Abstract
Down Syndrome (DS) is the most common genetic cause of intellectual disability resulting from trisomy of chromosome 21. The main feature of DS neuropathology includes early onset of Alzheimer's disease, with deposition of senile plaques and tangles. We hypothesized that apoptosis may be activated in the presence of AD neuropathology in DS, thus we measured proteins associated with upstream and downstream pathways of p53 in the frontal cortex from DS cases with and without AD pathology and from Ts65Dn mice, at different ages. We observed increased acetylation and phosphorylation of p53, coupled to reduced MDM2-mediated ubiquitination and lower levels of SIRT1. Activation of p53 was associated with a number of down-stream targets (bax, PARP1, caspase-3, heat shock proteins and PGC1α) that were modulated in both DS and DS/AD compared with age-matched controls. In particular, the most relevant changes (increased p-p53, acetyl-p53 and reduced formation of MDM2/p53 complex) were found to be modified only in the presence of AD pathology in DS. In addition, a similar pattern of alterations in the p53 pathway were found in Ts65Dn mice. These results suggest that p53 may integrate different signals, which can result in a pro-apoptotic-phenotype contributing to AD neuropathology in people with DS.
Keywords: apoptosis, bax, caspase, sirtuins, trisomy 21, Ts65Dn mouse model
INTRODUCTION
Down syndrome (DS) is the most frequent genetic disorder caused by the presence of all or part of a third copy of chromosome 21 (trisomy 21) affecting 1 in 1,000 infants. Characteristic features of DS are intellectual disability and the early appearance of Alzheimer's disease (AD) neuropathology by the age of 50 ys [1]. Among the molecular mechanisms proposed to be involved in the intellectual disability in DS individuals is the widespread hypo-cellularity observed in the brain. A reduction in neuronal number in the hippocampus, parahippocampal gyrus, cerebellum and neocortex of fetuses, children and adults with DS has been reported [2-4]. The brain cell loss may result from impaired neurogenesis during pre- and post-natal stages in fetal brain, also observed in the mouse model of DS [5-7] and defective proliferation of neural precursors. Thus, among putative mechanisms, it is likely that increased susceptibility of neurons to undergo apoptosis may be involved in the cell loss found in DS brain.
Within this scenario, a pivotal function in neuronal death is played by p53 as demonstrated by studies in several in vitro and in vivo models of neurodegeneration, showing increased p53 levels in affected neurons [8, 9]. The tumor suppressor protein p53 has been proposed “as the guardian of the genome” for its crucial role in regulating the transcription of a wide set of genes involved in cell cycle arrest, senescence, antioxidant system or apoptosis in response to various stress signals [10]. Although p53 promotes longevity by decreasing the risk of cancer through activation of apoptosis or cellular senescence, several findings suggest that the uncontrolled increase of its activity may have deleterious effects leading to “abnormal” aging phenotypes [11, 12].
Under normal, non-stressed physiological conditions, p53 protein is maintained at low levels within a cell by its negative regulator MDM2, an ubiquitin ligase responsible for p53 degradation [13]. Cellular stress affects the interaction between p53 and MDM2 leading to the accumulation of the former [14], and reactive oxygen (ROS) also modify p53 and its activity [15]. The regulatory events that affect the amount, stability and activity of p53 are in part derived from a variety of post-translational modifications, including phosphorylation, ubiquitination and acetylation [16].
The molecular basis underlying the choice between cell-cycle arrest and induction of apoptosis by p53 is not well understood. However, among the multiple posttranslational modifications of p53 that have been characterized, the acetylation of key lysine residues within the C-terminal region of p53 appears to be a determinant of activity [17]. Indeed, most studies suggest that acetylation stimulates p53 stabilization, sequence-specific binding activity, and activation of target genes [18, 19]. Further, following stress, p53 is also phosphorylated at multiple residues, thereby modifying its biochemical functions required for increased activity as a transcription factor. It has been reported that, other than being regulated by ROS, the activation of p53 leads to the generation of ROS [20, 21]. Studies from our groups and others support the idea that oxidative stress is a key “toxic” event, which may trigger neurodegenerative phenomena in DS brain accelerating the development of AD [22, 23]. We demonstrated that oxidative stress is an early event in DS phenotype [24] and we suggest that chronic, sublethal doses of ROS activates a plethora of signalling ultimately resulting in neuronal loss in the adult life.
Accumulating evidence suggests that defects in apoptosis may lead to neurodegenerative disorders. However, studies performed in different types of neurodegenerative diseases have led to some controversial results. It is unclear what role apoptotic processes have in DS. We hypothesize that p53 and its regulatory network in DS brain, is active prior to and after the development of AD pathology. Our findings suggest that activation of p53 may contribute to the development of AD neuropathology in DS brain.
MATERIALS AND METHODS
Subjects
Frozen frontal cortex samples from DS and control cases were obtained from the University of California-Irvine-ADRC Brain Tissue Repository, the NIH NeuroBioBank, and the University of Kentucky ADC. The human cases used in the present study are described in Table 1. DS cases were divided into two groups, with (DS) or without a neuropathology diagnosis of AD (DS/AD). All cases with both DS and AD were over the age of 40 years. Thus for the current study, controls were split into two groups, either less than or equal to 40 years (CTRY) or older than 40 years at death (CTRO). The post mortem interval (PMI) was different across groups (p<0.05).
Table 1.
Autopsy case demographics. A complete set of information is reported.
| GROUPS | CTRY | DS | CTRO | DS/AD |
|---|---|---|---|---|
| n. | 8 | 8 | 8 | 8 |
| Gender (m/f) | 4/4 | 5/3 | 5/3 | |
| Age years (avg. ± SD) | 24.8 ± 11.6 | 23.3 ± 16.8 | 57.2 ± 7.6 | 59.0 ± 3.2 |
| PMI (avg. ± SD) | 12.1 ± 4.7 | 13.4 ± 2.1 | 11.3 ± 6.9 | 4.3 ± 1.5 |
Mouse colonies
Mice were generated by repeatedly backcrossing Ts65Dn trisomic females with (C57BL/6JxC3H/ HeJ) F1 hybrid males, the parental generation was purchased from Jackson Laboratories. These breeding pairs produce litters containing both trisomic (Ts65Dn) and disomic (2N) offspring. The pups were genotyped to establish trisomy using standard PCR, as described by [25]. In addition, the recessive retinal degeneration 1 mutation (Pdeb rd1), which is segregates in the colony and results in blindness in homozygotes, was detected in all the Tg animals used in the present study by using a standard PCR. Mice were housed in clear plexiglass cages (20×22×20 cm) under standard laboratory conditions with a temperature of 22±2°C and 70% humidity, a 12-h light/dark cycle and free access to food and water. Mice were sacrificed at 6 and at 12 months of age and the frontal cortex region was collected (Table 2). All the experiments were performed in strict compliance with the Italian National Laws (DL 116/92), the European Communities Council Directives (86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
Table 2.
