ABSTRACT
GNbAC1 is a humanized IgG4 monoclonal antibody antagonist of Mulitple Sclerosis Retrovirus Envelope (MSRV-Env), a protein that could play a critical role in multiple sclerosis. This randomized placebo-controlled dose-escalation study evaluated the safety and pharmacokinetics of GNbAC1 in 21 healthy volunteers after single intravenous infusion at doses of 6, 18 and 36 mg/kg. Lumbar punctures were performed at days 2, 15 or 29 to measure GNbAC1 concentrations in cerebrospinal fluid (CSF). GNbAC1 was well tolerated. Serum data show a dose-linear pharmacokinetics. A mean CSF/serum ratio of 0.12% was observed at Day 2, increasing to 0.39% at Day 15 and 0.42% at Day 29. Linear regression analysis shows a relationship between GNbAC1 CSF/serum ratio and albumin CSF/serum ratio and a relationship at the limit of statistical significance with the timing of CSF sampling.
KEYWORDS: Cerebrospinal fluid, clinical trial, monoclonal antibody, multiple sclerosis, pharmacokinetics, safety
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating, neurodegenerative disorder of the central nervous system (CNS) whose etiology remains unknown. In MS pathogenesis, dysregulation of both innate and adaptive immune system is considered a main triggering or exacerbating factor. Pathological key features of MS could be due to the multiple sclerosis-associated retrovirus envelope protein (MSRV-Env), which is expressed in active brain lesions and has been shown to exert pro-inflammatory effects and myelination impairment by its interaction with the TLR4-receptor. MSRV-Env induces the release of pro-inflammatory cytokines such as interleukin (IL)-1ß, IL-6 or tumor necrosis factor1 from peripheral blood mononuclear cells (PBMC) in vitro, an effect that can be prevented by anti-toll-like receptor (TLR)4 antibodies. Furthermore, by interaction with the TLR4 receptor on oligodendrocyte precursor cells (OPC), MSRV-Env blocks their differentiation to mature oligodendrocytes necessary for remyelination.2 Based on this ability of MSRV-Env to activate the innate immune system, and given its direct toxicity on OPCs, MSRV-Env has emerged as a potential therapeutic target for MS.3 The aim of this approach is to target a potentially key factor for the disease without the need to modulate or suppress the immune system, which is currently the main approach for MS treatment.4,5
To explore the effects of targeting MSRV-Env in humans, the mAb (GNbAC1), which selectively binds with high affinity to the extracellular domain of the MSRV-Env, was selected for clinical development. GNbAC1 is a recombinant humanized monoclonal antibody (mAb) of the IgG4/kappa isotype (for a review of the GNbAC1 development, see ref. Three). Preclinical tests of GNbAC1 demonstrated its efficacy in MSRV-Env-induced experimental allergic encephalitis (EAE) in mice, as well as in in vitro cellular models. GNbAC1 is expected to neutralize the expression of MSRV-Env in MS plaques and on circulating lymphocytes, and prevent its inflammatory and neurodegenerative effects.
After a first-in-man study with single doses up to 6 mg/kg administered intravenously (i.v.) in 33 young healthy volunteers, GNbAC1 was tested in 10 MS patients in a randomized placebo controlled trial with a one year open label extension.6,7 Safety was favorable; GNbAC1 pharmacokinetics appears to be dose-linear. Encouraging pharmacodynamics responses in terms of target-related biomarkers and inflammation markers were observed.6,8 The CSF to serum ratio was estimated at 0.2% at one month post dosing in a single patient.7
According to target saturation estimation, a 6 mg/kg i.v. dose of GNbAC1 was estimated to be sufficient to ensure a full target occupancy.3 To ensure an optimal target access, evaluate possible overexpression of the target and maximize the benefit-risk of the product, investigation of higher dosages of GNbAC1 during further phases of its clinical development is planned.
The goals of this double-blind, placebo-controlled dose escalation Phase 1 study were to assess the safety profile of single higher i.v. doses of up to 36 mg/kg GNbAC1 in healthy subjects, and, in particular, to determine pharmacokinetic parameters in serum and GNbAC1 concentrations in CSF.
