Abstract
Objective:
This updated meta-analysis determines the effect of dipeptidyl peptidase-4 inhibitors on glycemic and tolerability outcomes in patients with type 2 diabetes mellitus and chronic kidney disease with glomerular filtration rate of ⩽60 mL/min or on dialysis.
Methods:
In all, 14 citations were identified from multiple databases. Qualitative assessments and quantitative analyses were performed.
Results:
There were 2261 participants, 49–79 years of age, 49% men and 44% Caucasians. In seven placebo-comparator studies, reduction in hemoglobin A1c at weeks 12–24 was 0.55% (95% confidence interval: −0.68 to −0.43), P < 0.00001). In three sulfonylurea-comparator studies, dipeptidyl peptidase-4 inhibitors did not significantly reduce hemoglobin A1c at weeks 52–54 (−0.15% (95% confidence interval: −0.32 to 0.02)). In one sitagliptin versus albiglutide study, albiglutide significantly reduced hemoglobin A1c in patients with moderate renal impairment (−0.51%). A similar reduction in hemoglobin A1c was seen with sitagliptin versus vildagliptin (−0.56% vs −0.54%). Compared with placebo or sulfonylurea, dipeptidyl peptidase-4 inhibitors did not significantly reduce hemoglobin A1c after 12 and 54 weeks in patients on dialysis. Hypoglycemia was reported by ~30% of patients in both dipeptidyl peptidase-4 inhibitors and placebo groups over 24–52 weeks. While hypoglycemia was more common with a sulfonylurea at 52–54 weeks (risk ratio: 0.46 (95% confidence interval: 0.18 to 1.18)), there was significant heterogeneity (I2 = 87%). Limitations included high drop-out rate from most studies and small number of active-comparator studies.
Conclusions:
Dipeptidyl peptidase-4 inhibitors in patients with chronic kidney disease caused a modest reduction in hemoglobin A1c versus placebo, but not when compared with sulfonylureas or albiglutide, or when used in patients on dialysis. Additional active-comparator studies are needed to further elucidate the role of dipeptidyl peptidase-4 inhibitors in patients with chronic kidney disease stages 3–5 or on dialysis.
Keywords: Meta-analysis, sitagliptin, linagliptin, saxagliptin, vildagliptin, dipeptidyl peptidase-4 inhibitors, chronic kidney disease, dialysis, type 2 diabetes mellitus
Introduction
Chronic kidney disease (CKD) is a gradual and permanent loss of kidney function caused by diabetes mellitus (DM), hypertension or other conditions. Estimates of glomerular filtration rate (eGFR) and albuminuria enable diagnosis.1 Patients with CKD stages 3–5 have either reduced eGFR (stage 3: 30–59 mL/min/1.73 m2; stage 4: 15–29 mL/min/1.73 m2 or stage 5: <15 mL/min/1.73 m2) with or without albuminuria (>30 mg of albumin/gram urine creatinine), for greater than 3 months.1,2 Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2006 showed that 39.6% of those diagnosed with DM, 41.7% of those with undiagnosed DM, 17.7% of those with pre-diabetes and 10.6% of those without DM had CKD.3 In 2011, DM was listed as the primary cause of renal failure in 44% of new cases in the United States4 and Medicare Part D costs for patients with CKD and DM was US$24.6 billion.5
Combination drug therapy is often required to attain glycemic goals to prevent diabetes-related complications; however, many anti-hyperglycemic agents (AHAs) are renally excreted and require dosage adjustment based on renal status.6 Sitagliptin, a dipeptidyl peptidase-4 inhibitor (DPP-4I), received Food and Drug Administration (FDA) approval in 2006 for treatment of type 2 DM (T2DM) and dose adjustments prior to and during treatment are recommended to reduce adverse renal events.7 Other currently available DPP-4I, saxagliptin, alogliptin and vildagliptin, require similar adjustments;8–10 however, linagliptin11 is exempt as it is mainly fecally eliminated. Patients receiving dialysis can receive sitagliptin,7 alogliptin8 or vildagliptin,12 regardless of timing of dialysis; however, with saxagliptin, it is recommended that it should be administered 4 hours post-dialysis session as dialysis will remove ~23% of the dose.9
Recently, a number of studies examining the clinical effect of DPP-4I in the CKD population have been published. Two meta-analyses have been performed on this topic; however, additional data are now available.13,14 This updated meta-analysis seeks to determine the effect of DPP-4I in patients with T2DM with CKD stages 3–5 or on dialysis. Where applicable, analyses will assess efficacy outcomes at 3-month intervals, by renal impairment (RI) status and by comparators (placebo vs active).
Methods
Data sources and searches
Two independent reviewers (D.S.F and E.T.C) identified pertinent articles from the following databases: MEDLINE, EMBASE, PUBMED, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Biomedical Reference Collection, CINAHL, Nursing and Allied Health Collection, Ageline and Clinicaltrials.gov website. Search terms and limiters (adults ⩾ 18 years, humans, English language and any year) are found on Supplementary file 1. Relevant journals, bibliographies and personal files were hand-searched for additional articles. The last search was performed on 1 May 2016 with all citations exported to EndNoteX7.4 (New York, NY, USA) where duplicates were eliminated.
Randomized, placebo- or active-controlled, clinical trials (parallel or cross-over), studying a DPP-4I in adult patients with T2DM and CKD, were included. Publications with pooled data were allowed if data were not duplicative. Studies with <10 patients/group, post-renal transplantation and non-original research articles were excluded.
Data extraction
Two independent reviewers (D.S.F and E.T.C) evaluated titles and abstracts of articles followed by full review to determine suitability for inclusion. Continuous data on hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG) were independently extrapolated from figures by D.S.F. and E.T.C. Discrepancies were resolved by consensus. Corresponding authors and manufacturers were contacted for data not provided within studies or when outcome data were presented in unusable format for meta-analysis (e.g. graphs only or mean difference between the groups only). While we received clarification of published data, we did not receive any additional data or were told requested data were unavailable.
Data synthesis and analysis
Primary analysis assessed effect of DPP-4I on HbA1c, achievement of HbA1c ⩽7% or ⩾0.5% reduction. Study duration of 12 weeks was required to adequately assess change in HbA1c. Secondary analyses were performed on FPG, urine albumin-to-creatinine ratio (UACR), weight and tolerability.
Statistical analysis
Effect size for all continuous data (change from baseline to time-point) was pooled using inverse variance method to calculate weighted mean difference (WMD). Tolerability results were given as count data (minimum of one event had to occur to include in analysis) and were presented as Mantel–Haenszel risk ratios (RR). All comparisons were made to placebo and/or active comparator. A 95% confidence interval (CI) was calculated for both types of data. Random effects model was employed with assumption that effect size will vary somewhat between studies due to differences in studied populations.
All authors independently assessed each study for risk of bias using Cochrane risk of bias tool criteria.15 Disagreements were resolved by consensus. Heterogeneity assessed by calculating Z score (Q test) and Chi-square statistic set at P < 0.10.16 I2 was calculated to quantify heterogeneity of results of studies and considered low, moderate or high if ⩽25%, 26%–74% or ⩾75%, respectively. Where applicable and appropriate, possible sources of heterogeneity were evaluated by conducting subgroup analyses. Funnel plots assessed publication bias. Analyses were performed using Review Manager 5 software (Review Manager (RevMan) (Computer program), Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were followed for reporting results (Table S1).17
Results
Studies analyzed
In all, 14 citations met inclusion criteria: five 24- to 54-week studies with sitagliptin;18–22 five 24- to 52-week studies with linagliptin;23–27 two with saxagliptin (12-week study followed by 40-week observational period)28,29 and three with vildagliptin (one 24-week study and another 24-week study followed by 28-week extension);12,30,31 no alogliptin studies met inclusion criteria (Figure 1). All studies were published from 2008 to 2015, conducted internationally at multiple sites, and manufacturer-sponsored with most authors employed by manufacturers of studied DPP-4I.
