Abstract
AIM: To analyze the effect of age-period and birth cohort on gastric cancer mortality, in Brazil and across its five geographic regions, by sex, in the population over 20 years of age, as well as make projections for the period 2010-2029.
METHODS: An ecological study is presented herein, which distributed gastric cancer-related deaths in Brazil and its geographic regions. The effects of age-period and birth cohort were calculated by the Poisson regression model and projections were made with the age-period-cohort model in the statistical program R.
RESULTS: Progressive reduction of mortality rates was observed in the 1980’s, and then higher and lower mortality rates were verified in the 2000’s, for both sexes, in Brazil and for the South, Southeast and Midwest regions. A progressive decrease in mortality rates was observed for the Northeast (both sexes) and North (men only) regions within the period 1995-1999, followed by rising rates.
CONCLUSION: Regional differences were demonstrated in the mortality rates for gastric cancer in Brazil, and the least developed regions of the country will present increases in projected mortality rates.
Keywords: Gastric neoplasms, Brazil, Projections, Mortality
Core tip: Currently there are no detailed predictions in Brazil per geographic region and this study will provide the means for the elaboration of public health actions. This study presents a high citation potential, due to the innovative methodology and to the scientific development of Brazil, which is among the countries with most publications nowadays.
INTRODUCTION
Gastric cancer incidence has been decreasing globally since 1950[1], however its aggressiveness, malignity and, consequently, its prognosis, remain unaltered. Gastric cancer is currently one of the main causes of cancer-related deaths worldwide, and appears as the fourth most frequent cancer in men and sixth in women, being the fifth cancer-related cause of death for both sexes in the world[1].
Regarding risk factors for the development of gastric cancer, a higher incidence is verified in men than in women, in a 2:1 proportion, approximately[2]. Also, researchers remark that the main etiological factors related to the development of gastric cancer are infection by Helicobacter pylori (H. pylori) and diet, as the elevated consumption of salted foods (preserved or smoked), meat, and refined carbohydrates is directly associated with the risk of developing this neoplasm, while a diet based on fiber, vegetables and fresh fruit presents the inverse association[3,4].
Gastric cancer is usually also associated with low socioeconomic conditions[5], an aspect that highlights the importance of analyzing mortality along with the geographic distribution of the population, especially in countries characterized by high socioeconomic inequalities.
Given the epidemiological importance of gastric cancer, essential measures to support public policy actions include the analysis of the age-period and birth cohort on the distribution of mortality and mortality rate projections. These analyses enable the evaluation of the role of risk factors as well as modifications in therapeutics and diagnosis methods in the evolution of mortality rates[6,7].
Brazil presents demographic and socioeconomic heterogeneity across its five geographic regions, which translates to different mortality and morbidity patterns due to non-transmissible chronic diseases. Addressing cancer, both in prevention and attention to patients, requires differentiated responses that should be adapted to each specific region. Monitoring the trends of incidence and mortality rates, as well as risk factor prevalence, is paramount for vigilance actions and planning of prevention and treatment policies. The objectives of this manuscript are to analyze the effect of age-period and birth cohort in gastric cancer mortality, for Brazil and its five geographic regions, by sex, for the population over the age of 20, and make projections for the period 2010-2029.
MATERIALS AND METHODS
Study design and population
An ecological study is presented herein, on the distribution of deaths by gastric cancer, in Brazil and its five geographic regions, per sex. The study included deaths classified as 151 (stomach neoplasm) and C16 (malignant stomach neoplasm) in the 9th and 10th edition of the International Classification of Diseases (ICD), respectively. The study population included Brazilian men and women over 20 years of age. Mortality data were obtained from the Mortality Information System (MIS/DATASUS). Population data were obtained from the Informatics Department of the Unified Health System (DATASUS), based on the population censuses of 1980, 1991, 2000 and 2010. Inter-census projections for populations on July, 1st of the inter-census years were estimated by the Brazilian Institute of Geography and Statistics (BIGS).
Study variables
After extraction, data were corrected, redistributing 50.0% of the registries classified as ill-defined causes (codes 780-789 in ICD-9, and R00-R99 in ICD-10), utilizing the redistribution methodology of the World Health Organization (WHO)[8]. After redistribution of ill-defined registries, deaths with incomplete diagnosis were redistributed proportionally by year and age group[9] (Mello et al[8], 2002). Herein gastric cancer deaths were corrected by two death groups with incomplete diagnosis: incomplete diagnosis for general cancer and incomplete diagnosis for gastric cancer. The following codes were considered as incomplete diagnosis for general cancer: C-77 to C-80 and C-97 in ICD-10; and codes 195, 197 to 199, 238 to 239 in ICD-9. For incomplete diagnosis of gastric cancer, the following codes were considered: 150 in ICD-9 and C-26 in ICD-10.
Once death data were corrected, mortality rates for gastric cancer were calculated per 100000 inhabitants, adjusted by the world population[10]. Age groups, periods and birth cohorts were grouped in five-year intervals, totaling 13 age groups (20-24 years of age to over 80 years of age), six periods (1980-1984 to 2005-2009) and 20 birth cohorts (1895-1899 to 2005-2009).
Data projections were made by sex for the periods 2010-2014, 2015-2019, 2020-2024 and 2025-2029 based on the three observed periods (1995-1999, 2000-2004 and 2005-2009), with results being presented in three age groups (0-39, 40-59 and ≥ 60 years of age) as well as the total result.
Statistical analysis
Age-period and birth cohort (APC) effects were calculated by the Poisson regression method. In this model, effects act in a multiplicative manner on the rates and the logarithm of the expected rate value is a linear function of the effect of age, period and cohort[6,7].
ln(E[rij]) = ln(θij/Nij) = μ + αi + βj + γk
Where is the mortality rate expected for age i and period j, θij is the number of deaths for age i and period j, and Nij expresses the population under risk of death in age i and period j; μ represents the average of the effect, αi represents the effect of group age i, βj represents the effect of period j, and γk is the effect of cohort k.