Results
Twenty-one subjects entered the study in accordance with the protocol and the treatment randomization (GNbAC1 n=15, placebo n=6) (Fig. 1); all completed the study as per protocol except one subject in the GNbAC1 6 mg/kg group who withdrew his consent during the study after being dosed. The three dose levels of GNbAC1 (6, 18, 36 mg/kg i.v.) were studied as planned. All subjects were Caucasian men aged between 21 and 55 years, both inclusive; the mean age was 40 y (SD 9.5), the mean height was 179 cm (SD 8.4) and the mean weight was 81.3 kg (SD 9.5). Demographic data by treatment groups are summarized in Table 1. The mean age and body mass index (BMI) were relatively similar for subjects across all treatment groups. All subjects satisfied the inclusion criteria prior to entry into the study. There were no findings of clinical concern in the medical history for any subjects. In addition, there were no baseline signs and symptoms of clinical concern prior to dosing for any subjects. The results of the urinary screening for drugs of abuse, of alcohol detection in breath and of serological tests at screening indicated that all subjects were suitable for inclusion in the study.
Figure 1.
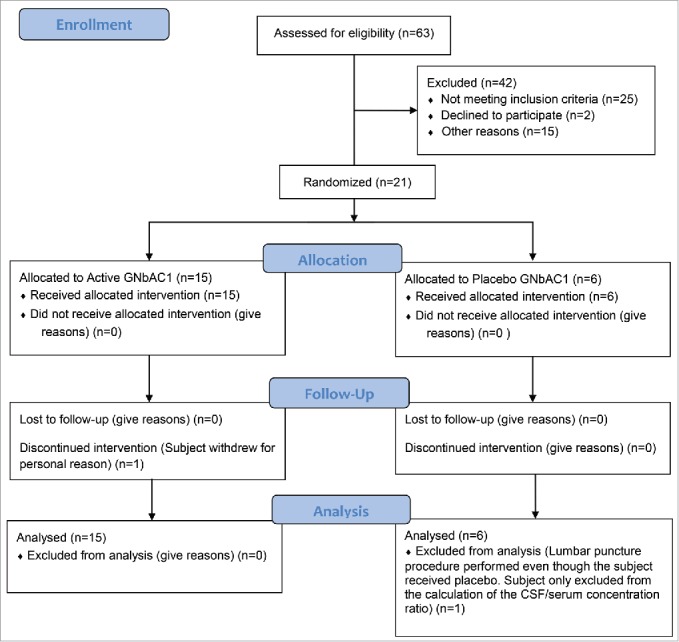
Study flow diagram.
Table 1.
Summary of screening demographic data by dose groups.
| Placebo (n=6) | 6 mg/kg (n=5) | 18 mg/kg (n=5) | 36 mg/kg (n=5) | |
|---|---|---|---|---|
| Age (years) | 39 (9.4) | 36 (9.2) | 44 (8.1) | 43 (12.0) |
| Height (cm) | 180 (5.3) | 176 (9.3) | 183 (11.6) | 178 (8.1) |
| Weight (kg) | 76.7 (9.2) | 81.8 (11.0) | 82.9 (9.6) | 84.8 (9.1) |
| BMI (kg/m2) | 23.7 (3.4) | 26.3 (1.3) | 24.8 (1.7) | 26.8 (1.9) |
Arithmetic mean (SD) data are presented
Abbreviations: BMI = body mass index; n = number of subjects studied
Twenty-one subjects received a single infusion of GNbAC1 or placebo. Single doses of GNbAC1 were well tolerated when administered at dose levels of 6, 18 and 36 mg/kg during 1, 2 and 4 hours infusion, respectively. The treatment emergent adverse events are summarized in Table 2. All adverse events were mild or moderate in severity. No serious adverse events were reported and no subjects were withdrawn as a result of adverse events. Overall, 13 events were reported by 7 subjects. Two adverse events were reported by 2 subjects who had received placebo. The most commonly reported adverse events belonged to the system organ classes “musculoskeletal and connective tissue disorders” and “infections and infestations.” Three adverse events (flu-like symptoms, musculoskeletal pain and postural dizziness) in one of the 36 mg/kg subject were considered to have a possible relationship to study drug. One single increase in alanine amino transferase (ALT) (up to 3 ULN) was reported in a patient receiving the 36 mg/kg dose and was considered as not related to GNbAC1 by the investigator. No changes in vital signs or electrocardiogram (ECG) data apparently related to the study drug were noticed.
Table 2.