Figure 1.
Literature search results.
Studies included 2261 participants, 49–79 years of age, 49% men,12,18–21,24–27,29,31 44% Caucasians,18–21,24,26,27,29,31 20% Asians,12,18–21,24,26,27,31 and 12% Hispanics18–21,31 (Table 1). Randomized, double-blind, placebo- or active-controlled design was employed by most studies;12,18–23,25,26,29,31 one study was a pooled analysis24 of three previously published randomized studies,32–34 and another was a retrospective analysis27 of two previously published studies.26,35 Outcomes-of-interest data from studies that included patients with renal clearance ⩾ 60 mL/min,22,23 and the pooled24 and retrospective27 analyses were included in meta-analyses if data were only of patients with CKD stage 3–5 and/or dialysis. Five studies included patients on dialysis;12,18,20,21,29 however, the majority of patients (57%) had moderate RI (clearance 30–59 mL/min), followed by severe RI (32%, clearance < 30 mL/min) and then dialysis (11%).
Table 1.
Patient demographics of included studies.
| Sitagliptin studies | N | Mean±SD age (y) % Male % W/A/O |
Renal status (% Pts) CL = 30–59 mL/min CL < 30 mL/min Pts on dialysis |
Background Meds |
Baseline HbA1c (%) |
Change in HbA1c at various time-points |
|---|---|---|---|---|---|---|
| Chan et al.20,a
PCB × 12W Glipizide × 42W |
26 | 65.3±9.7 62 31/27/42 |
58 23 19 |
Insulin allowed; oral agents discontinued |
7.8 ± 0.9 | W12: −0.1 ± 0.74 (n = 26) W54: −0.8 ± 0.97 (n = 25) |
| Sitagliptin | 65 | 68.9±9.8 48 34/31/35 |
57 25 18 |
7.6 ± 0.9 | W12: −0.6 ± 0.79 (n = 62) W54: −0.7 ± 1.07 (n = 51) |
|
| Ferreira et al. AJKD18,a
Glipizide Sitagliptin 2.5 mg/day |
65 | 58.5±9.9 56.9 27.7/49.2/23.1 |
All patients on dialysis | Oral agents discontinued; patients on insulin within 12 weeks of randomization excluded; Insulin allowed for rescue only |
7.8 ± 0.7 | W54: −0.87 ± 0.95 (n = 62) |
| 64 | 60.5±9.1 62.5 25/43.8/31.2 |
7.9 ± 0.7 | W54: −0.72 ± 0.9 (n = 59) | |||
| Ferreira et al.19 DCa
Glipizide |
142 | 64.3±9.2 54.9 28.2/58.5/13.3 |
74.6 25.4 – |
7.8 ± 0.7 | W12: −0.55 ± 0.69b
W24: −0.54 ± 0.8b W54: −0.6 ± 0.91 |
|
| Sitagliptin | 135 | 64.8±10.6 59.3 29.6/53.3/17.1 |
72.6 27.4 – |
7.8 ± 0.7 | W12: −0.56 ± 0.71b
W24: −0.66 ± 0.9b W54: −0.8 ± 0.89 |
|
| Kothny et al.21DIA Vildagliptin 50 mg/day Sitagliptin 25 mg/day |
83 | 66.7±8.8 50.6 61.4/NR/38.6 |
– 93 7 |
Background oral and/or insulin allowed |
7.5 ± 0.9 | W24: −0.54 ± 1.06 (n = 78) |
| 65 | 66.9±9.6 44.6 61.5/NR/38.5 |
– 91 9 |
7.8 ± 1.1 | W24: −0.56 ± 1.02 (n = 62) | ||
| Leiter et al.22,a
Albiglutide Sitagliptin |
249 | 63.2±8.37 54.6 45/34/21 |
41 7.6 – |
Background oral agents allowed |
8.13 ± 1.04 | Moderate RI W26: −0.88 ± 1 (n = 98) Severe RI W26: −1.08 ± 0.91 (n = 19) |
| 246 | 63.5±9.02 52.8 46/31/23 |
41.1 6.9 – |
8.23 ± 0.94 | Moderate RI W26: −0.37 ± 1.33 (n = 99) Severe RI W26: −0.65 ± 1.24 (n = 15) |
||
| Linagliptin studies | ||||||
| McGill et al.26 DC Placebo Linagliptin 5 mg/day |
65 | 64.9±9.6 53.8 69.2/16.9/13.9 |
21.5 78.5 – |
Stable doses ⩾ 12 weeks allowed |
8.2 ± 0.9 | W12: 0.01 ± 1.26 (n = 62) W24: 0.04 ± 1.10 (n = 62) W52: 0.01 ± 1.26 (n = 62) |
| 68 | 64±10.9 66.2 77.9/11.8/10.3 |
7.4 92.6 – |
8.2 ± 1.1 | W12: −0.71 ± 1.22c (n = 66) W24: −0.64 ± 1.06c (n = 66) W52: −0.71 ± 1.22c (n = 66) |
||
| Barnett et al.23
Placebo Linagliptin 5 mg/day |
79 | 74.9±4.2 62 96.2/2.5/1.3 |
26.6 1.3 – |
Stable doses ⩾ 8 weeks allowed |
7.7 ± 0.7 | Moderate RI W24: −0.05 ± 0.63 (n = 20) |
| 162 | 74.9±4.4 71.6 96.9/1.9/1.2 |
25.3 1.2 – |
7.8 ± 0.8 | Moderate RI W24: −0.7 ± 0.64c (n = 41) |
||
| Groop et al.24
Placebo Linagliptin 5 mg/day |
25 | 65.6±6.4 36 48/52/0 |
All patients with CL = 30–59 mL/min | Insulin not allowed | 8.2 ± 0.9 | W24: −0.03 ± 0.90 (n = 25) |
| 68 | 66.4±8 47.1 63.2/36.8/0 |
8.2 ± 1.0 | W24: −0.56 ± 1.08 (n = 68)d | |||
| McGill et al.27 DVDR Placebo Linagliptin 5 mg/day |
68 | 68±9.1 48.5 94/3/3 |
All patients with CL = 30–59 mL/min | Stable treatment with basal insulin ± metformin ± pioglitazone | 8.2 ± 0.8 (n = 66) |
W12: −0.008 ± 0.69b (n = 59) W24: 0.01 ± 0.87b (n = 45) W52: −0.17 ± 0.96b (n = 32) |
| 59 | 65.8±7.4 55.9 88.1/10.2/1.7 |
8.3 ± 0.9 (n = 58) |
W12: −0.5 ± 0.93b,e (n = 52) W24: −0.66 ± 0.83b,e (n = 50) W52: −0.46 ± 0.99b (n = 38) |
|||
| Laakso et al.25
PCB × 12W Glimepiride 1–4 mg/day × 40W |
122 | 65.9±9.4 64.8 NR |
63.1 36.9 – |
Insulin allowed | 8.03 ± 0.94 | W12: −0.11 ± 1.2 (n = 120) W24: −0.74 ± 1.42 (n = 120) W52: −0.50 ± 1.42 (n = 120) |
| Linagliptin 5 mg/day |
113 | 67.3±9.2 61.9 NR |
69 31 – |
8.08 ± 0.89 | W12: −0.53 ± 1.17c (n = 113) W24: −0.73 ± 1.28 (n = 113) W52: −0.64 ± 1.38 (n = 113) |
|
| Saxagliptin studies | ||||||
| Nowicki et al.28,29
Placebo Saxagliptin 2.5 mg/day |
85 | 66.2±9.1 48.2 100/0/0 |
49.4 27.1 23.5 |
Stable doses > 4 weeks with oral or insulin agents allowed | 8.09 ± 1.08 (n = 83) |
Moderate RI W12: −0.05 ± 0.90 (n = 42) W52: 0.19 ± 1.17 (n = 42) Severe RI W12: −0.5 ± 0.96 (n = 23) W52: −0.49 ± 1.20 (n = 23) Dialysis W12: −0.87 ± 1.03 (n = 18) W52: −0.99 ± 1.15 (n = 17) |
| 85 | 66.8±8.3 37.6 100/0/0 |
56.5 21.2 22.3 |
8.45 ± 1.22 (n = 81) |
Moderate RI W12: −0.64 ± 0.90 (n = 45) W52: −0.94 ± 1.19 (n = 44) Severe RI W12: −0.95 ± 0.97 (n = 18) W52: −0.8 ± 1.20 (n = 17) Dialysis W12: −0.84 ± 1.03 (n = 18) W52: −1.13 ± 1.15 (n = 17) |
||
| Vildagliptin studies | ||||||
| Lukashevich et al.31/Kothny et al.30
Placebo Moderate RI |
129 | 69.7±7.3 62 72.9/11.6/15.5 |
100 – – |
Stable doses > 4 weeks with oral/insulin agents allowed | 7.8 ± 0.9 | W24: −0.24 ± 1.13 (n = 128) W52: −0.14 ± 1.87 (n = 76) |
| Severe RI |
97 | 64.5±10.8 54.6 50.5/21.7/27.8 |
– 100 – |
7.7 ± 1.0 | W24: −0.34 ± 1.51 (n = 95) W52: −0.077 ± 1.71 (n = 59) |
|
| Vildagliptin 50 mg/day Moderate RI |
165 | 67.7±8.8 58.2 70.3/14.5/15.2 |
100 – – |
7.8 ± 1.0 | W24: −0.7 ± 1.25c (n = 157) W52: −0.6 ± 1.05f (n = 111) |
|
