-challenged control OT-II mice showed few or no signs of intestinal inflammation, OT-II(ΔRunx3) mice readily developed diarrhea and severe pathology, as confirmed by fecal lipocalin-2 levels (Fig. 4A–C). We concluded that prevention of ThPOK loss and CD4IEL differentiation resulted in a local inflammatory response towards dietary antigens, although the reduction in pTregs could also contribute to the exaggerated inflammatory phenotype observed in the large intestine of OT-II(ΔRunx3) mice. In contrast, conditionally targeting Thpok by administering tamoxifen to OVA-fed iOT-II(ΔThpok) mice (16) resulted in a severe impairment of pTreg development in all tissues examined with a concomitant increase in CD4IELs in the intestine, while no inflammatory phenotype was observed (fig. S4E–G). We then tested whether the OT-II(ΔRunx3) disease phenotype could be rescued, or prevented by wild-type or Thpok-deficient OT-II cells (16). We found that transferred wild-type CD45.1 OT-II cells, which could differentiate to both pTreg and CD4IELs, as well as CD4+ T cells from tamoxifen-treated iOT-II(ΔThpok) mice, which could only differentiate to CD4IELs, rescued the diarrhea and inflammatory phenotype observed in OT-II(ΔRunx3) mice (Fig. 4B, C and fig. S4H and I). These data indicate that a balance in ThPOK levels regulates inflammatory, CD4+ T cell-mediated responses to dietary antigens. Additionally, these results suggest that CD4IELs exert a cell-extrinsic control of local intestinal inflammation.
Fig. 4. Complementary roles for pTregs and CD4IELs in regulating local inflammatory response towards dietary antigens.
OVA-specific TCR transgenic mice (Rag1−/− background) were fed an OVA-containing diet for 7 days. (A–C) ThpokGFP OT-II(ΔRunx3) and control ThpokGFP OT-II mice were analyzed. (A) Hematoxylin and eosin staining of the small intestine jejunum (SI) (upper panels) and the large intestine colon (LI) (lower panels). Original magnification, 40x. Graphs represent histological scores of the SI (upper) and the LI (lower) (each symbol represents one mouse). (B, C) Sorted naïve OVA-specific TCR transgenic cells (TCRVα2+CD4+CD62L+CD44low) from wild-type OT-II or tamoxifen-treated iOT-II(ΔThpok) were transferred to host OT-II(ΔRunx3) prior to treatment as in (A). (B) Frequency of diarrhea-free mice after oral OVA challenge. (C) Quantification of fecal Lipocalin-2. (D–F) TBmc Foxp3sf (scurfy) and TBmc control treated as in (A), and injected with isotype control or anti-CD8α depleting antibody. (D) Hematoxylin and eosin staining of the SI (upper) and the LI (lower). Original magnification, 40x. Graphs represent histological scores of the SI (upper) and the LI (lower) (each symbol represents one mouse). (E) Frequency of diarrhea-free mice after oral OVA challenge. (F) Quantification of fecal Lipocalin-2. Data expressed as mean +SD or median ± interquartile range (A, D), representative of at least two independent experiments (n=3 to 8 per group). Scale bar, 200 μm. *P<0.05, **P<0.01, ***P<0.001 (Student’s t test or Mann-Whitney test (A), one-way ANOVA with Tukey post-test (C, F), Log-rank test (B, E) and Kruskal-Wallis with Dunns post-test (D)).
To specifically address the possibility that pTregs and CD4IELs play complementary anti-inflammatory in the intestine, we compared T cell responses to dietary OVA using BALB/c background monoclonal OVA-specific TCR strains, carrying either wild-type Foxp3 or a scurfy mutation (Foxp3sf)(16), which results in a Foxp3 loss of function (27). In contrast to OT-II mice (C57BL/6 background), TBmc Foxp3wt mice fed an OVA diet showed a high rate of pTreg induction in all tissues examined, but less ThPOK loss and CD4IELs in the epithelium (fig. S4A–C and J–N). Conversely, TBmc Foxp3sf showed a high degree of ThPOK loss and increased CD4IEL development in small intestine, in a frequency that mirrored the relative amounts of pTregs in TBmc Foxp3wt mice (fig. S4J–N). However, no inflammatory phenotype or diarrhea was observed even in the absence of functional Foxp3 in this monoclonal model (Fig. 4D–F). To directly address whether exaggerated CD4IEL differentiation could compensate for the absence of pTregs in TBmc Foxp3sf mice, we depleted CD4IELs using anti-CD8α antibodies during OVA feeding (fig. S4M and N). We found that TBmc Foxp3sf mice treated with anti-CD8α antibodies, but not TBmc Foxp3wt treated with anti-CD8α antibodies or TBmc Foxp3sf treated with control antibodies, showed severe intestinal inflammation and diarrhea (Fig. 4D–F and fig. S4O). These results support a model in which CD4IELs and pTregs cooperate in the regulation of local intestinal inflammation.
