Abstract
Guanylate-binding proteins (GBPs) are interferon-stimulated factors involved in the defense against cellular pathogens and inflammation. These proteins, particularly GBP-1, the most prominent member of the family, have been established as reliable markers of interferon-γ-activated cells in various diseases, including colorectal carcinoma (CRC) and inflammatory bowel diseases (IBDs). In CRC, GBP-1 expression is associated with a Th1-dominated angiostatic micromilieu and is correlated with a better outcome. Inhibition of tumor growth by GBP-1 is the result of its strong anti-angiogenic activity as well as its direct anti-tumorigenic effect on tumor cells. In IBD, GBP-1 mediates the anti-proliferative effects of interferon-γ on intestinal epithelial cells. In addition, it plays a protective role on the mucosa by preventing cell apoptosis, by inhibiting angiogenesis and by regulating the T-cell receptor signaling. These functions rely to a large extent on the ability of GBP-1 to interact with and remodel the actin cytoskeleton.
Keywords: Guanylate-binding proteins, Colorectal carcinoma, Inflammatory bowel diseases, Interferon
Core tip: Guanylate-binding proteins (GBPs) are interferon-stimulated factors involved in the defense against cellular pathogens and inflammation. In addition, guanylate-binding proteins have been established as reliable markers of interferon-γ-activated cells in various diseases including colorectal carcinoma and inflammatory bowel diseases. The GBP-1 is the best characterized member of the family. For instance, the expression of GBP-1 has been associated with a better outcome in colorectal carcinoma. The inhibition of tumor growth by GBP-1 is due to its strong anti-angiogenic activity as well as its direct anti-tumorigenic effect on tumor cells. In inflammatory bowel diseases, on the one hand GBP-1 mediates the anti-proliferative effects of interferon-γ on intestinal epithelial cells, and on the other hand, it protects the mucosa by preventing cell apoptosis, by inhibiting angiogenesis and by regulating the T-cell receptor signaling. These functions rely to a large extent on the ability of GBP-1 to interact with and remodel the actin cytoskeleton.
INTRODUCTION
Guanylate-binding proteins (GBPs) belong to the dynamin family of large GTPases and display two main particularities that distinguish them from small GTPases of the Ras superfamily. First, they hydrolyze GTP upon self-activation by oligomerization and therefore do not require the presence of GTPase-activating proteins (GAPs) or guanine nucleotide exchange factors (GEFs)[1-3]. Second, they are inducible proteins expressed in response of various stimuli, mainly to type 1 and type 2 interferons (IFNs) and, to a lower extent, to the inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)-α[4,5]. Up to now, seven human GBPs (GBP-1 to -7) and eleven mouse GBPs (mGBP-1 to -11) have been described[6,7]. Most of the available knowledge is related to human GBP-1. The structure of GBP-1 has been resolved and comprises two domains: (1) a large globular α/β domain displaying the canonical GTPase sequences at the N-terminus and (2) a long C-terminal part organized in an index finger-like domain composed exclusively of α-helices (7-13, Figure 1)[8]. Because of the high sequence homology between all GBPs, their respective structures are considered to be similar. In addition, GBP-1, GBP-2 and GBP-5 are C-terminally prenylated, allowing their attachment to cellular membranes[9].
Figure 1.
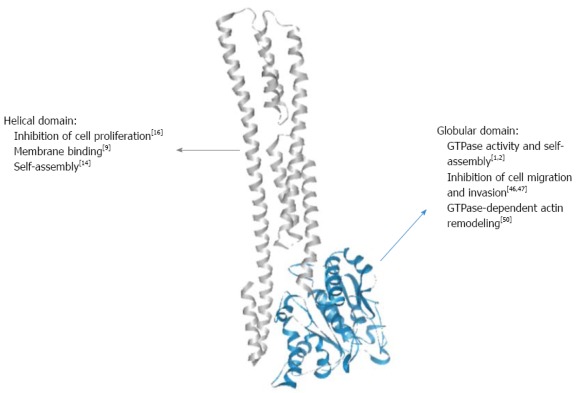
Structure-function relationships of guanylate-binding protein-1. The guanylate binding protein-1 can be roughly divided into two domains: a compact globular domain carrying the GTPase activity at the N-terminus (blue) and a long helical domain comprised of α-helices at the C-terminus (grey), which are responsible for different protein functions.
To date, biochemical as well as structural investigations have led to deeper insights into the molecular structure and enzymatic mechanism of human GBP-1. Dimer formation is induced by GTP binding leading to reorientation of a catalytic arginine side chain, thereby accelerating hydrolytic activity[1,10]. Moreover, the same catalytic machinery is employed to catalyze a second step of phosphate cleavage resulting in GMP as the major product[11,12]. While this is a unique feature among GTPases, another intriguing observation is the impact of this catalytic activity on changes in the C-terminal helical domain of GBP-1[2,13]. A salt bridge contact between the GTPase domain and the helical domain is switched such that a major reorientation of α-helices 12 and 13 allows for formation of a second contact area on the GBP-1 dimer resulting in association of the two α13 helices and thereby juxtaposition of the farnesyl groups attached to the C-termini[14]. Further biochemical experiments with farnesylated GBP-1 are required to disclose the role of this nucleotide hydrolysis-driven rearrangement for interaction with other GBP isoforms (see e.g., ref. 9) and with membranous compartments.