| Severe RI | 124 | 64.1±9.2 52.4 49.2/19.4/31.4 |
– 100 – |
7.7 ± 1.0 | W24: −0.9 ± 2.21c (n = 122) W52: −0.8 ± 1.87c (n = 87) |
|
| Ito et al.12,a
Control |
21 | 68±9.17 67 0/100/0 |
All patients on dialysis | Continued current oral agents |
6.7 ± 0.55 | W12: −0.02 ± 0.48b (n = 21) W24: −0.06 ± 0.48b (n = 21) |
| Vildagliptin 50–100 mg/day |
30 | 67±10.95 70 0/100/0 |
6.7 ± 0.46 | W12: −0.41 ± 0.67b,g (n = 30) W24: −0.60 ± 0.61b,g (n = 30) |
||
N: sample size; SD: standard deviation; Y: years; W/A/O: White/Asian/Other; Pts: patients; CL: renal clearance; Meds: medications; HbA1c: hemoglobin A1c; W: weeks; RI: renal impairment; NR: not reported.
Dosing regimens:
Chan: Sitagliptin dose for patients with CL = 30–50 mL/min was 50 mg/day; dose for patients with CL < 30 mL/min: 25 mg/day. Glipizide was initiated at 5 mg/day, but could be increased to 10 mg twice-daily at 2-week intervals.
Ferreira AJKD: Initial glipizide dose was 2.5 mg/day, but could be increased to 10 mg twice-daily at 2-week intervals.
Ferreira DC: Sitagliptin dose for patients with CL = 30–50 mL/min was 50 mg/day; dose for patients with CL < 30 mL/min: 25 mg/day. Initial glipizide dose was 2.5 mg/day, but could be increased to 10 mg twice-daily at 2-week intervals.
Leiter: Initial albiglutide dose was 30 mg subcutaneously weekly, but could be increased to 50 mg weekly. Sitagliptin dose for patients with CL = 30–50 mL/min was 50 mg/day; dose for patients with CL < 30 mL/min was 25 mg/day.
Ito: Initial vildagliptin dose 50 mg/day, but could be increased to 100 mg/day after 8 weeks if target HbA1c < 7% not reached. Pooled SD formula was used to calculate change in HbA1c at weeks 12 and 24.
Data extrapolated from figures.
P ⩽ 0.0001 versus placebo.
P < 0.01 versus placebo.
P < 0.001 versus placebo.
P = 0.005 versus placebo.
P < 0.05 versus control.
Seven studies compared DPP-4I with placebo,12,23,24,26,27,29,31 four compared DPP-4I with a sulfonylurea (glipizide or glimepiride)18–20,25 and two compared DPP-4I with other incretin-based therapies.21,22 For the incretin-comparator studies, sitagliptin was compared with vildagliptin or albiglutide.21,22 Two studies compared DPP-4I with placebo for initial 12 weeks and then switched to a sulfonylurea for an additional 40 or 42 weeks.20,25 Eight studies allowed patients to remain on insulin.20,21,23,25–27,29,31
Efficacy
Change in HbA1c
Change in HbA1c from baseline to various time-points was considered the primary outcome in nine studies,18,19,22–27,29 while safety was the primary outcome in three studies;20,21,31 all provided change in HbA1c values.
In placebo-comparator studies, reduction in HbA1c over 12–24 weeks was 0.55%, in favor of DPP-4I (P < 0.00001), and the effect remained significant up to 52 weeks (Figure 2, Table 2). In sulfonylurea-comparator studies, DPP-4I were no different than sulfonylureas in lowering HbA1c at 24 and 52–54 weeks. Both sitagliptin and vildagliptin caused a similar reduction in HbA1c by ~0.5%; in contrast, albiglutide was more effective than sitagliptin in reducing HbA1c in patients with moderate RI, but not more effective in patients with severe RI, with moderate but significant heterogeneity (I2 = 59%, P = 0.09).
Figure 2.
Change in hemoglobin A1c at primary endpoint.
MRI: moderate renal impairment (estimated clearance = 30–50mL/min); SRI: severe renal impairment (estimated clearance <30mL/min).
Table 2.
Sub-group analyses on the effect of DPP-4 inhibitors on selected outcomes.
| Comparator | Time-points (weeks) | References | DPP-4I (N) | Comparator (N) | MD (95% CI), P value | I2Q-test |
|---|---|---|---|---|---|---|
| Change in hemoglobin A1c (%) | ||||||
| Placebo | 12 | 20, 25–27, 29 | 356 | 332 | −0.52 (−0.66 to −0.37), P < 0.00001 | 0% |
| 24 | 23, 24, 26, 27, 31 | 504 | 375 | −0.58 (−0.73 to −0.44), P < 0.00001 | 0% | |
| 52 | 26–28, 30 | 363 | 294 | −0.62 (−0.88 to −0.36), P < 0.00001 | 33%, P = 0.19 | |
| Glipizide/glimepiride | 24 | 19, 25 | 248 | 262 | −0.09 (−0.26 to 0.09) | 0% |
| 52–54 | 19, 20, 25 | 299 | 287 | −0.15 (−0.32 to 0.02) | 0% | |
| MRI: placebo | 12–24 | 23, 24, 27, 29, 31 | 361 | 260 | −0.57 (−0.72 to −0.42), P < 0.00001 | 0% |
| SRI: placebo | 12–24 | 29, 31 | 140 | 118 | −0.51 (−0.9 to −0.13), P = 0.008 | 0% |
| Dialysis: placebo | 12 | 12, 29 | 48 | 39 | −0.29 (−0.64 to 0.06) | 18%, P = 0.11 |
| 24–52 | 12, 28 | 47 | 38 | −0.49 (−0.77 to −0.21), P = 0.0006 | 0% | |
| Dialysis: glipizide | 54 | 18 | 59 | 62 | 0.15 (−0.18 to 0.48) | NA |
| Comparator | Time-points (weeks) | References | DPP-4I (n/N) |
Comparator (n/N) | RR (95% CI), P value | I2Q-test |
| Proportion of patients reaching goal HbA1c ⩽ 7% | ||||||
| Placebo | 52 | 26 | 11/61 | 6/61 | 1.83 (0.72 to 4.64) | NA |
| Glipizide/glimepiride | 52–54 | 19, 25 | 87/239 | 72/252 | 1.35 (0.84 to 2.15) | 53%, P = 0.15 |
| Vildagliptin | 24 | 21 | 22/56 | 27/69 | 1 (0.65 to 1.56) | NA |
| Comparator | Time-points (weeks) | References | DPP-4I (N) | Comparator(N) | MD (95% CI), P value | I2Q-test |
| Change in fasting plasma glucose (mg/dL) | ||||||
| Placebo | 12 | 25, 26, 28 | 237 | 239 | −7.35 (−20.53 to 5.83) | 0% |
| 52 | 26, 28 | 125 | 120 | −6.80 (−21 to 7.4) | 0% | |
| Glipizide/glimepiride | 24 | 19, 25 | 247 | 261 | 3.73 (−5.09 to 12.56) | 0% |
| 52–54 | 19, 25 | 247 | 261 | 7.76 (−1.31 to 16.83) | 0% | |
| Dialysis: placebo | 52 | 28 | 15 | 18 | 49.2 (−12.49 to 110.89) | NA |
| Dialysis: glipizide | 54 | 18 | 59 | 60 | 4.6 (−11.12 to 20.32) | NA |
| Change in weight (kg) | ||||||
| Glipizide | 54 | 19, 20 | 199 | 173 | −1.59 (−2.34 to −0.84), P < 0.0001 | 2% |
| Dialysis: glipizide | 54 | 18 | 45 | 41 | −1 (−2.75 to 0.75) | NA |
DPP-4I: dipeptidyl peptidase-4 inhibitors; MD: mean difference; CI: confidence interval; MRI: moderate renal impairment (estimated clearance = 30–50 mL/min); SRI: severe renal impairment (estimated clearance < 30 mL/min); NA: not applicable; RR: Risk Ratio.