The single-layered intestinal epithelium constitutes a uniquely challenging location for immune regulatory processes, given its proximity to highly stimulatory luminal contents and limited spatial organization. It is currently thought that Tregs utilize several redundant and complementary mechanisms to suppress inflammatory responses, and their capacity to sense specific environmental cues plays a major role in their function (28–30). The physiological instability that we observe in the Treg lineage within the intestinal epithelium may represent an important modulation of regulatory activity that is coordinated by this particular environment (3, 20, 28, 31, 32). Although our targeting strategies do not discriminate between the function of “ex-Tregs” and “directly-converted” CD4IELs, natural or forced ThPOK downmodulation was previously associated with an impaired helper function in CD4+ T cells, including reduced production of pro-inflammatory cytokines and reduced expression of costimulatory molecules (8, 10, 33). The data presented here supports a cell-extrinsic suppressive role for CD4IELs, although the likely epithelium-specific mechanism employed by these cells to actively regulate or prevent tissue inflammation remains unclear. However, a role for CD4IELs in triggering inflammation via their cytotoxic activity, under specific contexts, is conceivable (8, 34). Nevertheless, the observation that particular environmental cues, such as the microbiota, induce plasticity of seemingly stable lymphocyte lineages may contribute to the understanding of how specialized tissue-adaptation pathways function to balance efficient immune protective responses with tissue tolerance.
Supplementary Material
Acknowledgments
We are indebted to K. Velinzon and N. Thomas for sorting cells, members of the Nussenzweig lab and The Rockefeller University employees for continuous assistance. We thank S. Hemmers (MSKCC) for generating Cd4CreER mice. We especially thank A. Rogoz for outstanding technical support. We thank members of our laboratory, particularly V. Pedicord, and D. Esterhazy, for discussions and critical reading and editing of the manuscript. The data reported in this manuscript are tabulated in the main paper and in the supplementary materials. This work was supported by Leona M. and Harry B. Helmsley Charitable Trust (T.S., D.vK., B.R., D.M.), Japan Foundation for Applied Enzymology and Uehara Memorial Foundation (T.S.), Alexandre Suerman Stipend, Royal Netherlands Academy of Sciences and the Prince Bernhard Cultural Foundation (D.vK.), the Crohn’s & Colitis Foundation of America (B.R., D.M.), the Irma T. Hirschl Award (D.M.), a National Institutes of Health NIH R01 DK093674 grant (D.M.). The Rockefeller University Bio-Imaging Resource Center is supported by the Empire State Stem Cell Fund through NYSDOH C023046. D.M. conceived; D.M and B.S.R. supervised this study; T.S., J.J.L., B.S.R. and D.M. designed experiments; T.S., M.L., D.vK., T.R., H.M.S. and B.S.R. performed and analyzed experiments; T.B. provided the Cd4CreER strain (23); T.S., M.L., D.vK. and B.S.R. prepared figures and helped with manuscript preparation; D.M. wrote the paper.
Footnotes
References
- 1.Shale M, Schiering C, Powrie F. CD4(+) T-cell subsets in intestinal inflammation. Immunological reviews. 2013 Mar;252:164. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 3.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013 Dec 19;504:446. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 4.Mucida D, et al. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005 Jul 1;115:1923. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013 Aug 2;341:569. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013 Dec 19;504:451. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013 Aug 8;500:232. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 8.Mucida D, et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013 Mar;14:281. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis BS, Hoytema van Konijnenburg DP, Grivennikov SI, Mucida D. Transcription Factor T-bet Regulates Intraepithelial Lymphocyte Functional Maturation. Immunity. 2014 Aug 21;41:244. doi: 10.1016/j.immuni.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat Immunol. 2013 Mar;14:271. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007 Aug 6;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011 Jul 22;35:13. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 Aug 6;204:1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010 Sep 24;329:1667. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelblum KL, et al. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proceedings of the National Academy of Sciences of the United States of America. 2012 May 1;109:7097. doi: 10.1073/pnas.1112519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Materials and methods are available as supplementary materials on Science Online.
- 17.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008 Apr;28:546. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012 Feb 24;36:262. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016 May;16:295. doi: 10.1038/nri.2016.36. [DOI] [PubMed] [Google Scholar]
- 20.Atarashi K, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science. 2010 Dec 23; doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KS, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016 Jan 28; doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 22.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008 Oct;9:1131. doi: 10.1038/ni.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sledzinska A, et al. TGF-beta signalling is required for CD4(+) T cell homeostasis but dispensable for regulatory T cell function. PLoS biology. 2013 Oct;11:e1001674. doi: 10.1371/journal.pbio.1001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinger M, et al. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. 2009 Apr 15;182:4581. doi: 10.4049/jimmunol.0900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maekawa Y, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008 Oct;9:1140. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 26.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007 May;26:579. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Curotto de Lafaille MA, et al. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008 Jul 18;29:114. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohnmacht C, et al. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015 Aug 28;349:989. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 30.Sefik E, et al. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015 Aug 28;349:993. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010 Mar;10:159. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 32.Bollrath J, Powrie FM. Controlling the frontier: regulatory T-cells and intestinal homeostasis. Seminars in immunology. 2013 Nov 30;25:352. doi: 10.1016/j.smim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Vacchio MS, et al. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nat Immunol. 2014 Oct;15:947. doi: 10.1038/ni.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol. 2015 Dec;15:771. doi: 10.1038/nri3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chassaing B, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PloS one. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.