Initially, GBPs were shown to be among the most highly induced proteins in human fibroblasts exposed to IFN-γ[4]. Subsequently, it was reported that GBP-1 expression can be induced in vitro by treatment with IFN-γ in many cell types including primary cells (endothelial cells, fibroblasts, keratinocytes, B-cells, T-cells and peripheral blood mononuclear cells), immortalized intestinal epithelial cells and several tumor cell lines[5,15]. In agreement with its induction by IFNs and inflammatory cytokines, GBP-1 has predominantly been detected in vivo in inflamed tissues associated with various diseases such as psoriasis, lupus erythematosus, adverse drug reactions and Kaposi’s sarcoma[5,16,17]. Consequently, GBP-1 has been established as a robust marker of inflammation, specifically to detect activation of cells by IFN-γ at the single cell level in tissues[16].
In addition to their association with inflammation, GBPs participate in cell-autonomous defense against bacteria, protozoa and viruses[18]. Moreover, elevated expression of GBP-1, together with other GBPs, has been observed in the colons of patients with inflammatory bowel disease (IBD) or in colorectal carcinoma (CRC)[15,19-21]. This review will focus on the pathophysiological roles of GBPs in CRC and IBD, and especially on GBP-1, the best described member of the GBP family to date.
CLINICAL RELEVANCE OF GBP EXPRESSION IN CRC AND IBD
GBP-1 is an independent positive prognostic factor in CRC
CRC is the third most frequent type of cancer in developed countries. The development of sporadic CRC has been considered the archetype of multistep carcinogenesis. Over the past two decades, major progress has been made in the characterization of CRC. New types of classifications have been proposed, which have allowed prognoses to be refined, and have opened new perspectives for improving therapeutic decisions. Various kinds of molecular subtypes have been described including chromosomal instability, microsatellite instability and CpG island methylator phenotype[22-24]. In addition, gene expression profiles that correlate with prognosis have been identified. For instance, CRCs with a stem cell gene expression signature are associated with a poor prognosis[24,25]. Besides molecular classifications, the host’s immune response has emerged as a powerful prognostic factor in CRC. Additionally, immune cell infiltration into the tumor and a polarized immune response have been shown to be of prognostic relevance. In particular, the presence of an active Th1 adaptive immune response in CRC correlates with a better outcome[26]. More precisely, the presence of cytotoxic and memory T-cells, together with the expression of Th1-associated factors (IFN-γ, IL-12 or IRF1), has been associated with a better prognosis in CRC[26,27]. In the meanwhile, such associations have been observed in a variety of solid tumors including breast, lung, ovarian and liver cancers[28]. Interestingly, molecular studies of CRCs also revealed the presence of an “inflammatory” gene expression signature enriched with interferon-stimulated genes (ISGs) and associated with improved prognosis[24,25]. Overall, the so-called Immunoscore has been shown to be better than the standard histo-pathological classification or the presence of microsatellite instability as a predictor of outcome for patients with CRC[26,29,30]. Expression of GBP-1, along with several other ISGs, has been detected in CRC[19,31]. Furthermore, a study involving 388 patients showed GBP-1 to be an independent prognostic marker in CRC, which correlates with prolonged 5-year cancer-specific survival[19]. This has subsequently been confirmed in a study of The Cancer Genome Atlas (TCGA) network, which also found that the expression of GBP-1 and GBP-4 in CRC was associated with a less aggressive phenotype, including tumor stage, lymph node invasion and metastasis[32]. Of note, an association has been found between expressions of GBP-1 and GBP-2 and the regression of melanoma metastasis after immunotherapy[33]. Furthermore, GBP-1 participates in a signature of immune function genes associated with recurrence-free survival in breast cancer patients[34]. In addition, GBP-2 has been shown to be expressed in esophagus squamous cell carcinomas and in breast cancer, where it is associated with T-cell infiltration and a better prognosis[35,36]. Hence, expression of GBPs appears to be generally associated with a prognostically favorable immunological response to cancer. Specific evidence has been obtained that GBP-1 mediates the anti-tumorigenic effects induced by a Th1-dominated micromilieu.
GBP-1 is a marker for IFN-γ-induced cell activation in IBDs
IBDs comprise two main forms of chronic relapsing disorders, namely ulcerative colitis (UC) and Crohn’s disease (CD)[37]. The pathogenesis of IBD is not yet fully understood, but it is generally believed to involve dysregulation of intestinal homeostasis, characterized by the loss of barrier function and an aberrant immune response leading to the loss of tolerance of enteric bacterial flora accompanied by acute inflammation[37,38]. Active IBD displays infiltration of the lamina propria by both innate and adaptive immune cells and increased local levels of cytokines[39-42]. Compared to healthy controls, specimens with active CD or UC show increased expression of GBP-1 at the RNA and protein levels[15,21,43]. In addition, GBP-1 mRNA expression correlated with the expression of IFN-γ in whole tissue samples, and GBP-1 expressing cells were found in the vicinity of IFN-γ-producing cells[21]. While UC has been considered as a Th2-mediated condition mostly driven by IL-13, Th1 cytokines such as IL-12 or IFN-γ have been described as primarily involved in CD. However, this paradigm has been revised and cytokines such as TNF-α, IFN-γ, IL-1, -6, -12 and -17 are considered to be involved in the pathogenesis of both diseases[42]. Accordingly, GBP-1 expression is also increased in both, and at similar levels (Table 1)[21]. In agreement with human data, elevated expression of GBP-1 was also detected in colonic tissues of mice undergoing colitis after treatment with dextran sodium sulfate (DSS)[21]. This increase was observed in both, acute and chronic DSS models and was associated with an elevation in IFN-γ tissue expression[21]. Hence, GBP-1 expression seems to be a useful marker for inflammation and IFN-γ-induced cell activation in IBD. Cytokines such as IFN-γ, TNF-α and IL-1β are not only involved in the recruitment and activation of immune cells but can also affect epithelial or endothelial cells, leading to an amplification of tissue damage and inflammation[39-42]. In IBD samples, GBP-1 expression was observed in infiltrating immune cells, in endothelial cells and in epithelial cells, confirming that IFN-γ indeed acts in vivo on both mesenchymal and epithelial cells[15,21].