Addition of DPP-4I in patients with moderate or severe RI caused a significant reduction in HbA1c, versus placebo, without heterogeneity (Table 2). Meta-analysis of data from two placebo-controlled dialysis studies at week 12 showed no difference in reduction of HbA1c with DPP-4I versus placebo. Over 24–52 weeks, DPP-4I significantly reduced HbA1c (−0.49%, P = 0.0006), without heterogeneity. However, this outcome was driven by Ito et al.,12 which reported a significant reduction in HbA1c by week 24 with vildagliptin (WMD: −0.54% (95% CI: −0.84 to −0.24)). In contrast, Nowicki et al.28 did not report a significant reduction with saxagliptin at 52 weeks (WMD: -0.14 (95% CI: −0.91 to 0.63)). In one active-comparator dialysis study, both sitagliptin and glipizide similarly reduced HbA1c by week 54 (−0.72% and −0.87%, respectively; WMD: 0.15%) (Table 2).
Proportion of patients reaching goal HbA1c
A similar proportion of patients receiving DPP-4I versus comparator achieved goal HbA1c ⩽ 7% (33.7% vs 27.5%) (Table 2). In three placebo-comparator studies, 61% versus 38% of patients in DPP-4I versus placebo groups had an HbA1c reduction ⩾0.5% after 12 weeks of treatment (RR: 1.59 (95% CI: 1.20 to 2.11), P = 0.001), but with significant heterogeneity, I2 = 58%, P = 0.09).25,26,29
Fasting plasma glucose
DPP-4I were not associated with a significant reduction in FPG at any time-point, regardless of comparator or RI status (Figure 3, Table 2).
Figure 3.
Change in fasting plasma glucose.
MRI: moderate renal impairment (estimated clearance = 30–50 mL/min); SRI: severe renal impairment (estimated clearance < 30 mL/min).
Seven studies18–22,26,30,31 described rescue protocols based on elevated FPG values (>240 to 280 mg/dL) and usable data from five studies showed more patients receiving comparator (placebo or glipizide) versus DPP-4I, regardless of renal status, required rescue for uncontrolled FPG (13% vs 8.5%, RR 0.63 (95% CI: 0.46 to 0.86), P = 0.004) without heterogeneity.
Weight
Meta-analysis of two glipizide-comparator studies showed a modest but significant weight loss associated with sitagliptin, without heterogeneity (Table 2), but this effect was not found in patients on dialysis. No other studies provided usable data for this outcome.
UACR
Meta-analysis for change in UACR could not be completed due to lack of data.
Tolerability
While proportion of patients experiencing adverse events (AEs) were reported by all studies, data were extracted for meta-analysis from studies that provided AEs only for patients with CKD stage 3–5 or dialysis. Majority of studies stated AEs were rated by study investigators for intensity and relationship to study drug.
Meta-analysis of seven studies showed a similar rate of completion between DPP-4I and comparator (Table 3). Regardless of comparator, dialysis or background insulin use, ~29% of patients did not complete their studies and AEs accounted for ~12% of discontinuations. Meta-analysis of data from two dialysis studies of patients randomized to DPP-4I versus placebo showed high heterogeneity (I2 = 78%); in Ito et al.,12 all patients completed the study, whereas in Nowicki et al.,28 only 14/39 patients with end-stage kidney disease (ESRD) completed their study. Serious AEs (23%), drug-related AEs (22%), any AEs (82%) and deaths (3.6%) occurred similarly between the groups over 12–54 weeks (Figure 4, Table 3). Other AEs such as gastrointestinal events occurred similarly between the groups. Heterogeneity was high with two AEs (musculoskeletal and infections) and removal of the study by Chan et al.20 from both meta-analyses resulted in no heterogeneity. With musculoskeletal AEs, the number of events was low for Chan et al.,20 and in favor of DPP-4I (one event vs four events), but the three other studies21,25,26 had a similar incidence with the control group (22% vs 17.4%). With infections, Chan et al.20 reported no events with DPP-4I group, whereas the other two studies18,21 had a similar incidence with the control group (22.5% vs 20.3%).
Table 3.
Proportion of patients reporting any adverse events.
| Comparators | References | DPP-4I (#events/N) |
Comparator (#events/N) | Risk ratio (95% CI) |
I2Q-test |
|---|---|---|---|---|---|
| Completers | |||||
| Placebo | 26, 28, 30 | 281/423 | 226/356 | 1.05 (0.94–1.16) | 3% |
| Glipizide/vildagliptin | 19–21, 25 | 358/454 | 343/443 | 1.03 (0.95–1.12) | 24%, P = 0.26 |
| Dialysis: placebo | 12, 28 | 36/49 | 30/41 | 0.87 (0.37–2.04) | 78%, P = 0.03 |
| Dialysis: glipizide | 18 | 47/64 | 45/65 | 1.06 (0.85–1.32) | NA |
| DC due to adverse events | |||||
| Placebo | 26–28, 30 | 43/428 | 35/371 | 1.09 (0.72–1.67) | 0% |
| Glipizide/glimepiride/vildagliptin | 19–21, 25 | 57/453 | 62/443 | 0.87 (0.62–1.22) | 0% |
| Dialysis: glipizide | 18 | 14/64 | 16/65 | 0.89 (0.47–1.67) | NA |
| Serious adverse events | |||||
| Placebo | 24, 26–28, 30 | 112/497 | 99/396 | 0.96 (0.76–1.22) | 0% |
| Glipizide/glimepiride/vildagliptin | 19–21, 25 | 97/453 | 103/443 | 0.88 (0.69–1.12) | 0% |
| Drug-related adverse events | |||||
| Placebo | 24, 26, 27, 30 | 109/412 | 86/311 | 0.99 (0.78–1.26) | 0% |
| Glipizide/vildagliptin | 19–21 | 49/340 | 58/321 | 0.8 (0.56–1.14) | 0% |
| Any adverse events | |||||
| Placebo, W12 | 20, 25, 28 | 176/263 | 152/233 | 1.04 (0.92–1.17) | 0% |
| Placebo, W24 | 24, 31 | 246/356 | 181/251 | 0.96 (0.87–1.07) | 0% |
| Vildagliptin, W24 | 21 | 56/65 | 68/83 | 1.05 (0.91–1.21) | NA |
| Deaths | 18–21, 26–28, 30 | 24/710 | 26/668 | 0.81 (0.46–1.45) | 0% |
| Gastrointestinal | 18–21, 25, 26, 30 | 116/801 | 92/726 | 1.16 (0.90–1.48) | 0% |
| Respiratory | 18–21, 25, 26, 28, 30 | 137/886 | 132/811 | 0.96 (0.79–1.18) | 0% |
| Central nervous system | 18–21, 25, 26, 30 | 116/801 | 91/726 | 1.11 (0.84–1.47) | 12%, P = 0.33 |
| Musculoskeletal | 20–21, 25–26 | 55/311 | 51/296 | 1.08 (0.63–1.86) | 51%, P = 0.1 |
| Vascular | 18–21, 25–28, 30 | 179/954 | 147/870 | 1.04 (0.84–1.29) | 14%, P = 0.31 |
| Urinary tract infections | 18–20, 25, 26, 28, 30 | 60/821 | 59/728 | 0.85 (0.6–1.22) | 0% |
| Infections | 18, 20, 21 | 29/194 | 34/174 | 0.84 (0.33–2.15) | 62%, P = 0.07 |
| Anemia | 18, 20, 28 | 11/214 | 14/176 | 0.62 (0.24–1.65) | 33%, P = 0.22 |
DPP-4I: dipeptidyl peptidase-4 inhibitors; #events/N: number of events per sample; CI: confidence interval; DC: discontinuations; NA: not applicable; MRI: moderate renal impairment (estimated clearance = 30–50 mL/min); SRI: severe renal impairment (estimated clearance < 30 mL/min); W: weeks.