Mice demographics
| GROUPS | Euploid 6M | Ts65Dn 6M | Euploid 12M | Ts65Dn 12M |
|---|---|---|---|---|
| n. | 8 | 8 | 8 | 8 |
| Gender (m/f) | 3/5 | 5/3 | 4/4 | 5/3 |
| Age months (avg. ± SD) | 6.3 ± 0.2 | 6.1 ± 0.1 | 12.1 ± 0.1 | 12.2 ± 0.2 |
| Weight (avg. ± SD) | 27.2 ± 2.3 | 28.8 ± 5.1 | 44.0 ± 6.4 | 34.3 ± 6.2 |
Samples preparation for Western Blot
Brain tissue (Frontal Cortex) from human and mouse samples were homogenized by sonication in RIPA buffer (pH 7.4) containing 50 mMTris-HCl (pH 7.4), 150 mMNaCl, 1% NP-40, 0.25 % sodium deoxycholate,1mM EDTA, 0,1% SDS, 1mM PMSF, 1mM NaF and 1 mM Na3VO4. was centrifuged at 14,000 ×g for 30 min to remove cellular debris. The supernatant was extracted to determine the total protein concentration by the BCA method (Pierce, Rockford, IL).
Western Blot
For Western blot, 30 μg of proteins were separated by 12% SDS– PAGE using Criterion Gel TGX Stain free (Bio-Rad) and blotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Before blotting, the gel image was used to obtain total protein load to normalize blot analysis. Membranes were blocked for 1h and 30 min with 3% bovine serum albumin in T-TBS at room temperature and then incubated with primary anti-body. The following antibodies were used: p53 (Human, 1:1000) and Acetyl-p53 (1:1000) from Millipore (Boston, MA, USA), from Abcam (Cambridge, MA, USA), Caspase-3 (1:500), p53 (Mouse, 1:1000), p-p53 (1:1000), Sirt1 and 2 (1:1000), Hsp70 (1:1000), Hsp27 (1:1000), PARP1 (1:1000), Bax (1:1000), PCG1alpha (1:1000), Mdm-2 (1:1000) from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The membranes were incubated for 1 h at room temperature with secondary antibody horseradish peroxidase-conjugated anti-rabbit, anti-mouse or anti-goat IgG (1:5000; Sigma–Aldrich, St Louis, MO, USA). Membranes were developed with the Clarity Western ECL Substrate (Bio-Rad) acquired with ChemiDoc XP (Bio-Rad) and analyzed using Image Lab software (Bio-Rad).
Immunoprecipitation
Briefly, 100 μg of protein extracts were dissolved in RIA buffer (10mM Tris, pH 7.6; 140mM NaCl; 0.5% NP40 including protease inhibitors) and then incubated with 1 μg of primary antibody at 4°C overnight. Immunocomplexes were collected by using protein A/G beads (Santa Cruz, CA, USA) for 2 h at 4°C. Immunoprecipitated protein was recovered by resuspending the pellets in reducing SDS buffers and subjected to electrophoresis on 12% gels followed by western blot analysis.
Statistical analysis
Data are expressed as mean ± SD per group. All statistical analyses were performed using a nonparametric one-way ANOVA with post hoc Bonferroni t-tests. p < 0.05 (*) was considered significant. All statistical analysis was performed using GraphPad Prism 5.0 software.
Results
All the results obtained from human autopsy samples were analysed by considering the difference in PMI among groups. Correlation analysis did not show any association between all the reported measures and PMI (supplementary Table 3).
p53: protein level and post-translational modifications
p53 is activated in response to a wide variety of stresses that can damage the cell genome; once it is bound to sites of DNA damage, p53 promotes DNA repair and simultaneously stimulates the transcription of direct effectors of cell-cycle arrest [26]. Our results show that p53 expression was not altered across the four groups, indicating that trisomy does not affect p53 expression levels (Figure 1, A.3). However, the activity of p53 as a sequence-specific transcription factor is highly regulated by post-translational modifications (PTMs), protein–protein interactions and protein stabilization [27].
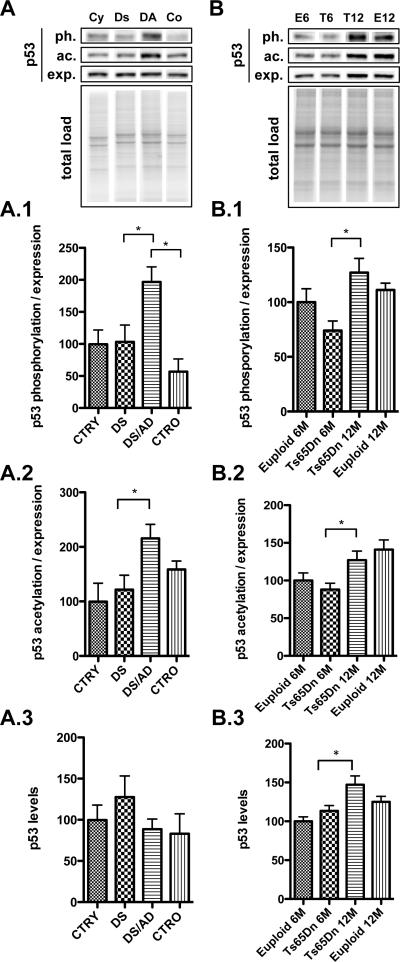
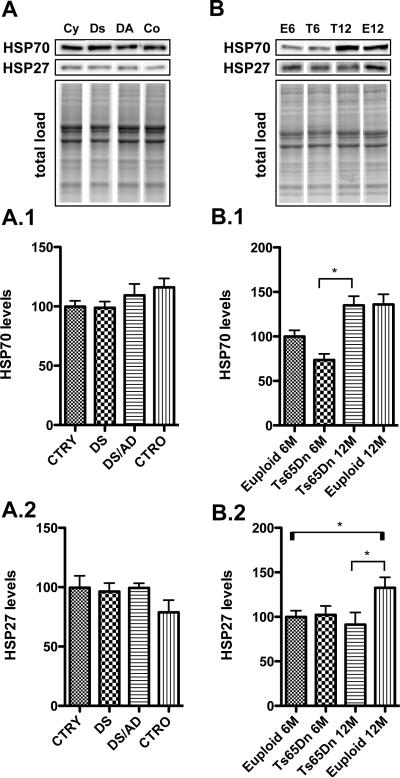
Figure 1. p53 protein levels and post-translational modifications.