Table 1.
Expression and functions of guanylate-binding protein-1
| GBP-1 expression | |
| Inflammatory bowel diseases | Expressed in UC and CD[15,20,21] |
| Expression in epithelial cells, endothelial cells and immune cells[15,21] | |
| Colorectal carcinoma | Expression in the stroma and partially in tumor cells[15,19] |
| Associated with Th1-dominated angiostatic micromilieu and improved survival[19,31] | |
| Functions of GBP-1 | |
| Endothelial cells | Anti-angiogenic, inhibits proliferation, migration, invasion and spreading; protection against apoptosis[16,44,46,47,50] |
| Intestinal epithelial cells | Inhibits proliferation; protection against apoptosis[20,43] |
| Colon tumor cells | Inhibits proliferation, migration, invasion and tumor growth in vivo; no induction of apoptosis (but partially required)[9] |
| T-cells | Inhibition of spreading and early T-cell receptor signaling[54] |
GBP: Guanylate-binding protein; UC: Ulcerative colitis; CD: Crohn’s disease.
GBP-1 FUNCTIONS IN STROMAL CELLS
GBP-1 mediates the anti-angiogenic effects of IFNs in CRC
Expression of GBP-1 has been detected in the stroma of CRCs[15,19]. In the course of tumorigenesis, the angiogenic switch allows tumors to grow beyond a critical size. In the tumor micro-environment, pro-angiogenic and anti-angiogenic factors co-exist and the resulting angiogenic balance determines the state of the vasculature. Based on the observation that the inflammatory cytokines IL-1β, TNF-α and IFN-γ inhibit proliferation and migration of endothelial cells, GBP-1 was identified by differential display RT-PCR as a gene induced by these angiostatic cytokines and repressed by the angiogenic factors VEGF and bFGF[16]. Subsequently, it has be shown that GBP-1 expression in tissues is closely associated with blood vessel endothelial cells that are exposed to these cytokines[5,16]. In agreement with these findings, in CRC, the expression of GBP-1 in vessels correlated with significantly repressed angiogenic activity and the presence of an angiostatic micro-environment, indicated by co-expression of the anti-angiogenic cytokines CXCL9, -10 and -11[19]. Because of its association with immune angiostasis in tissues in vivo, GBP-1 was investigated for directly exerting angiostatic activity. Indeed, it was shown to inhibit the proliferation of human umbilical vein endothelial cells (HUVECs) through its helical domain (Figure 1)[16]. It was also found to mediate the effects of IFN-α by protecting HUVECs from serum starvation-induced apoptosis[44]. Of note, in the same study, continuous exposure to IFN-α led to senescence of HUVECs. Unexpectedly, GBP-1 was shown to be secreted, however exclusively by endothelial cells[45]. As yet, the role of extracellular GBP-1 remains unknown. Interestingly, GBP-1 has been shown to inhibit the invasiveness and tube-forming capabilities of HUVECs by down-regulating MMP-1 expression[46]. This effect was found to be dependent on the GTPase activity of the protein. Furthermore, GBP-1 is able to inhibit migration and spreading of endothelial cells on a fibronectin matrix through expression of integrin-α4, indicating that its effect might depend on the extracelluar matrix composition of the surrounding micromilieu[47]. Mouse GBP-2 has similarly been shown to inhibit endothelial cell spreading[48,49]. Interestingly, GBP-1 has been shown to interact with other GBPs (GBP-2 to -5) and to recruit them in its own cellular compartment, suggesting that GBPs act in a cooperative manner and that GBP-1 is dominant over the other family members[9]. The effects of GBP-1 on cell migration, invasion and spreading can be explained by the fact that GBP-1 was found to bind β-actin[50]. More precisely, it has been found to mediate the IFN-γ-induced disruption of actin fibers in various tumor cell lines and in endothelial cells[50]. The disintegration of actin fibers by GBP-1 occurred through direct binding between both proteins, both in vivo and in vitro using purified recombinant GBP-1 and actin[50]. The interaction with β-actin required both self-assembly and the GTPase activity of GBP-1[50]. Taken together, it has been shown that GBP-1 exerts a powerful anti-angiogenic role by directly inhibiting endothelial cell proliferation, migration, invasion and spreading, together with protecting cells against apoptosis, thus creating an angiostatic state for the vessels (Table 1 and Figure 2). Thereby, GBP-1 links anti-tumor immune response and inhibition of angiogenesis in CRC as a major mediator of the anti-angiogenic effect of IFNs.
Figure 2.