Adverse events by dose groups (number of subjects with an AE) by preferred term.
| GNbAC1 | ||||
|---|---|---|---|---|
| System Organ Class##Preferred Term | Placebo##(n=6) | 6 mg/kg## (n=5) | 18 mg/kg## (n=5) | 36 mg/kg## (n=5) |
| General Disorders And Administration Site Conditions | 0 | 0 | 0 | 1 |
| Influenza-Like Illness | 0 | 0 | 0 | 1 |
| Infections And Infestations | 0 | 0 | 2 | 1 |
| Nasopharyngitis | 0 | 0 | 1 | 0 |
| Rhinitis | 0 | 0 | 0 | 1 |
| Upper Respiratory Tract Infection | 0 | 0 | 1 | 0 |
| Injury, Poisoning And Procedural Complications | 0 | 1 | 0 | 0 |
| Post Lumbar Puncture Syndrome | 0 | 1 | 0 | 0 |
| Investigations | 0 | 0 | 0 | 1 |
| Alanine Aminotransferase Increased | 0 | 0 | 0 | 1 |
| Musculoskeletal And Connective Tissue Disorders | 1 | 1 | 1 | 1 |
| Back Pain | 1 | 0 | 1 | 0 |
| Joint Effusion | 0 | 1 | 0 | 0 |
| Musculoskeletal Pain | 0 | 0 | 0 | 1 |
| Myalgia | 0 | 0 | 0 | 1 |
| Nervous System Disorders | 1 | 0 | 0 | 1 |
| Dizziness Postural | 0 | 0 | 0 | 1 |
| Headache | 1 | 0 | 0 | 0 |
The mean serum concentration time curves are presented in Fig. 2. Pharmacokinetic data are in Table 3. The geometric mean t1/2 was estimated between 500 to 621 h (20.8 to 25.9 days) across all dose levels. Similarly, geometric mean residence time (MRT) values by dose were calculated with a range from 682 to 871 h (28.4 to 36.3 days). The median tmax were observed at times ranging from 2 to 4 hours reflecting the increasing durations of drug infusion. Geometric mean clearance ranged from 0.082 to 0.088 mL/h/kg. GNbAC1 exposure increases with increasing doses in a dose proportional manner as illustrated by the ratios of the means for AUC0-tlast and AUC0∞ between doses of 18 mg/kg and 6 mg/kg i.v., which were 2.96 and 3.09, respectively, and the ratios for these parameters between doses of 36 mg/kg and 18 mg/kg i.v., which were 1.96 and 1.80, respectively.
Figure 2.
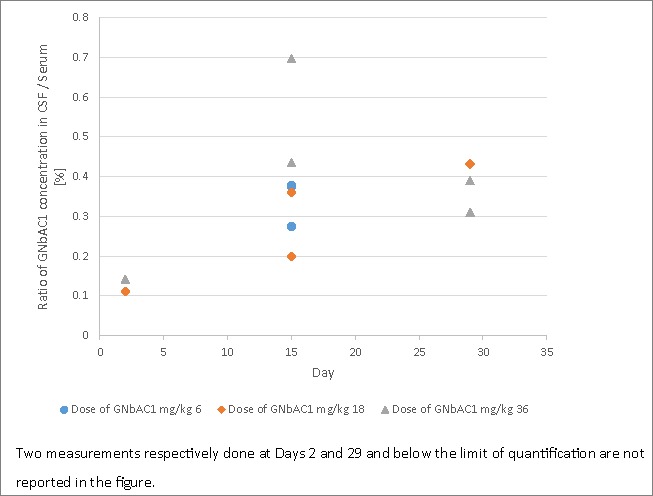
GNbAC1 mean serum pharmacokinetic profiles by dose group.
Table 3.
Geometric mean (CV%) of the pharmacokinetic parameters of GNbAC1 by doses following a single infusion with dose normalized values for Cmax and AUC.