p53 phosphorylation at Ser-20 (1)(ph.), acetylation at Lys382 (K382) (2)(ac.) and total expression (exp.) levels (3)were measured by Western Blot in the frontal cortex of controls and DS cases (Panel A) and in the frontal cortex of Ts65Dn mice at 6 and 12 months of age (T6, T12) compared to age-matched euploid animals (E6, E12 -Panel B). Densitometric values shown in the bargraph are the mean of 8 samples per group normalized to total protein load and are given as percentage of control (E6 mice), set as 100%. On the top a representative blot image with protein bands is shown (*p < 0.05).
To investigate the activation of p53, which results from multiple PTMs, we focused our attention on two different PTMs, phosphorylation and acetylation. Both phosphorylation at Ser20 and acetylation of at Lys382 residues within the C-terminal region of p53, are a determinant of its activity [28]. The p-(Ser20)p53 /p53 ratio, which represents the majority of nuclear p53 protein and weakens the interaction of p53 with MDM2, was significantly increased in DS/AD vs. DS (~2 fold increase, *p<0.05) and DS/AD vs. CTRO (~ 150% increase, *p<0.05) (Figure 1, A.1). Similarly, the levels of acetyl-p53 at Lys382 (K382)/p53 were increased in DS/AD vs. DS (~100% increase, *p<0,05). This increase was not statistically significant, in DS/AD vs. CTRO (Figure 1, A.2).
Interestingly, a similar increase in p53 phosphorylation and acetylation was observed in brain of Ts65Dn mice at 12 months of age compared with Ts65Dn at 6 months of age (~ 30% increase in Ts65Dn 12M vs. 6M p<0.05; Figure 1, B.1 and B:2) however the alterations were also accompanied by increased p53 expression levels (~ 40% in Ts65Dn 12M vs. 6M *p<0.05) (Figure 1, B.3).
Upstream regulation of p53: SIRTs and MDM2
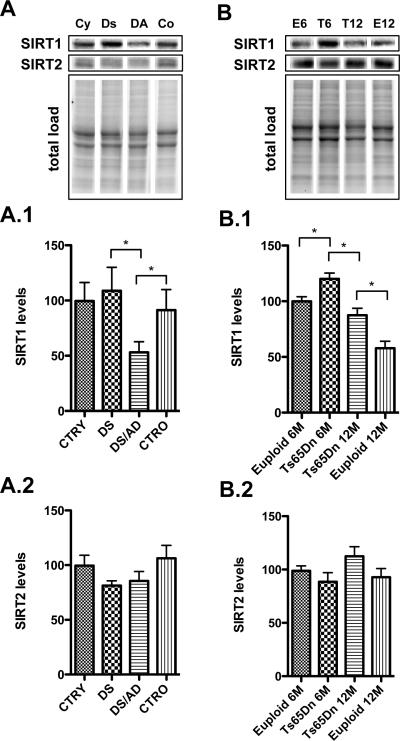
One major regulator of p53 activity is the sirtuin family [29]. By deacetylating p53, both SIRT1 and SIRT2 lead to the degradation of p53 suppressing apoptosis [30, 31]. We found that the levels of SIRT1 are significantly reduced in DS/AD samples vs. DS (*p<0.05) as well as in DS/AD vs. CTRO (~ 50% decrease, *p<0.05) (Figure 2, A.1). Decreased SIRT1 levels were also observed in 12 month-old Ts65Dn mice compared with 6 month-old Tg mice (~ 30% decrease, p<0.05) and also, between 12 month-old Ts65Dn mice and their age matched controls (~ 40% decrease, p<0.05) (Figure 2, B.1). Surprisingly, SIRT1 is expressed in higher amounts in 6 months Ts65Dn mice compared with euploid mice (Figure 2, B.1).
Figure 2. Upstream regulation of p53.
Sirtuins: SIRT1 (1) and SIRT2 (2) levels were measured by Western Blot in the frontal cortex of controls and DS cases (Panel A) and in the frontal cortex of euploid and Ts65Dn mice at 6 and 12 months of age (Panel B). Densitometric values shown in the bargraph are the mean of 8 samples per group normalized per total protein load and are given as percentage of control, set as 100%. On the top a representative blot image with protein bands is shown (*p < 0.05).
In contrast, SIRT2 did not show any significant decreases in both human and mouse samples, suggesting this protein may not be critically involved in DS or age associated AD neuropathology (Figure 2, A.2 and B.2).
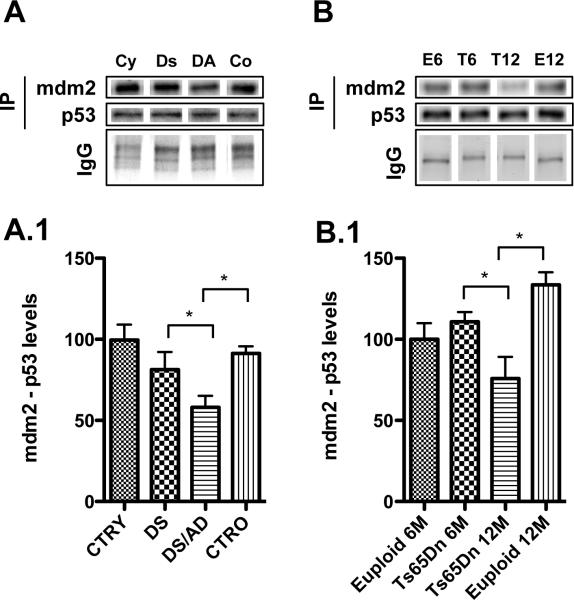
MDM2, a p53-specific E3 ubiquitin ligase, is the principal cellular antagonist of p53, acting to limit the p53 growth-suppressive function in unstressed cells by constantly mono-ubiquitinating p53. The interaction between p53 and MDM2 is conformation-based and is tightly regulated at multiple levels. Disruption of the p53-MDM2 complex by multiple routes is the pivotal event for p53 activation, leading to p53 induction and its biological response [32]. In the present study, we immunoprecipitated p53 and evaluated the levels of p53-MDM2 complexes. As shown in Figure 3, A.1 we found reduced levels of MDM2/p53 complex in DS/AD vs. DS as well as in DS/AD vs. CTRO (about 30% decrease, *p<0.05), thus suggesting that p53 is activated and there is reduced antagonism by the interaction with MDM2 (Figure 3, A.1).