The greatest limitation with the estimation of APC effect parameters is the linear relationship between the factors age, period and cohort, which hinders the estimation of the complete model. Methodologies have been proposed to address this issue; however, there is still no consensus in literature[6,7]. The APC effect parameters were estimated by estimable functions: deviations, curvatures, and drift, a method proposed by Holford[6,7].
A reference age group was selected (50-54 years of age), along with a reference period (1990-1994), and a reference cohort (which was the median value, as central cohorts are more stable). This manuscript utilized the 1930-1934[6,7] cohort as reference. The adjustment of the model was evaluated by the statistical function deviance, defined as twice the logarithm of the likelihood of the complete model in relation to the logarithm of the likelihood function of the estimated model. The contribution of effects was evaluated by the comparison between the deviance of the model with the specific effect in relation to the complete model (age-period-cohort). The results with P ≤ 0.05 were considered statistically significant.
The association measurements, respective confidence intervals (95%CI), and the adjustments of the models were calculated by statistics program R version 3.2.1, with the Epi 1.1.18 library (R Foundation for Computational Statistics, Viena, Austria http://www.r-project.org).
Projections were made for each period utilizing the age-period-cohort model of the Norpred program, inscribed within the program R. Data were compiled in 5-year blocks and the limit age group considered for analysis was the first with more than 10 cases for the combined period. The results of the projections are presented by the observed and expected deaths for each period, for each Brazilian state. Also, for each period, adjusted mortality rates were calculated based on the standard world population to enable comparison with international data, expressed per 100000 inhabitants per year (ASW/100000 inhab). Variations between the number of cases in the last projected year (2025-2029) and the last observed period (2005-2009) were calculated, considering the proportion of variation that occurred in terms of changes in risk or demographics (size and structure of population). Both components can be different from zero and present a positive or negative direction. Calculation is expressed as follows[11]:
Δ tot = Δ risk + Δ pop= (Nfff - Noff) + (Noff - Nooo)
Where Δ tot is the total change, Δ risk is change in function of risk, Δ pop is change in function of population, Nooo is the number of observed cases, Nfff is the number of projected cases, and Noff is the number of expected cases when mortality rates increase throughout the observed period.
RESULTS
Within the period 1980-2009, there were 314445 deaths registered in Brazil, corresponding to an average standardized mortality rate of 11.71 deaths per 100000 inhabitants. After correction of the number of deaths, there was an increase of 30.8% in the number of deaths (411558), representing an average mortality rate of 15.32 deaths per 100000 inhabitants for gastric cancer, in individuals over 20 years of age. It must be highlighted that, throughout the country, the highest proportion of deaths occurred in the male sex. Also, the highest mortality rates for gastric cancer were observed for the male sex in Brazil and in all geographic regions of the country.
Analysis of the evolution of gastric cancer mortality rates for the last 30 years indicated a progressive decrease in rates in the 1980’s, with higher and lower rates in the 2000’s for both sexes in Brazil and the South, Southeast and Midwest regions. In the Northeast region, for both sexes, and in the North region for the male sex, there was a progressive decrease in mortality rates until 1995-1999, followed by rising rates (Figure 1).
Figure 1.
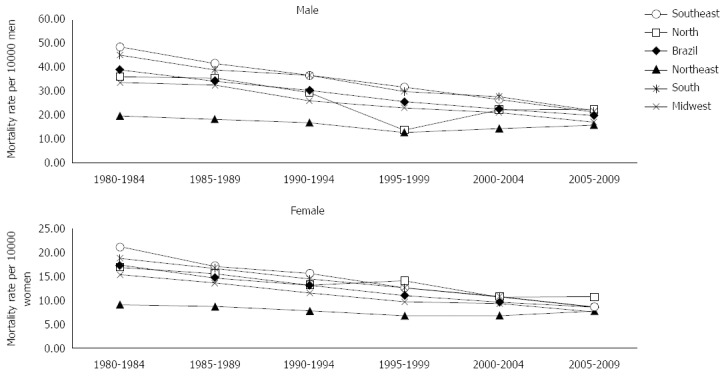
Mortality from gastric cancer according to sex and geographical regions, in Brazil, 1980 to 2009.
In Brazil, as well as in all geographic regions and both sexes, the mortality rates for gastric cancer increased considerably after the age group 55-59 years of age. The highest mortality rates were verified in the age group over 80 years of age. Analysis of mortality according to study periods evidenced decreasing trends for both sexes in the South, Southeast and Midwest regions.
Regarding the mortality rates per age group according to birth cohorts, decreasing trends were observed in the evolution of mortality rates, for both sexes, in Brazil and in the Midwest, Southeast and South regions. This reduction occurred after the 1920 birth cohort, and was observed in all age groups. However, in the North region, increasing mortality rates were identified, for both sexes, in the age groups 75-79 years of age and over 80 years of age, starting from the 1919-1924 birth cohort. A similar profile was verified in the Northeast region, for both sexes, in individuals born after the 1910-1914 cohort for age groups after 65-69 years of age.
Regarding death risk in the analyzed periods, Brazil presented a protection risk (relative risk, RR, under 1) when compared to the reference period 1990-1994. Analysis of death risk per geographic region pointed towards disparities between the Brazilian geographic regions. The South and Southeast regions showed decreases in death risks due to this neoplasm, with a protection effect (RR ≤ 1) especially in the period 2005-2009 for both sexes. The Midwest region presented a very specific profile, as despite RR was above 1 in all periods in relation to the reference period, there was a progressive reduction in death risk due to gastric cancer in the analyzed periods (Figure 1). The North and Northeast regions presented decreases in death risks for the period 1995-1999, but in the following periods there was an increase in risk (RR ≥ 1), for both sexes (Figure 2).
Figure 2.