Gastrointestinal (e.g. nausea, vomiting, abdominal pain, dyspepsia); respiratory (e.g. nasopharyngitis, respiratory tract infections, rhinitis); central nervous system (e.g. dizziness, headache, lethargy, fall); musculoskeletal (e.g. back pain, arthralgias, extremity pain, asthenia); vascular (e.g. hypertension, peripheral edema, major cardiovascular event).
Figure 4.
Proportion of patients with any adverse events over 52 weeks.
MRI: moderate renal impairment (estimated clearance = 30–50 mL/min); SRI: severe renal impairment (estimated clearance < 30 mL/min).
eGFR
Meta-analysis for change in eGFR could not be completed due to lack of data.
Hypoglycemia
All studies provided incidence of hypoglycemia; however, two studies22,23 did not provide data specifically for those with RI. Study investigators reviewed patient-reported hypoglycemic episodes with subsequent severity determination.
In placebo-comparator studies, ~30% of patients, regardless of treatment arm, reported hypoglycemia of any severity, over 24–52 weeks (Figure 5). In sulfonylurea-comparator studies, DPP-4I were associated with fewer hypoglycemic events (RR: 0.46), but with significant heterogeneity (I2 = 87%). Removal of the glimepiride-comparator study by Laakso et al.,25 reduced heterogeneity to zero as a similar proportion of patients in both groups reported hypogly-cemic events (63.7% vs 71.3%), compared with the other sulfonylurea-comparator studies.19,20 In sitagliptin versus vildagliptin study,21 90% of patients were on background insulin ± sulfonylureas, and 15% of patients in both groups experienced hypoglycemia of any severity.
Figure 5.
Proportion of patients with hypoglycemia of any severity.
MRI: moderate renal impairment (estimated clearance 30–50 mL/min); SRI: severe renal impairment (estimated clearance < 30 mL/min).
Regardless of CKD stage, a similar proportion of patients experienced hypoglycemia of any severity (Table 4) and a small proportion (~2.5%) reported severe hypoglycemia, regardless of comparator.
Table 4.
Proportion of patients reporting hypoglycemia.
| Comparators | References | DPP-4I (#events/N) | Comparator (#events/N) | Risk ratio (95% CI) | I2Q-test |
|---|---|---|---|---|---|
| Any hypoglycemia | |||||
| MRI: placebo (W24–52) | 24, 27, 28, 30 | 78/298 | 61/224 | 1.04 (0.74–1.45) | 20%, P = 0.29 |
| SRI: placebo (W52) | 28, 30 | 23/112 | 15/87 | 1.24 (0.69–2.23) | 0% |
| Dialysis: placebo (W52) | 28 | 4/19 | 5/20 | 0.84 (0.27–2.67) | NA |
| Dialysis: glipizide (W54) | 18 | 4/64 | 7/65 | 0.58 (0.18–1.89) | NA |
| Severe hypoglycemia | |||||
| Placebo: W12–24 | 24, 31 | 6/356 | 4/251 | 0.92 (0.26–3.19) | 0% |
| Placebo: W52–54 | 26–28, 30 | 8/428 | 12/371 | 0.62 (0.25–1.55) | 0% |
| Glipizide/glimepiride: W52–54 | 19, 20, 25 | 9/388 | 14/360 | 0.6 (0.21–1.74) | 29%, P = 0.25 |
| Vildagliptin: W24 | 21 | 2/65 | 1/83 | 2.55 (0.24–27.55) | NA |
DPP-4I: dipeptidyl peptidase-4 inhibitors; #events/N: number of events per sample; CI: confidence interval; NA: not applicable; MRI: moderate renal impairment (estimated clearance = 30–50 mL/min); SRI: severe renal impairment (estimated clearance < 30 mL/min); W: weeks.
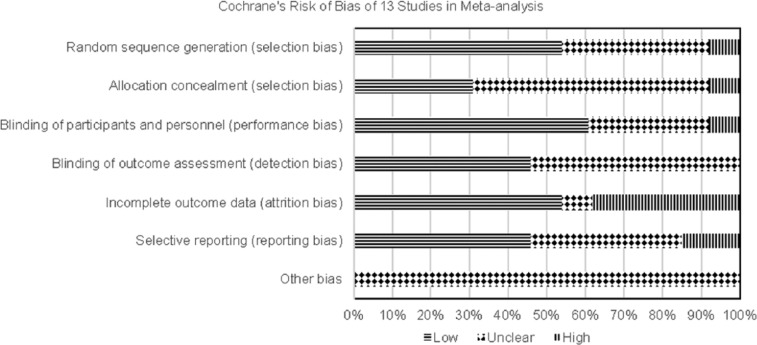
Bias
All studies used in the meta-analysis were reviewed for bias (Figures 6 and 7).
Figure 6.
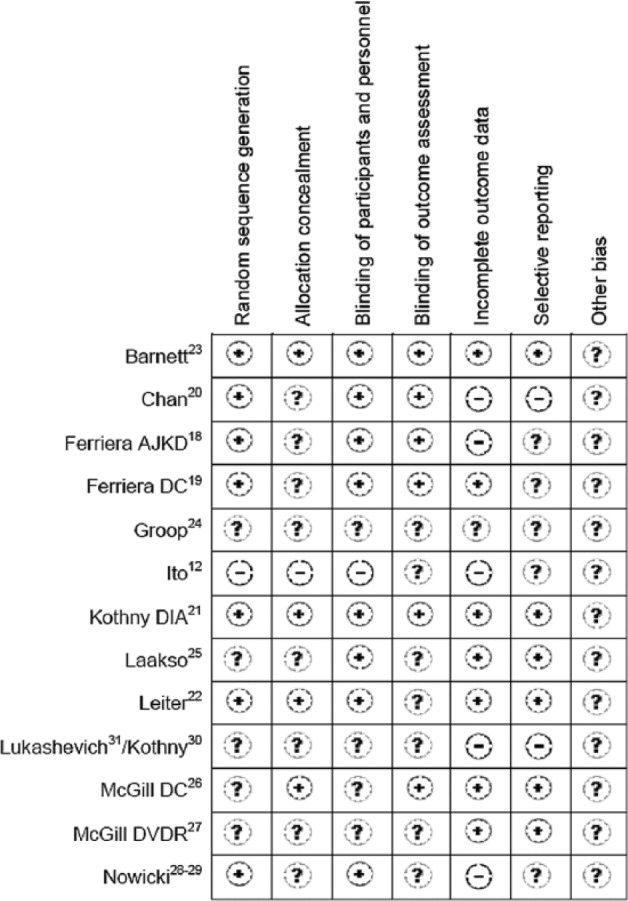
Risk of bias evaluation of each study.