Role of guanylate-binding protein-1 in colorectal carcinoma and inflammatory bowel diseases. In colorectal carcinoma (CRC), guanylate-binding protein (GBP)-1 expression is associated with a Th1-dominated micromileu and results in an angiostatic vasculature. In CRC tumor cells, expression of GBP-1 induces an anti-tumorigenic phenotype. Absence of expression in tumor cells in a GBP-1-positive context indicates a mechanism of resistance. In T-cells, GBP-1 participates in the modulation of the T-cell receptor signaling pathway. In inflammatory bowel disease (IBD), GBP-1 expression is elevated in active disease and inhibits the proliferation of intestinal epithelial cells, thereby exerting a protective effect against the loss of barrier function and apoptosis. Here again, GBP-1 is associated with angiostasis and T-cell regulation. INF: Interferon; IL: Interleukin.
Role of GBP-1 in IFN-induced vascular remodeling in IBD
As chronic IBD is established, the vascular system undergoes profound reorganization[51]. On the one hand, angiogenesis is induced through the production of VEGF. On the other hand, inflammatory stimuli activate endothelial cells, enhancing leukocyte binding, tissue infiltration and ultimately, chronic inflammation[52,53]. However, the resulting vasculature is dysfunctional. Expression of GBP-1 was observed in vascular cells and was associated with in situ IFN-γ expression in human IBD tissues and in DSS-induced mouse colitis samples, indicating that those vessels were in an anti-angiogenic state[21]. This was confirmed by the fact that neutralization of IFN-γ resulted in an increase in blood vessel density[21]. Hence, the angiostatic effects of IFN-γ mediated by GBP-1 seem to be a more general mechanism, because it also occurs in colitis. Of note, IFN-γ was found to increase vascular permeability in the same mouse colitis model. Thereby, IFN-γ is involved in establishing dysfunctional vasculature, potentially participating in the aggravation of tissue damage and in the increase of immune cell infiltration[21].
GBP-1 regulates T-cell receptor signaling
Expression of GBP-1 has been detected in vitro and in vivo in monocytes/macrophages and T-cells in response to IFN-γ[5,19,54]. In T-cells, GBP-1 has been found to mediate the interaction between early T-cell receptor signaling and the cytoskeleton[54]. More precisely, it was required for inhibiting T-cell spreading, down-regulating CD3 and CD45 at the cell surface, and decreasing IL-2 release. Its function in T-cells was mediated by interactions with the actin-regulating proteins plastin-2 and βII-spectrin[54]. Hence, GBP-1 might participate in a negative feedback loop in T-cells activated by IFN-γ resulting in reduced IL-2 expression during Th1 differentiation (Figure 2). Therefore, GBP-1 might prevent T-cells from over-activation, leading to a protective effect during IBD. It cannot be excluded that GBP-1 might also participate in T-cell anergy, which would result in a negative impact on the anti-tumor immune response in CRC. However, the overall impact of GBP-1 expression remains positive, which indicates that its effects on endothelial and tumor cells overcome the inhibitory functions in T-cells.
GBP-1 FUNCTIONS IN COLON EPITHELIAL AND TUMOR CELLS
GBP-1 exerts anti-tumorigenic effects in colon tumor cells
In CRC, GBP-1 expression is predominantly detected in the desmoplastic stroma. In about half the cases, GBP-1 expression is also detected in tumor cells[15]. This suggests that the stimulus responsible for GBP-1 expression, for instance IFN-γ, originates from infiltrating cells and that the tumor cells might have lost responsiveness to the respective factor and concomitantly the ability to express GBP-1. This suggests that the loss of GBP-1 expression may be a tumor escape mechanism fostered by the genetic and epigenetic instability of the cancer cells, which does not affect the adjacent stromal cells. In this framework, it is important to note that GBP-1, as shown for endothelial cells, can also inhibit proliferation, migration and invasion of CRC cell lines (Table 1, Figures 1 and 2)[15]. In addition, GBP-1 was able to reduce anchorage-independent growth and tumor growth in a mouse xenograft model[15]. Using RNA interference, it was confirmed that GBP-1 mediates the anti-proliferative, anti-migratory and anti-invasive effects of IFN-γ in CRC cell lines[15]. However, it did not induce apoptosis by itself, even if it was at least partially required for IFN-γ-induced apoptosis[15]. In this context GBP-1 must be regarded as a bona fide tumor suppressor gene. Nevertheless, its effects on tumor cells seem to be highly tumor type specific[55]. In accordance with its functions in CRC cells, GBP-1 has also been shown to inhibit mammary tumor growth in mice[56]. In contrast, GBP-1 expression has been associated with Paclitaxel resistance in ovarian cancer cell lines[57,58], docetaxel resistance of prostate cancer cells[59], and with radioresistance[60]. Other reports have shown that GBP-1 induces glioblastoma growth in mice through increased invasiveness but not proliferation of glioblastoma cells[61,62]. Similarly, it was found to induce invasion of oral squamous cell carcinoma cells[55]. Based on these findings it is clear that GBP-1 has anti-tumor functions in CRC but its activity may be converted to pro-tumorigenic functions in other tumor types. It remains to be determined in future studies whether this is dependent on specific cellular or micromilieu-derived co-factors or is partly the result of different experimental setups. Overall, GBP-1 has been shown to have a pleiotropic role in CRC, by mediating both the anti-angiogenic and anti-tumorigenic effects of IFN-γ.