| Parameter | 6 mg/kg (n=5) | 18 mg/kg (n=5) | 36 mg/kg (n=5) |
|---|---|---|---|
| AUC0tlast (µg·h/mL) | 57188 (24.5) | 169392 (18.0) | 335204 (8.8) |
| AUC0∞ (µg·h/mL) | 71465 (31.8) | 220723 (33.0) | 411381 (13.9) |
| Cmax (µg/mL) | 178.0 (8.6) | 452.3 (13.5) | 1018.5 (9.8) |
| tmaxa (h) | 2.02 (1.05-3.05) | 3.02 (2.05-8.02) | 4.06 (4.05-4.10) |
| t1/2 (h) | 500.4 (28.5) | 620.8 (40.3) | 606.0 (10.7) |
| MRT (h) | 682.0 (28.4) | 870.9 (39.1) | 783.1 (11.6) |
| CL (mL/h/kg) | 0.084 (31.8) | 0.082 (33.0) | 0.088 (13.9) |
| Vz (mL/kg) | 60.6 (6.8) | 73.0 (9.6) | 76.5 (6.7) |
| Dose norm AUC0tlast (µg·h/mL) | 9531.4 | 9410.6 | 9311.2 |
| Dose norm AUC0∞ (µg·h/mL) | 11910.9 | 12262.4 | 11427.3 |
| Dose norm Cmax (µg/mL) | 29.7 | 25.1 | 28.3 |
Median (range)
Lumbar punctures were performed in 13 of 15 subjects who received GNbAC1 (one subject withdrew his consent before puncture and one subject withdraw from the study before lumbar puncture). For subjects receiving placebo, a mock lumbar puncture was performed. Mean CSF/serum ratios are presented in Fig. 3 by sampling day and dose. A mean CSF/serum ratio of 0.12% was observed at Day 2, increasing to 0.39% at Day 15 and 0.42% at Day 29. GNbAC1 concentrations in CSF and serum and CSF/serum ratios for GNbAC1 and albumin are shown in Table 4: 2 subjects had albumin ratios higher than 9.0, suggesting a higher permeability of their blood brain barrier.9,10 The results of the linear regression of the CSF/serum GNbAC1 ratio by dose, sampling day, and albumin CSF/serum ratio show that the 2 latter predictors are statistically significant or close to statistical significance (regression F(3,8) = 5.4, p<0.025; albumin CSF/serum ratio coefficient t-test p = 0.030, sampling day coefficient t-test p = 0.058; dose coefficient t-test p = 0.35).
Figure 3.
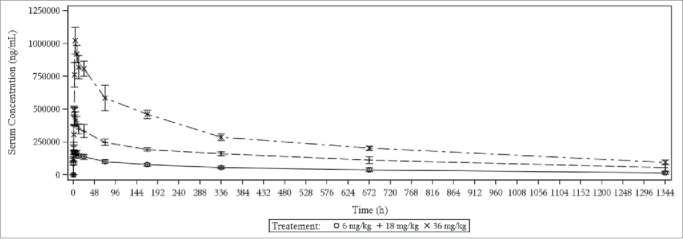
GNbAC1 CSF/serum concentration ratios in percent by dose and sampling day.
Table 4.
Summary of CSF and serum GNbAC1 concentrations, CSF/serum ratios for GNbAC1 and albumin by dose and sampling day
| Dose Level | Sampling Day | GNbAC1 CSF (ng/mL) | GNbAC1 Serum (ng/mL) | Ratio GNbAC1 CSF/Serum (%) | Ratio Albumin CSF/serum (x10−3) | Age (yrs) |
|---|---|---|---|---|---|---|
| 6 mg/kg | 15 | 171.8 | 45606.2 | 0.38 | 7.8 | 49 |
| 6 mg/kg | 2 | 150* | 153168.1 | n.a | 8.0 | 25 |
| 6 mg/kg | 15 | 187 | 68147.2 | 0.27 | 6.7 | 32 |
| 6 mg/kg | 29 | 150* | 27724.9 | n.a | n.a. | 41 |
| 18 mg/kg | 15 | 577.6 | 160181.7 | 0.36 | 7.3 | 52 |
| 18 mg/kg | 15 | 305.9 | 153705.3 | 0.20 | 4.8 | 47 |
| 18 mg/kg | 2 | 375.5 | 338064.5 | 0.11 | 10.7† | 36 |
| 18 mg/kg | 29 | 318.3 | 73672.2 | 0.43 | 7.8 | 49 |
| 36 mg/kg | 15 | 1148.7 | 263940.2 | 0.44 | 8.5 | 40 |
| 36 mg/kg | 29 | 675.9 | 218347.2 | 0.31 | 6.8 | 24 |
| 36 mg/kg | 15 | 1791.1 | 256774.9 | 0.70 | 14.6† | 55 |
| 36 mg/kg | 2 | 1037.7 | 732517.2 | 0.14 | 2.7 | 46 |
| 36 mg/kg | 29 | 838.5 | 215238.6 | 0.39 | 8.4 | 50 |
Concentrations in CSF below the limit of quantification were set to the lower limit of quantification (LLOQ) = 150 ng/mL.