Figure 7.
Overall evaluation of bias.
Selection bias was low for seven studies that used automation to generate allocation sequences;18–23,28,29,36 bias was high for one open-labeled study as it stated an independent investigator randomized subjects (but not how this randomization was performed) and there was purposeful group assignment to balance age, gender, dialysis duration.12 Allocation concealment bias was judged as unclear for most studies as only four studies described allocation concealment with use of an interactive voice-response system.21–23,28,29 Performance bias was low as most studies used double-blind techniques; however, three studies did not explain method used to maintain blinding.12,26,30,31 Detection bias was low for primary outcome (HbA1c) for six studies as these described using central laboratories for analyses.18–21,23,26 Attrition rates were judged to be high if they exceeded 20% and rates for the included studies were 21%–46%, with many ending up underpowered. However, incomplete outcome data were addressed by last-observation-carried-forward method for eight studies18,20–23,25,26,30,31 and two studies19,28,29 analyzed data from per-protocol populations. Overall, reporting bias was low or unclear except for two studies.20,30,31 These studies were judged as high risk because the post-hoc analysis was the basis for HbA1c assessment,20 sample sizes for various time-points were not provided within figures and change in HbA1c was not provided numerically but had to be extracted from figure30,31. Publication bias exists because all studies were manufacturer-sponsored.
Data from three studies32–34 used in the pooled analysis24 and one study35 used in the retrospective analysis27 were included in our meta-analysis. Bias for the three studies is described elsewhere.36 For the remaining study,35 selection, detection, attrition and reporting biases were judged as low and performance bias was unclear.
Sensitivity analysis removing high-risk studies had little impact on HbA1c effect size (lowered 0.01%). Funnel plot analysis for change in HbA1c shows both small and large studies were lacking. Among the placebo-controlled trials, there were no negative studies. This indicates the true effect size is probably smaller than our estimate.
Discussion
When compared with placebo, addition of renally adjusted doses of DPP-4I (or full-dose linagliptin) in patients with CKD stages 3–5 and not receiving dialysis, with a baseline HbA1c range of 7.5%–9%, reduced HbA1c modestly by 0.55% without excess AEs, including hypoglycemia. However, DPP-4I were not more effective than albiglutide or sulfonylureas in reducing HbA1c after 6 months of treatment. Additionally, patients receiving DPP-4I were not more likely to reach goal HbA1c ⩽ 7% and their FPG was not significantly improved. In dialysis patients, addition of sitagliptin (vs glipizide) or saxagliptin (vs placebo) was not associated with a significant change in HbA1c or FPG at any time-point. However, in one study, addition of vildagliptin (vs control) was associated with a significant reduction in HbA1c at weeks 12 and 24.
Two non-dialysis studies provided change in HbA1c stratified by baseline values. Arjona Ferreira et al.19 reported at 54 weeks a greater reduction in HbA1c in patients with higher baseline HbA1c (≥8%: −1.25% vs −1.10% in sitagliptin vs glipizide groups) and McGill et al.26 reported these findings at 52 weeks (>8%: −0.87% vs −0.04% in linagliptin vs placebo groups). Therefore, DPP-4I may lower HbA1c by up to one percentage point in patients with CKD stages 3–5 with higher levels of HbA1c.
Meta-analysis of studies with incretin-based comparators showed that efficacy of sitagliptin is similar to vildagliptin, although rationale for this study21 was that each agent slowed the inactivation of incretin hormones via different mechanisms (sitagliptin competitively but reversibly inhibits DPP-4 enzyme, whereas vildagliptin binds to the active site of the DPP-4 enzyme for a prolonged period of time), possibly resulting in different outcomes; however, this was not the case. Albiglutide, a glucagon-like peptide-1 agonist administered subcutaneously once-weekly, is not inactivated by DPP-4 enzyme and dose adjustment is not suggested for moderate or severe RI. In patients without RI and receiving metformin, albiglutide was more effective at reducing HbA1c and FPG compared with sitagliptin or glimepiride at 104 weeks, with a similar rate of any AEs, except hypoglycemia (more common with glimepiride).37 Meta-analysis showed that albiglutide was significantly more effective than sitagliptin at reducing HbA1c and FPG in patients with moderate RI, but not in those with severe RI; only 36 included patients had severe RI.
Initial post-marketing experience with sitagliptin reported worsening renal function (including acute renal failure sometimes requiring dialysis) and review of a subset of these cases showed patients received incorrect doses based on renal status.7 Therefore, monitoring of renal clearance at baseline and periodically with subsequent dosage reduction is recommended. Although meta-analysis could not be conducted, most studies reported no clinically meaningful changes in clearance from baseline to endpoint in both DPP-4I and control groups.19,24–26,28–31 In the recently published TECOS study evaluating cardiovascular (CV) outcomes with dose-adjusted sitagliptin (N = 14,671), change in eGFR was a pre-specified outcome.38 At 48 months, the mean change from baseline was greater with sitagliptin versus placebo (−4.0 ± 18.4 vs −2.8 ± 18.3 mL/min/1.73 m2, respectively). The lower eGFR with sitagliptin remained consistent over all post-randomization visits, with an estimated least-squares mean difference of −1.34 mL/min/1.73 m2 (95% CI: −1.76 to −0.91, P < 0.001).38 While those with eGFR < 30 mL/min/1.73 m2 were excluded, 9.3% of patients had eGFR < 50 mL/min/1.73 m2; however, change in eGFR was not provided specifically for this subgroup. In the EXAMINE trial evaluating the CV safety profile of dose-adjusted alogliptin versus placebo in patients with recent acute coronary syndrome (N = 5380), ~29% had eGFR < 60 mL/min/1.73 m2.39 After a median follow-up of 18 months, mean changes from baseline in eGFR in those with moderate (1.1 vs 2.1 mL/min/1.73 m2) and severe RI (0.2 vs 1.6 mL/min/1.73 m2) were low in both groups, respectively. While patients on dialysis were excluded from both TECOS and EXAMINE, <1.5% of all patients (with or without CKD) transitioned to renal failure/dialysis.