GBP-1 inhibits the proliferation of intestinal epithelial cells
Expression of GBP-1 in intestinal epithelial cells has been observed in human colon specimens of IBD and in the inflamed colonic mucosa of DSS-treated mice[15,21,43]. In addition, immortalized human primary colon epithelial cells are able to express GBP-1 in vitro after treatment with IFN-γ, IL-1β or TNF-α[15]. When induced by IFN-γ/TNF-α, GBP-1 was described as inhibiting cell proliferation of the colonic epithelial cell lines SKCO15 and T84, which harbor an enterocyte-stereotypic differentiation, are polarized and therefore are considered valuable models for studying intestinal epithelial cells[43]. In these cell lines, GBP-1 was able to reduce β-catenin protein levels and β-catenin serine 552 phosphorylation in a proteasome-independent mechanism[43]. On the other hand, it was found to have a protective effect against IFN-γ-induced apoptosis and loss of barrier function in the colonic epithelium (Figure 2)[20].
GBPS AND CELLULAR RESPONSE TO INTESTINAL PATHOGENS
Several human and mouse GBPs have been shown to be involved in the response against intracellular pathogens including viruses such as vesicular stomatitis virus, encephalomyocarditis virus, hepatitis C virus, influenza A virus, dengue virus, swine fever virus and human immunodeficiency virus-1[63-69]. GBPs have also been shown to mediate host’s defense against bacterial and mycobacterial infectious agents such as Listeria monocytogenes, Chlamydia trachomatis, Salmonella typhimurium, and Mycobacterium bovis[70-72]. Finally, GBPs are involved in intracellular immunity against Toxoplasma gondii[73]. In particular, GBP-1 has been shown to be up-regulated in a colon cell line after rotavirus infection[74]. Furthermore, GBP-1 was found to be up-regulated differently in two mouse strains after colonization with commensal bacteria, and it is thought to participate, together with other interferon-stimulated genes, to host-specific responses to commensal gut bacteria, thereby shaping the microbiota[75]. Actually, IFN-α expression induced by commensal nonpathogenic Escherichia coli in the intestine of newborn mice was shown to mediate GBP-1 expression[76]. Furthermore, GBP-1 was required for the anti-apoptotic effects of IFN-α in immature human colon epithelia treated with staurosporine, indicating that it might play a role in the prevention of intestinal epithelial apoptosis induced by commensal bacteria[76].
CONCLUSION
Overall, expression of GBPs and in particular GBP-1 has been shown to be associated with infection by intracellular pathogens, Th1-driven inflammation during IBD and the Th1-dominated anti-tumor immune response in CRC. Additionally, GBP-1 exerts strong anti-proliferative, anti-migratory and anti-invasive effects on various cell types, resulting in the creation of angiostatic vasculature in IBD and CRC and in direct anti-tumorigenic effects on CRC cells.
Footnotes
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Germany
Peer-Review Report Classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare having no conflict-of-interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 5, 2016
First decision: May 12, 2016
Article in press: June 13, 2016
P- Reviewer: Arora Z, Larson BK S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Ghosh A, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature. 2006;440:101–104. doi: 10.1038/nature04510. [DOI] [PubMed] [Google Scholar]
- 2.Vöpel T, Syguda A, Britzen-Laurent N, Kunzelmann S, Lüdemann MB, Dovengerds C, Stürzl M, Herrmann C. Mechanism of GTPase-activity-induced self-assembly of human guanylate binding protein 1. J Mol Biol. 2010;400:63–70. doi: 10.1016/j.jmb.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 3.Wehner M, Kunzelmann S, Herrmann C. The guanine cap of human guanylate-binding protein 1 is responsible for dimerization and self-activation of GTP hydrolysis. FEBS J. 2012;279:203–210. doi: 10.1111/j.1742-4658.2011.08415.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng YS, Colonno RJ, Yin FH. Interferon induction of fibroblast proteins with guanylate binding activity. J Biol Chem. 1983;258:7746–7750. [PubMed] [Google Scholar]
- 5.Lubeseder-Martellato C, Guenzi E, Jörg A, Töpolt K, Naschberger E, Kremmer E, Zietz C, Tschachler E, Hutzler P, Schwemmle M, et al. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am J Pathol. 2002;161:1749–1759. doi: 10.1016/S0002-9440(10)64452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olszewski MA, Gray J, Vestal DJ. In silico genomic analysis of the human and murine guanylate-binding protein (GBP) gene clusters. J Interferon Cytokine Res. 2006;26:328–352. doi: 10.1089/jir.2006.26.328. [DOI] [PubMed] [Google Scholar]
- 7.Degrandi D, Konermann C, Beuter-Gunia C, Kresse A, Würthner J, Kurig S, Beer S, Pfeffer K. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J Immunol. 2007;179:7729–7740. doi: 10.4049/jimmunol.179.11.7729. [DOI] [PubMed] [Google Scholar]