CSF/serum albumin ratio higher than 9.0 indicates a higher blood brain barrier permeability9,10 (other reference19 proposes lower threshold values: 6.5 for subjects younger than 40 y and 8.0 for subjects younger than 60 years)
CSF: cerebrospinal fluid. n.a.: not available
There was no evidence of antibody production against GNbAC1 throughout the entire study period. One subject was positive at baseline but the antibody titers became negative during treatment. The data indicate that single ascending i.v. infusions of GNbAC1 did not induce an antibody response in healthy subjects.
Discussion
We report here the results of a Phase 1 study testing high doses of the IgG4 mAb GNbAC1, the first mAb targeting an endogenous retroviral protein, MSRV-Env, which may play a critical role in MS. In this study testing single i.v. doses of GNbAC1 in healthy volunteers, the safety profile of GNbAC1 appears favorable. All reported adverse events were of mild to moderate intensity. A single transient elevation of transaminases was observed in one subject receiving the highest dose of GNbAC1: the liver enzyme perturbation was considered as unrelated by the investigator, but its origin remains unexplained. Based on the data, one can conclude that single i.v. administration of GNbAC1 is well tolerated and safe in healthy subjects up to a dose of 36 mg/kg.
Pre-existing antibodies were detected in 1 subject, but the detection became negative during treatment. These results are in line with the absence of immunogenicity observed so far in early stage clinical trials.6,7,11 Nonetheless, the limited sample size and short duration of the study calls for caution in this conclusion.
The observed pharmacokinetic profile of GNbAC1 confirms earlier findings observed at lower doses with GnbAC1. Geometric mean t1/2 values ranged from 21 to 26 d. These values are in line with the half-lives observed in healthy subjects at low doses11 as well as in MS patients.6 Geometric mean clearance by dose ranges from 0.082 to 0.088 mL/h/kg or 2.0 to 2.1 mL/day/kg are slightly lower than that of the 3.5 mL/day/kg value published for endogenous IgG in humans with normal IgG concentrations, which suggests that even at these doses the Fc-Rn mediated recycling capacity is not saturated.12 The observed geometric mean volume of distribution by dose ranged from 60.7 to 76.6 mL/kg. These values are similar to the ones found at lower doses with GNbAC1 in healthy subjects,11 and are in line with the limited distribution by diffusion of mAbs from the blood to the extracellular space, leading to relatively low volumes of distribution.13 The exposure to GNbAC1 increases with the administered dose in a linear fashion. Taking into account the available small sample size, one can conclude that there is a dose-proportionality of GNbAC1 pharmacokinetics in healthy subjects at high doses, as observed at lower doses. As the subjects were slightly overweight in this study, extrapolation of the pharmacokinetic data to future MS patients should be done with caution.
To our knowledge, this is the first clinical study assessing sequentially concentrations of a mAb in the CSF. In a paper on rituximab kinetics in the CSF, 2 MS patients were followed-up with repeated lumbar punctures, and it was possible to see increasing ratios over the first weeks post administration; peak CSF/serum ratios of 0.24% and 0.16% were observed after 3 and 16 weeks, respectively, in these patients.14 In a recent paper on the IgG1 BIIB033 anti-LINGO, CSF concentrations were assessed in healthy subject and MS patients at 2 weeks post-dose. The mean CSF/serum ratios were observed to lie between 0.03% and 0.22%.15 For the same dosages (>10 mg/kg of BIIB033), the ratios observed in healthy subjects (between 0.03% and 0.08%) were lower than those of MS patients (between 0.1% to 0.2%), which may be due to blood-barrier disruption in CNS inflammatory disorders such as MS16 In this study, CSF/serum ratios were on average at 0.13% at day 2 post dosing and ˜0.4% at days 15 and 29 post-dose. The linear regression analysis show that there is a statistically significant correlation between CSF/serum ratios for GNbAC1 and albumin, reflecting the blood-brain barrier permeability, as well as a correlation at the limit of statistical significance with the timing of sampling. These results were also in line with a first observation of a ratio of 0.2% in one MS patients receiving 6 mg/kg GNbAC1.7
In conclusion, single doses of GNbAC1 were well tolerated by male healthy subjects up to a dosage of 36 mg/kg i.v. The dose-proportional pharmacokinetics in the tested dose range was in line with the pharmacokinetics data obtained at lower dosages. The blood-brain penetration was assessed by the CSF/serum ratios, and were comprised between averages of 0.13% and 0.4% according to the days of sampling. This ratio is somewhat higher than the few ratio data available in MS, such as those with rituximab or BIIB033. It should be noted that the CSF/serum ratios for albumin were high among these healthy subjects, which may be explained by the slight overweight observed in the sample;17 this observation may represent a limitation in the extrapolation of the CSF results. Overall, the present pharmacokinetic data of GNbAC1 in serum and in CSF are extremely important for a better understanding of the mAb penetration in the CNS, and represent a key element in the rationale of the future study dose planning in order to bind optimally to the intra-CNS targets.