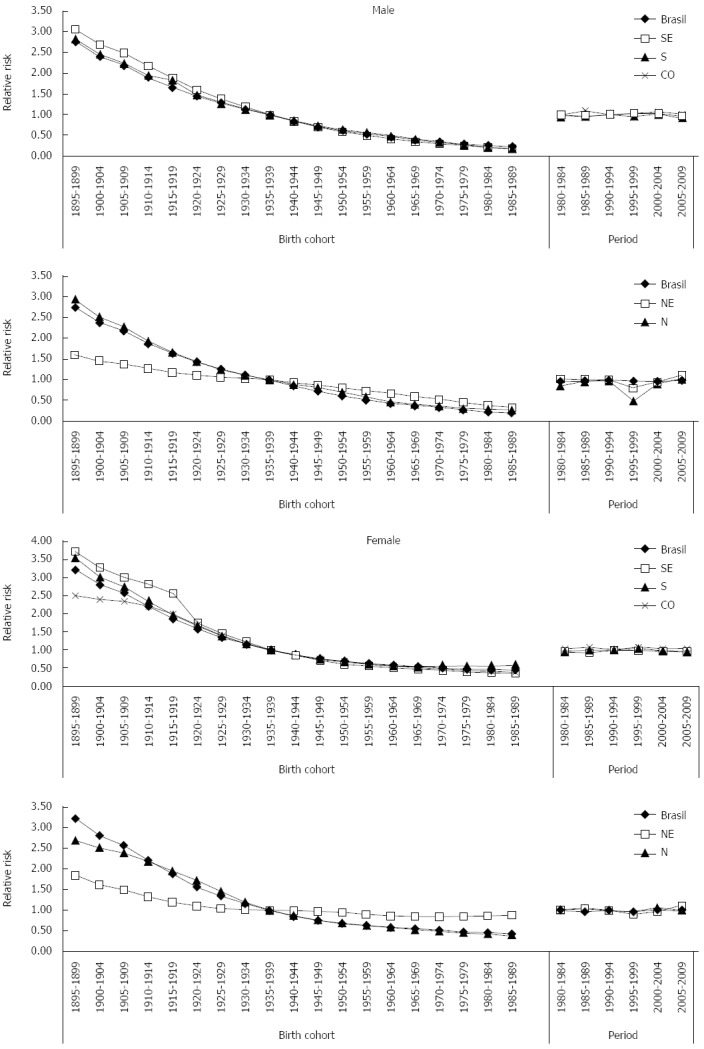
Results of the Age-period-cohort model, adjusted for gastric cancer mortality, according to to sex and geographical regions, in Brazil, 1980 to 2009.
Brazil and the Midwest, North, Southeast and South regions presented reductions in death risks for gastric cancer for both sexes, with a protection effect for birth cohorts after 1940-1944, when compared to the reference cohort. This reduction was more expressive in the South and Southeast regions (Figure 2).
Table 1 shows the deviance changes in the sequential construction of APC models. In the evolution of rates for both sexes, in Brazil and its geographic regions, the model with three factors (APC) presented best fit, except for the Midwest region and female sex, for which the most explanatory model was age-cohort (AC).
Table 1.
Deviance changes in the sequential construction of age-period and birth cohort models
| Models | Brazil |
|||||
| Female |
Male |
|||||
| Resid.DF | Res.Dev | P value | Resid.DF | Res.Dev | P value | |
| Age | 72 | 9069.3 | 72 | 15752.0 | ||
| Age-drift | 71 | 661.6 | < 0.00001 | 71 | 515.1 | < 0.00001 |
| Age-cohort | 67 | 390.5 | < 0.00001 | 67 | 403.0 | < 0.00001 |
| Age-period-cohort | 64 | 372.5 | 0.001 | 64 | 370.6 | < 0.00001 |
| Age-period | 68 | 642.8 | < 0.00001 | 68 | 485.9 | < 0.00001 |
| Age-drift | 71 | 661.6 | 0.001 | 71 | 515.1 | < 0.00001 |
| Midwest | ||||||
| Models | ||||||
| Age | 72 | 399.77 | 72 | 899.91 | ||
| Age-drift | 71 | 88.88 | < 0.00001 | 71 | 116.80 | < 0.00001 |
| Age-cohort | 67 | 72.14 | 0.002 | 67 | 111.94 | 0.302 |
| Age-period-cohort | 64 | 66.18 | 0.200 | 64 | 95.83 | 0.003 |
| Age-period | 68 | 83.03 | 0.002 | 68 | 100.13 | 0.366 |
| Age-drift | 71 | 88.88 | 0.210 | 71 | 116.80 | 0.002 |
| North | ||||||
| Models | ||||||
| Age | 72 | 325.04 | 72 | 1310.75 | ||
| Age-drift | 71 | 140.37 | < 0.00001 | 71 | 779.28 | < 0.00001 |
| Age-cohort | 67 | 129.65 | 0.030 | 67 | 751.44 | < 0.00001 |
| Age-period-cohort | 64 | 110.22 | 0.001 | 64 | 178.57 | 0.003 |
| Age-period | 68 | 121.64 | 0.022 | 68 | 194.68 | < 0.00001 |
| Age-drift | 71 | 140.37 | 0.001 | 71 | 779.28 | < 0.00001 |
| Northeast | ||||||
| Models | ||||||
| Age | 72 | 566.75 | 72 | 1038.52 | ||
| Age-drift | 71 | 405.46 | < 0.00001 | 71 | 656.22 | < 0.00001 |
| Age-cohort | 67 | 269.65 | < 0.00001 | 67 | 597.78 | < 0.00001 |
| Age-period-cohort | 64 | 142.85 | < 0.00001 | 64 | 155.70 | < 0.00001 |
| Age-period | 68 | 230.83 | < 0.00001 | 68 | 208.92 | < 0.00001 |
| Age-drift | 71 | 405.46 | < 0.00001 | 71 | 656.22 | < 0.00001 |
| South | ||||||
| Models | ||||||
| Age | 72 | 1903.85 | 72 | 3090.98 | ||
| Age-drift | 71 | 219.90 | < 0.00001 | 71 | 207.96 | < 0.00001 |
| Age-cohort | 67 | 152.41 | < 0.00001 | 67 | 189.07 | 0.001 |
| Age-period-cohort | 64 | 124.92 | < 0.00001 | 64 | 129.74 | < 0.00001 |
| Age-period | 68 | 200.58 | < 0.00001 | 68 | 143.44 | < 0.00001 |
| Age-drift | 71 | 219.90 | 0.001 | 71 | 207.96 | < 0.00001 |
| Southeast | ||||||
| Models | ||||||
| Age | 72 | 6993.8 | 72 | 11604.0 | ||
| Age-drift | 71 | 447.6 | < 0.00001 | 71 | 399.5 | < 0.00001 |
| Age-cohort | 67 | 278.8 | < 0.00001 | 67 | 290.5 | < 0.00001 |
| Age-period-cohort | 64 | 217.6 | < 0.00001 | 64 | 209.5 | < 0.00001 |
| Age-period | 68 | 389.4 | < 0.00001 | 68 | 284.7 | < 0.00001 |
| Age-drift | 71 | 447.6 | < 0.00001 | 71 | 399.5 | < 0.00001 |
When comparing the evolution of standardized mortality rates for gastric cancer, increases were observed for the male sex in the North and Northeast regions, and in the female sex in the Northeast region. The projections for the Midwest, South and Southeast regions as well as the pooled analysis for Brazil indicated a reduction in mortality rates for the male sex and stability for the female sex. The number of cases per region, the adjusted rates for the observed period, and projections per sex are presented in Table 2.