Figure 3. p53-MDM2 complex.
Levels of p53-MDM2 complex were measured by immunoprecipitation/Western Blot in the frontal cortex of controls and DS cases (panel A) and in the frontal cortex of euploid and Ts65Dn mice at 6 and 12 months of age (Panel B). Densitometric values shown in the bargraph are the mean of 8 samples per group and are given as percentage of control, set as 100%. On the top a representative blot image with protein bands is shown (*p < 0.05).
The same trend of reduced MDM2 bound to p53 was also observed in Ts65Dn mice as a function of age, as showed in Figure 3, B.1. A significant reduction of MDM2/p53 complex is observed in 12-month old Ts65Dn compared with 6-month old mice (~ 40% decrease, *p<0.05) but also compared with 12-month old euploid mice (Figure 3, B.1). These data suggest that in “old” Ts65Dn mice, p53 is likely less degraded and possibly transcriptionally active.
p53-down stream targets: apoptotic pathway (BAX, PARP1 and caspase 3), HSPSs and PGC1α
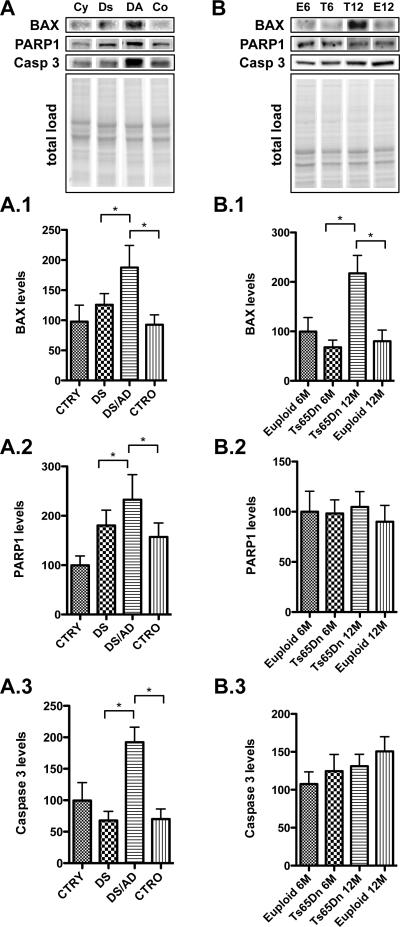
Western blot experiments show that the mitochondrial pro-apoptotic protein BAX was increased in DS/AD vs. CTRO and also in DS/AD vs DS (~ 100% and 80% increased respectively, p<0.05) (Figure 4, A.1). As showed in Figure 4 B.1, the same trend was also observed in Ts65Dn mice, once again showing a significant increase in 12-month old Ts65Dn mice vs. 6-month old Ts65Dn mice as well as vs. 12-month-old euploid mice (~ 100% increase, *p<0.05).
Figure 4. Bax, PARP1 and caspase-3 down stream targets of p53.
Proteins associated with the apoptotic pathway including bax (1), PARP1 (2) and Caspase-3 (3) levels were measured by Western Blot in the frontal cortex of controls and DS cases (Panel A) and in the frontal cortex of Ts65Dn mice at 6 and 12 months of age compared to age-matched euploid animals (Panel B). Densitometric values shown in the bargraph are the mean of 8 samples per group normalized per total protein load and are given as percentage of control, set as 100%. On the top a representative blot image with protein bands is shown (*p < 0.05).
Another apoptotic effector activated in response to oxidative/metabolic stress is the DNA-binding enzymes PARP-1 and PARP-2, which are involved in base excision repair. We found that PARP1 protein levels are increased in DS/AD vs. CTRO and DS/AD vs. DS (~ 30% and 40% increase respectively, p<0.05) (Figure 4, A.2). Conversely, no significant increases were observed in older Ts65Dn mice (Figure 4, B.2).
The apoptotic executioner Caspase-3 was increased in DS/AD compared with DS and also with CTRO (~ 120% increase for both, p<0.05) (Figure 4, A.3), while no age changes were observed in Ts65Dn mice (Figure 4, B.3).
In order to address the presence of a pro-apoptotic phenotype in cerebral cortex of DS as a function of AD pathology, we measured the levels of specific heat shock proteins (HSPs) that are induced/repressed during different types of stress. As shown in Figure 5 Hsp70, that has an anti-apoptotic function, was not modulated in DS human samples (Figure 5, A.1), while an increase of approximately 50% (p<0.05) was observed in 12-month old Ts65Dn mice compared with 6-month-old Ts65Dn mice (Figure 5, B.1). However, this increase is likely due to an aging phenotype rather then specific of the trisomic phenotype.
Figure 5. Heat shock protein in response to p53 activation.
Two heat shock proteins Hsp70 (1) and Hsp27 (2) were measured by Western Blot in the frontal cortex of controls and DS cases (Panel A) and in the frontal cortex of Ts65Dn mice at 6 and 12 months of age compared to age-matched euploid animals (Panel B). Densitometric values shown in the bargraph are the mean of 8 samples per group normalized per total protein load and are given as percentage of control, set as 100%. On the top a representative blot image with protein bands is shown (*p < 0.05).
Consistent with Hsp70, Hsp27 levels, with anti-apoptotic activity, were unchanged in DS human brain (Figure 5, A.2), while euploid mice a slight increase is observed as a function of age (Figure 5, B.2).