Patients and methods
This study was a Phase 1, single-center, inpatient, randomized, double-blind, placebo-controlled, dose-escalating study to evaluate the safety, tolerability and pharmacokinetic profiles of single i.v. infusions of GNbAC1 over 1, 2 and 4 hours for doses of 6, 18, and 36, mg/kg, respectively, in healthy subjects. The study was doubleblind to avoid bias in the collection and evaluation of data during its conduct. As lumbar punctures were deemed unethical for patients receiving placebo, specific dummy procedures were performed to keep the blind during lumbar punctures. Study drug was administered via i.v. infusion because this is the intended clinical route of administration of GNbAC1. The prepared solution for infusion was stored at room temperature and used within 4 hours after dose preparation.
Subjects were eligible if they: 1) were healthy males subjects as determined by medical history, physical examination, vital signs, ECG and clinical laboratory evaluations, of any ethnic origin, and aged between 18 and 55 y inclusive; 2) had clinically acceptable supine blood pressure and pulse rate; 3) had a body mass index (BMI) between 18.0 and 29.9 kg/m2 inclusive and a body weight between 50 – 100 kg; 4) had no need for regular concomitant medication; 5) agreed to use adequate contraception during, and for at least the 4 weeks after administration of study medication; 6) had the ability to communicate well with the investigator and comply with the requirements of the study; and 7) had given written informed consent to participate in the study. Subjects were excluded if they had: 1) a history of serious adverse reactions or hypersensitivity to any drug; 2) had a presence or history of allergy requiring treatment; 3) had abnormal physical findings of clinical significance at the screening or baseline examination which would interfere with the objectives of the study; 4) needed any prescription medication within 15 d prior to the administration of the drug and/or nonprescription medication within 7 d prior to the administration of the drug or anticipated need for any concomitant medication during the study; 5) had participated in a clinical trial during the previous 3 months before current trial; 6) had a loss of 500 mL blood or more during the 3 month period before the trial; 7) had any surgical or medical condition that may interfere with the subject safety, the distribution, metabolism or excretion of the drug (e.g., impaired renal or hepatic function, diabetes mellitus, cardiovascular abnormalities); 8) had symptoms of a significant somatic or mental illness in the 2 week period preceding drug administration; 9) had a history or clinical evidence of a significant disease in particular disorder of coagulation or bleeding disorder; 10) had a history of positive serology for hepatitis B or C or a positive HIV serology; 11) had a history of serious mental disorders including alcohol or drug abuse; 12) were heavy smokers (i.e., more than 5 cigarettes per day); 13) had positive results of the drug abuse screening; 14) needed a vaccination from screening until the end of study; or 15) were considered as vulnerable subjects (e.g., in detention, under guardianship)
Prior to the start of the study, the study protocol and informed consent form were reviewed and approved by the Ethics Committee of the Land of Berlin and the German Paul Ehrlich Institute. The study was conducted at the Early Phase Clinical Unit of Parexel Ltd, Berlin, Germany, in accordance with the relevant articles of the “Declaration of Helsinki,” the International Conference on Harmonization (ICH) GCP consolidated guidelines, and the German laws.
All subjects enrolled in this study had signed the written informed consent form prior to any study procedures being performed.
Twenty-one subjects were sequentially assigned to one of 3 escalating dose cohorts (6 mg, 18 mg, 36 mg of GNbAC1 per kg of body weight). Subjects were randomized to receive a single i.v. dose of either GNbAC1 or placebo with the ratio 5:2. Doses were calculated based on body weight at baseline. All subjects were randomized by cohort in dose escalating order between May 2015 and June 2015.
The starting dose was the dose of 6 mg/kg i.v., which has been tested in MS patients in a Phase 2a study.6,7 We plan to test repeated monthly doses up to 18 mg/kg i.v. in future clinical trials. Thus, with an accumulation index of about 2 calculated based on a monthly administration, an estimated average half-life of 28 d and assuming a linear pharmacokinetics for GNbAC1,18 it was necessary to assess the tolerance of a dose up to 36 mg/kg of GNbAC1 with a single i.v. administration.