- 8.Prakash B, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature. 2000;403:567–571. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- 9.Britzen-Laurent N, Bauer M, Berton V, Fischer N, Syguda A, Reipschläger S, Naschberger E, Herrmann C, Stürzl M. Intracellular trafficking of guanylate-binding proteins is regulated by heterodimerization in a hierarchical manner. PLoS One. 2010;5:e14246. doi: 10.1371/journal.pone.0014246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Praefcke GJ, Kloep S, Benscheid U, Lilie H, Prakash B, Herrmann C. Identification of residues in the human guanylate-binding protein 1 critical for nucleotide binding and cooperative GTP hydrolysis. J Mol Biol. 2004;344:257–269. doi: 10.1016/j.jmb.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Kunzelmann S, Praefcke GJ, Herrmann C. Transient kinetic investigation of GTP hydrolysis catalyzed by interferon-gamma-induced hGBP1 (human guanylate binding protein 1) J Biol Chem. 2006;281:28627–28635. doi: 10.1074/jbc.M604911200. [DOI] [PubMed] [Google Scholar]
- 12.Rani A, Pandita E, Rahman S, Deep S, Sau AK. Insight into temperature dependence of GTPase activity in human guanylate binding protein-1. PLoS One. 2012;7:e40487. doi: 10.1371/journal.pone.0040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vöpel T, Kunzelmann S, Herrmann C. Nucleotide dependent cysteine reactivity of hGBP1 uncovers a domain movement during GTP hydrolysis. FEBS Lett. 2009;583:1923–1927. doi: 10.1016/j.febslet.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Vöpel T, Hengstenberg CS, Peulen TO, Ajaj Y, Seidel CA, Herrmann C, Klare JP. Triphosphate induced dimerization of human guanylate binding protein 1 involves association of the C-terminal helices: a joint double electron-electron resonance and FRET study. Biochemistry. 2014;53:4590–4600. doi: 10.1021/bi500524u. [DOI] [PubMed] [Google Scholar]
- 15.Britzen-Laurent N, Lipnik K, Ocker M, Naschberger E, Schellerer VS, Croner RS, Vieth M, Waldner M, Steinberg P, Hohenadl C, et al. GBP-1 acts as a tumor suppressor in colorectal cancer cells. Carcinogenesis. 2013;34:153–162. doi: 10.1093/carcin/bgs310. [DOI] [PubMed] [Google Scholar]
- 16.Guenzi E, Töpolt K, Cornali E, Lubeseder-Martellato C, Jörg A, Matzen K, Zietz C, Kremmer E, Nappi F, Schwemmle M, et al. The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J. 2001;20:5568–5577. doi: 10.1093/emboj/20.20.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naschberger E, Wenzel J, Kretz CC, Herrmann M, Stürzl M, Kuhn A. Increased expression of guanylate binding protein-1 in lesional skin of patients with cutaneous lupus erythematosus. Exp Dermatol. 2011;20:102–106. doi: 10.1111/j.1600-0625.2010.01160.x. [DOI] [PubMed] [Google Scholar]
- 18.MacMicking JD. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol. 2012;12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naschberger E, Croner RS, Merkel S, Dimmler A, Tripal P, Amann KU, Kremmer E, Brueckl WM, Papadopoulos T, Hohenadl C, et al. Angiostatic immune reaction in colorectal carcinoma: Impact on survival and perspectives for antiangiogenic therapy. Int J Cancer. 2008;123:2120–2129. doi: 10.1002/ijc.23764. [DOI] [PubMed] [Google Scholar]
- 20.Schnoor M, Betanzos A, Weber DA, Parkos CA. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-gamma and regulates barrier function through effects on apoptosis. Mucosal Immunol. 2009;2:33–42. doi: 10.1038/mi.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haep L, Britzen-Laurent N, Weber TG, Naschberger E, Schaefer A, Kremmer E, Foersch S, Vieth M, Scheuer W, Wirtz S, et al. Interferon Gamma Counteracts the Angiogenic Switch and Induces Vascular Permeability in Dextran Sulfate Sodium Colitis in Mice. Inflamm Bowel Dis. 2015;21:2360–2371. doi: 10.1097/MIB.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 22.Laghi L, Malesci A. Microsatellite instability and therapeutic consequences in colorectal cancer. Dig Dis. 2012;30:304–309. doi: 10.1159/000337003. [DOI] [PubMed] [Google Scholar]
- 23.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S, Ostos LC, Lannon WA, Grotzinger C, Del Rio M, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. 2013;19:619–625. doi: 10.1038/nm.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadanandam A, Wang X, de Sousa E Melo F, Gray JW, Vermeulen L, Hanahan D, Medema JP. Reconciliation of classification systems defining molecular subtypes of colorectal cancer: interrelationships and clinical implications. Cell Cycle. 2014;13:353–357. doi: 10.4161/cc.27769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 27.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 28.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 29.Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Grenz S, Naschberger E, Merkel S, Britzen-Laurent N, Schaal U, Konrad A, Aigner M, Rau TT, Hartmann A, Croner RS, et al. IFN-γ-driven intratumoral microenvironment exhibits superior prognostic effect compared with an IFN-α-driven microenvironment in patients with colon carcinoma. Am J Pathol. 2013;183:1897–1909. doi: 10.1016/j.ajpath.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carretero R, Wang E, Rodriguez AI, Reinboth J, Ascierto ML, Engle AM, Liu H, Camacho FM, Marincola FM, Garrido F, et al. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int J Cancer. 2012;131:387–395. doi: 10.1002/ijc.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascierto ML, Kmieciak M, Idowu MO, Manjili R, Zhao Y, Grimes M, Dumur C, Wang E, Ramakrishnan V, Wang XY, et al. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Cancer Res Treat. 2012;131:871–880. doi: 10.1007/s10549-011-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guimarães DP, Oliveira IM, de Moraes E, Paiva GR, Souza DM, Barnas C, Olmedo DB, Pinto CE, Faria PA, De Moura Gallo CV, et al. Interferon-inducible guanylate binding protein (GBP)-2: a novel p53-regulated tumor marker in esophageal squamous cell carcinomas. Int J Cancer. 2009;124:272–279. doi: 10.1002/ijc.23944. [DOI] [PubMed] [Google Scholar]