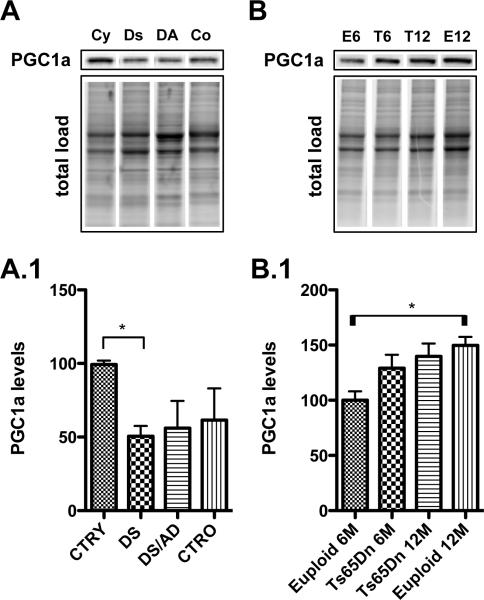
Last, we hypothesized that PGC1α, a downstream target of p53, that is a member of a family of transcriptional co-activators that plays a central role in the regulation of cellular energy metabolism, may be reduced in DS. Recent reports indicate that PGC1α could be a potential biomarker of AD, as reduced PGC1α mRNA and protein levels were detected in AD brains [33, 34]. We found that PGC1α protein levels were reduced only in DS vs. CTRY (~ 50% decrease, *p<0.05), with no further decrease in the presence of AD pathology (Figure 6, A.1). The opposite effect was observed in Ts65Dn mice, PGC1α was not decreased in Ts65Dn mice but a slight increase was found in euploid mice with increasing age (~ 30% increase, p<0.05) (Figure 6, B.1).
Figure 6. PGC1α, a down stream target of p53.
PGC1α levels were measured by Western Blot in the frontal cortex of controls and DS cases (Panel A) and in the frontal cortex of Ts65Dn mice at 6 and 12 months of age compared to age-matched euploid animals (Panel B). Densitometric values shown in the bargraph are the mean of 8 samples per group normalized per total protein load and are given as percentage of control, set as 100%. On the top a representative blot image with protein bands is shown (*p < 0.05).
Discussion
People with DS consistently develop AD neuropathology, which includes deposition of senile plaques, neurofibrillary tangles, and neuronal loss following middle age [35]. Apoptosis has been proposed as a potential mechanism leading to the neuronal death observed in several neurodegenerative disorders, including AD [36, 37]. Increased immunoreactivity for activated caspase-3 is observed in neurons of AD and DS [38]; however, the underlying mechanism remains to be unraveled. In addition, p53 levels are up-regulated and aberrantly modified in the brains of patients with AD and Parkinsons’ disease [39-42]. Elevated gene expression of apoptotic factors including the p53 protein was also detected in the brains of people with DS [43]. In the present study, we did not observe any significant changes in p53 protein levels in the frontal cortex of cases with DS, prior to and after development of AD pathology or in Ts65Dn mice. However, p53 undergoes PTMs that critically control its stability and function, including phosphorylation, acetylation, sumoylation, oxidation and others. We focused our attention on the acetylation of key lysine residues (Lys382) within the C-terminal region of p53 that appear to be a determinant of activity [18, 45-47]. In addition, under different stress stimuli p53 is also phosphorylated at multiple residues. Specifically the phosphorylation of p53 at Ser20 residue is essential for the interaction between MDM2 and p53 [48]. Nevertheless, acetylation or phosphorylation alone are likely not sufficient for p53 activation but their coupled increase might result in the stimulation of an apoptotic pathway. Indeed, we found that, despite no change in the levels of p53, both acetylation and phosphorylation levels were significantly increased in DSAD vs. DS thus suggesting the either age-dependent or AD-dependent activation of p53.
Initial stabilization of p53 occurs through the disruption of the p53 and MDM2 interaction and the balance between acetylation, phosphorylation and ubiquitination appears to be critical to regulate p53 transcriptional activity of antiapoptotic genes. A reduced level of the p53/MDM2 complex was observed in DSAD vs. DS as well as vs. CTRO, confirming that p53 is transcriptionally active. The activation of p53 observed in this study may be in part regulated by the activity of SIRTs, a class of enzyme that deacetylate a variety of proteins, resulting in a robust, protective cellular response. By deacetylating p53, SIRT1 acts as a negative regulator of its activity thus inhibiting apoptosis. Reduced levels of SIRT1 were observed in DSAD compared with younger DS cases, which also supports higher levels of p53 acetylation. In addition, overexpression of SIRT1 is reported to reduce both Aβ production and plaques, whereas removing SIRT1 results in an increase of Aβ levels [49].
Moreover, SIRT1 can promote tau accumulation, by deacetylation [52]. In the presence of reduced SIRT1 levels and p53/MDM2 complex, the steady-state levels of p53 and its target genes could be not efficiently regulated [53]. Surprisingly, the constitutive levels of SIRT1 are increased in Ts65Dn mice compared with euploid mice. Although a decrease in SIRT1 in Ts65Dn with aging was observed, but is higher overall compared to euploid mice suggesting a constitutively higher expression independent of age. Taken together, our results suggest that p53 is transcriptionally active in the presence of AD pathology in DS, suggesting that molecular cascades associated with apoptosis are induced in the neurodegenerative process affecting DS individuals.
To better understand if activation of p53 translates to increased apoptosis, we have evaluated p53-downstream targets. Among these, the levels of the pro-apoptotic factor Bax were increased in DSAD relative to DS and to CTRO. In agreement with our hypothesis, the up-regulation of Bax and the down-regulation of antiapoptotic Bcl-2 factors are observed in different cortical and cerebellar regions of DS foetuses [54, 55].
PARP-1 levels were also increased in the presence of AD pathology in DS brain and associated with the activation of caspase-3. Activation of PARP-1 is another early response event after DNA damage in neurons and mediates a variety of complex mechanisms involved in DNA repair or apoptotic signalling. Accordingly, a previous study by Salemi and colleagues [56] showed increased PARP1 expression in 72% of DS cases compared to controls. Increased immunoreactivity of activated caspase-3 [38] and TUNEL assay labelling [57] are observed in the brains of adults with DS. In addition, Sun et al. [58] showed that overexpression of RCAN1 in primary neurons activates caspase-9 and caspase-3, and subsequently induces neuronal apoptosis..