Each subject received a single i.v. dose of either GNbAC1 or placebo during the study; in each dose cohort, 5 subjects received GNbAC1 and 2 subjects received placebo. All doses of GNbAC1 and placebo were administered in the fasted state. There was a need for a follow-up of at least 7 days in at least 6 subjects in a dose cohort to allow satisfactory review of the safety data prior to progression to the next dose cohort. For the cohorts at 18 and 36 mg/kg i.v., there was a sentinel pair of subjects – one receiving the active treatment, the other one receiving the placebo, who were observed for at least 24 h before the other subjects received their dose. Screening was performed in the 3 week period prior to admission of the subjects to the study center. Each subject participated in one study period only and remained resident in the clinical unit from the morning of Day –1 (the day before dosing) until Day 2. Dosing occurred on Day 1 for each subject. The Investigator checked on all subjects' wellbeing prior to their discharge from the clinical unit. Subjects returned to the clinical unit at Days 4, 8, 15, 29 and 57 after dosing for their end-of-study assessments. Lumbar punctures were performed on Days 2, 15 or 29: each subject on active treatment underwent only one lumbar puncture randomly at one of those visits and subjects under placebo underwent one dummy lumbar puncture; all subjects remained in the clinical unit for 24 hours.
Blood and urine samples were collected for clinical laboratory evaluations at specific times during the study, and when judged to be clinically appropriate. High sensitivity C-reactive protein (hsCRP) vital signs (sitting blood pressure and pulse, respiratory rate and aural body temperature) were determined. At each visit during the study, except day 4, a 12lead resting ECG was taken; ECG was monitored every hours during infusion and up to 4 hours after the end of the infusion.
Blood samples were collected for pharmacokinetic assessment at: Pre-dose (0 hours) and after start of infusion at 0.5, 1, 2, 3, 4, 8, 12, 24, 72, 168, 336, 672 and 1344 hours (14 samples per subject).
The analysis of GNbAC1 was based on a competitive electrochemiluminescence (ECL) based immunoassay using an anti-idiotypic mAb (Mab1E4F7H6) against GNbAC1 as capture antibody.9 Pharmacokinetic parameters were determined from the serum concentrations of GNbAC1 using noncompartmental procedures. The following pharmacokinetic parameters for GNbAC1 were determined: 1) area under the serum concentration versus time curve extrapolated to infinity (AUC0∞); 2) area under the serum concentration vs. time curve from time zero to the last data point tlast above the limit of quantitation (AUC0tlast); 3) the maximum observed serum concentration (Cmax); 4) the time to the maximum observed serum concentration (tmax); 5) the corresponding half-life calculated from the loglinear terminal slope of the serum concentration versus time (t1/2); 6) the MRT; 7) the total body clearance (CL); and 8) the volume of distribution based on the terminal phase (Vz).
Blood samples were taken before GNbAC1 administration and at days 29 and 57 post infusion to determine the immunogenic potential of GNbAC1. The screening for binding antibodies against GNbAC1 was performed by ECL using a bridging assay.9 Positive samples were further analyzed by a titration assay. The albumin concentrations in CSF and serum were measured by a routine nephelometric assay (Behring AG, Germany).
Sample size considerations for this study were based on the usual sample size for safety assessment in Phase 1 studies. Designs ranging from 4 subjects on active and 2 subjects on placebo per dose level to 6 subjects on active and 3 subjects on placebo per dose level are usually implemented. Summary statistics are presented for the pharmacokinetics, safety, and tolerability data, as appropriate. Summary statistics are presented for the pharmacokinetic data using geometric means. Descriptive statistics were determined using SAS® Version 9.2 or higher (SAS Institute, Cary, North Carolina, USA). The pharmacokinetic analysis was performed using WinNonlin Version 6.3 or higher (Pharsight Corporation, Mountain View, California, USA). A post-hoc linear regression analysis of the CSF/serum GNbAC1 ratios over sampling days, doses and CSF/serum albumin ratios were performed using Stata software 14.0 (StataCorp Inc., College Station, Texas, USA).
Disclosure of potential conflicts of interest
FC, VV, CB, ABL, and HP are employees of Geneuro SA.