In addition to determining eGFR to stage CKD, it is also recommended that evidence of kidney damage (i.e. persistent albuminuria) be assessed as albuminuria is a strong predictor of mortality and CV events independent of eGFR.1 While meta-analysis could not be carried out for change in UACR, results from two sitagliptin versus glipizide studies showed conflicting results.19,20 A search of the literature found several studies with DPP-4I in patients with T2DM that suggests DPP-4I may be associated with a further reduction in albuminuria.40–44 However, these studies were of short duration (4–24 weeks) with small sample sizes and varying degrees of glycemic control and RI (majority of patients with eGFR ⩾ 60 mL/min/1.73 m2). In TECOS, the median baseline UACR was 10.6 mg/g and ~8% of patients in both treatment groups had micro-albuminuria at endpoint.38 In SAVOR-TIMI 53, which evaluated the CV effects of saxagliptin versus placebo (N = 16,496), ~37% of patients had a UACR ⩾ 3.4 mg/mmol.45 After a median duration of 2 years, there was a significant relationship between changes in UACR levels and use of saxagliptin versus placebo in patients with eGFR > 50 mL/min/1.73 m2 or 30–50 mL/min/1.73 m2.46 Patients receiving saxagliptin showed an improvement in UACR (and less worsening) versus placebo. No such relationships were found between the two groups in patients with eGFR < 30 mL/min/1.73 m2. Several prospective, randomized-controlled studies are underway to determine the effect of linagliptin on renal endpoints (i.e. albuminuria, renal death, ESRD, change in eGFR) in patients with varying degrees of renal function.47–49
Regardless of treatment arm, RI status, background AHA and dialysis use, a similar proportion of patients reported AEs, including vascular events. Recently, in a pre-specified secondary analysis of SAVOR-TIMI 53, median change in HbA1c at 1 year was significantly lower in saxagliptin versus placebo in patients with eGFR > 50, 30–50 and <30 mL/min/1.73 m2, respectively.46 However, regardless of treatment group, 2-year risk of CV death, myocardial infarction or ischemic stroke increased as renal function decreased (6.4%, 11.2% and 15.9%, respectively). Heart failure (HF) hospitalization was significantly elevated in patients with eGFR 30–50 mL/min/1.73 m2 and receiving saxagliptin (hazard ratio (HR): 1.46 (95% CI: 1.07 to 2), P = 0.02 vs placebo).46 In EXAMINE, 3.9% versus 3.3% of patients (with or without a history of HF) treated with alogliptin versus placebo, respectively, were hospitalized for HF (HR: 1.19 (95% CI: 0.9 to 1.58)).50 Hospital admission for those with a history of HF was similar between the groups (HR: 1 (95% CI: 0.71 to 1.42)), but higher for those without a history of HF in the alogliptin group (2.2% vs 1.3% (HR: 1.76 (95% CI: 1.07 to 2.90), P = 0.026).50 Labeling for both saxagliptin and alogliptin have been updated to include this safety concern.51 Increased risk of HF hospitalizations has not been found with sitagliptin,38 vildagliptin52 or linagliptin;53 additional studies are ongoing for linagliptin.48,49
Patients with CKD stages 4–5 or receiving dialysis are at increased risk for hypoglycemia due to decreased clearance of insulin and impaired kidney gluconeogenesis.6 Therefore, patients may require insulin or sulfonylurea dose reduction within the first year of starting dialysis.54 The two sulfonylureas studied, glipizide and glimepiride, can be used in CKD patients; glipizide does not have active metabolites and its clearance and elimination half-life are not affected by renal function.55 With glimepiride, low doses are recommended due to renal elimination following hepatic metabolism.56 While meta-analysis of hypoglycemic events from three 52–54 week studies showed significantly more hypoglycemic episodes with sulfonylureas, there was significant heterogeneity. This was most likely due to data from Laakso et al.,25 where a high but similar proportion of patients in DPP-4I versus glimepiride group (63.7% vs 71.3%) reported hypoglycemia. When assessing effect of DPP-4I on change in HbA1c and incidence rates of hypoglycemia, regardless of background AHA (metformin, sulfonylureas, insulin) use and comparator (placebo or sulfonylureas), addition of a DPP-4I caused a modest reduction in HbA1c but similar hypoglycemia rates (27% vs 32%) as those in the comparator group. In SAVOR-TIMI 53, 2-year Kaplan–Meier rate estimates showed a higher risk for major hypoglycemia (assistance of another person required) with saxagliptin in patients with eGFR 30–50 mL/min/1.73 m2 (HR: 1.91 (95% CI: 1.27 to 2.92)) versus placebo.46 Therefore, patients should be vigilant with blood glucose monitoring and symptom detection if the decision is made to initiate DPP-4I in patients with CKD stages 3–5, with or without background sulfonylurea/insulin. DPP-4I would seem to be of most benefit in fine-tuning rather than getting to the goal range because clearly those with HbA1c > 9% need insulin management and those between 7% and 9% need to balance benefits of further reducing micro- and macro-vascular damage and hypoglycemia.57–59
Our updated meta-analysis is different from those previously published for several reasons. First, additional studies with usable data became available, thus increasing the sample by 350 participants compared with Cheng et al.13 and by 800 participants compared with Li et al.14 Second, we performed individual analyses of efficacy outcomes at 3-month intervals, by comparator (placebo vs sulfonylureas or other incretin-based therapies) and by dialysis-only versus non-dialysis studies. Conflicting outcomes on the effect of DPP-4I versus placebo on FPG, where data from dialysis and non-dialysis studies were combined, were reported by the two meta-analyses. However, in our meta-analysis, effect of DPP-4I on FPG in non-dialysis patients in placebo-only studies was not significantly different between the groups. Patients on dialysis differ from patients not on dialysis as there is uncertainty regarding the accuracy of HbA1c, a marker of blood glucose control over the previous 2–3 months. Shortened red blood cell survival, erythropoietin deficiency, use of erythropoiesis-stimulating agents, blood transfusion, phlebotomy and blood loss in the dialyzer circuit are factors that may lead to decreased time for glucose and erythrocytes to interact.60–63 Therefore, it may not be appropriate to combine data from dialysis and non-dialysis studies, or placebo and active-comparator studies, although heterogeneity may be absent.
This meta-analysis had several limitations: (1) two reviewers independently extracted data and calculated an average so there is potential for incorrect estimation; (2) results not generalizable to other populations as majority of patients were Caucasian or Asian; (3) most studies had fewer than 250 participants; (4) only six active-comparator studies (sulfonylureas and albiglutide) were available; (5) high drop-out rate reported by most studies; (6) only three studies with dialysis-only patients were available and (7) publication bias found in meta-analyses of HbA1c change because small and large studies were lacking. Direction of bias for HbA1c change overestimates the impact of DPP-4I on this outcome.
In conclusion, renally adjusted doses of DPP-4I (except linagliptin) will lower HbA1c in patients with T2DM and CKD stages 3–5 and who are not on dialysis, but their role is limited due to modest efficacy; DPP-4I did not appear to benefit patients who were already on dialysis. Appropriate monitoring for hypoglycemia remains a counseling point. Labeling of both saxagliptin and alogliptin now includes a warning about increased risk of HF hospitalizations. There are multiple DPP-4I and comparators available, but the predominant comparator in our meta-analysis was placebo; additionally, the overall drop-out rate was high. To gain a better understanding of the role of individual DPP-4I in patients with CKD stages 3–5 and/or dialysis, large studies with active comparators are needed.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. KDIGO CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation of chronic kidney disease. Kidney Int 2013; 3: 1–150. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Microvascular complications and foot care. Diabetes Care 2016; 39(Suppl. 1): S72–S80. [DOI] [PubMed] [Google Scholar]
- 3. Plantinga LC, Crews DC, Coresh J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010; 5: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Diabetes Statistics Report, 2014. Estimates of diabetes and its burden in the United States, http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (accessed 19 October 2015).
- 5. US Renal Data System. USRDS 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013. [Google Scholar]
- 6. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60: 850–886. [DOI] [PubMed] [Google Scholar]
- 7. Januvia (package insert). Whitehouse Station, NJ: Merck & Co., Inc, 2010. [Google Scholar]
- 8. Nesina (package insert). Deerfield, IL: Takeda Pharmaceuticals America, Inc, 2015. [Google Scholar]
- 9. Onglyza (package insert). Wilmington, DE: AstraZeneca Pharmaceuticals LP, 2015. [Google Scholar]
- 10. Galvus Summary of Product Characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf (accessed 1 October 2015).