- 36.Godoy P, Cadenas C, Hellwig B, Marchan R, Stewart J, Reif R, Lohr M, Gehrmann M, Rahnenführer J, Schmidt M, et al. Interferon-inducible guanylate binding protein (GBP2) is associated with better prognosis in breast cancer and indicates an efficient T cell response. Breast Cancer. 2014;21:491–499. doi: 10.1007/s12282-012-0404-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 41.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Bouguen G, Chevaux JB, Peyrin-Biroulet L. Recent advances in cytokines: therapeutic implications for inflammatory bowel diseases. World J Gastroenterol. 2011;17:547–556. doi: 10.3748/wjg.v17.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capaldo CT, Beeman N, Hilgarth RS, Nava P, Louis NA, Naschberger E, Stürzl M, Parkos CA, Nusrat A. IFN-γ and TNF-α-induced GBP-1 inhibits epithelial cell proliferation through suppression of β-catenin/TCF signaling. Mucosal Immunol. 2012;5:681–690. doi: 10.1038/mi.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pammer J, Reinisch C, Birner P, Pogoda K, Sturzl M, Tschachler E. Interferon-alpha prevents apoptosis of endothelial cells after short-term exposure but induces replicative senescence after continuous stimulation. Lab Invest. 2006;86:997–1007. doi: 10.1038/labinvest.3700461. [DOI] [PubMed] [Google Scholar]
- 45.Naschberger E, Lubeseder-Martellato C, Meyer N, Gessner R, Kremmer E, Gessner A, Stürzl M. Human guanylate binding protein-1 is a secreted GTPase present in increased concentrations in the cerebrospinal fluid of patients with bacterial meningitis. Am J Pathol. 2006;169:1088–1099. doi: 10.2353/ajpath.2006.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guenzi E, Töpolt K, Lubeseder-Martellato C, Jörg A, Naschberger E, Benelli R, Albini A, Stürzl M. The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression. EMBO J. 2003;22:3772–3782. doi: 10.1093/emboj/cdg382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinländer K, Naschberger E, Lehmann MH, Tripal P, Paster W, Stockinger H, Hohenadl C, Stürzl M. Guanylate binding protein-1 inhibits spreading and migration of endothelial cells through induction of integrin alpha4 expression. FASEB J. 2008;22:4168–4178. doi: 10.1096/fj.08-107524. [DOI] [PubMed] [Google Scholar]
- 48.Balasubramanian S, Messmer-Blust AF, Jeyaratnam JA, Vestal DJ. Role of GTP binding, isoprenylation, and the C-terminal α-helices in the inhibition of cell spreading by the interferon-induced GTPase, mouse guanylate-binding protein-2. J Interferon Cytokine Res. 2011;31:291–298. doi: 10.1089/jir.2010.0056. [DOI] [PubMed] [Google Scholar]
- 49.Messmer-Blust AF, Balasubramanian S, Gorbacheva VY, Jeyaratnam JA, Vestal DJ. The interferon-gamma-induced murine guanylate-binding protein-2 inhibits rac activation during cell spreading on fibronectin and after platelet-derived growth factor treatment: role for phosphatidylinositol 3-kinase. Mol Biol Cell. 2010;21:2514–2528. doi: 10.1091/mbc.E09-04-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostler N, Britzen-Laurent N, Liebl A, Naschberger E, Lochnit G, Ostler M, Forster F, Kunzelmann P, Ince S, Supper V, et al. Gamma interferon-induced guanylate binding protein 1 is a novel actin cytoskeleton remodeling factor. Mol Cell Biol. 2014;34:196–209. doi: 10.1128/MCB.00664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS. Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol. 2011;17:578–593. doi: 10.3748/wjg.v17.i5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 53.Cromer WE, Ganta CV, Patel M, Traylor J, Kevil CG, Alexander JS, Mathis JM. VEGF-A isoform modulation in an preclinical TNBS model of ulcerative colitis: protective effects of a VEGF164b therapy. J Transl Med. 2013;11:207. doi: 10.1186/1479-5876-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forster F, Paster W, Supper V, Schatzlmaier P, Sunzenauer S, Ostler N, Saliba A, Eckerstorfer P, Britzen-Laurent N, Schütz G, et al. Guanylate binding protein 1-mediated interaction of T cell antigen receptor signaling with the cytoskeleton. J Immunol. 2014;192:771–781. doi: 10.4049/jimmunol.1300377. [DOI] [PubMed] [Google Scholar]
- 55.Yu CJ, Chang KP, Chang YJ, Hsu CW, Liang Y, Yu JS, Chi LM, Chang YS, Wu CC. Identification of guanylate-binding protein 1 as a potential oral cancer marker involved in cell invasion using omics-based analysis. J Proteome Res. 2011;10:3778–3788. doi: 10.1021/pr2004133. [DOI] [PubMed] [Google Scholar]