References
- 1.Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol 2006; 176(12):7636-44; PMID:16751411; http://dx.doi.org/ 10.4049/jimmunol.176.12.7636 [DOI] [PubMed] [Google Scholar]
- 2.Kremer D, Schichel T, Förster M, Tzekova N, Bernard C, van der Valk P, van Horssen J, Hartung HP, Perron H, Küry P. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann Neurol 2013; 74(5):721-32; PMID:23836485; http://dx.doi.org/ 10.1002/ana.23970 [DOI] [PubMed] [Google Scholar]
- 3.Curtin F, Perron H, Kromminga A, Porchet H, Lang AB. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs 2015; 7(1):265-75; PMID:25427053; http://dx.doi.org/ 10.4161/19420862.2014.985021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh J, O'Connor PW. Novel and imminently emerging treatments in relapsing-remitting multiple sclerosis. Curr Opin Neurol 2015 Jun; 28(3):230-6; PMID:25887773; http://dx.doi.org/26380353 10.1097/WCO.0000000000000203 [DOI] [PubMed] [Google Scholar]
- 5.D'Amico E, Caserta C, Patti F. Monoclonal antibody therapy in multiple sclerosis: critical appraisal and new perspectives. Expert Rev Neurother 2015 Mar; 15(3):251-6; PMID:25708308; http://dx.doi.org/26380353 10.1586/14737175.2015.1008458 [DOI] [PubMed] [Google Scholar]
- 6.Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, Du Pasquier R, Schluep M, Desmeules J, Lang AB, et al.. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus in multiple sclerosis patients. Mult Scler 2015 Jun; 21(7):885-93; PMID:25392325; http://dx.doi.org/26380353 10.1177/1352458514554052 [DOI] [PubMed] [Google Scholar]
- 7.Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, Du Pasquier R, Schluep M, Desmeules J, Lang AB, et al.. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients - A twelve month follow-up. J Neuroimmunol 2015 Aug 15; 285:68-70; PMID:26198921; http://dx.doi.org/26380353 10.1016/j.jneuroim.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann M, Sanderson NS, Rasenack M, Lalive PH, Lang AB, Curtin F, Lindberg RL, Kappos L, Derfuss T. Immunologic monitoring during a phase 2a trial of the GNbAC1 antibody in patients with MS. Neurol Neuroimmunol Neuroinflamm 2015 Aug 20; 2(5):e144; PMID:26380353; http://dx.doi.org/ 10.1212/NXI.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischbach F, Dunning MB. A Manual of Laboratory and Diagnostic Tests 8th Edition Wolters Kluver/Lippincott Williams and Wilkins, Philadelphia: 2009; p.333 [Google Scholar]
- 10.Burtis CA, Ashwood ER, Bruns DE Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th Edition Elsevier; 2013; p.613 [Google Scholar]
- 11.Curtin F, Lang AB, Perron H, Laumonier M, Vidal V, Porchet HC, Hartung HP. GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin Ther 2012 Dec; 34(12):2268-78; PMID:23200102; http://dx.doi.org/7874779 10.1016/j.clinthera.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008 Nov; 84(5):548-58; PMID:18784655; http://dx.doi.org/7874779 10.1038/clpt.2008.170 [DOI] [PubMed] [Google Scholar]
- 13.Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010 Aug; 49(8):493-507; PMID:20608753; http://dx.doi.org/7874779 10.2165/11531280-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.Petereit HF, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler 2009 Feb; 15(2):189-92; PMID:18971221; http://dx.doi.org/7874779 10.1177/1352458508098268 [DOI] [PubMed] [Google Scholar]
- 15.Tran JQ1, Rana J1, Barkhof F1, Melamed I1, Gevorkyan H1, Wattjes MP1, de Jong R1, Brosofsky K1, Ray S1, Xu L1, et al.. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurol Neuroimmunol Neuroinflamm 2014 Aug 21; 1(2):e18; PMID:25340070; http://dx.doi.org/7874779 10.1212/NXI.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leech S, Kirk J, Plumb J, McQuaid S. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol 2007 Feb; 33(1):86-98; PMID:172390117874779 [DOI] [PubMed] [Google Scholar]
- 17.Brettschneider J, Claus A, Kassubek J, Tumani H. Isolated blood-cerebrospinal fluid barrier dysfunction: prevalence and associated diseases. J Neurol 2005 Sep; 252(9):1067-73; PMID:15789126; http://dx.doi.org/7874779 10.1007/s00415-005-0817-9 [DOI] [PubMed] [Google Scholar]
- 18.Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications Third Edition, Lippinkott Williams and Wilkind Philadelphia; 1995. [Google Scholar]
- 19.Reiber H. External quality assessment in clinical neurochemistry: survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin Chem 1995; 41(2):256-263; PMID:7874779 [PubMed] [Google Scholar]