- 11. Tradjenta (package insert). Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc, 2015. [Google Scholar]
- 12. Ito M, Abe M, Okada K, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Endocr J 2011; 58: 979–987. [DOI] [PubMed] [Google Scholar]
- 13. Cheng D, Fei Y, Liu Y, et al. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta-analysis. PLoS One 2014; 9: e111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li R, Wang R, Li H, et al. Short-term and long-term effects of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with renal impairment: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. Epub ahead of print 3 October 2015. DOI: 10.1002/dmrr.2731. [DOI] [PubMed] [Google Scholar]
- 15. Higgins J, Altman D, Sterne J. Chapter 8. Assessing risk of bias in included studies. In: Higgins J, Green S. (eds) Cochrane handbook for systematic reviews of interventions: the cochrane collaboration (Cochrane book series). Chichester: John Wiley & Sons Ltd, 2011. Available at: http://handbook.cochrane.org/ [Google Scholar]
- 16. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions: the cochrane collaboration, 2011. Available at: http://handbook.cochrane.org
- 17. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arjona Ferreira JC, Corry D, Mogensen CE, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis 2013; 61: 579–587. [DOI] [PubMed] [Google Scholar]
- 19. Arjona Ferreira JC, Marre M, Barzilai N, et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 2013; 36: 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan JC, Scott R, Arjona Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab 2008; 10: 545–555. [DOI] [PubMed] [Google Scholar]
- 21. Kothny W, Lukashevich V, Foley J, et al. Comparison of vildagliptin and sitagliptin in patients with type 2 diabetes and severe renal impairment: a randomised clinical trial. Diabetologia 2015; 58: 2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leiter LA, Carr MC, Stewart M, et al. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care 2014; 37: 2723–2730. [DOI] [PubMed] [Google Scholar]
- 23. Barnett AH, Huisman H, Jones R, et al. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet 2013; 382: 1413–1423. [DOI] [PubMed] [Google Scholar]
- 24. Groop PH, Del Prato S, Taskinen MR, et al. Linagliptin treatment in subjects with type 2 diabetes with and without mild-to-moderate renal impairment. Diabetes Obes Metab 2014; 16: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laakso M, Rosenstock J, Groop PH, et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care 2015; 38: e15–e17. [DOI] [PubMed] [Google Scholar]
- 26. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care 2013; 36: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGill JB, Yki-Järvinen H, Crowe S, et al. Combination of the dipeptidyl peptidase-4 inhibitor linagliptin with insulin-based regimens in type 2 diabetes and chronic kidney disease. Diab Vasc Dis Res 2015; 12: 249–257. [DOI] [PubMed] [Google Scholar]
- 28. Nowicki M, Rychlik I, Haller H, et al. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract 2011; 65: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 29. Nowicki M, Rychlik I, Haller H, et al. Saxagliptin improves glycaemic control and is well tolerated in patients with type 2 diabetes mellitus and renal impairment. Diabetes Obes Metab 2011; 13: 523–532. [DOI] [PubMed] [Google Scholar]
- 30. Kothny W, Shao Q, Groop PH, et al. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obes Metab 2012; 14: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 31. Lukashevich V, Schweizer A, Shao Q, et al. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab 2011; 13: 947–954. [DOI] [PubMed] [Google Scholar]
- 32. Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 2011; 13: 258–267. [DOI] [PubMed] [Google Scholar]
- 33. Owens DR, Swallow R, Dugi KA, et al. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med 2011; 28: 1352–1361. [DOI] [PubMed] [Google Scholar]
- 34. Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2011; 13: 65–74. [DOI] [PubMed] [Google Scholar]
- 35. Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ⩾52-week randomized, double-blind study. Diabetes Care 2013; 36: 3875–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh-Franco D, McLaughlin-Middlekauff J, Elrod S, et al. The effect of linagliptin on glycaemic control and tolerability in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2012; 14: 694–708. [DOI] [PubMed] [Google Scholar]
- 37. Ahrén B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014; 37: 2141–2148. [DOI] [PubMed] [Google Scholar]
- 38. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373: 232–242. [DOI] [PubMed] [Google Scholar]
- 39. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 40. Fujita H, Taniai H, Murayama H, et al. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1alpha in type 2 diabetic patients with incipient nephropathy. Endocr J 2014; 61: 159–166. [DOI] [PubMed] [Google Scholar]
- 41. Groop PH, Cooper ME, Perkovic V, et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 2013; 36: 3460–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kawasaki I, Hiura Y, Tamai A, et al. Sitagliptin reduces the urine albumin-to-creatinine ratio in type 2 diabetes through decreasing both blood pressure and estimated glomerular filtration rate. J Diabetes 2015; 7: 41–46. [DOI] [PubMed] [Google Scholar]
- 43. Mori H, Okada Y, Arao T, et al. Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J Diabetes Investig 2014; 5: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tani S, Nagao K, Hirayama A. Association between urinary albumin excretion and low-density lipoprotein heterogeneity following treatment of type 2 diabetes patients with the dipeptidyl peptidase-4 inhibitor, vildagliptin: a pilot study. Am J Cardiovasc Drugs 2013; 13: 443–450. [DOI] [PubMed] [Google Scholar]
- 45. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 46. Udell JA, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 trial. Diabetes Care 2015; 38: 696–705. [DOI] [PubMed] [Google Scholar]
- 47. Boehringer Ingelheim Pharmaceuticals. MARLINA—T2DM: efficacy, safety & modification of albuminuria in type 2 diabetes subjects with renal disease with LINAgliptin. In: ClinicalTrials.gov (Identifier: NCT01792518). Bethesda, MD: National Library of Medicine; (US), 2000, http://www.clinicaltrials.gov/ct2/show/NCT01792518?term=01792518&;rank=1 (accessed 28 November 2015). [Google Scholar]
- 48. Boehringer Ingelheim Pharmaceuticals. Cardiovascular and renal microvascular outcome study with linagliptin in patients with type 2 diabetes mellitus (CARMELINA). In: ClinicalTrials.gov (Identifier: NCT01897532). Bethesda, MD: National Library of Medicine; (US), 2000, http://www.clinicaltrials.gov/ct2/show/NCT01897532?term=01897532&;rank=1 (accessed 28 November 2015). [Google Scholar]
- 49. Boehringer Ingelheim Pharmaceuticals. CAROLINA: cardiovascular outcome study of linagliptin versus glimepiride in patients with type 2 diabetes. In: ClinicalTrials.gov (Identifier: NCT01243424). Bethesda, MD: National Library of Medicine; (US), 2000, http://www.clinicaltrials.gov/ct2/show/NCT01243424?term=01243424&;rank=1 (accessed 9 December 2015). [Google Scholar]
- 50. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015; 385: 2067–2076. [DOI] [PubMed] [Google Scholar]
- 51. FDA Drug Safety Communication. FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin, http://www.fda.gov/Drugs/DrugSafety/ucm486096.htm (accessed 26 April 2016).
- 52. McInnes G, Evans M, Del Prato S, et al. Cardiovascular and heart failure safety profile of vildagliptin: a meta-analysis of 17000 patients. Diabetes Obes Metab 2015; 17: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 53. Rosenstock J, Marx N, Neubacher D, et al. Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol 2015; 14: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Biesenbach G, Bodlaj G, Ebner S, et al. Metabolic control and vascular diseases under oral antidiabetic drug versus insulin therapy and/or diet alone during the first year of hemodialysis in type 2 diabetic patients with ESRD. Int Urol Nephrol 2011; 43: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 55. Glipizide, http://www.uptodate.com.ezproxylocal.library.nova.edu (accessed 30 April 2015).
- 56. Glimepiride, http://www.uptodate.com.ezproxylocal.library.nova.edu (accessed 30 April 2015).
- 57. Hill CJ, Maxwell AP, Cardwell CR, et al. Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Am J Kidney Dis 2014; 63: 84–94. [DOI] [PubMed] [Google Scholar]
- 58. Papademetriou V, Lovato L, Doumas M, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int 2015; 887: 649–659. [DOI] [PubMed] [Google Scholar]
- 59. Shurraw S, Hemmelgarn B, Lin M, et al. Association bet-ween glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med 2011; 171: 1920–1927. [DOI] [PubMed] [Google Scholar]
- 60. Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis 2004; 44: 715–719. [PubMed] [Google Scholar]
- 61. Robinson TW, Freedman BI. Assessing glycemic control in diabetic patients with severe nephropathy. J Ren Nutr 2013; 23: 199–202. [DOI] [PubMed] [Google Scholar]
- 62. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis 2014; 64: 510–533. [DOI] [PubMed] [Google Scholar]
- 63. Williams ME, Garg R. Glycemic management in ESRD and earlier stages of CKD. Am J Kidney Dis 2014; 63: S22–S38. [DOI] [PubMed] [Google Scholar]