Table 2.
Observed and predicted number of deaths by age and world age-standardized rates in Brazil
| Males |
Observed |
Predicted |
|||||||
| 1995-1999 | 2000-2004 | 2005-2009 | 2010-2014 | 2015-2019 | 2020-2024 | 2025-2029 | |||
| Males | |||||||||
| North | Age | 0-39 | 99 | 126 | 156 | 200 | 243 | 277 | 296 |
| 40-59 | 700 | 818 | 929 | 1119 | 1247 | 1478 | 1670 | ||
| ≥ 60 | 1287 | 1597 | 2151 | 2792 | 3579 | 4332 | 5195 | ||
| Total | 2086 | 2541 | 3236 | 4110 | 5069 | 6087 | 7160 | ||
| ASW | 13.6 | 13.3 | 14.3 | 15.0 | 15.2 | 14.8 | 14.2 | ||
| Northeast | Age | 0-39 | 310 | 332 | 405 | 388 | 377 | 366 | 365 |
| 40-59 | 1662 | 2041 | 2633 | 3226 | 3745 | 4135 | 4155 | ||
| ≥ 60 | 4086 | 5624 | 7020 | 9070 | 11415 | 14018 | 16827 | ||
| Total | 6058 | 7997 | 10058 | 12685 | 15537 | 18519 | 21347 | ||
| ASW | 7.5 | 8.7 | 9.8 | 10.9 | 11.4 | 11.6 | 11.4 | ||
| Midwest | Age | 0-39 | 94 | 98 | 102 | 102 | 105 | 97 | 92 |
| 40-59 | 684 | 707 | 753 | 779 | 813 | 941 | 1126 | ||
| ≥ 60 | 1404 | 1705 | 1827 | 2039 | 2371 | 2784 | 3362 | ||
| Total | 2182 | 2510 | 2682 | 2920 | 3289 | 3821 | 4580 | ||
| ASW | 13.5 | 12.2 | 10.6 | 9.3 | 8.4 | 7.9 | 7.8 | ||
| Southeast | Age | 0-39 | 801 | 616 | 660 | 563 | 576 | 619 | 524 |
| 40-59 | 6255 | 6404 | 6304 | 5748 | 5629 | 6052 | 7537 | ||
| ≥ 60 | 16611 | 16790 | 14648 | 14316 | 15277 | 17650 | 21598 | ||
| Total | 23667 | 23810 | 21612 | 20627 | 21482 | 24320 | 29658 | ||
| ASW | 18.7 | 15.5 | 12.0 | 9.6 | 8.3 | 7.9 | 8.3 | ||
| South | Age | 0-39 | 250 | 235 | 241 | 188 | 137 | 120 | 108 |
| 40-59 | 2245 | 2356 | 2403 | 2361 | 2410 | 2444 | 2649 | ||
| ≥ 60 | 5515 | 6052 | 6062 | 6392 | 7041 | 8199 | 9745 | ||
| Total | 8010 | 8643 | 8706 | 8941 | 9588 | 10762 | 12502 | ||
| ASW | 18 | 16.2 | 13.6 | 11.5 | 10.3 | 9.7 | 9.5 | ||
| Brazil | Age | 0-39 | 1660 | 1403 | 1567 | 1796 | 2105 | 2502 | 2736 |
| 40-59 | 13382 | 12196 | 12944 | 13909 | 15114 | 16962 | 19978 | ||
| ≥ 60 | 32344 | 31439 | 34145 | 37297 | 41994 | 48255 | 56146 | ||
| Total | 47386 | 45038 | 48656 | 53002 | 59213 | 67718 | 78861 | ||
| ASW | 16.7 | 13.3 | 12.2 | 11.2 | 10.4 | 10.0 | 10.0 | ||
| Females | |||||||||
| North | Age | 0-39 | 110 | 122 | 127 | 159 | 163 | 174 | 180 |
| 40-59 | 324 | 375 | 460 | 557 | 659 | 790 | 909 | ||
| ≥ 60 | 678 | 792 | 1040 | 1327 | 1739 | 2244 | 2870 | ||
| Total | 1112 | 1289 | 1627 | 2044 | 2561 | 3208 | 3959 | ||
| ASW | 7.1 | 6.4 | 6.8 | 6.9 | 7.0 | 7.1 | 7.0 | ||