However, changes in PARP-1 and Caspase-3 levels were not observed in Ts65Dn mice, thus neuronal loss in these Tg mice may be caused only partially by programmed cell death. This may not be entirely surprising, as Ts65Dn mice do not develop AD neuropathology with age. Similarly, decreased expression levels of PGC1α were observed only in human autopsy samples while in Tg mice its levels showed an opposite trend. PGC1α, besides regulating mitochondrial biogenesis and function [59], it is involved in a wide range of cellular metabolic processes and thereby influences cell survival on multiple levels [60]. Interestingly, expression of PGC1α is reduced in AD patients and in the Tg2576 mouse model of AD [33, 34].
This scenario fits with the concept that one of the major contributors to the increased susceptibility of neurons to death is the elevation of oxidative stress, which occurs at early ages in DS brain [36, 61, 62]. Increased oxidative stress conditions in DS may be caused by the triplication of some of the genes encoded on chromosome 21, which directly or indirectly lead to increased free radical release [63-65]. In addition, both in vitro and in vivo studies suggest that in neurons, p53 plays a role in ROS-mediated apoptotic signalling (reviewed in [44]). However, the response of p53 to oxidative damage may depend on the extent of damage. Initially, at low levels of oxidative stress, p53 has primarily antioxidant functions and target genes of p53 are induced to counteract oxidative damage. This could explain the reason why we did not observe significant induction of apoptosis in young DS. However, when oxidative stress overwhelms antioxidant defences, p53 changes its function and serves as a prooxidant by stimulating pro-oxidative genes. This concept is reinforced by the finding that HSPs, a well-recognized protective and pro-survival response, are not induced in DS and DSAD in agreement with previous studies form our group showing that one of the member of HSP family, HO-1, is transcriptionally repressed by the trisomic gene Bach-1 [63].
p53 is activated by the orchestrated action of multiple PTMs, as observed in our study. It also interesting to note that PTMs of p53 may vary between neuronal cell type, depending on the source and severity of the “stress”, and may change during development and aging. However, with the accumulation of oxidative damage over the lifespan, and the consequent impairment of selected proteins [22, 66] as demonstrated by redox proteomics studies from our group, p53 transcriptional activity is increased and a pro-apoptotic phenotype prevails driving the accelerated development of AD neuropathology in DS.
In conclusion, the current study suggests that p53 is transcriptionally active in the brains of DS individuals and is associated with an increase in apoptotic markers in the presence of AD pathology. It is likely that Aβ-induced neurotoxicity and OS– mediated damage are chronic stimuli in neurons and may be responsible for increased susceptibility of neurons to death. We suggest that p53 is able to integrate different signals that lead to a pro-apoptotic phenotype that contributes to the development of AD neuropathology in DS.
Data obtained on Ts65Dn mice confirm the majority of the results obtained by human DS autopsy samples, however some discrepancies exist. We suggest that activation of apoptosis is associated with the development of AD in DS aging brain. Although Ts65Dn mice exhibit many of the neurodegenerative DS phenotypes, they do not display the neuropathological hallmarks of AD - plaques and tangles. Furthermore, differences between studies performed in DS individuals and in Ts65Dn mice, as well as in other trisomic models, could be due to the fact that many of the orthologous genes found in Hsa21 are not triplicated in these models. Additionally, some genes that are not overexpressed in DS are found in trisomy in these mice. Collectively, mouse models have certainly contributed to unravel putative molecular mechanisms responsible of learning and memory deficits in DS and to identify neuronal targets to be tested in clinical trial. Novel Tg mouse models would provide further understanding of the complexity of DS phenotypes.
Supplementary Material
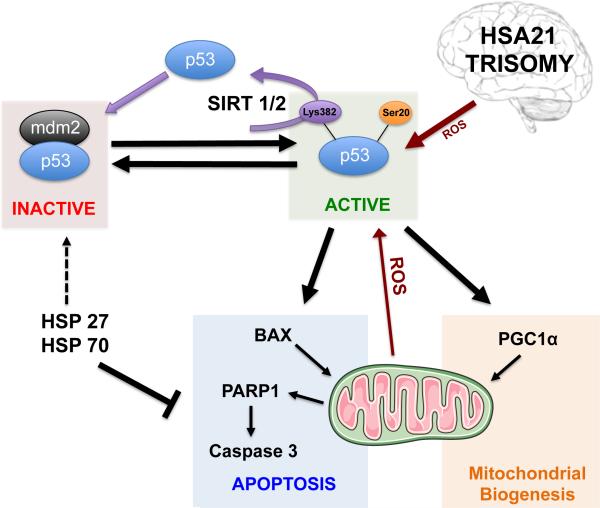
Figure 7. Putative scenario of p53 network in Down Syndrome.
Accumulation and transcriptional activation of p53 occurs in response to a wide variety of insults such as DNA damage, oxidative stress, excitotoxicity, etc. p53 undergoes PTMs that critically control its stability and function, including phosphorylation, acetylation, ubiquitination, oxidation and others. Cellular stress affects the interaction between p53 and MDM2, an ubiquitin ligase responsible for p53 degradation. In parallel, transcriptional activity of p53 requires acetylation and/or phosphorylation that may promote cell death pathway through the activation of downstream targets (Bax, Puma, PARP1, Caspase 3, PGC-1α and others). HSPs (27 and 70) act as pro-survival signals by interacting with pro-apoptotic mediators and stabilizing p53 inactive form (p53/MDM2 complex).
Acknowledgements
This work was partially supported by Fondi di Ateneo Sapienza to Dr. Perluigi and Dr. Di Domenico and funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant agreement n° 624341 to E.B. and M.P.
Brain tissue was acquired by EH under funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Aging Grant NIH/NICHD RO1HD064993. Additional autopsy tissue was obtained from the UCI-ADRC (P50AG16573), from the UK ADC (P30AG28383) and from the NIH NeuroBioBank (www.neurobiobank.nih.gov). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributions.
M.P., D.A.B and F.DD conceived and designed the experiments. A.T., G.P., D.B. and A.A. performed the experiments. E.B., C.L and C.B. performed statistics and analyzed the data. M.P. and A.T. wrote the paper. E.H. and D.A.B. critically revised the manuscript. All the authors read and discussed the manuscript. Study supervision: M.P. and F.DD.