- 56.Lipnik K, Naschberger E, Gonin-Laurent N, Kodajova P, Petznek H, Rungaldier S, Astigiano S, Ferrini S, Stürzl M, Hohenadl C. Interferon gamma-induced human guanylate binding protein 1 inhibits mammary tumor growth in mice. Mol Med. 2010;16:177–187. doi: 10.2119/molmed.2009.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan Z, Foster R, Brakora KA, Yusuf RZ, Seiden MV. GBP1 overexpression is associated with a paclitaxel resistance phenotype. Cancer Chemother Pharmacol. 2006;57:25–33. doi: 10.1007/s00280-005-0026-3. [DOI] [PubMed] [Google Scholar]
- 58.De Donato M, Mariani M, Petrella L, Martinelli E, Zannoni GF, Vellone V, Ferrandina G, Shahabi S, Scambia G, Ferlini C. Class III β-tubulin and the cytoskeletal gateway for drug resistance in ovarian cancer. J Cell Physiol. 2012;227:1034–1041. doi: 10.1002/jcp.22813. [DOI] [PubMed] [Google Scholar]
- 59.Desarnaud F, Geck P, Parkin C, Carpinito G, Makarovskiy AN. Gene expression profiling of the androgen independent prostate cancer cells demonstrates complex mechanisms mediating resistance to docetaxel. Cancer Biol Ther. 2011;11:204–212. doi: 10.4161/cbt.11.2.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukumoto M, Amanuma T, Kuwahara Y, Shimura T, Suzuki M, Mori S, Kumamoto H, Saito Y, Ohkubo Y, Duan Z, et al. Guanine nucleotide-binding protein 1 is one of the key molecules contributing to cancer cell radioresistance. Cancer Sci. 2014;105:1351–1359. doi: 10.1111/cas.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Mukasa A, Inda MM, Zhang J, Chin L, Cavenee W, Furnari F. Guanylate binding protein 1 is a novel effector of EGFR-driven invasion in glioblastoma. J Exp Med. 2011;208:2657–2673. doi: 10.1084/jem.20111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lan Q, Wang A, Cheng Y, Mukasa A, Ma J, Hong L, Yu S, Sun L, Huang Q, Purow B, et al. Guanylate binding protein-1 mediates EGFRvIII and promotes glioblastoma growth in vivo but not in vitro. Oncotarget. 2016;7:9680–9691. doi: 10.18632/oncotarget.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Itsui Y, Sakamoto N, Kakinuma S, Nakagawa M, Sekine-Osajima Y, Tasaka-Fujita M, Nishimura-Sakurai Y, Suda G, Karakama Y, Mishima K, et al. Antiviral effects of the interferon-induced protein guanylate binding protein 1 and its interaction with the hepatitis C virus NS5B protein. Hepatology. 2009;50:1727–1737. doi: 10.1002/hep.23195. [DOI] [PubMed] [Google Scholar]
- 64.Anderson SL, Carton JM, Lou J, Xing L, Rubin BY. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology. 1999;256:8–14. doi: 10.1006/viro.1999.9614. [DOI] [PubMed] [Google Scholar]
- 65.Tietzel I, El-Haibi C, Carabeo RA. Human guanylate binding proteins potentiate the anti-chlamydia effects of interferon-gamma. PLoS One. 2009;4:e6499. doi: 10.1371/journal.pone.0006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan W, Zuo X, Feng T, Shi X, Dai J. Guanylate-binding protein 1 participates in cellular antiviral response to dengue virus. Virol J. 2012;9:292. doi: 10.1186/1743-422X-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Z, Shi Z, Yan W, Wei J, Shao D, Deng X, Wang S, Li B, Tong G, Ma Z. Nonstructural protein 1 of influenza A virus interacts with human guanylate-binding protein 1 to antagonize antiviral activity. PLoS One. 2013;8:e55920. doi: 10.1371/journal.pone.0055920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li LF, Yu J, Li Y, Wang J, Li S, Zhang L, Xia SL, Yang Q, Wang X, Yu S, et al. Guanylate-Binding Protein 1, an Interferon-Induced GTPase, Exerts an Antiviral Activity against Classical Swine Fever Virus Depending on Its GTPase Activity. J Virol. 2016;90:4412–4426. doi: 10.1128/JVI.02718-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krapp C, Hotter D, Gawanbacht A, McLaren PJ, Kluge SF, Stürzel CM, Mack K, Reith E, Engelhart S, Ciuffi A, et al. Guanylate Binding Protein (GBP) 5 Is an Interferon-Inducible Inhibitor of HIV-1 Infectivity. Cell Host Microbe. 2016;19:504–514. doi: 10.1016/j.chom.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 70.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 71.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 72.Al-Zeer MA, Al-Younes HM, Lauster D, Abu Lubad M, Meyer TF. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG-inducible guanylate binding proteins. Autophagy. 2013;9:50–62. doi: 10.4161/auto.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HW, Macmicking JD, Sibley LD. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 2013;9:e1003320. doi: 10.1371/journal.ppat.1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuadras MA, Feigelstock DA, An S, Greenberg HB. Gene expression pattern in Caco-2 cells following rotavirus infection. J Virol. 2002;76:4467–4482. doi: 10.1128/JVI.76.9.4467-4482.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brodziak F, Meharg C, Blaut M, Loh G. Differences in mucosal gene expression in the colon of two inbred mouse strains after colonization with commensal gut bacteria. PLoS One. 2013;8:e72317. doi: 10.1371/journal.pone.0072317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirpuri J, Brazil JC, Berardinelli AJ, Nasr TR, Cooper K, Schnoor M, Lin PW, Parkos CA, Louis NA. Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. J Immunol. 2010;184:7186–7195. doi: 10.4049/jimmunol.0903116. [DOI] [PMC free article] [PubMed] [Google Scholar]