| Northeast | Age | 0-39 | 274 | 314 | 380 | 460 | 554 | 527 | 524 |
| 40-59 | 943 | 1114 | 1394 | 1773 | 2105 | 2490 | 2754 | ||
| ≥ 60 | 2551 | 3152 | 4318 | 5723 | 7392 | 9309 | 11348 | ||
| Total | 3768 | 4580 | 6092 | 7955 | 10051 | 12326 | 14626 | ||
| ASW | 4 | 4 | 4.7 | 5.2 | 5.6 | 5.8 | 5.9 | ||
| Midwest | Age | 0-39 | 82 | 92 | 96 | 93 | 87 | 82 | 79 |
| 40-59 | 277 | 333 | 382 | 442 | 547 | 634 | 717 | ||
| ≥ 60 | 603 | 771 | 875 | 1028 | 1217 | 1515 | 1947 | ||
| Total | 962 | 1196 | 1353 | 1562 | 1850 | 2232 | 2743 | ||
| ASW | 5.8 | 5.3 | 4.7 | 4.3 | 4.0 | 3.9 | 3.9 | ||
| Southeast | Age | 0-39 | 576 | 652 | 675 | 674 | 685 | 627 | 562 |
| 40-59 | 2413 | 2778 | 3008 | 2983 | 3140 | 3569 | 4168 | ||
| ≥ 60 | 8058 | 9652 | 9619 | 9950 | 10667 | 12047 | 14398 | ||
| Total | 11047 | 13082 | 13302 | 13606 | 14493 | 16243 | 19127 | ||
| ASW | 6.8 | 6.4 | 5.4 | 4.6 | 4.2 | 4.1 | 4.2 | ||
| South | Age | 0-39 | 215 | 217 | 258 | 257 | 280 | 238 | 220 |
| 40-59 | 984 | 949 | 1052 | 1150 | 1283 | 1546 | 1857 | ||
| ≥ 60 | 3005 | 3106 | 3143 | 3268 | 3578 | 4111 | 4939 | ||
| Total | 4204 | 4272 | 4453 | 4675 | 5142 | 5895 | 7016 | ||
| ASW | 7.6 | 6.2 | 5.3 | 4.7 | 4.4 | 4.4 | 4.6 | ||
| Brazil | Age | 0-39 | 1313 | 1376 | 1511 | 1811 | 2107 | 2268 | 2515 |
| 40-59 | 5540 | 5458 | 6274 | 7075 | 8168 | 9879 | 11902 | ||
| ≥ 60 | 16991 | 17450 | 18982 | 20635 | 23267 | 27116 | 32678 | ||
| Total | 23844 | 24284 | 26767 | 29522 | 33542 | 39263 | 47095 | ||
| ASW | 6.9 | 5.6 | 5.2 | 4.8 | 4.7 | 4.7 | 4.9 | ||
ASW: Age-standardized rates.
For Brazilian men, an increase is expected in the number of deaths (30203) when comparing the last observed period with the last projected period, representing a 62% growth, of which 101% is due to population increase and -39% is due to reduction in risk. For women, the expected increase in the number of deaths is 20308, with 99% growth due to changes in population and -23% in reduction of risk. Figure 3 shows the changes in the number of deaths, in function of risk and population increase, when comparing the last observed period and the last projected period for gastric cancer in the Brazilian regions.
Figure 3.
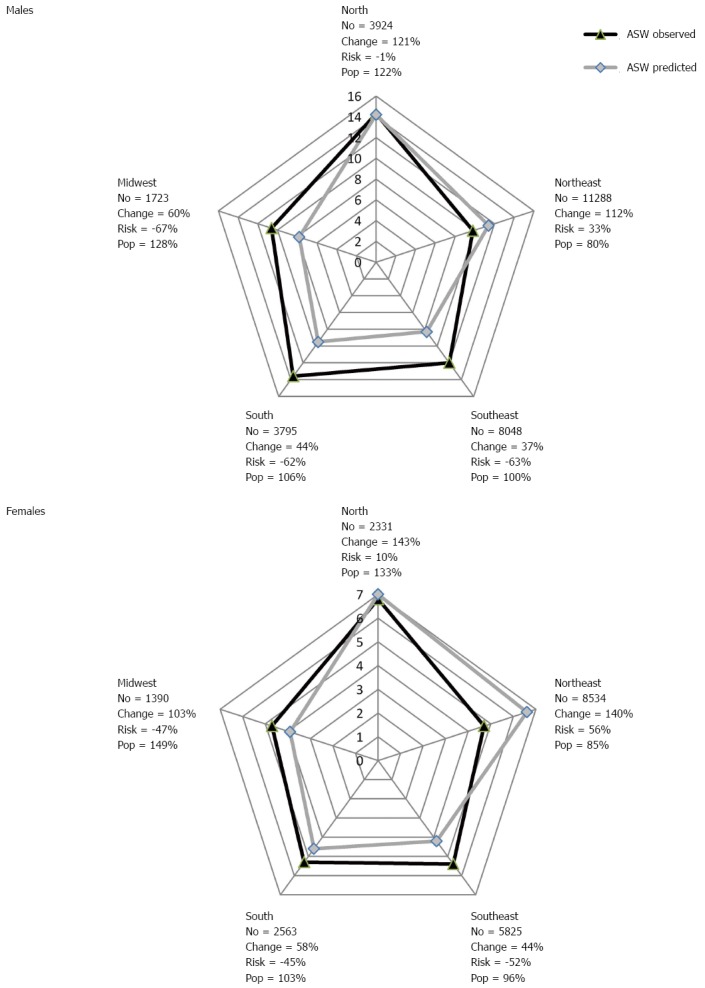
World age-standardized rates, changes in numbers of deaths relative change due to risk and changed population, between 2005-2009 (observed) and 2025–2029 (predicted) of gastric cancer mortality in Brazil. ASW: Age-standardized rates; Pop: Population.