Competing interests.
The authors declare no competing financial interests.
References
- 1.Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH. The changing survival profile of people with Down's syndrome: implications for genetic counselling. Clin Genet. 2002;62:390–393. doi: 10.1034/j.1399-0004.2002.620506.x. [DOI] [PubMed] [Google Scholar]
- 2.Rueda N, Florez J, Martinez-Cue C. Apoptosis in Down's syndrome: lessons from studies of human and mouse models. Apoptosis. 2013;18:121–134. doi: 10.1007/s10495-012-0785-3. [DOI] [PubMed] [Google Scholar]
- 3.Golden JA, Hyman BT. Development of the superior temporal neocortex is anomalous in trisomy 21. J Neuropathol Exp Neurol. 1994;53:513–520. doi: 10.1097/00005072-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Guidi S, Bonasoni P, Ceccarelli C, Santini D, Gualtieri F, Ciani E, Bartesaghi R. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol. 2008;18:180–197. doi: 10.1111/j.1750-3639.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contestabile A, Fila T, Ceccarelli C, Bonasoni P, Bonapace L, Santini D, Bartesaghi R, Ciani E. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice. Hippocampus. 2007;17:665–678. doi: 10.1002/hipo.20308. [DOI] [PubMed] [Google Scholar]
- 6.Contestabile A, Fila T, Cappellini A, Bartesaghi R, Ciani E. Widespread impairment of cell proliferation in the neonate Ts65Dn mouse, a model for Down syndrome. Cell Prolif. 2009;42:171–181. doi: 10.1111/j.1365-2184.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rueda N, Mostany R, Pazos A, Florez J, Martinez-Cue C. Cell proliferation is reduced in the dentate gyrus of aged but not young Ts65Dn mice, a model of Down syndrome. Neurosci Lett. 2005;380:197–201. doi: 10.1016/j.neulet.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 9.Checler F, Alves da Costa C. p53 in neurodegenerative diseases and brain cancers. Pharmacol Ther. 2014;142:99–113. doi: 10.1016/j.pharmthera.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- 12.Fiorini A, Sultana R, Barone E, Cenini G, Perluigi M, Mancuso C, Cai J, Klein JB, St Clair D, Butterfield DA. Lack of p53 affects the expression of several brain mitochondrial proteins: insights from proteomics into important pathways regulated by p53. PLoS One. 2012;7:e49846. doi: 10.1371/journal.pone.0049846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- 15.Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell. 2011;2:456–462. doi: 10.1007/s13238-011-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura S, Roth JA, Mukhopadhyay T. Multiple lysine mutations in the C-terminal domain of p53 interfere with MDM2-dependent protein degradation and ubiquitination. Mol Cell Biol. 2000;20:9391–9398. doi: 10.1128/mcb.20.24.9391-9398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A. 2004;101:2259–2264. doi: 10.1073/pnas.0308762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardaci S, Filomeni G, Rotilio G, Ciriolo MR. Reactive oxygen species mediate p53 activation and apoptosis induced by sodium nitroprusside in SH SY5Y cells. Mol Pharmacol. 2008;74:1234–1245. doi: 10.1124/mol.108.048975. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Nikulenkov F, Zawacka-Pankau J, Li H, Gabdoulline R, Xu J, Eriksson S, Hedstrom E, Issaeva N, Kel A, Arner ES, Selivanova G. ROS-dependent activation of JNK converts p53 into an efficient inhibitor of oncogenes leading to robust apoptosis. Cell Death Differ. 2014;21:612–623. doi: 10.1038/cdd.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Domenico F, Pupo G, Tramutola A, Giorgi A, Schinina ME, Coccia R, Head E, Butterfield DA, Perluigi M. Redox proteomics analysis of HNE-modified proteins in Down syndrome brain: clues for understanding the development of Alzheimer disease. Free Radic Biol Med. 2014;71:270–280. doi: 10.1016/j.freeradbiomed.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perluigi M, Di Domenico F, Buttterfield DA. Unraveling the complexity of neurodegeneration in brains of subjects with Down syndrome: insights from proteomics. Proteomics Clin Appl. 2014;8:73–85. doi: 10.1002/prca.201300066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterfield DA, Di Domenico F, Swomley AM, Head E, Perluigi M. Redox proteomics analysis to decipher the neurobiology of Alzheimer-like neurodegeneration: overlaps in Down's syndrome and Alzheimer's disease brain. Biochem J. 2014;463:177–189. doi: 10.1042/BJ20140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinholdt LG, Ding Y, Gilbert GJ, Czechanski A, Solzak JP, Roper RJ, Johnson MT, Donahue LR, Lutz C, Davisson MT. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm Genome. 2011;22:685–691. doi: 10.1007/s00335-011-9357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva AR, Santos AC, Farfel JM, Grinberg LT, Ferretti RE, Campos AH, Cunha IW, Begnami MD, Rocha RM, Carraro DM, de Braganca Pereira CA, Jacob-Filho W, Brentani H. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer's disease. PLoS One. 2014;9:e99897. doi: 10.1371/journal.pone.0099897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks CL, Gu W. New insights into p53 activation. Cell Res. 2010;20:614–621. doi: 10.1038/cr.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Leeuwen I, Lain S. Sirtuins and p53. Adv Cancer Res. 2009;102:171–195. doi: 10.1016/S0065-230X(09)02005-3. [DOI] [PubMed] [Google Scholar]
- 30.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann G, Breitenbucher F, Schuler M, Ehrenhofer-Murray AE. A novel sirtuin 2 (SIRT2) inhibitor with p53-dependent pro-apoptotic activity in non-small cell lung cancer. J Biol Chem. 2014;289:5208–5216. doi: 10.1074/jbc.M113.487736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanni C, Uberti D, Racchi M, Govoni S, Memo M. Unfolded p53: a potential biomarker for Alzheimer's disease. J Alzheimers Dis. 2007;12:93–99. doi: 10.3233/jad-2007-12109. [DOI] [PubMed] [Google Scholar]