DISCUSSION
This study evidenced disparities in the evolution of mortality rates due to gastric cancer in Brazilian geographic regions, within the analyzed periods. There was an increase in death risk by this neoplasm in the North and Northeast regions starting from the 2000’s, when compared to the reference period.
Gastric cancer, despite the decrease presented in incidence and mortality rates, is still one of the main cancer-related causes of death globally[12]. This disease presents a bad prognosis, with 5-year survival rates under 20%[13].
The mortality rates presented in Brazil, in all regions and for both sexes, are located at intermediate levels, similar to the rates of Venezuela and Argentina, but still superior to those of developed countries such as the United States, Spain, France and Canada. However, Brazilian rates are lower than the mortality rates verified for China, Japan and South Korea[12-20].
This neoplasm can be classified according to its location, in cardia and non-cardia gastric cancer. These diseases present different risk factors and population distributions. While non-cardia gastric cancer presents main risk factors such as H. pylori infection, consumption of salt-preserved food, low consumption of fruit and vegetables, consumption of alcohol and use of tobacco, cardia gastric cancer (esophagogastric junction adenocarcinoma) is a pathology related to obesity, gastroesophageal reflux and Barrett esophagus. Besides, this disease is more common in developed countries and white race individuals, while non-cardia gastric cancer is more incident in Afro-Americans and in developing countries[19,21,22]. There have been important global reductions in incidence and mortality rates for non-cardia gastric cancer; however, an increasing trends have been observed for esophagogastric junction adenocarcinoma, which represents currently 50% of new diagnosed cases of gastric cancer. The prognosis of this type of cancer is worst than non-cardia gastric cancer, as esophagogastric junction adenocarcinoma can disseminate to the abdominal region as well as to the lymph nodes of the thoracic region. Due to the absence of symptoms at the beginning of the disease, esophagogastric junction adenocarcinoma is usually diagnosed in advanced stages[23].
As in other parts of the world, the Brazilian and per region rates for the male sex were approximately twice higher than mortality rates for the female sex[19,21,22]. Some authors believe that this difference is due to the co-existence of other risk factors and that there is unequal exposition according to sex[5,21]. Other authors mention that this reality is related to the fact that women are more aware of health issues than men[18].
Mortality due to this neoplasm in Brazil and in the Midwest, South and Southeast regions presented decreasing trends, along with France, Italy, Spain, Germany, South Korea, Japan and China[1,14]. In the United States and United Kingdom, half of gastric cancers are located in the cardia area, and in the last 30 years, globally, incidence of this disease has increased between 5 and 6 times[22].
A reduction in death risk was evidenced in Brazil and in the South and Southeast regions, starting from the 1990’s, and increased risk was observed in the North and Northeast regions after the 2000’s. Changes in mortality rates of diseases can reflect changes in exposition to risk factors (environmental and/or associated with lifestyle), as well as improvements in diagnosis, treatment, verification and certification of deaths. The differences presented between the North-Northeast regions and other regions of Brazil, especially in the 2000’s, can be a result of the period effect, due to improvement in death registry data and better access to health services. Due to an increase in the possibilities of diagnosing this neoplasm, even if in advanced stages of the disease, these regions presented higher mortality rates in the 2000’s.
Regarding birth cohorts, a progressive reduction in death risk was verified starting in the XX century, in all regions and for both sexes. This result was similar to the results of South Korea, Japan, United States, and Spain[15,17,24,25]. This reality can be explained by the prevalence reduction of H. pylori infection, use of refrigerators (which increased the consumption of fruit and vegetables)[26-29], besides the reduction in the consumption of salt-preserved foods[30] and better sewage collection and treatment, which contributes to reducing the transmission of H. pylori, especially in children and teenagers[19,22]. Reduction of mortality can also be related to new therapeutic strategies implemented within the last decade for the treatment of gastric cancer. These are based on neoadjuvant/adjuvant chemotherapy treatment, which can be associated with radiotherapy[31]. A literature review study with meta-analysis evidenced that patients treated with adjuvant chemotherapy presented better global survival (HR = 0.82; 95%CI: 0.76-0.90; P < 0.001) and disease-free survival (HR = 0.82; 95%CI: 0.75-0.90, P < 0.001) when compared to patients submitted to surgical treatment only[32]. However, there is still no consensus within literature on the best treatment to be utilized, and no studies were found in Brazil that evaluated the implementation of these new therapeutic measures on the survival of patients.
Survival rates are affected by early diagnosis, standardized surgery techniques, nutritional therapy, availability of beds in intensive care units, and the existence of specialized health teams for cancer treatment[13,17]. In this way, the pronounced inequity between Brazilian geographic regions (large urban centers vs the interior) regarding access to cancer diagnosis and treatment services can influence the evolution of mortality rates for this disease. The study by Oliveira et al[33] evidenced sanitary gaps related to breast cancer treatment, especially in North Brazil, with half of health assistance concentrated in few capital cities. The cities of Rio de Janeiro and São Paulo (Southeast Brazil) were responsible for one fifth of attendances, mostly for the resident population. Brazil has a public and universal health system that faces limitations regarding funding and access to diagnosis and treatment services. This reality should not be exclusive to breast cancer treatment and highlights the difficult access to health services that most cancer patients suffer in Brazil[34].
The projections indicated that the least developed regions of Brazil (North and Northeast) will present increments in gastric cancer mortality rates. These regions present structural challenges in oncology services[34-36]. Also in the North and Northeast regions of the country, the increased mortality can be explained by higher difficulty in the access to diagnosis and treatment services. The ratio medical doctor/inhabitant is lower in the North and Northeast regions (in 2010, approximately 1 doctor per 1000 inhabitants) than in the South and Southeast regions (respectively 2.5 and 2.0 doctors per 1000 inhabitants). The distribution of oncology specialists and oncology hospitals is also unbalanced[37]. Also, there is concentration of oncology services in large urban centers, as a consequence of internal migration and development of these areas. The search for appointments with specialists and access to diagnosis and treatment services generates dislocation from rural areas to large urban centers, which delays diagnosis and therefore, entail in worst prognosis for these patients[32].