- 33.Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem. 2012;120:419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lott IT, Head E. Down syndrome and Alzheimer's disease: a link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7:172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- 36.Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 37.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 38.Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Bruck W, Jellinger K, Lassmann H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease. Evidence for apoptotic cell death. Am J Pathol. 1999;155:1459–1466. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esposito E, Cuzzocrea S. New therapeutic strategy for Parkinson's and Alzheimer's disease. Curr Med Chem. 2010;17:2764–2774. doi: 10.2174/092986710791859324. [DOI] [PubMed] [Google Scholar]
- 40.Cenini G, Sultana R, Memo M, Butterfield DA. Elevated levels of pro apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer's disease. J Cell Mol Med. 2008;12:987–994. doi: 10.1111/j.1582-4934.2008.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Domenico F, Cenini G, Sultana R, Perluigi M, Uberti D, Memo M, Butterfield DA. Glutathionylation of the pro-apoptotic protein p53 in Alzheimer's disease brain: implications for AD pathogenesis. Neurochem Res. 2009;34:727–733. doi: 10.1007/s11064-009-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cenini G, Sultana R, Memo M, Butterfield DA. Effects of oxidative and nitrosative stress in brain on p53 proapoptotic protein in amnestic mild cognitive impairment and Alzheimer disease. Free Radic Biol Med. 2008;45:81–85. doi: 10.1016/j.freeradbiomed.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de la Monte SM. Molecular abnormalities of the brain in Down syndrome: relevance to Alzheimer's neurodegeneration. J Neural Transm Suppl. 1999;57:1–19. doi: 10.1007/978-3-7091-6380-1_1. [DOI] [PubMed] [Google Scholar]
- 44.Olsson A, Manzl C, Strasser A, Villunger A. How important are post- translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 2007;14:1561–1575. doi: 10.1038/sj.cdd.4402196. [DOI] [PubMed] [Google Scholar]
- 45.Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell. 2001;8:1243–1254. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 46.Liu G, Chen X. Regulation of the p53 transcriptional activity. J Cell Biochem. 2006;97:448–458. doi: 10.1002/jcb.20700. [DOI] [PubMed] [Google Scholar]
- 47.Wang DB, Kinoshita C, Kinoshita Y, Morrison RS. p53 and mitochondrial function in neurons. Biochim Biophys Acta. 2014;1842:1186–1197. doi: 10.1016/j.bbadis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson T, Tovar C, Yang H, Carvajal D, Vu BT, Xu Q, Wahl GM, Heimbrook DC, Vassilev LT. Phosphorylation of p53 on key serines is dispensable for transcriptional activation and apoptosis. J Biol Chem. 2004;279:53015–53022. doi: 10.1074/jbc.M410233200. [DOI] [PubMed] [Google Scholar]
- 49.Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5:344–352. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura T, Lipton SA. S-nitrosylation of critical protein thiols mediates protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Antioxid Redox Signal. 2011;14:1479–1492. doi: 10.1089/ars.2010.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration. J Alzheimers Dis. 2010;20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 54.Helguera P, Pelsman A, Pigino G, Wolvetang E, Head E, Busciglio J. ets-2 promotes the activation of a mitochondrial death pathway in Down's syndrome neurons. J Neurosci. 2005;25:2295–2303. doi: 10.1523/JNEUROSCI.5107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seidl R, Cairns N, Lubec G. The brain in Down syndrome. J Neural Transm Suppl. 2001:247–261. doi: 10.1007/978-3-7091-6262-0_20. [DOI] [PubMed] [Google Scholar]
- 56.Salemi M, Barone C, Romano C, Ridolfo F, Gulotta E, Scavuzzo C, Salluzzo MG, Giambirtone M, Caraci F, Romano C, Bosco P. Differential expression of PARP1 mRNA in leucocytes of patients with Down's syndrome. J Genet. 2011;90:469–472. doi: 10.1007/s12041-011-0074-x. [DOI] [PubMed] [Google Scholar]
- 57.Anderson AJ, Stoltzner S, Lai F, Su J, Nixon RA. Morphological and biochemical assessment of DNA damage and apoptosis in Down syndrome and Alzheimer disease, and effect of postmortem tissue archival on TUNEL. Neurobiol Aging. 2000;21:511–524. doi: 10.1016/s0197-4580(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Wu Y, Chen B, Zhang Z, Zhou W, Tong Y, Yuan J, Xia K, Gronemeyer H, Flavell RA, Song W. Regulator of calcineurin 1 (RCAN1) facilitates neuronal apoptosis through caspase-3 activation. J Biol Chem. 2011;286:9049–9062. doi: 10.1074/jbc.M110.177519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 60.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 61.Perluigi M, Butterfield DA. Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr Gerontol Geriatr Res. 2012;2012:724904. doi: 10.1155/2012/724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lott IT, Head E, Doran E, Busciglio J. Beta-amyloid, oxidative stress and down syndrome. Curr Alzheimer Res. 2006;3:521–528. doi: 10.2174/156720506779025305. [DOI] [PubMed] [Google Scholar]
- 63.Di Domenico F, Pupo G, Mancuso C, Barone E, Paolini F, Arena A, Blarzino C, Schmitt FA, Head E, Butterfield DA, Perluigi M. Bach1 overexpression in Down syndrome correlates with the alteration of the HO-1/BVR-a system: insights for transition to Alzheimer's disease. J Alzheimers Dis. 2015;44:1107–1120. doi: 10.3233/JAD-141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guedj F, Pennings JL, Ferres MA, Graham LC, Wick HC, Miczek KA, Slonim DK, Bianchi DW. The fetal brain transcriptome and neonatal behavioral phenotype in the Ts1Cje mouse model of Down syndrome. Am J Med Genet A. 2015 doi: 10.1002/ajmg.a.37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guedj F, Pennings JL, Wick HC, Bianchi DW. Analysis of adult cerebral cortex and hippocampus transcriptomes reveals unique molecular changes in the Ts1Cje mouse model of down syndrome. Brain Pathol. 2015;25:11–23. doi: 10.1111/bpa.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Domenico F, Coccia R, Cocciolo A, Murphy MP, Cenini G, Head E, Butterfield DA, Giorgi A, Schinina ME, Mancuso C, Cini C, Perluigi M. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer's disease neuropathology: redox proteomics analysis of human brain. Biochim Biophys Acta. 2013;1832:1249–1259. doi: 10.1016/j.bbadis.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.