A limitation to be considered in the study is the impossibility of separating cardia and non-cardia locations for gastric cancer, which seem to present different behaviors according to most recent studies, as the Brazilian Mortality Information System does not differentiate gastric cancers according to histological type[21]. Another important fact to consider is the lack of historical series for risk factor prevalence, which could aid in the analysis of the observed changes. Finally, it must be highlighted that this is an ecological population study, and intra-regional differences can be found in Brazil due to its large continental dimensions, especially regarding the quality of death registries, which the authors attenuated after correcting death data. Limitations related to the APC models must also be mentioned, as these are still under development and there is no consensus in literature on the best methodology to correct the issue of non-identification of the complete model[6,7].
The projections made must be interpreted with caution, as the diagnostic and therapeutic conditions can change in the future, and consequently the mortality trends could be slightly modified. The projection of mortality rates is very important to support the planning of public health measures, as well as to control modifiable risk factors at short and long terms, on the burden of the disease to the population[33]. The main objective of the projections made herein was to provide the required information for health planning purposes, aiming at the selection of vigilance actions for gastric cancer.
COMMENTS
Background
The evolution of gastric cancer mortality has evidenced decreasing trends for Brazil in the South, Southeast and Midwest regions of the country, for both genders. However, after the 1990’s, increasing trends were observed in the Northeast (both genders) and North (in men only) regions. After the 1940-1944 birth cohort, reduction in death risk was observed for this neoplasm, in both genders, for Brazil and its South, Southeast and Midwest regions. The opposite situation was verified for the Northeast region, which presented a progressive death risk for cohorts born from the 1940-1944 cohort. Regarding mortality projections until 2030, increased mortality rates were evidenced for both genders, in the North and Northeast regions of the country.
Research frontiers
The study evidenced a progressive reduction in risk of death from gastric cancer in birth cohorts after the 1940’s, in the most developed regions of Brazil, with the opposite occurring in the poorer geographic regions. These findings are similar to those in studies carried out in South Korea, Denmark, Japan, United States, England, Italy, Switzerland and Spain, and evidences the important role played by basic sanitation and access to health services in gastric cancer mortality.
Innovations and breakthroughs
In most epidemiological studies, trend analysis of mortality rates is based on the evaluation of mortality by age group and death date. The present study analyzed the effect of age-period-birth cohort on the evolution of gastric cancer mortality rates and enabled the evaluation of factors related to age and period as well as whether modifications in mortality trends for this disease were associated to changes in exposition to long-term risk factors (birth cohort effect).
Applications
The results suggest flaws in gastric cancer prevention and control measures in the North and Northeast regions of Brazil. The findings will help plan Brazilian public policies directed to the promotion of primary, secondary and tertiary prevention, to reduce mortality rates for gastric cancer in Brazil and its geographic regions.
Terminology
South region: this Brazilian geographic region presents the best human development indices. This region includes the states of Rio Grande do Sul, Santa Catarina and Paraná. Southeast region: this is the most populous and rich region of the country, and 85% of industry-related jobs are located in this region. This region comprehends São Paulo, Minas Gerais, Rio de Janeiro and Espírito Santo. Midwest region: the main economic activity of this region is farming and livestock. This region presents the second lowest demographic density of the country, and is constituted by Mato Grosso, Mato Grosso do Sul, Goiás and Distrito Federal. Northeast region: this Brazilian region presents one of the worst human development indices, and is characterized by the presence of a semi-arid region within its territory, and is one of the poorer areas of the country. This region comprises nine states: Alagoas, Bahia, Ceará, Maranhão, Paraíba, Pernambuco, Piauí, Rio Grande do Norte and Sergipe. North region: this Brazilian region presents the lowest demographic density, as well as the second worst human development index. This region spans the Amazon Forest, which is an important ecosystem for the world, and includes the states of Amazonas, Amapá, Pará, Tocantins, Roraima and Rondônia. Age effect: evaluates the influence of age on the evolution of mortality rates. Period effect: evaluates the impacts of changes in diagnostic methods, treatment protocols, as well as changes in death certification and improvement of mortality information systems on the evolution of mortality trends. Birth cohort effect: analyzes whether modifications in mortality trends were associated with changes in exposition to long-term risk factors.
Peer-review
In this study the authors report trends in the incidence and mortality of gastric cancer over the last decades and make a prediction on the mortality in the upcoming years. All in all the study is well conducted and has interesting results, needs some minor revisions.
Footnotes
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Brazil
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of the Federal University of Rio Grande do Norte, Brazil.
Informed consent statement: This is an ecological-type observational study that utilized public domain data from the Brazilian government website. All details that might disclose the identity of the subjects were omitted or anonymized.
Conflict-of-interest statement: The authors declare no conflicting interests.
Data sharing statement: Data sharing statement is not applicable, as this is an ecological-type observational study based on public domain data from the Brazilian government website.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 3, 2016
First decision: April 1, 2016
Article in press: June 13, 2016
P- Reviewer: Cho JY, Schmidt T, Velasco I S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302–308. [PubMed] [Google Scholar]
- 4.Matsuzaka M, Fukuda S, Takahashi I, Shimaya S, Oyama T, Yaegaki M, Shimoyama T, Sakamoto J, Nakaji S, Umeda T. The decreasing burden of gastric cancer in Japan. Tohoku J Exp Med. 2007;212:207–219. doi: 10.1620/tjem.212.207. [DOI] [PubMed] [Google Scholar]
- 5.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 7.Robertson C, Gandini S, Boyle P. Age-period-cohort models: a comparative study of available methodologies. J Clin Epidemiol. 1999;52:569–583. doi: 10.1016/s0895-4356(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 8.Mello JMH, Gotlieb SLD, Laurenti RO. Sistema de informações sobre mortalidade: problemas e propostas para o seu enfrentamento I-mortes por causas naturais.Rev. bras. Epidemiol. 2002;5:197–211. [Google Scholar]
- 9.Mathers CD, Bernard C, Iburg KM, Inoue M, Fat DM, Shibuya K, Stein C, Tomijima N, Xu H. Global Burden of Disease in 2002: data sources, methods and results. Global Programme on Evidence for Health Policy Discussion Paper No. 54. Geneva: World Health Organization; 2004. [Google Scholar]
- 10.Doll R, Payne PM, Waterhouse JAH. Cancer incidence in five countries. International Union Against Cancer. Berlin: Springer-Verla; 1966. [Google Scholar]
- 11.Møller B, Fekjaer H, Hakulinen T, Tryggvadóttir L, Storm HH, Talbäck M, Haldorsen T. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev. 2002;11 Suppl 1:S1–96. [PubMed] [Google Scholar]
- 12.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Guimarães RM, Muzi CD. Trend of mortality rates for gastric cancer in Brazil and regions in the period of 30 years (1980-2009) Arq Gastroenterol. 2012;49:184–188. doi: 10.1590/s0004-28032012000300003. [DOI] [PubMed] [Google Scholar]
- 14.Yako-Suketomo H, Katanoda K. Comparison of time trends in stomach cancer mortality (1990-2006) in the world, from the WHO mortality database. Jpn J Clin Oncol. 2009;39:622–623. doi: 10.1093/jjco/hyp107. [DOI] [PubMed] [Google Scholar]
- 15.García-Esquinas E, Pérez-Gomes B, Polláns M, Boldo E, Férnandez-Navarro P, Lopez V. Gastric cancer mortality trends in Spain, 1976-2005, differences by autonomous region and sex. BMC Cancer. 2009;9:1–9. doi: 10.1186/1471-2407-9-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song M, Kang D, Yang JJ, Choi JY, Sung H, Lee Y, Yoon HS, Choi Y, Kong SH, Lee HJ, et al. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric Cancer. 2015;18:580–589. doi: 10.1007/s10120-014-0411-x. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Ioka A, Nakayama T, Tsukuma H, Nakamura T. Comparison of trends in cancer incidence and mortality in Osaka, Japan, using an age-period-cohort model. Asian Pac J Cancer Prev. 2011;12:879–888. [PubMed] [Google Scholar]
- 18.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22:375–387. doi: 10.1007/s10552-010-9707-2. [DOI] [PubMed] [Google Scholar]
- 19.El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50:368–372. doi: 10.1136/gut.50.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Weber A, Graham DY. Age, period, and cohort effects on gastric cancer mortality. Dig Dis Sci. 2015;60:514–523. doi: 10.1007/s10620-014-3359-0. [DOI] [PubMed] [Google Scholar]
- 21.Inoue M, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Daily total physical activity level and total cancer risk in men and women: results from a large-scale population-based cohort study in Japan. Am J Epidemiol. 2008;168:391–403. doi: 10.1093/aje/kwn146. [DOI] [PubMed] [Google Scholar]
- 22.Eslick GD. Helicobacter pylori infection causes gastric cancer? A review of the epidemiological, meta-analytic, and experimental evidence. World J Gastroenterol. 2006;12:2991–2999. doi: 10.3748/wjg.v12.i19.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronnier C, Piessen G, Mariette C. Diagnosis and treatment of non-metastatic esophagogastric junction adenocarcinoma: what are the current options? J Visc Surg. 2012;149:e23–e33. doi: 10.1016/j.jviscsurg.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Seoane-Mato D, Aragonés N, Ferreras E, García-Pérez J, Cervantes-Amat M, Fernández-Navarro P, Pastor-Barriuso R, López-Abente G. Trends in oral cavity, pharyngeal, oesophageal and gastric cancer mortality rates in Spain, 1952-2006: an age-period-cohort analysis. BMC Cancer. 2014;14:254. doi: 10.1186/1471-2407-14-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801–804. doi: 10.3748/wjg.v7.i6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 28.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 29.Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373–2379. doi: 10.1111/j.1572-0241.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 30.Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 31.Miceli R, Tomasello G, Bregni G, Di Bartolomeo M, Pietrantonio F. Adjuvant chemotherapy for gastric cancer: current evidence and future challenges. World J Gastroenterol. 2014;20:4516–4525. doi: 10.3748/wjg.v20.i16.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira EX, Melo EC, Pinheiro RS, Noronha CP, Carvalho MS. [Access to cancer care: mapping hospital admissions and high-complexity outpatient care flows. The case of breast cancer] Cad Saude Publica. 2011;27:317–326. doi: 10.1590/s0102-311x2011000200013. [DOI] [PubMed] [Google Scholar]
- 34.Curado MP, de Souza DL. Cancer burden in Latin America and the Caribbean. Ann Glob Health. 2014;80:370–377. doi: 10.1016/j.aogh.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Barbosa IR, de Souza DL, Bernal MM, do C C Costa Í. Cancer mortality in Brazil: Temporal Trends and Predictions for the Year 2030. Medicine (Baltimore) 2015;94:e746. doi: 10.1097/MD.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souza DL, Jerez-Roig J, Cabral FJ, de Lima JR, Rutalira MK, Costa JA. Colorectal cancer mortality in Brazil: predictions until the year 2025 and cancer control implications. Dis Colon Rectum. 2014;57:1082–1089. doi: 10.1097/DCR.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 37.Demografia Médica no Brasil, v. 2/Coordenação de Mário Scheffer; Equipe de pesquisa: Alex Cassenote, Aureliano Biancarelli. São Paulo: Conselho Federal de Medicina; 2013. [Google Scholar]


